Abstract
Background
Although warfarin is widely recommended to prevent atrial fibrillation-related thromboembolism, many eligible patients do not take warfarin. The objective of this study was to describe factors associated with warfarin discontinuation in people newly starting warfarin for atrial fibrillation.
Methods and Results
We identified 4,188 subjects newly starting warfarin in the ATRIA Study and tracked longitudinal warfarin use using pharmacy and laboratory databases. Data on patient characteristics, international normalized ratio (INR) tests, and incident hospitalizations for hemorrhage were obtained from clinical and laboratory databases. Multivariable Cox regression analysis was used to identify independent predictors of prolonged warfarin discontinuation, defined as ≥ 180 consecutive days off warfarin.
Within one year after warfarin initiation, 26.3% of subjects discontinued therapy despite few hospitalizations for hemorrhage (2.3% of patients). The risk of discontinuation was higher in patients aged < 65 years (adjusted hazard ratio and 95%CI [HR] 1.33 [1.03-1.72] compared to age ≥ 85 years), patients with poorer anticoagulation control (HR 1.46 [1.42-1.49] for every 10% decrease in time in therapeutic INR range) and lower stroke risk (HR 2.54 [1.86-3.47] for CHADS2 stroke risk index of 0 compared to 4-6).
Conclusions
More than one in four individuals newly starting warfarin for atrial fibrillation discontinued therapy in the first year despite a low overall hemorrhage rate. Individuals deriving potentially less benefit from warfarin, including those with younger age, fewer stroke risk factors, and poorer INR control, were less likely to remain on warfarin. Maximizing the benefits of anticoagulation for atrial fibrillation depends upon determining which patients are most appropriately initiated and maintained on therapy.
Keywords: anticoagulation, atrial fibrillation, discontinuation, stroke prevention, warfarin
INTRODUCTION
Atrial fibrillation is an increasingly common condition among older adults and one that substantially increases the risk of ischemic stroke[1,2]. Oral vitamin K antagonists such as warfarin sodium significantly reduce the risk of atrial fibrillation-associated thromboembolism, and consequently are widely recommended for people with atrial fibrillation who have additional risk factors for stroke[3]. Despite the high efficacy of warfarin, however, multiple cross-sectional studies show that a substantial proportion of people with atrial fibrillation do not take warfarin[4-6]. It is unclear from such studies whether rates of warfarin non-use were due to a lack of warfarin initiation at the time of atrial fibrillation diagnosis, or due to people starting warfarin and subsequently discontinuing therapy. To better delineate the pattern of warfarin discontinuation, we followed individuals in the large AnTicoagulation and Risk Factors In Atrial fibrillation (ATRIA) Study who were newly started on warfarin for atrial fibrillation and describe rates of warfarin discontinuation and clinical risk factors associated with discontinuation.
METHODS
Cohort Assembly
The ATRIA Study followed 13,559 adults with diagnosed nonvalvular atrial fibrillation who received care within Kaiser Permanente of Northern California, a large integrated healthcare delivery system[7]. Patients with a diagnosis of atrial fibrillation between July 1, 1996 and December 31, 1997 were identified by searching automated inpatient, outpatient, and electrocardiographic databases for physician-assigned International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) diagnosis of atrial fibrillation (427.31). The cohort was followed until September 30, 2003 (median 6.0 years, interquartile range [IQR] 3.1 to 6.7 years). Patients with diagnoses of mitral stenosis, documented valvular repair or replacement, transient post-operative atrial fibrillation, or concurrent hyperthyroidism were excluded so as to focus on non-transient, nonvalvular atrial fibrillation. In a random sample of 115 cohort members with at least 3 available electrocardiograms, 21% of patients had evidence of paroxysmal atrial fibrillation in that 2 electrocardiograms with atrial fibrillation were separated by at least one that did not demonstrate atrial fibrillation. We searched clinical inpatient and ambulatory (outpatient clinics and emergency department) databases during the five years before each subject’s index date to identify previously diagnosed ischemic stroke, heart failure, coronary heart disease, and hypertension using relevant ICD-9 codes. A validated, comprehensive health plan diabetes registry was used to identify patients with diabetes mellitus. Risk factors were also used to calculate a CHADS2 stroke risk score for each patient, a risk index that assigns 1 point for a history of Congestive heart failure, Hypertension, Age ≥75 years, or Diabetes mellitus, and 2 points to a history of ischemic Stroke or transient ischemic attack[8]. Ascertainment of individual stroke risk factors was validated against a review of samples of outpatient medical records; the crude agreement between ICD-9 codes and medical record review for individual risk factors was high (78-96%) and the corresponding kappa statistics ranged from 0.51 to 0.89[7,9]. We did not assess for aspirin exposure because aspirin is available without prescription and thus not systematically captured in our database.
