Abstract
A 28-year-old man treated with the antitumour necrosis factor α (TNFα) monoclonal antibody infliximab for Crohn's disease developed pulmonary tuberculosis (TB), despite testing negative for latent TB prior to treatment. On starting anti-TB treatment and withdrawal of the anti-TNFα therapy, he deteriorated both clinically and radiologically. He was diagnosed with a flare of Crohn's disease, and immune reconstitution inflammatory syndrome (IRIS) in his right upper lobe and mediastinal lymph nodes, and commenced on oral prednisolone. Anti-TNFα therapy was re-introduced, and prednisolone weaned, following 4 months of anti-TB treatment without complication. He made a full recovery from TB, although his Crohn's symptoms continue to be troublesome. There has been no reactivation of TB to date, after 2 years follow-up.
Background
This case highlights that a negative screen for latent tuberculosis (TB) may be falsely reassuring in patients subsequently receiving antitumour necrosis factor α (anti-TNFα) therapy, who can still develop de novo infection, and therefore increased vigilance is prudent. In the event that a patient does develop TB, treatment usually involves standard anti-tuberculous therapy in addition to withdrawal of anti-TNFα therapy. In this case, this may have contributed to the development of immune reconstitution inflammatory syndrome (IRIS), and certainly contributed to a flare in his Crohn's disease. Early reintroduction of anti-TNFα therapy was done safely and effectively, and there is increasing evidence that it may even be beneficial in the management of IRIS associated with TB.
Case presentation
A 28-year-old Caucasian man with a 2-year history of peri-oral and peri-anal Crohn's disease well controlled with azathioprine and anti-TNFα (infliximab) presented in June 2008 with a dry cough, a short duration of feverish illness and 5 kg weight loss. Examination was unremarkable. He did not have any other co-morbidities and was not on any other medications. There was no history of known TB contacts. He lived in an area of low TB prevalence, had not travelled to an area of high TB prevalence and had received BCG vaccination as an adolescent. The original diagnosis of Crohn's disease was based on typical clinical features and biopsy appearance, absence of organisms on Ziehl–Neelsen staining and negative stool cultures. Symptoms had been well controlled on immunosuppression as noted above. Of note, standard screening for active and latent TB, with chest x-ray (CXR) and interferon γ release assay (IGRA; T-spot.TB) was negative prior to initiation of infliximab, April 2008. There had been no response to conventional antibiotics.
Investigations
His inflammatory markers were modestly elevated (CRP 64, WCC normal). CXR showed mediastinal widening and CT thorax (figure 1A) confirmed mediastinal adenopathy. Endobronchial ultrasound guided fine needle aspiration (EBUS-FNA) revealed acid and alcohol fast bacilli (AAFB) within the right paratracheal lymph node, and extended culture confirmed fully sensitive Mycobacterium tuberculosis.
Figure 1.
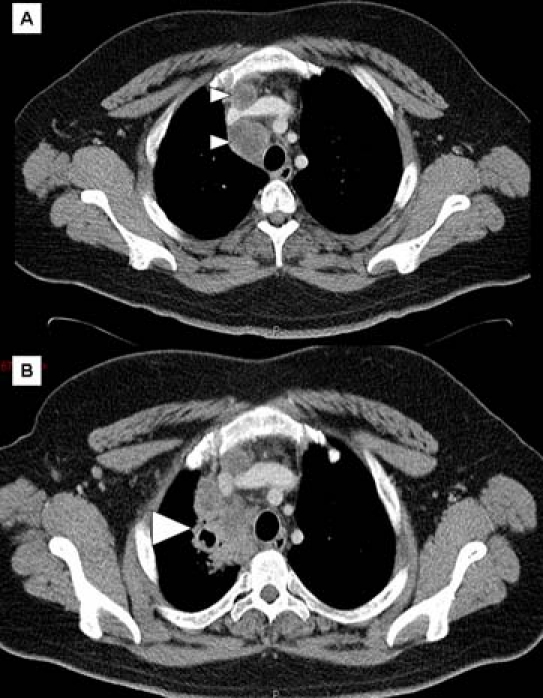
CT scans of chest. (A) At onset of symptoms showing enlarged mediastinal lymph nodes (small arrow heads). (B) After commencing antituberculous therapy and stopping anti-TNFα therapy, showing new consolidation and cavitation (large arrow head).
