Abstract
Jun N-terminal kinases or JNKs play a critical role in death receptor-initiated extrinsic as well as mitochondrial intrinsic apoptotic pathways. JNKs activate apoptotic signaling by the upregulation pro-apoptotic genes via the transactivation of specific transcription factors or by directly modulating the activities of mitochondrial pro- and anti-apoptotic proteins through distinct phosphorylation events. This review analyzes our present understanding of the role of JNK in apoptotic signaling and the various mechanisms by which JNK promotes apoptosis
Keywords: JNK, apoptosis, cell death, MAP3K, MKK4, MKK7
Introduction
JNKs belong to the superfamily of MAP-kinases involved in the regulation of cell proliferation, differentiation, and apoptosis. Analyses of pathways regulated by JNKs have shown that JNKs are indispensable for both cell proliferation and apoptosis. Whether the activation of JNKs leads to cell proliferation or apoptosis is dependent on the stimuli and the cell-type involved in such activation (Lin & Dibling, 2002; Liu & Lin, 2005). JNK-family of MAP-kinases was initially identified as UV-responsive protein kinases involved in the transactivation of c-Jun by phosphorylating the N-terminal Ser-63 and Ser-73 residues (Hibi et al., 1993; Derijard et al., 1994). While initial studies have shown that JNKs can be activated by several different stimuli including growth factors (Hibi et al., 1993; Prasad et al., 1995), cytokines (Westwick et al., 1994), and stress factors (Cano et al., 1994), the observations that inflammatory cytokines and many different cytotoxic as well as genotoxic reagents stimulate JNKs unraveled the critical role of JNKs in mediating apoptotic signaling (Sluss et al., 1994; Cano & Mahadevan, 1995; Dai et al., 1995).
Signaling pathways that initiate apoptosis has been broadly classified into two: 1) extrinsic pathway initiated by death receptors such as those of TNF-a, TRAIL, and FAS-L and 2) intrinsic pathway initiated by mitochondrial events (Elmore, 2007). JNK has been observed to play a central role in both of these pathways. To date, multiple splice variants of JNKs encoded by three distinct genes, namely JNK1, JNK2, and JNK3, have been identified (Davis, 2000; Johnson & Nakamura, 2007). JNKs form the last tier of the three-tier kinase module consisting of MAP kinase kinase kinase (MAP3K), MAP kinase kinase (MAP2K), and MAP kinase (MAPK) (Dhanasekaran & Reddy, 1998; Dhanasekaran et al., 2007). In response to specific stimuli, the penultimate dual-specificity kinase of the tier, either MKK4 or MKK7, activates JNKs by phosphorylating the Thr- and Tyr-residues of the TXY motif within the activation loop of the respective JNKs (Dhanasekaran & Reddy, 1998). While MKK4 can activate both p38MAPK and JNK, MKK7 is specifically involved in the activation of JNKs. Both anti-apoptotic and pro-apoptotic signals converge on activating MKK4-JNK or MKK7-JNK signaling node via specific MAP3Ks. JNKs in turn activate apoptotic signaling either through the upregulation pro-apoptotic genes via the transactivation of specific transcription factors including c-Jun or by directly modulating the activities of mitochondrial pro- and anti-apoptotic proteins through phosphorylation events. Although the apoptotic stimuli can also involve the stimulation of p38MAPK, this review analyzes our current understanding of the mechanisms through which JNK-signaling module is involved in mediating apoptosis.