Identification of New Starts on Warfarin
We identified new starts on warfarin with at least 4 weeks of follow-up after warfarin initiation. New starts were defined as subjects with a new prescription for warfarin during the study period who had been continuously enrolled in the health plan for at least 12 months and who had no prior identified warfarin prescription and fewer than 2 outpatient measurements of the international normalized ratio (INR) in the previous 12 months. Longitudinal warfarin exposure was assessed using a validated algorithm that was based on the number of days supplied per prescription as well as intervening INR measurements. Continuous warfarin exposure was assumed for periods of time where the second of any two consecutive filled prescriptions began within 60 days of the last day supplied by the previous prescription. For periods of time between consecutive warfarin prescriptions that were longer than 60 days, continuous warfarin therapy was assumed if there were intervening INR measurements at least every 42 days. If INR measurements were less frequently obtained, the subject was considered to be not taking warfarin from day 31 after the end date of the first prescription until the start date of the next prescription. This grace period of 30 days at the end of each warfarin period was given to accommodate reductions in warfarin dose and skipped doses, and the validity of this approach compared to chart review has been described previously[7]. During the study period, approximately 85% of warfarin users were managed by pharmacist or nurse-led anticoagulation clinics. For patients experiencing a hospitalization for hemorrhage during the follow-up period, warfarin therapy was presumed to be discontinued on the day of hospital admission and to not resume until the next new prescription of warfarin.
Because warfarin may be administered for transient periods around the time of cardioversion of atrial fibrillation, we excluded 28 subjects whose warfarin start date was within 4 weeks before or after the presence of a Current Procedural Terminology code for cardioversion (92960 or 92961), and who did not refill a warfarin prescription after cardioversion within 8 weeks after the supply from the first prescription ended.
Identification of Hemorrhage Outcomes
Hospitalization and billing databases were searched electronically through September 30, 2003 for primary and secondary discharge diagnoses of intracranial hemorrhage, including intraparenchymal, subdural and subarachnoid hemorrhage, as well as primary discharge diagnoses of non-intracranial hemorrhage, such as gastrointestinal and genitourinary bleeds (codes available on request). Trained medical record analysts obtained the relevant medical records using a structured protocol. The hospitalization medical records of all potential outcome events were reviewed and validated by a physician clinical outcomes committee using a formal study protocol and standardized criteria[9]. Hemorrhages that did not lead to a hospitalization or that occurred as a complication of a hospitalization for another problem were not included. Since Kaiser Permanente is an integrated healthcare system, hemorrhagic events that incurred medical bills were identified even if they occurred at non-Kaiser Permanente medical facilities.
Statistical Analysis
Subjects were followed until they reached the primary outcome of prolonged warfarin discontinuation (defined as a period of at least 180 consecutive days in which there were no filled warfarin prescriptions and no sequential INR measurements), or were censored by death, disenrollment in the health plan, or reached the end of the study’s follow-up period (September 30, 2003). We used the 180 day criterion to be more specific for true discontinuation of warfarin therapy, since short discontinuations may reflect temporary cessation of warfarin, such as is recommended for certain procedures. Kaplan-Meier tables were used to estimate the proportion of patients with discontinuation and the proportion of patients who restarted warfarin after discontinuation over time.
The association of time to warfarin discontinuation with categorical clinical characteristics, including age group and risk factors for stroke, was assessed using log-rank tests, while association with continuous variables such as INR control was tested using simple Cox regression analysis. INR control was measured as the percentage time spent in a therapeutic INR range (TTR) of 2.0-3.0 based on linear interpolation methods, excluding periods of time where the interval between INR tests exceeded 8 weeks[10]. Multivariable Cox regression models were used to identify the independent effects of individual variables.
