Abstract
Excited states of one-electron oxidized guanine in DNA are known to induce hole transfer to the sugar moiety and on deprotonation result in neutral sugar radicals that are precursors of DNA-strand breaks. This work carried out in homogeneous aqueous glass (7.5 M LiCl) at low temperatures (77 to 175 K) shows the extent of photoconversion of one-electron oxidized guanine and the associated yields of individual sugar radicals and are crucially controlled by photon energy, protonation state, and strandedness of the oligomer. In addition to forming sugar radicals, highly oxidizing excited states of one-electron oxidized guanine are produced with 405 nm light at pH 5 and below that are able to oxidize chloride ion in the surrounding solution to form Cl2•− via an excited state hole transfer process. Among the various DNA model systems studied in this work, the maximum amount of Cl2•− is produced with ds (double stranded) DNA where the one-electron oxidized guanine exists in its cation radical (G•+:C) form. Thus, via excited state hole transfer, the dsDNA is apparently able to protect itself from cation radical excited states by transfer of damage to the surrounding environment.
Introduction
Electronic excitation of a species often substantially increases its oxidation potential 1, 2 thereby creating an excellent one-electron oxidizing agent. From the known values of redox potentials 3, 4, it is evident that neither the guanine cation radical (G•+), also known as the “hole”, nor its deprotonated neutral form G(−H)• are able to oxidize a nucleoside sugar moiety via one-electron oxidation. However, our experimental and theoretical efforts have established that the photoexcitation of DNA base cation radicals form neutral sugar radicals in DNA systems by a proton-coupled hole transfer process.5 - 13a The formation of sugar radicals via photoexcited base cation radicals is found to be influenced by various factors, e.g., wavelength of the incident light, pH of the solution, length and the sequence of the oligomer, and the site of phosphate substitution (3′- or 5′-). 5 - 8
In double stranded (ds) highly polymerized DNA, photo-excitation of one-electron oxidized guanine produces C1′• in the wavelength range 310 – 480 nm and no significant sugar radical formation was observed at wavelengths above 520 nm.9 However, photoexcitation of G•+ in 2′-deoxyguanosine (dGuo) showed no wavelength dependence towards the formation of sugar radicals (C1′•, C3′•, C5′•) over the entire UVA-vis range from 330 to 800 nm.9 Theoretical calculations employing time dependent density functional theory (TD-DFT B3LYP/6-31G(d)) were carried out for G•+ in dGuo and in the dinucleoside phosphate TpdG. These calculations predicted that the light-induced excitations occur from inner MOs to the single occupied molecular orbital (SOMO) take place throughout 330 – 880 nm for G•+ in dGuo.9 However, TD-DFT calculations for G•+ in TpdG 10a predict that at longer wavelengths base-to-base hole transfer occurs whereas base-to-sugar hole transfer is predicted to occur at shorter UVA-vis wavelengths leading to sugar radical formation after deprotonation.
To find the experimental evidence of base-to-base hole transfer via photo-excitation of G•+, these studies were extended to single stranded (ss) and ds DNA-oligomers. Increasing the length of the oligomer from a 6 mer TTGGTT to a 10 mer i.e., in TTGGTTGGTT, resulted in a 50 % decrease in the initial yield of the sugar radical observed after the first 20 min of the visible light exposure and a relative increase in the yield of C1′• vs. (C3′• and C5′•) found after prolonged exposure.11
The formation of sugar radicals via photoexcitation of one-electron oxidized guanine in DNA and RNA monomers is found to depend on the protonation state of guanine. 8, 9, 13a At pH ≥ 9 where the guanine in dGuo or in Guanosine (Guo) exists in its deprotonated [G(N1-H)•] form in our system (i.e., glassy solution at low temperature) 14a, 15, only small or negligible yields of sugar radicals are found via photoexcitation.8, 9 On the other hand, in dGuo 9 or in Guo8, complete photoconversion of G•+ to sugar radicals occurs in the pH range (2 to 6) where protonated G•+ is found (pKa (G•+) ca. 5) in the homogeneous aqueous glass) 15.