Role of MAP3Ks in coupling stress stimuli to JNK
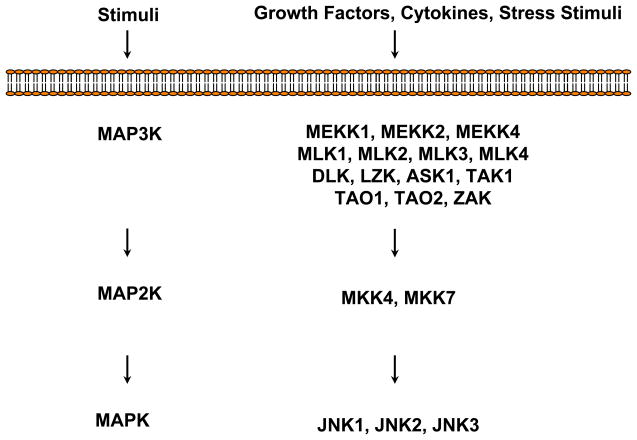
Of the twenty MAP3Ks that have been identified, fourteen have been observed to be involved in the activation JNKs via MKK4 or MKK7 (Fanger et al., 1997; Raman et al., 2007; Johnson & Nakamura, 2007) (Fig. 1). MEKK1 was the first member to be identified to be involved in promoting apoptosis via JNK signaling pathway (Widmann et al., 1998). Subsequent studies have shown that all of the MAP3Ks that can activate MKK4 or MMK7 can initiate JNK-mediated apoptotic pathway in a cell-type and stimuli specific manner. It has been observed that MLK-family of kinases including MLK1, MLK2, MLK3, and DLK are involved in transmitting signals from Rac/CDC42 to MKK4/7-JNK to induce neuronal cell apoptosis in response to the deprivation of NGF (Xu et al., 2001). It has also been observed that DLK mediated activation of JNK is required for calphostin-c induced apoptosis of breast cancer cell line (Robitaille et al., 2008).
Figure 1. MAP3Ks and MAP2Ks involved in the activation of JNKs.
At least fourteen MAP3Ks have been shown to activate the MAP2Ks MKK4 and/or MKK7. MKK4 can activate JNKs as well as p38MAPKs (not shown), whereas MKK7 specifically activates JNKs. MKK4 and MKK7 can activate all of the three isoforms of JNKs by phosphorylating the Thr- and Tyr-residues of the TXY motif.
Similarly, Zak-activated JNK has been shown to be involved in the apoptosis of hepatoma cell lines (Liu et al., 2000). Of these different MAP3Ks that link JNK-mediated apoptotic pathways to upstream stress stimuli, the mechanism by which ASK1 link diverse apoptotic stimuli to JNK is well characterized (Matsuzawa & Ichijo, 2001; Nagai et al., 2007). Apoptosis signal-regulating kinase 1 (ASK1) ASK1 is activated by diverse apoptotic stimuli such as tumor necrosis factor (TNF)-a, reactive oxygen species (ROS), lipopolysaccharide (LPS), and endoplasmic reticulum (ER)-stress. During TNF-a mediated apoptotic signaling, it appears that ROS-dependent activation of ASK1 by TNF-receptor-associated factor 2 (TRAF-2) leads to the subsequent stimulation of JNK-module and apoptosis (Matsuzawa & Ichijo, 2001; Nagai et al., 2007). Similar mechanism involving TRAF-2 has been proposed for the activation of ASK1-MKK-JNK mediated apoptotic signaling. In the case of LPS, TRAF6, closely related to TRAF2, has been shown to link LPS stimuli to the activation of ASK1 (Nagai et al., 2007). Oxidative stress has been shown to recruit both TRAF2 and TRAF6 in order to activate ASK1 and the downstream JNK-signaling (Liu et al., 2000; Fujino et al., 2007). Although studies with Ask−/− embryonic fibroblasts have indicated that ASK1 is not required for the transient activation of JNK, it is required for the persistent activation of JNK and apoptois induced by TNF-α (Tobiume et al., 2001). Activation of ASK1 and the subsequent stimulation JNK has been implicated in cisplatin induced apoptosis of ovarian carcinoma cells (Chen et al., 1999).
Role of JNKs in apoptotic signaling
All the three JNKs have been shown to involved in stimulating apoptotic signaling. The primary evidence that the activation of JNK-1 can be correlated with apoptosis came from the initial studies investigating γ-ray induced apoptosis (Chen et al., 1996). These studies have indicated that the γ-ray stimulates a rather delayed but persistent activation of JNK-1 in Jurkat cells with a concomitant increase in DNA fragmentation. Although these initial studies failed to establish a cause and effect relationship between JNK and DNA-fragmentation, the results indicated an underlying the differences between the growth factor induced, presumably ant-apoptotic JNK activation versus the g-ray induced pro-apoptotic activation. While g-ray induced JNK-activation was delayed – similar to UV-induced JNK-activation, the growth factor induced JNK-activation was rapid and transient. Subsequent studies have clearly established the role of JNK in TNF-α, Fas-ligand, X-ray, and UV-ray induced apoptosis (Verheij et al., 1996; Wilson et al., 1996: Zanke et al., 1996).