RESULTS
Magnitude and pattern of warfarin discontinuation
We identified 4,188 people with atrial fibrillation in the ATRIA Study who were newly started on warfarin therapy and who had at least 4 weeks of follow-up in the cohort after initiation of warfarin. The median time of follow-up of these individuals was 4.6 years [IQR 2.4 – 6.2], the mean age was 71.8 years, and 43% were 75 years or older. Most subjects (70%) had at least one risk factor for atrial fibrillation-related ischemic stroke (heart failure, hypertension, diabetes mellitus, or prior stroke) in addition to age ≥ 75 years. Few subjects had diagnosed risk factors for hemorrhage, such mechanical fall diagnosed during a hospitalization, or prior gastrointestinal hemorrhage (Table 1).
Table 1.
Baseline clinical characteristics of 4,188 subjects with atrial fibrillation newly started on warfarin
| Subject Characteristic | N (%) |
|---|---|
| Age (years) | |
| < 65 | 951 (22.7) |
| 65-74 | 1428 (34.1) |
| 75-84 | 1477 (35.3) |
| ≥ 85 | 332 (7.9) |
| Female | 1769 (42.2) |
| Prior ischemic stroke | 351 (8.4) |
| Heart failure | 1122 (26.8) |
| Hypertension | 2304 (55.0) |
| Diabetes mellitus | 703 (16.8) |
| Prior gastrointestinal bleed | 161 (3.8) |
| Diagnosed dementia | 105 (2.5) |
| Prior fall during hospitalization | 159 (3.8) |
| CHADS2*score | |
| 0 | 834 (19.9) |
| 1 | 1332 (31.8) |
| 2 | 1147 (27.4) |
| 3 | 581 (13.9) |
| 4-6 | 294 (7.0) |
CHADS2: risk score assigning 1 point for congestive heart failure, hypertension, age≥75 years, diabetes mellitus, and 2 points for stroke/TIA[8]
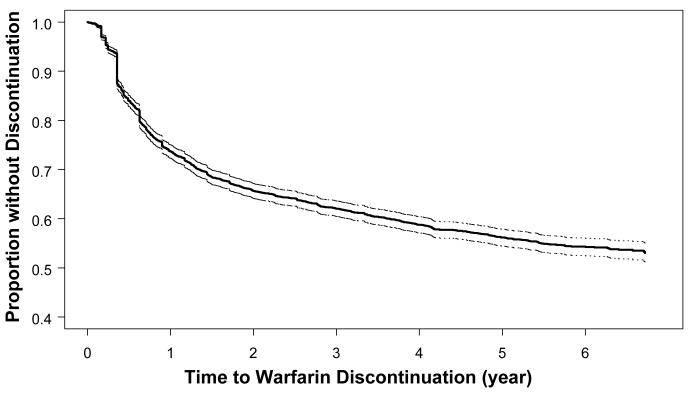
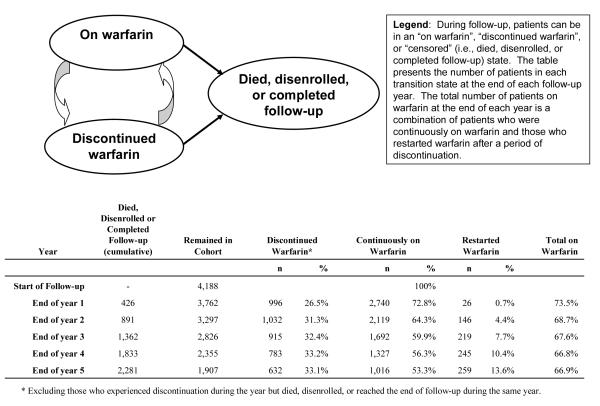
Kaplan-Meier estimates indicated that 26.3% (95% confidence interval [CI]: 25.0-27.7%) of patients discontinued warfarin within the first year of therapy (Figure 1). However, patients who remained on warfarin throughout the first year of therapy were more likely to remain on therapy in subsequent years. By the end of the second year of follow-up, only an additional 8.0% of patients discontinued warfarin, while an additional 3.6% discontinued warfarin by the end of the third year. Among the 1,538 subjects who discontinued warfarin for at least 180 days, 25.2% [95% CI: 23.0-27.6%] had evidence of restarting warfarin (at least one filled warfarin prescription) during the two years after initial discontinuation. Because of this dynamic process of stopping and restarting warfarin, the overall proportion of cohort members taking warfarin at any given time remained relatively stable after the first year of therapy: 73.5% at the end of year 1, 68.7% at the end of year 2, and 66.9% at the end of year 5 of follow-up (Figure 2).