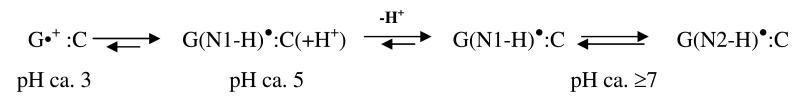
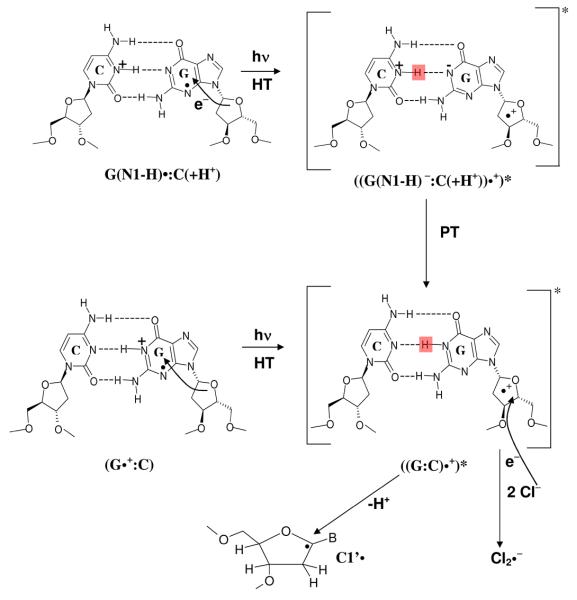
In dsDNA oligomers containing G, facile intra-base pair proton transfer occurs to form only (G(N1-H) •:C(+H+)) in low temperature (77 – 155 K) aqueous glasses at pH 4-6 (scheme 1).14a, 15 Thus, from photoexcitation results obtained using monomers, no sugar radical formation is expected in ds DNA via photo-excitation of one-electron oxidized guanine even at pH 5 and above. As expected for d[TGCGCGCA]2 at pH ca. 3, the initial rate of sugar radical formation was considerable, and at pH ca. 5, the initial rate of sugar radical formation was still substantial although less than that found at pH ca. 3. Only, at pH ≥ 7, at which the one-electron oxidized GC base pair exists in the deprotonated state [as (G(N1-H)•:C) and (G(N2-H)•:C)], the initial rate of formation of sugar radicals was found to be very low.13a These experimental observations have led to the hypothesis that formation of sugar radical requires the one-electron oxidized GC base pair to be in its cation radical (G•+: C) state, 13a or for (G(N1-H)•:C(+H+)), the reprotonation of the guanine base in the excited state is proposed to precede the deprotonation from the sugar moiety.13a In homogeneous aqueous glasses at 175 K, at pH 7 to 9, one-electron oxidized G in d[TGCGCGCA]2 is reported to exist in an equilibrium mixture of G(N2-H)•:C and G(N1-H)•:C forms. 13a Thus, for the formation of sugar radical via photo-excitation of either G(N1-H)•:C or G(N2-H)•:C, the proton must return from the surrounding solvent to reprotonate the guanine which explains the lack of significant sugar radical formation on photoexcitation.
Scheme 1.
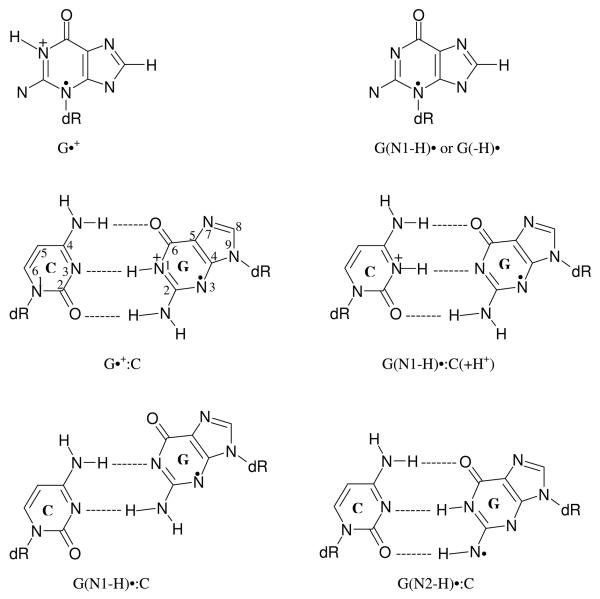
Schematic representation of the states of one-electron oxidized guanine involved in the protropic equilibria in dsDNA oligomers observed in our work at low temperatures (77 – 175 K). 13a, 14a The pH values at which the various prototropic forms of one-electron oxidized guanine in DNA are stable in our system homogeneous glassy system at low temperature (77 – 175 K), have been indicated. 13a (G•+ :C) denotes the cation radical state, whereas G(N1-H)•:C(+H+) corresponds to the intra-base pair proton transferred form. 13a, 14a G(N1-H)•:C and G(N2-H)•:C denote the deprotonated states where duplex-to-solvent deprotonation occurs from the N1- atom in the guanine ring or from the exocyclic amine N-atom (N2). The structures of these radicals are shown in scheme 3.