Treatment
Standard quadruple anti-TB chemotherapy was commenced, and all immunosuppression, including infliximab, withheld.
Outcome and follow-up
Anti-TB treatment was tolerated well, despite increased gastrointestinal (GI) symptoms in the form of peri-oral ulceration. However, he re-presented in September 2008 with supraclavicular lymphadenopathy. Neck ultrasound revealed a lymph node abscess, and aspirate showed AAFB (extended culture not requested). CT thorax revealed marked deterioration with significant enlargement of the mediastinal adenopathy and new right upper lobe cavitation/consolidation (see figure 1B). A diagnosis of IRIS was made. Prednisolone 40 mg was commenced and infliximab restarted in October 2008, on completion of 4 months of anti-TB therapy. He completed 9 months of anti-TB treatment with full clinical and radiological resolution. Despite ongoing symptomatic Crohn's disease, ongoing treatment with anti-TNFα therapy and intermittent oral steroids, he has had no further active TB.
Discussion
Differentiating Crohn's disease from intestinal TB can be difficult, particularly in endemic TB areas, as the presentation can be similar. However, in this case the diagnosis of Crohn's disease was supported by the chronicity of the predominantly peri-oral and peri-anal symptoms, the biopsy appearances, and the fact that the GI symptoms improved with immunosuppression, and worsened during anti-TB treatment. In addition, the patient lives in an area of low TB prevalence, but high prevalence of Crohn's disease.1 2
Novel biologic therapies against inflammatory mediators have proved effective treatment for inflammatory skin, joint and bowel diseases not fully controlled with standard immunosuppression. Among the most successful are those targeting TNFα, including monoclonal antibodies against TNFα itself (infliximab and adalimumab) and a decoy receptor (etanercept). As with other immunosuppressants, there is increased risk of opportunistic infection. In particular, patients taking anti-TNFα therapies have increased risk of re-activation of latent TB, usually within 3 months of treatment, and de novo active TB infection,3 4 thought in part to be due to the crucial role of TNFα in regulating TB granuloma integrity. The risk with infliximab is perhaps higher, and extrapulmonary TB is seen more commonly. The British Thoracic Society (BTS) recommends screening for active and latent TB prior to all anti-TNFα therapy.5 Our patient underwent screening with a focused history and examination and a normal CXR. He did not undergo TB skin testing (TST) as he was already on immunosuppressive therapy, which can lead to false negatives.
The recently developed IGRAs (T-spot. TB; Oxford Immunotec, Abingdon, UK; QuantiFERON-TB-Gold; Cellestis, Valencia, California, USA) may offer improved screening efficacy in immunosuppressed patients. They have equal, if not higher, sensitivity and specificity to TST but are less susceptible to immunosuppression, and are not influenced by previous BCG vaccination.6 There is increasing evidence that all patients on immunosuppressive therapy offered anti-TNFα should undergo IGRA for detection of latent TB, and this is now recommended practice.7 Our patient had a negative T-spot test and developed TB after only 3 months treatment, suggesting either a false negative and missed latent TB, or de novo infection, which cannot be prevented by screening. In the event that active TB develops while on anti-TNFα therapy there is a lack of data, and therefore conflicting guidance, regarding stopping treatment. The BTS recommend continuing treatment if clinically indicated, whereas the American College of Rheumatology recommends discontinuation.8 In our patient, the anti-TNFα was stopped, and he subsequently deteriorated, with a flare of Crohn's disease, and worsening TB on standard treatment.