Unequivocal evidence that JNK1 and JNK2 are involved in apoptotic signaling came from studies using embryonic fibroblasts derived from JNK1−/−JNK2−/− mice (Tournier et al., 2000). These JNK−/− mouse embryonic fibroblasts (MEFs) showed resistance to apoptosis in response to UV irradiation, DNA-alkylating agent methyl methanesulfate, and translation inhibitor anisomycin. The defect in apoptosis can be correlated with the lack of mitochondrial depolarization, cytochrome C release, and the activation of caspases. Interestingly, these cells were responsive to Fas-mediated apoptosis suggesting that JNK1 and JNK2 are not required for the Fas-induced apoptosis of these fibroblasts. It has also been observed that the MEFs carrying non-phosphorylatable alanine mutations in c-Jun showed similar resistance to UV-induced apoptosis (Behrens et al., 1999). Together, these studies suggest that JNK1 and JNK2 and their phosphorylation of c-Jun are required for UV-induced apoptosis. The role for JNK3 in apoptosis was also established using JNK3−/− mice (Yang et al., 1997). It is known that the excitotoxic agents such as kainite and glutamate induce the apoptosis of the hippocampal neurons in wild-type mice. In a striking contrast, JNK3−/− mice were observed to be quite resistant to glutamate induced apoptosis of their hippocampal neurons. Interestingly MEFs from JNK−/− JNK2−/− mice did not show such resistance to excitotoxic appotosis. In contrast, Miffs from mice carrying non-phosphorylatable alanine mutations at Ser63 and Ser73 exhibited resistance to excitotoxic apoptosis similar to that seen with JNK3-deficiency, thereby suggesting the critical role of JNK3 and its phosphorylation of c-Jun in excitotoxic cell death. In addition to these direct evidences that have established a role for JNKs in pro-apoptotic signaling, studies using JNK-specific inhibitors have also corroborated the pro-apoptotic role of JNKs. JNK-specific inhibitors have been shown to attenuate the apoptosis of hepatocytes, and sinusoidal endothelial cells during hepatic I/R injury (Uehara et al., 2005). Likewise, JNK inhibitor has been shown to inhibit the apoptosis of cardiomyocytes using a rat cardiac I/R model animal model (Ferrandi et al., 2004).
While the pro-apoptotic role of JNKs is beginning to be understood, it should be noted here that the pro-apoptotic role of JNK is often determined by other cellular factors. These may include the parallel activation of cell survival or anti-apoptotic pathway and the overall apoptotic signaling strength. It appears that the sustained activation of JNK is associated with apoptosis whereas the acute and transient activation of JNK is involved in cell proliferative or survival pathway (Sánchez-Perez et al., 1998: Chen & Tan, 2000).
Nuclear signaling of JNK in the regulation of apoptosis
Upon activation by the upstream MAP2Ks, the phosphorylated JNK translocates to nucleus where it phosphorylates and transactivates c-Jun (Davis 2000; Chang & Karin 2001). Phosphorylation of c-Jun leads to the formation of AP-1, which is involved in the transcription of a wide variety of proteins, some of them being known pro-apoptotic proteins (Dhanasekaran & Johnson, 2007; Raman et al., 2007; Turjanski et al., 2007). It has been noted that the JNK-AP-1 pathway is involved in the increased expression of pro-apoptotic genes such as TNF-a, Fas-L, and Bak (Fan & Chambers, 2001). JNK can also phosphorylate several other transcription factors including JunD, ATF2, ATF3, Elk 1, Elk-3, p53, RXRα, RARα, AR, NFAT4, HSF-1, and c-Myc (Johnson & Nakamura, 2007). Thus in the context of apoptosis, the nuclear activity of JNK can potentially lead to an increase in the expression of pro-apoptotic genes and/or a decrease in the expression of pro-survival genes (Fig. 2). There is sufficient evidence that the nuclear activity of JNK such as its translocation to nucleus and the transactivation of c-Jun are required for its apoptotic activity. It has been observed that JNK is required for the apoptosis of central nervous system neurons and the expression of dominant negative inhibitors of nuclear JNK confer resistance to their apoptosis following trophic support (Bjorkblom et al., 2008). These findings indicate the important role of nuclear JNK in promoting apoptotic signaling. It is significant to note here that the non-phosphorylatable mutants of c-Jun confer resistance to apoptosis of MEFs in response to UV-irradiation. Together, these results may be indicative of a role for JNK-c-Jun/Ap1 mediated expression of pro-apoptotic genes in JNK-mediated apoptosis. However, this may not be a universal mechanism. It has been noted that the silencing of c-Jun and ATF2 using small interfering RNA does not protect the central nervous system neurons against apoptosis in response to trophic hormone withdrawal (Björkblom et al., 2008), suggesting these transcription factors may not play a role in the apoptosis of at least central nervous system neuron. In contrast, the apoptosis of hippocampal neurons, cerebellar granule neuron (Mei et al., 2008), or developing neurons (Barone et al., 2008) appear to require a mechanism involving the activation of c-Jun by JNK. Thus it appears whether JNK activation of c-Jun is required for the apoptotic signaling appears to be very much dependent on the cell type in which JNK is activated.