Our previous experimental work has shown that a wavelength dependence exists for the formation of sugar radicals in ss DNA oligomers 11 and in dTGCGCGA]2 13a and in highly polymerized DNA9. In this work, we present evidence that photoexcitation of one-electron oxidized guanine in the cation radical (G•+) state in monomers (dGuo) or in ss or ds DNA-oligomers, oxidizes the surrounding matrix (a homogeneous 7.5 M LiCl solution) thereby resulting in the formation of Cl2•− in a wavelength and pH dependent manner. Our results suggest that the reduction potential of excited G•+ is higher than that of excited G(N1-H)• as well as that of the ground state reduction potential of Cl2•−. Furthermore, for dsDNA at pH 5 and below, employing ds DNA-oligomers (8 mer d[TGCGCGCA]2 and the 14-mer -d[TATAGCGGCCGCTATA]2) and lasers at wavelengths - 405 nm and 640 nm in this work, we show that the formation of specific sugar radicals formed via photo-excitation is dependent on the wavelength of the incident light - specially, in dGuo, we find increased formation of C5′• at 405 nm with a decrease in the yield of C1′• owing to the photoconversion of C1′• to C5′• at 405 nm.
Materials and Methods
Compounds
2′-Deoxyguanosine (dGuo) was obtained from Sigma Chemical Company (St. Louis, MO). The ss DNA oligomer TGT and the ds DNA oligomers d[TGCGCGCA]2 and d[TATAGCGGCCGCTATA]2 were procured from SYNGEN, Inc. (Hayward, CA). These DNA oligomers were lyophilized, desalted and column purified.
Potassium persulfate (crystal) was obtained from Mallinckrodt, Inc. (Paris, KY) and Lithium chloride (99% anhydrous, Sigma Ultra) was purchased from Sigma (St. Louis, MO). Deuterium oxide (99.9 atom % D) was obtained from Aldrich Chemical Company Inc. (Milwaukee, WI). All compounds were used without any further purification.
Preparation of solutions
As per our earlier works with the monomers, 8, 9, 12 - 16, ~3 mg of dGuo was dissolved in 1 ml of 7.5 M LiCl in D2O in the presence of 5 to 6 mg K2S2O8 as the electron scavenger. Following our ongoing work regarding DNA-oligomers, 10, 11, 13a, 14, 16 the homogeneous solutions of TGT and of the ds DNA-oligomers were prepared by dissolving ca. 1.5 mg of compound in 0.50 ml of 7.5 M LiCl/D2O in the presence of 4 to 5 mg K2S2O8 as the electron scavenger.
The use of K2S2O8 as the electron scavenger enabled us to study only the one-electron oxidized radicals.
Our previous work showed that the ds DNA 8-mer d[TGCGCGCA]2 is double stranded in 7.5 M LiCl in D2O up to 48°C.13a We note that in homogeneous aqueous (H2O) glasses (10 M LiCl), the dsDNA oligomers have been reported to be in the B-conformation.13b
Adjustment of pH and pD
Following our works,8, 9, 12 - 16 the pHs/pDs of the solutions of dGuo, TGT, and the ds DNA oligomers mentioned above were adjusted by quickly adding the adequate micromole amounts of 0.1 M to 1 M NaOH in D2O and 0.1 M to 1 M HCl in D2O under ice-cooled conditions. Owing to the high ionic strength (7.5 M LiCl) of these solutions and also due to the use of pH papers, all the pH/pD values reported in this work are approximate.
Subsequently, these homogenous solutions were degassed by bubbling thoroughly with the nitrogen gas at room temperature.
Preparation of glassy samples and their storage
Following our work, 8 - 16 these homogeneous solutions of dGuo, TGT, and the ds DNA oligomers were drawn into 4 mm Suprasil quartz tubes (Catalog no. 734-PQ-8, WILMAD Glass Co., Inc., Buena, NJ) and these tubes containing these solutions were rapidly cooled to 77 K. This rapid cooling of these liquid solutions resulted into the transparent glassy solutions which were later used for the irradiation, annealing, and the subsequent photoexcitation experiments.
All samples were stored at 77 K in Teflon containers in the dark.
γ- irradiation of glassy samples and their storage
As per our work, 13a the Teflon containers with these transparent glassy samples were placed in a 400 ml Styrofoam dewar under liquid nitrogen (77 K). The γ irradiation of the glassy samples was performed at 77 K with the help of the 109 – GR 9 irradiator which contains a properly shielded 60Co gamma source.
The glassy samples of these DNA oligomers were γ-irradiated with an absorbed dose of 2.5 kGy.
Annealing of the samples
Following our previous work with dGuo 9, 14, 15 and TGT 11, the γ-irradiated glassy samples of dGuo and TGT used in this report were annealed at 155 K for 15 to 20 min. Following our works regarding DNA oligomers,13a, 14a the annealing studies of the γ-irradiated glassy samples of the ds DNA oligomers were carried out in the range 155 K to 175 K.
The annealing studies for all these above-mentioned samples were carried out by using a variable temperature assembly (Air products) via cooled nitrogen gas in the dark which regulated the gas temperature within 4 °C. To monitor the sample temperature during annealing, we used a copper–constantan thermocouple in direct contact with the sample.