Figure 1.
Kaplan-Meier graph of time to first warfarin discontinuation period of ≥ 180 days among 4,188 subjects with atrial fibrillation starting warfarin. Dotted lines indicate 95% confidence intervals.
Figure 2.
Warfarin status transitions at the end of each follow-up year in a cohort of 4,188 subjects with atrial fibrillation starting warfarin.
Univariate predictors of warfarin discontinuation
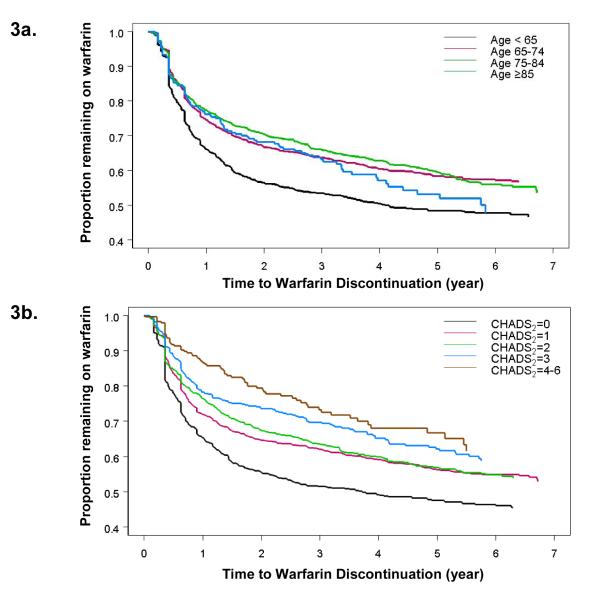
When stratified by age, the youngest patients (< 65 years of age) were the most likely to discontinue warfarin: 33.9% [95% CI: 30.9-37.1%] of patients younger than 65 years discontinued warfarin within the first year of therapy (p<0.001 compared with the other 3 age groups, Figure 3a). However, age subgroups of patients older than 65 years did not differ significantly in terms of the proportion who discontinued therapy within the first year: 23.8% [95% CI: 19.4-29.1%] of patients aged ≥ 85 years, compared to 22.7% [95% CI: 20.5-25.0%] of patients aged 75-84 years, and 25.4% [95% CI: 23.2-27.8] of patients aged 65-74 years (p=0.49).
Figure 3.
Kaplan-Meier graph of time to first warfarin discontinuation period of ≥ 180 days among 4,188 subjects with atrial fibrillation starting warfarin
a. Stratified by patient age
b. Stratified by CHADS2 score
Other univariate predictors of warfarin discontinuation in the first year included being male and not having risk factors for ischemic stroke, such as prior stroke, diagnosed heart failure, hypertension, and diabetes mellitus (Table 2). As CHADS2 scores increased, indicating higher stroke risk, the likelihood of discontinuing warfarin decreased monotonically, where only 13.8% of individuals with a CHADS2 score of 4-6 discontinued warfarin at 1 year (Figure 3b). Poorer INR control was associated with warfarin discontinuation as well; the median TTR in the overall cohort was 61% [IQR: 45-73%] and the likelihood of subsequent warfarin discontinuation increased by a HR of 1.44 [1.41-1.48] for every 10% absolute decrease in TTR.
Table 2.
Univariate association of clinical characteristics with warfarin discontinuation at 1 year among 4,188 subjects with atrial fibrillation newly starting warfarin.
