Abstract
Enterocutaneous fistulae (ECF) are challenging problem commonly encountered by surgeons and cause significant complications. They not only prolong hospital stay, but also restrict a patient’s activities of daily living. The authors report a case of successful treatment of multiple intractable ECF in a patient with decreased coagulation factor XIII (FXIII) activity using intravenous FXIII treatment. A 74-year-old man with multiple ECF was referred. Although no factors involved in impaired wound were initially identified, he developed ECF after multiple surgical interventions with repeated wound dehiscence. FXIII activity was below the normal value. A definitive operation was performed and FXIII was administrated for 5 days postoperatively. There was no fistula recurrence and no other complications. Preoperative assessment of factors related to wound repair, such as FXIII activity, may be important for patients with wound dehiscence and subsequent fistula development and should be considered in patients who are resistant to standard treatments.
Background
Enterocutaneous fistulae (ECF) are abnormal communications between the gastrointestinal tract and the skin. Most ECF form after abdominal surgery as a result of bowel injury, inadvertent enterotomy or anastomotic leakage.1 2 Postoperative ECF cause life-threatening complications such as electrolyte and fluid imbalance, sepsis and malnutrition.3 Despite advances in conservative management, the morbidity and mortality after the formation of ECF remain high (mortality ranges from 4.4% to 19%).1 2 4 5 Definitive surgery remains the mainstay of ECF treatment in patients with non-healing ECF after intensive medical treatment. However, the type of operative procedure, timing of surgery and perioperative patient management remain controversial.2 Coagulation factor XIII (FXIII) is a transglutaminase that is the last step in the coagulation cascade and it plays an important role in wound healing by stabilising the fibrin clot. Decreased FXIII activity has been observed in patients with surgical stress, inflammation and infection.6 7 Patients with decreased FXIII tend to have an increased risk of postoperative bleeding and delayed wound healing due to the instability of fibrin clot.8 It has been also reported that FXIII reduces vascular permeability and is associated with a pro-angiogenic state.6 9 Fujita et al reported that FXIII activity in patients with anastomotic leaks and non-healing fistulae after gastrointestinal surgery were below the normal. They concluded that systemic administration of FXIII might accelerate wound healing by increasing circulating growth factors, such as epidermal growth factor and transforming growth factor β, which play a role in proliferation of fibroblasts.6 10 Freeze-dried blood coagulation FXIII, derived from human plasma, is available for patients with decreased FXIII activity to potentially support and promote wound healing.
In this report, we describe a case of repeated wound dehiscence and subsequent development of multiple ECF in the context of a decrease of FXIII activity that was successfully treated with definitive surgery, nutritional support, management of sepsis and additional supportive administration of FXIII.
Case presentation
A 74-year-old man with multiple ECF was admitted to our hospital for nutritional support and definitive surgical treatment. He had previously undergone a defunctioning transverse colostomy at a regional general hospital for an obstructing sigmoid colon cancer. After decompression of the intestine, an extended left hemicolectomy, stoma closure and resection of the small intestine were performed. On the fourth postoperative day, the intestinal anastomosis leaked and generalised peritonitis occurred. The patient underwent emergency laparotomy and a stoma formed. Wound dehiscence occurred after this surgery and was followed by the development of multiple ECFs (figure 1A).
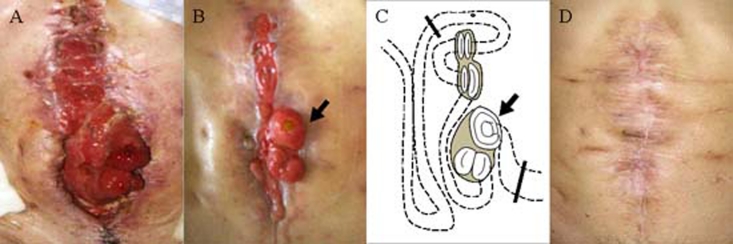
Figure 1.
(A) Wound dehiscence after the third operation; (B) wound after VAC treatment. Skin surrounding fistulae appears normal. (C) Scheme of connection between enterocutaneous fistulae and small intestine. Line of surgical resection (bold line). High-output jejunostomy (black arrow); (D) wound after definitive surgery.
Investigations
The patient did not have any underlying comorbidities such as anaemia, malnutrition, diabetes mellitus, liver failure, renal failure and was not receiving immunosuppressants, steroids or chemo-radiation. In addition, there were no abnormalities in coagulation parameters such as prothrombin time and activated partial thromboplastin time. We suspected reduced FXIII activity and laboratory measurement showed that FXIII activity was 68% of predicted.
Contrast radiographic imaging using gastrografin showed that there was 80 cm of healthy jejunum distal to the ligament of Treitz, the jejunum then communicated with the fistula complex and atrophic small intestine was identified distal to the ECF.