The annealing of the sample softens the glass which allows the radicals e.g. Cl2•− to migrate and react with solute to produce one-electron oxidized guanine.9
Photoexcitation
For photoexcitation experiments, a thermo-electrically cooled blue laser (TECBL-30G-405, World Star Tech., Lot 6880, λ = 405 nm, 30 mW) and a red laser (UH5-40G-640C, World Star Tech., Lot 6880, λ = 640 nm, 40 mW) were used as light sources.
Following our works regarding photoexcitation, 8 - 16 the same temperature assembly that had already been used to maintain the temperatures during the annealing processes was also employed to maintain the temperature during the photoexcitation of these glassy samples. The temperature during photo-excitation was maintained at 143 ± 2 K.
Electron Spin Resonance
Following γ-irradiation, annealing, and photoexcitation using lasers of different wavelengths, these glassy samples were immersed immediately in liquid nitrogen, and an electron spin resonance (ESR) spectrum was recorded at 77 K and at 45 dB (6.3 μW). Fremy's salt (with g (center) = 2.0056, AN = 13.09 G) was used for field calibration.17 We have recorded these ESR spectra using a Varian Century Series ESR spectrometer operating at 9.3 GHz with an E-4531 dual cavity, 9-inch magnet and with a 200 mW klystron. 17 All the ESR spectra are recorded at 77 K.
Analyses of experimental ESR spectra
The fractional compositions of radicals used as benchmarks (Figure 1) in the experimentally recorded ESR spectrum were determined with the help of least square fitting using programs viz. ESRPLAY and ESRADSUB developed in our laboratory. By employing the doubly integrated areas of the benchmark spectra, we have estimated the percentage (or, fractional) contribution of a particular radical in the experimentally found ESR spectrum. The number of spins of each radical is directly proportional to the doubly integrated area in its ESR spectrum.
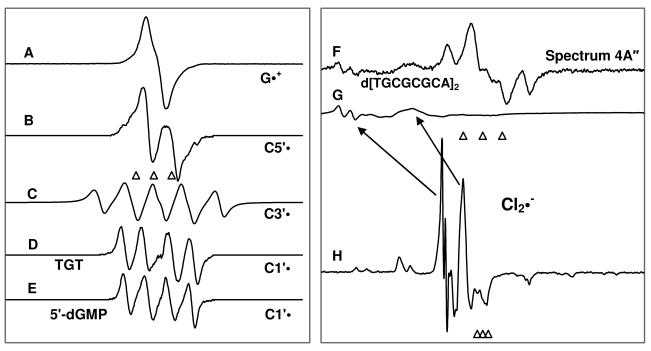
Figure 1.
Benchmark spectra used for computer analysis. (A) G•+ produced from dGuo via one-electron oxidation by Cl2•− at pD ca. 3. 14, 15 (B) C5′•, formed via photo-excitation of G•+ in 8-D-3′-dGMP.9 (C) C3′•, produced via photo-excitation of G•+ in dGuo.9 (D) C1′•, produced via photo-excitation of G•+ in TGT. 11 (E) C1′•, produced via photo-excitation of G•+ in 5′-dGMP.9 (F) As an example of the formation of the matrix radical (Cl2•−) owing to 405 nm photoexcitation of one-electron oxidized G (hole transfer to the matrix), the spectrum 4A″ is shown here. (G) Partial spectrum (220 G scan) of the total Cl2•− spectrum (1000 G scan) shown in spectrum 1(H). The scan range of Cl2•− spectrum shown in Figure 1(G) matches those of 1(A) to 1(E). (H) the Cl2•− spectrum obtained via γ-irradiation of 7.5 M LiCl/D2O solution containing persulfate to capture electrons followed by annealing at 143 K for 20 min.18
The three reference markers (open triangles) in this Figure and in other Figures show the position of Fremy's salt resonance with the central marker at g = 2.0056. Each of these markers is separated from each other by 13.09 G.
The benchmark spectra of the radicals that were used to analyze the experimental spectra recorded after annealing, and after photoexcitations using lasers are shown in the Figure 1.