| % subjects who discontinued warfarin by the end of the first year [95% confidence interval] |
P value* | |
|---|---|---|
| Sex | 0.006 | |
| Female | 23.4 [21.4-25.5] | |
| Male | 28.5 [26.7-30.4] | |
| Prior ischemic stroke | <0.001 | |
| Yes | 15.1 [11.6-19.5] | |
| No | 27.4 [26.0-28.9] | |
| Diagnosed heart failure | 0.001 | |
| Yes | 21.0 [18.7-23.7] | |
| No | 28.3 [26.7-30.0] | |
| Diagnosed hypertension | <0.001 | |
| Yes | 24.2 [22.5-26.1] | |
| No | 29.0 [27.0-31.2] | |
| Diabetes mellitus | 0.018 | |
| Yes | 23.0 [20.0-26.4] | |
| No | 27.1 [25.6-28.6] | |
| CHADS2 Score† | <0.001 | |
| 0 | 34.7 [31.6-38.1] | |
| 1 | 28.1 [25.8-30.7] | |
| 2 | 23.7 [21.2-26.3] | |
| 3 | 21.9 [18.6-25.7] | |
| 4-6 | 13.8 [10.2-18.6] | |
| History of gastrointestinal bleed | 0.47 | |
| Yes | 28.2 [21.3-36.6] | |
| No | 26.4 [25.0-27.8] | |
| Diagnosed dementia | 0.95 | |
| Yes | 17.3 [10.9-26.8] | |
| No | 26.6 [25.3-28.1] | |
| Mechanical fall diagnosis during prior hospitalization |
0.57 | |
| Yes | 27.6 [20.9-35.8] | |
| No | 26.4 [25.0-27.8] |
p values calculated using log rank tests comparing survival curves.
CHADS2: stroke risk score assigning 1 point for congestive heart failure, hypertension, age≥75 years, diabetes mellitus, and 2 points for stroke/TIA[8]
We identified 263 patients with incident hospitalizations for hemorrhage after warfarin initiation in the study period. Hospitalization for hemorrhage was relatively uncommon overall, with only 2.3% (95% CI: 1.8-2.8%) of cohort members experiencing an event in the first year after warfarin initiation. However, hospitalizations for hemorrhages led to subsequent warfarin discontinuation in 65% (95%CI: 59-70%) of the patients.
Multivariable predictors of warfarin discontinuation
Since the majority of patients experiencing a hemorrhage event discontinued warfarin and because we were interested in assessing other factors that contribute to discontinuation, we performed a multivariable analysis of warfarin discontinuation excluding the 263 patients who were hospitalized for hemorrhage. Table 3 presents the independent effects of baseline clinical features that were statistically significantly related to warfarin discontinuation or were components of the CHADS2 score. The presence of risk factors for stroke, assessed both as individual risk factors as well as by the CHADS2 score, were associated with lower risk of warfarin discontinuation. Patients younger than 65 years were the age category most likely to discontinue warfarin, even adjusting for other risk factors for stroke (Table 3).
Table 3.
Multivariable analysis of clinical factors associated with discontinuation of warfarin in 4,188 subjects newly starting warfarin
| Hazard Ratio [95% confidence interval] | |
|---|---|
| Age (years) | |
| <65 | 1.33 [1.03-1.72] |
| 65-74 | 1.14 [0.88-1.46] |
| 75-84 | 1.01 [0.79-1.30] |
| ≥ 85 | Referent |
| Female | 0.86 [0.76-0.96] |
| Prior ischemic stroke | 0.51 [0.38-0.67] |
| Diagnosed heart failure | 0.71 [0.62-0.81] |
| Diagnosed hypertension | 0.92 [0.82-1.03] |
| Diabetes mellitus | 0.77 [0.65-0.91] |
| Percent time in therapeutic INR range (per 10% increment) |
1.46 [1.42-1.49] |
| Substituting diagnosed heart failure, hypertension, age, diabetes mellitus, and prior ischemic stroke with CHADS2 score | |
| CHADS2 score | |
| 0 | 2.54 [1.86-3.47] |
| 1 | 1.98 [1.46-2.70] |
| 2 | 1.65 [1.20-2.25] |
| 3 | 1.22 [0.87-1.71] |
| 4-6 | Referent |
| Female | 0.86 [0.76-0.96] |
| Percent time in therapeutic INR range | 1.46 [1.42-1.49] |
Multivariable model included all listed variables in the table
DISCUSSION
Maintaining appropriate patients with atrial fibrillation on warfarin therapy is vitally important because clinical outcomes are strongly dependent upon remaining on anticoagulation[11]. Conversely, there may be individuals at low stroke risk or with elevated risk from warfarin in whom discontinuation of therapy is appropriate. In our ATRIA cohort, more than one in four patients with atrial fibrillation newly started on warfarin discontinued therapy within the first year of treatment. Although there was a high rate of discontinuation in the first year, people who remained on therapy at the end of the first year were then less likely to discontinue warfarin in subsequent years. In addition, sizable fractions of people who discontinued warfarin eventually restarted therapy, leading to a relatively stable prevalence of warfarin use in the overall cohort over time.