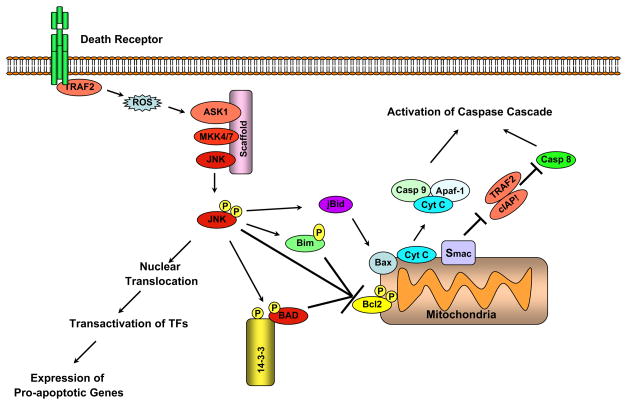
Figure 2. Nucleus- and mitochondria-targeted signaling by JNK.
JNK can promote apoptosis by two distinct mechanisms. In the first mechanism targeted at the nuclear events, activated JNK translocated to nucleus and transactivates c-Jun and pother target transcription factors (TF). JNK can promote apoptosis by increasing the expression of pro-apoptotic genes via the transactivation of c-Jun/AP1 dependent or p53/73-protein dependent mechanisms (see text for details). In pathways directed at mitochondrial apoptotic proteins, activated JNK translocates to mitochondria. There, JNK can phosphorylate BH3-only family of Bcl2 proteins to antagonize the anti-apoptotic activity of Bcl2 or Bcl Xl. In addition JNK can stimulate the release cytochrome C (Cyt C) from mitochondrial inner membrane via Bid-Bax dependent mechanism, promoting the formation of apaptosomes consisting of cytochrome C, caspase-9 (Casp 9), and Apaf-1. This complex initiates the activation of caspase-9 dependent caspase cascade. In another mechanism JNK can promote the release of Smac/Diablo (Smac) that can inhibit the TRAF2/IAP1 inhibitory complex, thereby relieving the inhibition on caspase-8 to initiate caspase activation. In addition by phosphorylating BAD and it sequestering partner 14-3-3, JNK can promote BAD-mediated neutralization of Bcl2 family of anti-apoptotic proteins. Finally, JNK can phosphorylate Bcl2 to suppress its anti-apoptotic activity (see text for details). These nuclear and mitochondrial events regulated by JNK need not be mutually exclusive.
In addition, the type of apoptotic stimuli plays a greater role in defining whether JNK-activated c-Jun is required for apoptosis. This is significantly exemplified in JNK-dependent apoptosis induced by two distinct microtubule inhibitors vinblastine and Taxol in breast cancer cells (Kolomeichuk et al., 2008). Both vinblastine and Taxol induce mitotic arrest and subsequent apoptotic cell death with similar kinetics. While both the drugs activate JNK and induce c-Jun protein and c-jun mRNA expression, only vinblastine induced c-Jun phosphorylation and c-jun transcriptional activation whereas Taxol failed to do so. However, inhibition of JNK or knock-down of JNK conferred resistance to both vinblastine- and Taxol-induced apoptosis. Thus, these results strongly suggest that although JNK-activation plays a critical role in apoptosis induced by both vinblastine and Taxol, the requirement for c-Jun/AP1 activation differs according to the apoptotic stimuli. Considering the possibility that JNK can phosphorylate and transactivate other transcription factors, it is likely that c-Jun is not the only obligatory substrate in all the cellular or physiological contexts pertaining to apoptosis.
