Abstract
In this report, an 8-year-old girl is presented with the complaint of progressive night blindness. The authors have performed eye funduscopy, which showed chorioretinal atrophy in gyrate shape. A high level of plasma ornithine was determined. Urinary excretion of ornithine as well as lysine and cystine were increased. Patient was treated with high dose pyridoxine supplement (500 mg/dl). The night blindness condition of the patient improved. After 1 month of pyridoxine therapy ornithine level of her plasma was successfully reduced and blindness improved.
Background
Gyrate atrophy (GA) of the choroid and retina is a rare, autosomal recessive inherited disease causing progressive chorioretinal degeneration resulting in blindness. The primary defect is due to a deficiency of the enzyme ornithine Δ-aminotransferase, which is responsible for markedly elevated levels of ornithine in plasma and other body fluids.
Case presentation
GA is a rare, autosomal recessive degenerative disease of the choroid and retina and is accompanied by defective ornithine metabolism.1–7 Simell and Takki2 demonstrated the association with hyperornithinemia in 1973. The main metabolic features are those of hyperornithinemia and ornithuria caused by a deficiency of the mitochondrial matrix enzyme, ornithine aminotransferase. Ophthalmological features of the disease are myopia, night blindness, constricted visual field and complicated cataracts.8 9 We report a rare case of GA, in which the patient’s high level of plasma ornithine, massive lysinuria and cystinuria was responsive to therapy with vitamin B6 supplement.
Investigations
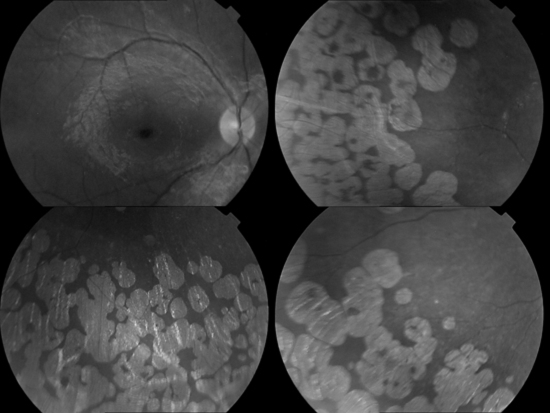
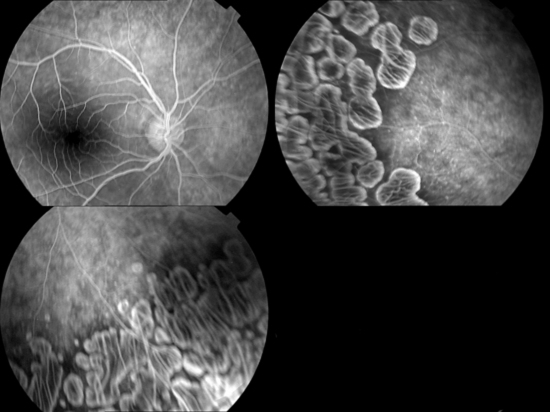
An 8-year-old girl is presented with the complaint of night blindness and visual loss during the past 3 years. She was the first child of healthy, consanguineous parents. She had no siblings. Her family history revealed that her parents were first-degree cousins and her grandfather had gradual visual loss that developed blindness at about age 50. Her refractive error measured −1.00 for the right eye and −0.75 for the left eye. Her best-corrected visual acuity was 3/10 in the right and left eye. Fundus examination of both eyes revealed sharply demarcated areas of choroid and retinal atrophy in gyrate shape and involving the midperiphery with the macular oedema (figure 1). Fundus fluorescein angiogram shows leakage from the left fovea, bilateral hyperfluorescence in macula-temporal area and cystoid macular oedema (figure 2). Amino acid analysis revealed a high plasma ornithine level: 12.616 mg/dl with the normal range being 0.25–1.06 mg/dl. Urinary excretion of ornithine, as well as lysine and cystine increased. The level of those amino acids were as follows: ornithine: 3732.78 µmol/24 h (normal: trace), lysine: 869.309 µmol/24 h (normal: 64–642) and cystine: 82.309 µmol/24 h (normal: 21–28). EEG showed diffuse slow activity. Her intelligence was normal.
Figure 1.
Fundus view of eye shows sharply demarcated areas of choroid and retinal atrophy in gyrate shape and involving the midperiphery with the macular oedema.
Figure 2.
Fundus fluorescein angiogram shows leakage from the left fovea, bilateral hyperfluorescence in macula-temporal area and cystoid macular oedema.
Outcome and follow-up
Fundus examination and laboratory findings of our patient confirmed the diagnosis of GA of the choroid and retina with hyperornithinemia, cystinuria and lysinuria. A 2-week trial of pyridoxine therapy (300–600 mg/day) is recommended for all newly diagnosed patients to determine their responsiveness. Our patient was also treated with vitamin B6 at a dose of 500 mg/day.
After 1 month, this vitamin supplementation successfully reduced her plasma ornithine level to nearly normal level (4.950 mg/dl) and urine ornithine level reduced by more than 50%–1226 µmol/24 h. It was difficult to apply a natural low-protein diet to our patient. To delay the progression of the chorioretinal changes, UCD-2 (low arginine diet) was added to her normal diet.
Since arginine is the precursor of ornithine, providing a low arginine content special diet (UCD-2) makes sense. To increase discharge from kidneys, l-lysine and α-aminoisobutyric acid could be recommended. Also to remedy reduced plasma levels, additional proline and creatinine could be provided as support.