It is challenging to determine what proportion of warfarin persistence vs. discontinuation was clinically appropriate. Patients with low stroke risk may not derive much benefit from warfarin. We found that people with the lowest predicted stroke risk were more likely to discontinue warfarin than those with additional stroke risk factors and higher CHADS2 scores, an observation noted in some prior studies of warfarin use[7,12]. These results may reflect a rational assessment of the net benefit of anticoagulation by prescribing clinicians and their patients.
There may also be clinically appropriate reasons to discontinue therapy. Our study found that individuals with lower proportions of time spent in the therapeutic INR range were more likely to discontinue warfarin. We were unable to determine the precise reasons for poorer INR control. Poor INR control may have been a marker for non-adherence to warfarin leading to subsequent discontinuation, or poor control may have led to concerns about the safety of continuing warfarin[13,14].
As expected, discontinuation of warfarin was high after hospitalizations for a hemorrhagic event, but because the overall rate of hemorrhage hospitalizations in the cohort was low, such events could only account for a small fraction of discontinuations. It is possible that more minor, outpatient hemorrhagic events resulted in additional patients discontinuing therapy. The youngest patients were the most likely to discontinue warfarin therapy, a finding that has been observed in other analyses of warfarin adherence [12,15]. Patients age 85 years and older were not more likely to discontinue warfarin than those aged 65-84 years old, despite the higher risk of hemorrhage among older adults[16].
Although warfarin discontinuation rates have varied across randomized clinical trial settings[17-19], it was notably high in the older patients enrolled in the recent Birmingham Atrial Fibrillation Treatment of the Aged Trial (BAFTA). After a mean follow-up period of 2.7 years, 33% of elderly patients discontinued warfarin compared to a 24% discontinuation rate on aspirin[20]. Of note, a large number of potential subjects in the BAFTA trial were excluded because their physicians thought warfarin would be beneficial, which may have selected for less suitable warfarin candidates. The BAFTA study also did not provide discontinuation rates stratified by age group, being study of older patients. Observational studies have found that warfarin discontinuation is frequently due to concerns about bleeding complications or safe anticoagulation management, as well as issues of patient adherence and preference[12,21,22]. One study of 651 patients prescribed oral anticoagulants after ischemic stroke observed a 22% discontinuation rate after 1 year. Bleeding was the reason cited for 36.4% of those stopping anticoagulants, and “patient request” or “hassle to visit anticoagulation clinic” were the reasons cited in another 31.5% of patients[22]. Another study found that among 153 patients aged ≥ 80 years, 26% discontinued warfarin within 1 year (excluding patients who died or who were in sinus rhythm), and the primary reason for discontinuation was due to concerns about the safety of anticoagulation[21]. There was a high bleeding complication rate observed in this study which may have been related to a large proportion of patients who were recruited during an acute hospitalization. In addition, there was a 40% rate of concomitant warfarin and aspirin use, which may have led to increased bleeding complications. Although our study found low rates of hospitalizations for hemorrhage, it is possible that minor hemorrhages, such as epistaxis or superficial bruising that did not lead to hospitalization, could have contributed to warfarin discontinuation. A more in-depth investigation of the impact of minor bleeding while on anticoagulants would give a richer perspective to the reasons that people discontinue warfarin.
Our results highlight the dynamic nature of adherence to anticoagulant therapy. Even after discontinuing warfarin for at least 180 days, a substantial proportion of people restarted warfarin at some point over subsequent years. The balance of discontinuations and resumption of therapy resulted in a fairly stable prevalence of warfarin use after the first 2 years of warfarin anticoagulation. Nonetheless, the large fraction of patients who discontinue warfarin permanently has important implications for quality improvement programs. Programs that are primarily directed towards more widespread adoption of warfarin therapy in patients with atrial fibrillation must work to improve not only the initiation of therapy, but the persistence of therapy in appropriate patients[23]. The observation that patients with lower stroke risk and worse INR control were more likely to come off therapy, while patients with additional risk factors for stroke were more likely to remain on warfarin, suggests that clinicians have significant influence on whether patients continue to take warfarin or not. Quality measurements that rely on cross-sectional assessments of the proportion of patients taking warfarin will not be able to capture the dynamic process of warfarin initiation and discontinuation in individual patients.