An alternate pathway contributing to JNK-mediated apoptosis involves the phosphorylation of p53 family of proteins by JNK (Fuchs et al., 1998). It has been suggested that the phosphorylation of p53 at Ser6 by JNK inhibits ubiquitin-mediated degradation of p53 and thereby stabilizing the levels of p53. Recent studies have shown that the phosphorylation of p53 at Ser6 by JNK2 is critically required for the apoptotic pathway (Oleinik et al., 2007). It has been observed that the expression of FDH (10-formyltetrahydrofolate dehydrogenase) is drastically reduced in tumors and its elevation induces p53-dependent apoptosis. Further analyses of this apoptotic event indicated that FDH induces direct phosphorylation of p53 at Ser6 involving both JNK1 and JNK2. Treatment of FDH-expressing cells with JNK inhibitor SP600125 or silencing of JNK1/2 or inhibited the phosphorylation of p53 at Ser6 as well as p53-dependent apoptosis in response to DNA-damage. Since p53 is involved in the expression of several pro-apoptotic genes such as Bax (Bcl2-associated X protein) and PUMA (p53 up-regulated modulator of apoptosis), it is quite possible that apoptotic pathway activated by JNK involves p53-mediiataed upregulation of pro-apoptotic genes.
JNK is also involved in the regulation of apoptosis involving another member of p53 family of transcription factors (Jones et al., 2007). DNA damage that activates JNK also causes the stabilization and activation of p73, a member of the p53 family of transcription factors. Similar to p53, p73 induces apoptosis by increasing the expression of pro-apoptotic genes including Bax and PUMA. Analysis of this pathway has shown that JNK is required for p73-mediated apoptosis induced by the DNA damaging agent cisplatin and it involves the phosphorylation of p73 at several serine and threonine residues. Consistent with the notion that JNK and its activity are required for p73-mediated apoptosis, mutation JNK-phosphorylation in p53 abrogates cisplatin-induced stabilization of p73 along with a marked reduction in p73- transcriptional activity, and cisplatin-induced apoptosis. Thus, JNK can stimulate the expression of pro-apoptotic genes and decrease the expression pro-survival genes via multiple transcription factors in cell-type and stimuli specific manner.
Mitochondrial signaling of JNK in the regulation of apoptosis
Diverse apoptotic signals such as DNA-damage, oxidative stress, deprivation of growth factors, and irradiation finally converge on mitochondria to release the apoptotic proteins from the inner membrane space. In addition to the canonical nuclear signaling that leads to the upregulation of pro- apoptotic and/or downregulation of anti-apoptotic genes, JNKs play an essential role in modulating the functions of pro- and anti-apoptotic proteins located in mitochondria (Aoki et al., 2002; Schroeter et al., 2003). Accordingly, following activation by apoptotic stimuli, JNK readily translocates to mitochondria (Kharbanda et al., 2000; Chauhan et al., 2003). The crucial mitochondrial event initiating apoptosis is the release of cytochrome C from the inner membrane space of mitochondria. Released cytochrome C, in combination with Apaf-1 and caspase-9 form the apoptosomes, which activate the capase-9 cascade (Chinnaiyan, 1999; Hill et al., 2004). JNK has been shown to be critically required for the release of cytochrome C from mitochondria during the UV-induced apoptosis of MEFs since JNK1−/−JNK2-/2-MEFs failed to show the release of cytochrome C in response to UV-irradiation (Tournier et al., 2000). Although the precise mechanism by which JNK mediates cytochrome C release in not fully understood, several lines evidence indicate that JNK-induced cleavage of Bid, a pro-apoptotic, BH3-only member of Bcl2-family apoptotic proteins, plays a role in this process (Madesh wt al., 2002). In the case of extrinsic apoptotic pathway initiated by death receptors induce caspase-8 dependent cleavage of Bid and the resultant 15.5 kDa C-terminal fragment of Bid, known as tBid, translocates