Discussion
GA of the choroid and retina is a rare degenerative disease, characterised biochemically by a marked increase in blood ornithine levels, due to deficiency of ornithine Δ-aminotransferase.1–7 Chorioretinal dystrophy that begins in paediatric population (age range 0–16) often leads to blindness during the fourth to seventh decade of life.8 9 The mechanism of GA remains unknown; however, the adverse effects of creatinine or pyroline-5-carboxylate deficiency on retinal function are thought to be a causative factor.10
The administration of pharmacologic doses of vitamin B6 in a disorder caused by decreased activity of a B6-dependent enzyme is an established procedure.10 11 The disease is more prevalent in the Finnish population.12 In the past, well over 150 cases of GA have been documented. Nearly 1/3 of these cases were from Finland and only seven of them (less than 5%) have been reported to be responsive to vitamin B6 supplementation.13 Our patient is a rare case of GA, such that her plasma ornithine level decreased from 12.616 mg/dl to 4.950 mg/dl (nearly normal level: 0.25–1.06 mg/dl) after administration of 500–mg/day vitamin B6 as dietary supplementation. The night blindness condition of the patient improved. Although several therapeutic regimens have been proposed; the reduction in ornithine accumulation obtained by reducing the intake of its precursor arginine (semi-synthetic low arginine diet) is the one most practiced.14 15
We suspect that GA might be the reason for blindness in grandfather as well, because, it was reported that his blindness was started as ‘night blindness’, when he was in early age, similar to our current patient. Grandfather was living in a rural area village and passed away long before our patient reported to us, no further investigation was possible to make about grandfather. Accordingly, we suspect of genetic link in GA, due to the occurrence of similar illness in the grandfather and granddaughter.
Learning points.
-
▶
Child patients applying to doctor with night blindness complaint, could also be suspected for GA.
-
▶
When GA is observed, next step should be suspicion of ornithine metabolism disorder.
-
▶
To prevent blindness in adult population (age 40 and above), high dose vitamin B6 treatment should be tried in all cases.
-
▶
To prevent ornithine build-up in the metabolism, a diet with low arginine level should be recommended.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Kaiser-Kupfer MI, Valle D, Del Valle LA. A specific enzyme defect in gyrate atrophy. Am J Ophthalmol 1978;85:200–4 [DOI] [PubMed] [Google Scholar]
- 2.Simell O, Takki K. Raised plasma-ornithine and gyrate atrophy of the choroid and retina. Lancet 1973;1:1031–3 [DOI] [PubMed] [Google Scholar]
- 3.Takki K, Simell O. Genetic aspects in gyrate atrophy of the choroid and retina with hyperornithinaemia. Br J Ophthalmol 1974;58:907–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang T, Lawler AM, Steel G, et al. Mice lacking ornithine-delta-aminotransferase have paradoxical neonatal hypoornithinaemia and retinal degeneration. Nat Genet 1995;11:185–90 [DOI] [PubMed] [Google Scholar]
- 5.Vannas-Sulonen K, Sipilä I, Vannas A, et al. Gyrate atrophy of the choroid and retina. A five-year follow-up of creatine supplementation. Ophthalmology 1985;92:1719–27 [DOI] [PubMed] [Google Scholar]
- 6.Kellner U, Weleber RG, Kennaway NG, et al. Gyrate atrophy-like phenotype with normal plasma ornithine. Retina (Philadelphia, Pa) 1997;17:403–13 [DOI] [PubMed] [Google Scholar]
- 7.Kaiser-Kupfer MI, Kuwabara T, Askanas V, et al. Systemic manifestations of gyrate atrophy of the choroid and retina. Ophthalmology 1981;88:302–6 [DOI] [PubMed] [Google Scholar]
- 8.Kaiser-Kupfer M, Kuwabara T, Uga S, et al. Cataract in gyrate atrophy: clinical and morphologic studies. Invest Ophthalmol Vis Sci 1983;24:432–6 [PubMed] [Google Scholar]
- 9.Kaiser-Kupfer MI, Ludwig IH, de Monasterio FM, et al. Gyrate atrophy of the choroid and retina. Early findings. Ophthalmology 1985;92:394–401 [DOI] [PubMed] [Google Scholar]
- 10.Weleber RG, Kennaway NG. Clinical trial of vitamin B6 for gyrate atrophy of the choroid and retina. Ophthalmology 1981;88:316–24 [DOI] [PubMed] [Google Scholar]
- 11.Mudd SH. Pyridoxine-responsive genetic disease. Fed Proc 1971;30:970–6 [PubMed] [Google Scholar]
- 12.Sipilä I, Valle D, Mitchell GA, et al. [Hyperornithinemia and gyrate atrophy: ornithine aminotransferase gene error causing a Finnish disease]. Duodecim 1994;110:681–6 [PubMed] [Google Scholar]
- 13.Valle D, Simell O. The hyperornithinemias. Scriver CR, Beaudet AL, Sly WS, The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill; 2001:1875–95 [Google Scholar]
- 14.Kaiser-Kupfer MI, Caruso RC, Valle D, et al. Use of an arginine-restricted diet to slow progression of visual loss in patients with gyrate atrophy. Arch Ophthalmol 2004;122:982–4 [DOI] [PubMed] [Google Scholar]
- 15.Santinelli R, Costagliola C, Tolone C, et al. Low-protein diet and progression of retinal degeneration in gyrate atrophy of the choroid and retina: a twenty-six-year follow-up. J Inherit Metab Dis 2004;27:187–96 [DOI] [PubMed] [Google Scholar]