Definitive operation was planned as spontaneous fistula closure was not expected and the high-output jejunostomy, producing more than 1000 ml of fluid per day, presented difficulty in maintaining the patient’s electrolyte balance and nutritional status using only total parenteral nutrition and intravenous fluids.
Treatment
The patient’s nutrition was initially supported by the total parenteral route to reduce fistula output until definitive surgery. In addition, intravenous lansoprazole was administrated; however, fistula output remained at approximately 1000 ml daily. Octreotide was not administrated as the drug is not licensed for this indication in Japan.
The ECF were further managed by a vacuum-assisted closure device and meticulous skin care to promote wound healing (figure 1B).
Resection of multiple ECF en bloc leaving approximately 2 m of small intestine was performed. Intestinal continuity was restored using the Gambee single layer through-and-through technique (figure 1C).
Freeze-dried blood coagulation FXIII, derived from human plasma (1500 U/day), was administered for 5 days beginning on the first postoperative day.
Outcome and follow-up
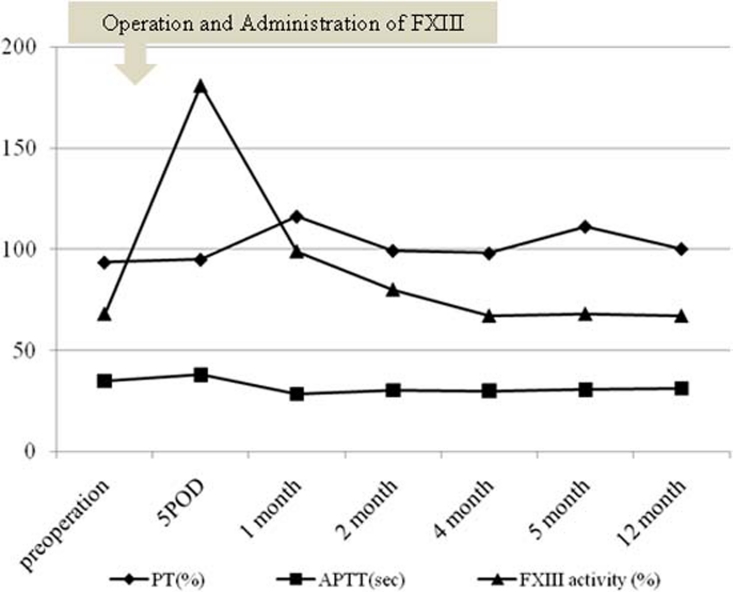
The patient’s postoperative course was uneventful. There was no anastomotic leak or wound dehiscence and no recurrence of an ECF (figure 1D). However, although FXIII activity was significantly elevated immediately after the administration of the freeze-dried factor FXIII, FXIII activity had decreased to 67% of normal by 4 months after the FXIII administration (figure 2). There was no evidence of fistula recurrence after 36 months of follow-up.
Figure 2.
Changes in prothrombin time (PT), activated partial thromboplastin time (APTT) and coagulation factor XIII (FXIII) activity. POD, postoperative day.
Discussion
ECF are a challenging problem often encountered by surgeons.1 2 4 11 ECF frequently occur in the context of previous surgical procedures2 12 13 and often cause sepsis, malnutrition, electrolyte abnormalities and fluid deficits. They also result in prolonged hospital admissions and restrict activities of daily living. Spontaneous fistula closure rates are low and range from 20–38%.2 11–15
Factors that significantly preclude spontaneous closure include jejunal fistula, multiple fistulas, sepsis, high fistula output and electrolyte deficits at diagnosis or referral.1 11 14 16 In addition, there are reports that high output, jejunal site and multiple ECF increase the mortality associated with fistula complications.1 2 16 Our case involved a jejunal site, multiple fistulas and high output on admission; therefore, we considered that spontaneous closure was unlikely and a surgical approach was undertaken.
The successful closure rate of ECFs treated by definitive operation is reported to be greater than 85%.1 2 12–15 However, the decision when to operate and the choice of surgical procedure are important and difficult as they may alter the incidence of further complications and the risk of fistula recurrence. Surgical closure should be performed when spontaneous closure using conservative treatments is no longer thought likely.
Surgical closure should be delayed for at least 3 months, because major abdominal surgery stimulates the formation of dense adhesions and this reaction is maximal from the third to tenth postoperative week. Definitive surgery during this time is more likely to be complicated by fistula recurrence.4 14 15 17 In addition, during this period sepsis should be aggressively managed and nutrition optimised. However, the interval between the previous and definitive surgery should not be extended unnecessarily particularly because the patient’s general condition, including nutritional status, is difficult to maintain over a prolonged period as excessive delay can cause the fistula recurrence rate to increase.15 Therefore, we emphasise that conservative treatments such as nutritional support and wound care, followed by definitive surgery, are absolutely essential.