to mitochondria where it activates Bax. The activated Bax forms membrane channels through which apoptogenic proteins such as cytochrome C can be released (Bossy-Wetzel & Greene, 1999; Kuwana et al., 2002; Madesh et al., 2002). It is significant to note here that during TNF-α induced apoptosis of HeLa cells, JNK has been shown to induce caspase-8 independent cleavage of Bid and the resultant 21 kDa fragment of Bid (jBid), which translocates to mitochondria and selectively promotes the release of the pro-apoptotic protein Smac/DIABLO (Deng et al., 2003). Although the mechanism through which JNK induces the cleavage of Bid is still elusive, it is more likely that the activated JNK stimulates the release of cytochrome C from mitochondria through an analogous pathway involving the pro-apoptotic proteins Bid and Bax. It is quite possible that a similar mechanism underlies the release of Smac/DIABLO during stress-induced apoptosis of multiple myeloma cells (Chauhan et al., 2003). It is worth noting here that the ability of JNK to induce Bid cleavage and thereby promoting the release of Smac/DIABLO links it to another mechanism through which it can mediate apoptotic signaling. During apoptosis induced by TNF-α, JNK promotes the release of jBID as described above (Deng et al., 2003). The released Smac/DIABLO has been proposed to disrupt the TRAF2-cIAP1 complex, which is involved in the inhibition of caspase-8 activation (Fig. 2). Thus JNK appears to play a critical role in the extrinsic pathway from mitochondrial events.
JNK has also been observed to modulate the activities of other pro-apoptotic BH3 –only subgroup of Bcl2 family of proteins such as Bim and Bmf, (Lei & Davis, 2003). During UV-induced apoptosis of HEK293T cells, the phosphorylation of Bim and Bmf by JNK releases them from the hold of the sequestering dynein and myosin V motor complexes. Once they are released the phosphorylated Bim and Bmf can activate Bax and/or Bak to initiate apoptosis (Marani et al., 2002; Letai et al., 2002). Alternatively, the phosphorylated Bim can bind and neutralize the anti-apoptotic activities of Bcl2 and Bcl XL, thereby promoting apoptosis (Puthalakath et al., 1999: Puthalakath & Strasser, 2002). Another BH3-only pro-apoptotic member of Bcl2 family targeted by JNK to promote apoptosis is Bad. JNK specifically phosphorylates the Ser128 of BAD and promotes its apoptotic effect of BAD in the primary granule neurons of the rat cerebellum (Donovan et al., 2002). BAD promotes cell death by interacting with and inhibiting the pro-survival Bcl-2 proteins (Gross et al., 1999). Normally, the pro-survival kinases such as Akt-1, PAK-1, and PKA inhibit the pro-apoptotic activity of BAD by phosphorylating it at serine 136, serine 112, or both. The Ser112/136-phosphorylated BAD was sequestered by 14-3-3 family of proteins (Gross et al., 1999). Apparently, Ser128 phosphorylation of BAD inhibits its interaction with 14-3-3 proteins so that the BAD can antagonize the anti-apoptotic Bcl2 proteins to promote apoptosis (Wang et al., 2007). JNK seems to further potentiate the pro-apoptotic role of BAD by phosphorylating 14-3-3ζ protein at Ser184 (Tsuruta et al., 2004), following which 14-3-3 releases the sequestered BAD (Sunayama et al., 2005). Thus JNK appears to ensure the pro-apoptotic signaling by phosphorylating the Ser128 of Bad and Ser184 of 14-3-3ζ.
In addition to stimulating the activities of pro-apoptotic proteins, especially those of BH3-only family of proteins, JNK promote apoptosis by inhibiting the anti-apoptotic proteins such as Bcl2. During microtubule-damaging agent-induced apoptosis of breast cancer cells, it has been shown that JNK is activated and the activated JNK is involved in the phosphorylation of Bcl2 at Ser70 (Yamamoto et al., 1999; Srivastava et al., 1999). MKP-1 mediated dephosphorylation of phospho-JNK or the mutation of Ser70 to Ala inhibited paclitaxel-induced apoptosis of these cells. Together, these finding suggest that JNK phosphorylates Bcl2 to suppress its anti-apoptotic activity.