There are several limitations to our study. The primary limitation is that we were unable to directly determine the specific reason for discontinuation. Some studies have found that concerns about safety and adherence are strongly associated with avoidance or discontinuation of warfarin[13,14,21]. Although it is likely that such concerns influenced warfarin discontinuation in our study, we lacked direct assessments of individual decision-making. Our study was also unable to capture outpatient hemorrhagic events that did not lead to hospitalization, but may have led to concerns about the safety of continuing on warfarin. Our measure of warfarin use and discontinuation may be subject to some errors in timing because we relied on an algorithm based on the number of pills prescribed to determine the length of warfarin therapy along with interim INR testing. In addition, our assessments of clinical factors associated with stroke risk were obtained using computerized clinical databases, which may introduce some level of misclassification. Our analysis was not able to capture comprehensive aspirin use, since it was available without a prescription. Since our study period largely preceded the publication of the results of the Atrial Fibrillation Follow-up Investigation of Rhythm Management trial in 2002[24], it is possible that some physicians discontinued warfarin in patients with long periods of sinus rhythm; our study was not able to determine which patients had only a single episode of atrial fibrillation followed by prolonged sinus rhythm[25-27].
Warfarin largely reverses the risk of stroke attributable to atrial fibrillation. Multiple guidelines recommend warfarin therapy in patients with atrial fibrillation with additional stroke risk factors, a clinical profile describing the majority of patients with atrial fibrillation[28-30]. In this large, community-based cohort of patients newly starting warfarin for atrial fibrillation, more than one-fourth discontinued therapy within the first year. Although hemorrhage events leading to hospitalization greatly increased the risk of warfarin discontinuation, the rate of such hemorrhages was too low to account for more than a small fraction of all discontinuations. It is probable that difficult warfarin control and concern about bleeding risk led patients and/or clinicians to stop warfarin. Quality indicators that rely simply on rates of warfarin use for atrial fibrillation will be unlikely to capture appropriate vs. inappropriate discontinuation of anticoagulation. Stroke prevention programs should consider the net benefit of warfarin for individual patients with atrial fibrillation, weighing the risks and benefits, and be attentive towards maintaining anticoagulation in appropriate patients.
What is Known.
Warfarin is widely recommended for the prevention of atrial fibrillation-related stroke
Cross-sectional studies show that many patients with atrial fibrillation are not on warfarin, but few describe which patients stop taking warfarin
What this Article Adds.
More than one in four patients newly starting warfarin in this large, community-based cohort discontinued therapy within 1 year
Patients with fewer stroke risk factors and poorer warfarin control were more likely to discontinue therapy, suggesting that a portion of those who discontinue warfarin might be doing so because of limited benefits or excess risks
Stroke-prevention efforts should address the appropriate persistence of warfarin therapy in patients with atrial fibrillation
Acknowledgments
Funding Sources: Funding for the study was provided by the National Institute on Aging (R01 AG15478 and K23 AG28978), the National Heart, Lung and Blood Institute (U19 HL91179 and RC2HL101589), and by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital (Boston, MA). The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of this manuscript.
Abbreviations
- ATRIA Study
AnTicoagulation and Risk factors In Atrial fibrillation Study
- CHADS2 Risk Index
acronym for Congestive heart failure, Hypertension, Age≥75 years, Diabetes mellitus, and Stroke/transient ischemic attack Risk Index
- CI
confidence interval
- HR
hazard ratio
- ICD-9
International Classification of Diseases, Ninth Revision, Clinical Modification
- INR
International Normalized Ratio
- IQR
Interquartile range
- TTR
time in therapeutic international normalized ratio range
Footnotes