Paradoxical clinical and radiological worsening of TB is well recognised following the introduction of anti-TB therapy, particularly in extrapulmonary disease. It is thought to arise from increased T cell response to TB antigens. This phenomenon is commonly seen in HIV with TB co-infection following the introduction of highly active antiretroviral therapy (HAART) and recovery of the CD4 T cell population. It is known as IRIS. As in our patient, an IRIS-like phenomenon has been recognised in patients with TB who have their anti-TNFα therapy withheld.9 As with paradoxical worsening of extrapulmonary TB and IRIS in HIV, steroid suppression of the overexuberant immune response has been used safely, although good supporting evidence is lacking. Controversy also exists over the timing of the reintroduction of anti-TNFα therapy. Several case reports have shown early reintroduction to be safe,10 11 and may even improve resolution of the inflammatory response,12 although discontinuation should be considered in life-threatening IRIS. In our case, anti-TNFα therapy was successfully reintroduced at 4 months with improvement in both Crohn's symptoms and resolution of TB.
Our case demonstrates observation for active TB infection in patients receiving anti-TNFα therapy is vital, even if screening for latent TB is negative; false negatives and de novo infection can arise. In addition, if anti-TNFα therapy is withdrawn during treatment for active TB, IRIS is a potential complication. Early reinstitution of anti-TNFα therapy during treatment for TB is likely to be safe, and in future continuing anti-TNFα therapy may be preferred to reduce the likelihood of IRIS, and maintain control of the underlying disease.
Learning points.
-
▶
Patients who are to receive anti-TNFα therapy a negative screen for latent TB may be falsely negative if they are already on immunosuppression, and they may still develop de novo infection. Therefore, increased vigilance is prudent.
-
▶
Patients on anti-TNFα therapy should be advised about the risks of travelling to areas with high TB prevalence.
-
▶
Those patients who do develop TB, and in whom anti-TNFα therapy is withdrawn, may experience a flare in the underlying disease.
-
▶
They may also be at increased risk of IRIS, with both clinical and radiological deterioration during the initial treatment phase.
-
▶
Although conventional treatment of IRIS with glucocorticoids is acceptable, early reintroduction of anti-TNFα therapy can be done safely, and may even be of benefit.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Almadi MA, Ghosh S, Aljebreen AM. Differentiating intestinal tuberculosis from Crohn's disease: a diagnostic challenge. Am J Gastroenterol 2009;104:1003–12 [DOI] [PubMed] [Google Scholar]
- 2.Makharia GK, Srivastava S, Das P, et al. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol 2010;105:642–51 [DOI] [PubMed] [Google Scholar]
- 3.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001;345:1098–104 [DOI] [PubMed] [Google Scholar]
- 4.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35–45 [DOI] [PubMed] [Google Scholar]
- 5.British Thoracic Society Standards of Care Committee BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-{alpha} treatment. Thorax 2005;60(10):800–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diel R, Loddenkemper R, Meywald-Walter K, et al. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube assay, and T-Spot.TB test in contact investigations for tuberculosis. Chest 2009;135:1010–18 [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Clinical Excellence Tuberculosis. Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. London, UK: Royal College of Physicians; 2006 [PubMed] [Google Scholar]
- 8.Pablos JL. Aspirin antiplatelet therapy and nonsteroidal antiinflammatory drugs: comment on the 2002 update of the American College of Rheumatology Guidelines for the Management of Rheumatoid Arthritis. Arthritis Rheum 2002;46:328–46 [DOI] [PubMed] [Google Scholar]
- 9.Belknap R, Reves R, Burman W. Immune reconstitution to Mycobacterium tuberculosis after discontinuing infliximab. Int J Tuberc Lung Dis 2005;9:1057–8 [PubMed] [Google Scholar]
- 10.Matsumoto T, Tanaka T, Kawase I. Infliximab for rheumatoid arthritis in a patient with tuberculosis. N Engl J Med 2006;355:740–1 [DOI] [PubMed] [Google Scholar]
- 11.Aslanidis S, Pyrpasopoulou A, Douma S, et al. Is it safe to readminister tumor necrosis factor alpha antagonists following tuberculosis flare? Arthritis Rheum 2008;58:327–8 [DOI] [PubMed] [Google Scholar]
- 12.Blackmore TK, Manning L, Taylor WJ, et al. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis 2008;47:e83–5 [DOI] [PubMed] [Google Scholar]