Conclusion
An overview of apoptotic pathway indicates that JNK signaling is involved in the extrinsic apoptotic pathway initiated by death receptors as well as the intrinsic pathway initiated at the mitochondria. In response to both the extrinsic and intrinsic apoptotic stimuli, JNK plays an essential role through its ability to interact and modulate the activities of diverse pro- and anti-apoptotic proteins. Through the coordinated regulation of the nuclear- and mitochondrial events, JNK ensures the efficient execution of apoptosis. With the identification of primary apoptotic signaling nodes regulated by JNK, the finer details of JNK-signaling in apoptosis specifically in relation to other growth stimulating stimuli is finally emerging and this should unravel novel therapeutic targets for diverse pathological conditions such as Alzheimer’s disease and cancer.
Acknowledgments
Studies presented in authors’ laboratories were supported by the National Institutes of Health Grants CA 123233 (to DND) and AG22022 (to EPR).
References
- Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- Barone MC, Desouza LA, Freeman RS. Pin1 promotes cell death in NGF-dependent neurons through a mechanism requiring c-Jun activity. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05427.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- Björkblom B, Vainio JC, Hongisto V, Herdegen T, Courtney MJ, Coffey ET. All JNKs Can Kill, but Nuclear Localization Is Critical for Neuronal Death. J Biol Chem. 2008;283:19704–19713. doi: 10.1074/jbc.M707744200. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Green DR. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J Biol Chem. 1999;274:17484–17490. doi: 10.1074/jbc.274.25.17484. [DOI] [PubMed] [Google Scholar]
- Cano E, Hazzalin CA, Mahadevan LC. Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and -2 are implicated in the induction of c-fos and c-jun. Mol Cell Biol. 1994;14:7352–7362. doi: 10.1128/mcb.14.11.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Munshi N, Kharbanda S, Anderson KC. JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem. 2003;278:17593–17596. doi: 10.1074/jbc.C300076200. [DOI] [PubMed] [Google Scholar]
- Chen YR, Meyer CF, Tan TH. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J Biol Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- Chen YR, Tan TH. The c-Jun N-terminal kinase pathway and apoptotic signaling. Int J Oncol. 2000;16:651–662. doi: 10.3892/ijo.16.4.651. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM. The apoptosome: heart and soul of the cell death machine. Neoplasia. 1999;1:5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Rubie E, Franklin CC, Kraft A, Gillespie DA, Avruch J, Kyriakis JM, Woodgett JR. Stress-activated protein kinases bind directly to the delta domain of c-Jun in resting cells: implications for repression of c-Jun function. Oncogene. 1995;10:849–855. [PubMed] [Google Scholar]
- Davis RJ. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Dérijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Johnson GL. MAPKs: function, regulation, role in cancer and therapeutic targeting. Oncogene. 2007;26:3097–3099. doi: 10.1038/sj.onc.1210395. Review. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. Review. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran N, Reddy EP. Signaling by dual specificity kinases. Oncogene. 1998;17:1447–1755. doi: 10.1038/sj.onc.1202251. [DOI] [PubMed] [Google Scholar]
- Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem. 2002;277:40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Chambers TC. Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resist Updat. 2001;4:253–267. doi: 10.1054/drup.2001.0214. [DOI] [PubMed] [Google Scholar]
- Fanger GR, Gerwins P, Widmann C, Jarpe MB, Johnson GL. MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr Opin Genet Dev. 1997;7:67–74. doi: 10.1016/s0959-437x(97)80111-6. [DOI] [PubMed] [Google Scholar]
- Ferrandi C, Ballerio R, Gaillard P, Giachetti C, Carboni S, Vitte PA, Gotteland JP, Cirillo R. Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte apoptosis and infarct size after myocardial ischemia and reperfusion in anaesthetized rats. Br J Pharmacol. 2004;142:953–960. doi: 10.1038/sj.bjp.0705873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27:8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SY, Adler V, Pincus MR, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci U S A. 1988;95:10541–10546. doi: 10.1073/pnas.95.18.