In Figure 1, spectrum A is the benchmark spectrum of G•+ obtained via one-electron oxidation by Cl2•− in dGuo at pD ca. 3 in 7.5 M LiCl/D2O. 14, 15 The ESR spectrum of C5′• (Figure 1B) and of C3′• (Figure 1C) have been obtained via photoexcitation of G•+ in dGuo in 7.5 M LiCl/D2O ( pD ca. 5). 9
Two benchmark spectra of C1′• have been used to determine the fraction of C1′• present in the experimentally recorded spectra (Figures 2 to 5). The C1′• spectrum shown in Figures 1D has been obtained from TGT 11 whereas the C1′• spectrum presented in Figure 1E was from 5′-dGMP 9. The β-hyperfine couplings of C2′-H atoms for C1′• varied slightly in these two spectra - (16 G (1 βH), 32 G (1 βH) – for C1′• from 5′-dGMP 9 and 15 G (1 βH), 34.5 G (1 βH) – for C1′• from TGT 11). Spectrum 1D has been used to analyze the percentage contribution of C1′• in the experimental spectra obtained from TGT (Figure 3) and from the dsDNA oligomers (Figures 4 and 5). On the other hand, spectrum 1E has been employed to analyze the percentage contribution of C1′• in the experimental spectrum (Figure 2) obtained from the dGuo samples. For spectra 1A to 1E, the three ESR lines (shown as open triangles) of the Fremy's Salt are each separated by 13.09 G with g(center) = 2.0056. All the spectra (Figures 1 to 5 along with supporting information Figures S1 and S2) are stored as 1000 point arrays, and the Fremy field markers in all these spectra are at 440, 500, 560, except for the spectrum shown in Figure 1H which are at 487, 500, 513.
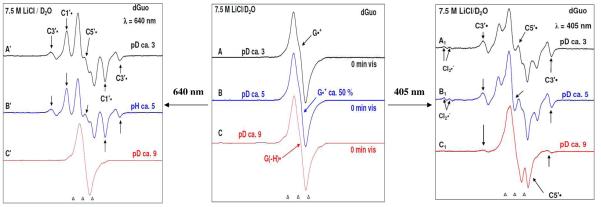
Figure 2.
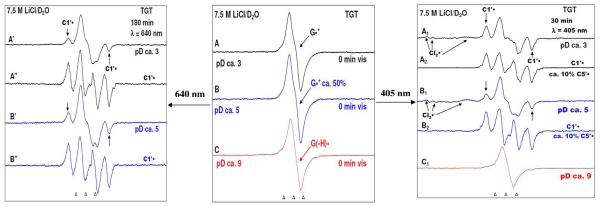
ESR spectra of one-electron oxidized dGuo formed by the attack of Cl2•− in the presence of electron scavenger K2S2O8 in 7.5 M LiCl/D2O at (A) pD ca. 3 (B) pD ca. 5 and (C) pD ca. 9. After exposure of these samples to the 405 nm laser (right panel) for 1 h (spectra A1 and B1) and for 3 h 30 min (spectrum A′) and 5 h 30 min (spectrum B′) to 640 nm laser (left panel), the formation of sugar radicals are observed mainly C5′• and C3′• along with the matrix radical Cl2•− (with 405 nm laser) and C5•′, C3′•, and C1′• (with 640 nm laser). No sugar radicals are observed at pD ca. 9 with 640 nm laser but a small amount (ca. 20%) is observed with 405 nm for 30 min.
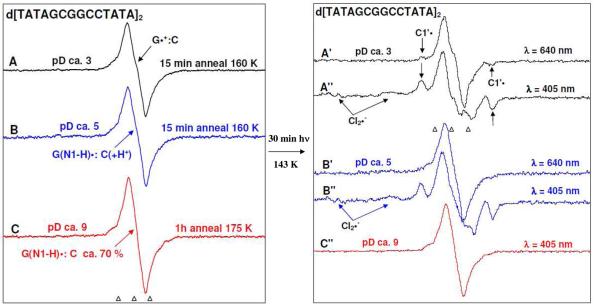
Figure 5.
Left panel: ESR spectra of one-electron oxidized d[TATAGCGGCCGCTATA]2 (3 mg/ml) formed via one-electron oxidation by Cl2•− via thermal annealing the sample at the temperature range 155 to 175 K, in the presence of electron scavenger K2S2O8 in 7.5 M LiCl/D2O. Spectrum (A, black) at pD ca. 3 is assigned to the cation radical state (G•+:C); spectrum (B, blue) at pD ca. 5 to the intra-base pair proton transferred state (G(N1-H)•:C(+H+)) and spectrum (C, red) at pD ca. 9 to the deprotonated state (G(N1-H)•:C (ca. 70%) and (G(N2-H)•:C (ca. 30%)) (see supporting information Figure S1). Right panel: Spectra are obtained after exposure of duplicate samples at each pD to 640 nm for 30 min (40 mW) (Spectrum A′, black, pD ca. 3 and Spectrum B′, blue, pD ca. 5) and to 405 nm (30 mW) for 30 min (Spectrum A″, black, pD ca. 3, Spectrum B″, blue, pD ca. 5, and spectrum C″, red, pD ca. 9) lasers at 143 K. Photoexcitation of one-electron oxidized Guanine in d[TGCGCGCA]2, at pDs ca. 3 and 5 using 405 nm laser led to loss of one-electron oxidized guanine (35 to 40% remaining) with the concomitant formation of sugar radicals having predominant C1′• and a small extent (ca. 5%) of C5′ •. Only in the case of 405 nm photoexcitation, formation of matrix radical (Cl2•−) is observed. No photo-conversion is found at pD ca. 9 as shown in spectrum C″. Spectra A′ (pD ca. 3) shows the small amount (ca. 10 %) of formation of C1•′ via 640 nm photoexcitation of the cation radical state (G•+:C) whereas spectrum B′ shows very little photoconversion at pD ca. 5.