Preoperative FXIII activity was below the normal value in our patient. FXIII deficiency is usually detected as a bleeding diathesis. However, the slight decrease in FXIII activity as seen in our patient does not cause symptoms under normal circumstances. The apparent low incidence of this condition may be a result of the fact that conventional coagulation testing does not identify decreased FXIII activity. FXIII levels are reduced by some conditions such as surgical stress, inflammation, infection and advanced cancer.6–8 The case of decreased FXIII activity in our case was unclear although we strongly suspected that the decreased FXIII activity had a role in the wound dehiscence and consequent fistula formation. We suggest that an asymptomatic decrease in FXIII activity is an additional risk factor for surgery and the development of unexpected complications.
In conclusion, a multidisciplinary approach is critical for the definitive management of ECF. Preoperative assessment of factors related to wound repair such as FXIII activity may also be important for patients with repeated wound dehiscence and subsequent development of ECF. Perioperative administration of FXIII may facilitate healing after surgical treatment of intractable complex ECF.
Learning points.
-
▶
A multidisciplinary approach is necessary for definitive surgery of ECF.
-
▶
Preoperative assessment of FXIII activity in patients with repeated wound dehiscence and subsequent development of ECF may be helpful.
-
▶
Supportive administration of FXIII may facilitate successful closure of intractable and multiple ECF in patients with decreased FXIII activity.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Martinez JL, Luque-de-Leon E, Mier J, et al. Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes. World J Surg 2007;32:436–43 [DOI] [PubMed] [Google Scholar]
- 2.Draus JM, Jr, Huss SA, Harty NJ, et al. Enterocutaneous fistula: are treatments improving? Surgery 2006;140:570–6 [DOI] [PubMed] [Google Scholar]
- 3.Lloyd DA, Gabe SM, Windsor AC. Nutrition and management of enterocutaneous fistula. Br J Surg 2006;93:1045–55 [DOI] [PubMed] [Google Scholar]
- 4.Evenson AR, Fischer JE. Current management of enterocutaneous fistula. J Gastrointest Surg 2006;10:455–64 [DOI] [PubMed] [Google Scholar]
- 5.Altomare DF, Serio G, Pannarale OC, et al. Prediction of mortality by logistic regression analysis in patients with postoperative enterocutaneous fistulae. Br J Surg 1990;77:450–3 [DOI] [PubMed] [Google Scholar]
- 6.Inbal A, Dardik R. Role of coagulation factor XIII (FXIII) in angiogenesis and tissue repair. Pathophysiol Haemost Thromb 2006;35:162–5 [DOI] [PubMed] [Google Scholar]
- 7.Born P, Lippl F, Ulm K, et al. Reduced levels of coagulation factor XIII in patients with advanced tumor disease. Hepatogastroenterology 2000;47:194–8 [PubMed] [Google Scholar]
- 8.Ichinose A. Physiopathology and regulation of factor XIII. Thromb Haemost 2001;86:57–65 [PubMed] [Google Scholar]
- 9.Dardik R, Loscalzo J, Inbal A. Factor XIII (FXIII) and angiogenesis. J Thromb Haemost 2006;4:19–25 [DOI] [PubMed] [Google Scholar]
- 10.Fujita I, Kiyama T, Mizutani T, et al. Factor XIII therapy of anastomotic leak, and circulating growth factors. J Nippon Med Sch 2006;73:18–23 [DOI] [PubMed] [Google Scholar]
- 11.Haffejee AA. Surgical management of high output enterocutaneous fistulae: a 24-year experience. Curr Opin Clin Nutr Metab Care 2004;7:309–16 [DOI] [PubMed] [Google Scholar]
- 12.Hollington P, Mawdsley J, Lim W, et al. An 11-year experience of enterocutaneous fistula. Br J Surg 2004;91:1646–51 [DOI] [PubMed] [Google Scholar]
- 13.Li J, Ren J, Zhu W, et al. Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J 2003;116:171–5 [PubMed] [Google Scholar]
- 14.Lynch AC, Delaney CP, Senagore AJ, et al. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg 2004;240:825–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner M, Clayton JL, Tillou A, et al. Risk factors for recurrence after repair of enterocutaneous fistula. Arch Surg 2009;144:500–5 [DOI] [PubMed] [Google Scholar]
- 16.Mulholland MW, Delaney JP. Proximal diverting jejunostomy for compromised small bowel. Surgery 1983;93:443–7 [PubMed] [Google Scholar]
- 17.Hill GL. Operative strategy in the treatment of enterocutaneous fistulas. World J Surg 1983;7:495–501 [DOI] [PubMed] [Google Scholar]