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. Embo J. 2004;23:2134–2145. doi: 10.1038/sj.emboj.7600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EV, Dickman MJ, Whitmarsh AJ. Regulation of p73-mediated apoptosis by c-Jun N-terminal kinase. Biochem J. 2007;405:617–623. doi: 10.1042/BJ20061778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Kolomeichuk SN, Terrano DT, Lyle CS, Sabapathy K, Chambers TC. Distinct signaling pathways of microtubule inhibitors--vinblastine and Taxol induce JNK-dependent cell death but through AP-1-dependent and AP-1-independent mechanisms, respectively. FEBS J. 2008;275:1889–1899. doi: 10.1111/j.1742-4658.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Lei K, Davis RJ. NK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Lin A, Dibling B. The true face of JNK activation in apoptosis. Aging Cell. 2002;1:112–116. doi: 10.1046/j.1474-9728.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Liu TC, Huang CJ, Chu YC, Wei CC, Chou CC, Chou MY, Chou CK, Yang JJ. Cloning and expression of ZAK, a mixed lineage kinase-like protein containing a leucine-zipper and a sterile-alpha motif. Biochem Biophys Res Commun. 2000;274:811–816. doi: 10.1006/bbrc.2000.3236. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madesh M, Antonsson B, Srinivasula SM, Alnemri ES, Hajnóczky G. Rapid kinetics of tBid-induced cytochrome c and Smac/DIABLO release and mitochondrial depolarization. J Biol Chem. 2002;277:5651–5659. doi: 10.1074/jbc.M108171200. [DOI] [PubMed] [Google Scholar]
- Marani M, Tenev T, Hancock D, Downward J, Lemoine NR. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22:3577–89. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Ichijo H. Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1. J Biochem. 2001;130:1–8. doi: 10.1093/oxfordjournals.jbchem.a002947. [DOI] [PubMed] [Google Scholar]
- Mei Y, Yuan Z, Song B, Li D, Ma C, Hu C, Ching YP, Li M. Activating transcription factor 3 up-regulated by c-Jun NH(2)-terminal kinase/c-Jun contributes to apoptosis induced by potassium deprivation in cerebellar granule neurons. Neuroscience. 2008;151:771–779. doi: 10.1016/j.neuroscience.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Buchsbaum D, Oliver P, Makhija S, Kimberly R, Zhou T. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene. 2003;22:2034–2044. doi: 10.1038/sj.onc.1206290. [DOI] [PubMed] [Google Scholar]
- Oleinik NV, Krupenko NI, Krupenko SA. Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway. Oncogene. 2007;26:7222–7230. doi: 10.1038/sj.onc.1210526. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O’Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Robitaille K, Daviau A, Lachance G, Couture JP, Blouin R. Calphostin C induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.77. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sánchez-Perez I, Murguía JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16:533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Boyd CS, Ahmed R, Spencer JP, Duncan RF, Rice-Evans C, Cadenas E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem J. 2003;372:359–369. doi: 10.1042/BJ20030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluss HK, Barrett T, Dérijard B, Davis RJ. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RK, Mi QS, Hardwick JM, Longo DL. Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci U S A. 1999;96:3775–3780. doi: 10.1073/pnas.96.7.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski AG, Vaqué JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- Uehara T, Bennett B, Sakata ST, Satoh Y, Bilter GK, Westwick JK, Brenner DA. JNK mediates hepatic ischemia reperfusion injury. J Hepatol. 2005;242:850–859. doi: 10.1016/j.jhep.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Wang XT, Pei DS, Xu J, Guan QH, Sun YF, Liu XM, Zhang GY. Opposing effects of Bad phosphorylation at two distinct sites by Akt1 and JNK1/2 on ischemic brain injury. Cell Signal. 2007;19:1844–1856. doi: 10.1016/j.cellsig.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Weitzel C, Minden A, Karin M, Brenner DA. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994;269:26396–26401. [PubMed] [Google Scholar]
- Wilson DJ, Fortner KA, Lynch DH, Mattingly RR, Macara IG, Posada JA, Budd RC. JNK, but not MAPK, activation is associated with Fas-mediated apoptosis in human T cells. Eur J Immunol. 1996;26:989–994. doi: 10.1002/eji.1830260505. [DOI] [PubMed] [Google Scholar]
- Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol. 2001;21:4713–4724. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DD, Kuan CY, Whitmarsh AJ, Rincón M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Zanke BW, Boudreau K, Rubie E, Winnett E, Tibbles LA, Zon L, Kyriakis J, Liu FF, Woodgett JR. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]