Figure 3.
ESR spectra of one-electron oxidized TGT (3 mg/ml) formed by attack of Cl2•− via thermal annealing the sample at 155 K, in the presence of electron scavenger K2S2O8 in 7.5 M LiCl/D2O, (A) at pD ca. 3. (B) at pD ca. 5 and (C) at pD ca. 9. Spectra are obtained after exposure of duplicate samples at each pD to 640 nm (40 mW) (left panel) for 3 to 5 h and to 405 nm (30 mW) (right panel) lasers for 30 min at 143 K. Photoexcitation of one-electron oxidized Guanine in TGT, with both 405 nm and 640 nm lasers lead to loss of one-electron oxidized guanine with the concomitant formation of sugar radicals and only in the case of 405 nm photoexcitation, formation of matrix radical (Cl2•−) is observed. Subtraction of matrix radicals (Cl2•−) and remaining one-electron oxidized guanine (ca. 25%) results in the spectra A2 and B2 which show the formation of mainly C1′• and a very small amount of C5•′. No photo-conversion from G(-H)• is found at pD ca. 9 as shown in spectrum C1. Spectra A″ and B″ (left panel) are obtained after the subtraction of remaining one-electron oxidized Guanine, G•+ (ca. 65 %) which shows the predominant formation of C1•′.
Figure 4.
Left panel: ESR spectra of one-electron oxidized d[TGCGCGCA]2 (3 mg/ml) formed via one-electron oxidation by Cl2•− via thermal annealing the sample at the temperature range 155 to 175 K, in the presence of electron scavenger K2S2O8 in 7.5 M LiCl/D2O. Spectrum (A, black) at pD ca. 3 is assigned to the cation radical state (G•+:C); spectrum (B, blue) at pD ca. 5 to the intra-base pair proton transferred state (G(N1-H)•:C(+H+)) and spectrum (C, red) at pD ca. 9 to the deprotonated state (G(N2-H)•:C (ca. 60%) and (G(N1-H)•:C (ca. 40%)) 9. Right panel: Spectra are obtained after exposure of duplicate samples at each pD to 640 nm for 1h (40 mW) (Spectrum A′, black, pD ca. 3 and Spectrum B′, blue, pD ca. 5) and to 405 nm (30 mW) for 30 min (Spectrum A″, black, pD ca. 3, Spectrum B″, blue, pD ca. 5, and spectrum C″, red, pD ca. 9) lasers at 143 K. Photoexcitation of one-electron oxidized Guanine in d[TGCGCGCA]2, at pDs ca. 3 and 5 using 405 nm laser led to loss of one-electron oxidized guanine (20% remaining) with the concomitant formation of sugar radicals having predominant C1′• and a small extent (ca. 5%) of C5′ •. Only in the case of 405 nm photoexcitation, formation of matrix radical (Cl2•−) is observed. No photo-conversion is found at pD ca. 9 as shown in spectrum C”. Spectra A′ (pD ca. 3) shows the small amount (ca. 15 %) of formation of C1•′ via 640 nm photoexcitation of the cation radical state (G•+:C) whereas spectrum B′ shows very little photoconversion at pD ca. 5.
In this work, we have found that via 405 nm photoexcitation of one-electron oxidized G, results in hole transfer to the matrix resulting in the formation of Cl2•− (see Figures 2 to 5). In the Figure 1F, we have taken spectrum 4A″ (i.e., 405 nm photoexcitation of one-electron oxidized d[TGCGCGCA]2) as an example of the formation of the matrix radical (Cl2•−). In the Figure 1H, we present the full spectrum of Cl2•− over 1000 G as shown in previous work.18 Hence, comparison of spectrum 1G (partial Cl2•− spectrum) with spectrum 1H (complete Cl2•− spectrum) shows that only the two large low field resonances from Cl2•− are observed in spectrum 1G and these are indicated by the arrows. To analyze the percentage composition of each sugar radical in Figures 2 to 5, we have subtracted the Cl2•− line components by employing the Cl2•− spectrum presented in Figure 1G (partial Cl2•− spectrum over same scan range as A through F (220 G scan)). To analyze the percentage of Cl2•− produced via 405 nm photoexcitation of one-electron oxidized G, for example in spectrum 1F, we simulated spectra that matched the height of the large low field resonances from Cl2•− (spectrum 1H).