Conflicts of Interest Disclosures: In the past two years, D.E. Singer has received a research grant from Daiichi Sankyo, Inc., consulted for Boehringer Ingelheim, Daiichi Sankyo, Inc., Johnson and Johnson, Inc., Merck and Co., and Sanofi Aventis, Inc. A.S. Go has received a research grant from Johnson & Johnson, Inc.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Hart R, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of Internal Medicine. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 4.Fang MC, Stafford RS, Ruskin JN, Singer DE. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Archives of Internal Medicine. 2004;164:55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, Radford MJ. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 6.Walker A, D B. Epidemiology and outcomes in patients with atrial fibrillation in the United States. Heart Rhythm. 2008;4:1365–1372. doi: 10.1016/j.hrthm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Annals of Internal Medicine. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 10.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thrombosis & Haemostasis. 1993;69:236–239. [PubMed] [Google Scholar]
- 11.Sorensen SV, Dewilde S, Singer DE, Goldhaber SZ, Monz BU, Plumb JM. Cost-effectiveness of warfarin: trial versus “real-world” stroke prevention in atrial fibrillation. Am Heart J. 2009;157:1064–1073. doi: 10.1016/j.ahj.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher AM, Rietbrock S, Plumb J, Van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? Journal of Thrombosis and Haemostasis. 2008;6:1500–1506. doi: 10.1111/j.1538-7836.2008.03059.x. [DOI] [PubMed] [Google Scholar]
- 13.Ingelgård A, Hollowell J, Reddy P, Gold K, Tran K, Fitzmaurice D. What are the barriers to warfarin use in atrial fibrillation? Development of a questionnaire. Journal of Thrombosis and Thrombolysis. 2006;21:257–265. doi: 10.1007/s11239-006-5633-2. [DOI] [PubMed] [Google Scholar]
- 14.Bungard TJ, Ghali WA, McAlister FA, Buchan AM, Cave AJ, Hamilton PG, Mitchell LB, Shuaib A, Teo KK, Tsuyuki RT. The relative importance of barriers to the prescription of warfarin for nonvalvular atrial fibrillation. Canadian Journal of Cardiology. 2003;19:280–284. [PubMed] [Google Scholar]
- 15.Arnsten JH, Gelfand JM, Singer DE. Determinants of compliance with anticoagulation: A case-control study. American Journal of Medicine. 1997;103:11–17. doi: 10.1016/s0002-9343(97)90048-6. [DOI] [PubMed] [Google Scholar]
- 16.Fang MC, Go AS, Hylek EM, Chang Y, Henault LE, Jensvold NG, Singer DE. Age and the risk of warfarin-associated hemorrhage: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. Journal of the American Geriatrics Society. 2006;54:1231–1236. doi: 10.1111/j.1532-5415.2006.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen P, Boysen G, Godtfredsen J, Andersen E, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: The Copenhagen AFASAK Study. Lancet. 1989;1:175–178. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 18.Stroke Prevention in Atrial Fibrillation Investigators Stroke Prevention in Atrial Fibrillation study: Final results. Circulation. 1991;84:527–539. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- 19.Stroke Prevention in Atrial Fibrillation Investigators Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633–638. [PubMed] [Google Scholar]
- 20.Mant J, Hobbs R, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E, on behalf of the BAFTA Investigators and the Midland Research Practices Network (MidReC) Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 21.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 22.De Schryver E, van Gijn J, Kappelle L, Koudstaal P, Algra A. Non-adherence to aspirin or oral anticoagulants in secondary prevention after ischaemic stroke. Journal of Neurology. 2005;252:1316–1321. doi: 10.1007/s00415-005-0858-0. [DOI] [PubMed] [Google Scholar]
- 23.ACC/AHA/Physician Consortium Clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter. Journal of the American College of Cardiology. 2008;51:865–884. doi: 10.1016/j.jacc.2008.01.006. 2008. [DOI] [PubMed] [Google Scholar]
- 24.AFFIRM Investigators A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. New England Journal of Medicine. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 25.Laupacis A, Albers G, Dalen J, Dunn M, Jacobsen A, Singer D. Antithrombotic therapy in atrial fibrillation. Chest. 1998;114:579S–589S. doi: 10.1378/chest.114.5_supplement.579s. [DOI] [PubMed] [Google Scholar]
- 26.Albers G, Dalen J, Laupacis A, Manning W, Petersen P, Singer DE. Antithrombotic Therapy in Atrial Fibrillation. Chest. 2001;119:194S–206S. doi: 10.1378/chest.119.1_suppl.194s. [DOI] [PubMed] [Google Scholar]
- 27.ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation. Journal of the American College of Cardiology. 2001;38:1231–1266. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 28.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, Lip GYH, Manning WJ. Antithrombotic Therapy in Atrial Fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:546S–592. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 29.National Collaborating Centre for Chronic Conditions . Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary Care. Royal College of Physicians; London: 2006. [PubMed] [Google Scholar]
- 30.ACC/AHA/ESC Writing Committee Guidelines for the Management of Patients With Atrial Fibrillation. Journal of the American College of Cardiology. 2006 doi: 10.1093/europace/eul097. 2006. [DOI] [PubMed] [Google Scholar]