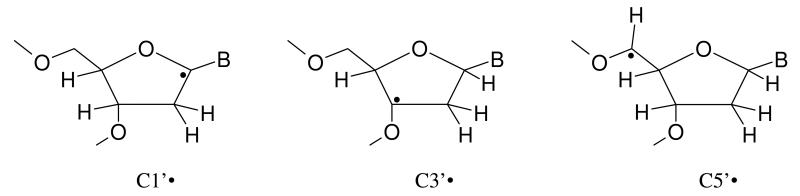
The structures of the various sugar radicals and the DNA-base radicals studied in this work are mentioned in schemes 2 and 3 respectively.
Scheme 2.
The structures of the sugar radicals used in this work; ‘B’ stands for the DNA base.
Scheme 3.
The structures of the DNA base radicals used in this work. The numbering scheme for G:C cation radical is also shown.
Results and Discussion
1. Protonation states of the one-electron oxidized compounds studied at pDs ca. 3, 5, and 9
dGuo
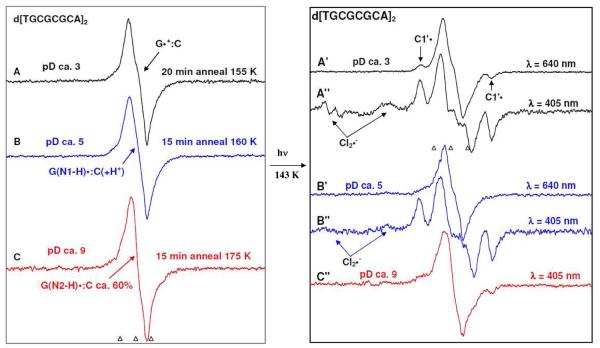
In Figures 2 (A), (B), and (C), the ESR spectra of one-electron oxidized guanine in dGuo formed via thermal annealing of the sample at 155 K in the presence of electron scavenger K2S2O8 in 7.5 M LiCl glass are shown. As reported earlier,14, 15 one-electron oxidized guanine in dGuo at pD ca. 3, exists in its “pristine” cation radical form (G•+) (spectrum 2A, black), at native pD of 7.5 M LiCl/D2O (pD ca. 5) in half of its protonated form (G•+) and half of its deprotonated form (G (N1-H)• or G(-H)•) (spectrum 2B, blue), and at pD ca. 9 exists in the deprotonated form (G(−H)•) (spectrum 2C, red).
TGT
In Figure 3(A), (B) and (C), the ESR spectra of one-electron oxidized TGT at different pDs ca. 3, 5 and 9 in 7.5 M LiCl/D2O are shown. The one-electron oxidation of TGT is carried out by Cl2•− via thermal annealing of the sample at 155 K. The spectrum 3A is found to be identical to the G•+ spectrum (Figure 2A, black) in dGuo. Using the benchmark spectrum shown in Figure 1A and also from our previous study using 8-deuteroguanine incorporated TGT,14 it is clear that one-electron oxidized guanine in TGT exists in cation radical form (G•+) at pD ca. 3 (spectrum 3A, black), in equal amount of its G•+ and G(−H)• forms at pD ca. 5 (for simplicity, it will be referred to as G•+ at this pD) (spectrum 3B, blue), and in its deprotonated form G(−H)• at pD ca. 9 (spectrum 3C, red).
d[TGCGCGCA]2
The ESR spectra of one-electron oxidized d[TGCGCGCA]2 at different pDs ca. 3, and 9 in 7.5 M LiCl/D2O are presented in the Figure 4(A), (B) and (C). The one-electron oxidation of d[TGCGCGCA]2 was carried out by Cl2•− via thermal annealing of the sample in the temperature range 155 to 175 K. Spectrum 4A of one-electron oxidized d[TGCGCGCA]2 at pD ca. 3 matches that of the G•+ benchmark spectrum (spectrum 1A). Our previous work which established the nature of protonation states vs. pH in dsDNA oligos,13a,14a allows us to assign one-electron oxidized d[TGCGCGCA]2 to exist in the following forms: pD 3, cation radical form (G•+:C) (spectrum 4A, black), pD 5, intra-base pair proton transferred form G(N1-H)•:C(+H+)• (spectrum 4B, blue), and at pD 9 in a mixture of its deprotonated forms (spectrum 4C, red) G(N2-H)•:C (ca. 60%) and G(N1-H)•:C (ca. 40%).. At pD 9, the deprotonation takes place from the duplex to the surrounding solvent i.e., D2O (see scheme 1).
d[TATAGCGGCCGCTATA]2
Being an 8-mer, d[TGCGCGCA]2 does not have a complete turn of the double helix. As a result, the proton on N1 in one-electron oxidized d[TGCGCGCA]2 may have direct access to solvent.. Hence, we have extended the studies regarding protonation states of one-electron oxidized guanine in a 16-mer, d[TATAGCGGCCGCTATA]2.
In the left panel of Figure 5 (Figure 5(A), (B), and (C)), the ESR spectra of one-electron oxidized d[TATAGCGGCCGCTATA]2 after thermal annealing to temperatures in the range 155 to 175 K at different pDs ca. 3, 5 and 9 in 7.5 M LiCl/D2O are shown. Following our work regarding characterization of the various protonation states of one-electron oxidized G in dsDNA oligos,13a,14a and the studies shown in Figure 4 in this work, we assign that at pD ca. 3, the one-electron oxidized d[TATAGCGGCCGCTATA]2 exists in its cation radical form (G•+:C) (spectrum 5A, black) and at pD 5, the one-electron oxidized d[TATAGCGGCCGCTATA]2 exists in the intra-base pair proton transferred form G(N1-H)•:C(+H+)• (spectrum 5B, blue). However, employing the reported authentic spectra of dG(N1-H)• and dG(N2-H)• derived from one-electron oxidized dGuo at pD ca. 9 15 and 1-methyl-guanosine (1-Me-Guo) at pD ca. 9 13a respectively as benchmarks, the spectrum of one-electron oxidized d[TATAGCGGCCGCTATA]2 at pD ca. 9 obtained via annealing at 175 K (spectrum 5C, red) was found to be an equilibrium mixture of its duplex-to-solvent deprotonated forms G(N1-H)•:C (ca. 70%) and G(N2-H)•:C (ca. 30%) (supporting information Figure S1)..
2. Photoexcitation studies
The results of the photoexcitation of the compounds (Figures 2 to 5) are summarized in Table 1 below.
Table 1.
Formation of Cl2•− and sugar radicals via photoexcitation of one-electron oxidized guanine in DNA systems by 405 and 640 nm at 143 K and at different pHs a
| Compound | λ (nm) |
pH | Percent convertedb (Time (h)) |
Radicals produced (%) | |||
|---|---|---|---|---|---|---|---|
| Cl2•− | C1′• | C3′• | C5′• | ||||
| dGuo | 405 | 3 | 90 (1h) | 15 | - | 25 | 50 |
| 5 | 90 (1h) | 10 | - | 35 | 45 | ||
| 9 | 20 (0.5) | - | - | 5 | 15 | ||
| 640 | 3 | 75 (3.5) | - | 15 | 30 | 30 | |
| 5 | 80 (5.5) | - | 15 | 35 | 30 | ||
| 9 | - (0.5) | - | - | - | - | ||
| TGT | 405 | 3 | 70 (0.5) | 30 | 35 | - | 5 |
| 5 | 70 (0.5) | 30 | 35 | - | 5 | ||
| 9 | - | - | - | - | - | ||
| 640 | 3 | 30 (3) | - | 30 | - | - | |
| 5 | 30 (5) | - | 30 | - | - | ||
| d[TGCGCGCA]2 | 405 | 3 | 85 (0.5) | 60 | 20 | - | 5 |
| 5 | 85 (0.5) | 30 | 50 | - | 5 | ||
| 9 | - (0.5) | - | - | - | - | ||
| 640 | 3 | 15 (1h) | - | 15 | - | - | |
| 5 | ca. 5 (1h) | - | ca. 5 | - | - | ||
| d[TATAGCGGCCTATA]2 | 405 | 3 | 60 (0.5) | 30 | 25 | - | 5 |
| 5 | 60 (0.5) | 20 | 35 | - | 5 | ||
| 9 | - (0.5) | - | - | - | - | ||
| 640 | 3 | 10 (0.5) | - | 10 | - | - | |
| 5 | ca.5 (0.5) | - | ca.5 | - | - | ||
Radical percentages are ±10% relative error 14a
Percentage conversion of one-electron oxidized G to various sugar radicals and Cl2•−. The total spectral intensities before and after photoexcitation were found to be nearly identical.
Analyses of the data presented in Table 1 result in the following important findings:
- pH and wavelength dependent formation of the matrix radical (Cl2•−) via photoexcited one-electron oxidized G in monomer (dGuo) and in ss and ds DNA oligomers
-
In Figures 2 and 3, and in Table 1, we present the evidence that only photoexcitation by 405 nm of one-electron oxidized guanine in its cation radical (G•+) state in each of the DNA systems investigated oxidizes the surrounding matrix (homogeneous LiCl solution) resulting in Cl2•− formation. However, in case of ds DNA-oligomers, Cl2•− formation is observed via 405 nm photoexcitation of both cation radical (G•+:C) and the intra-base pair proton transferred (G(N1-H)•:C(+H+)) forms (see Figures 4 and 5, and Table 1). Moreover, among the various types of DNA model systems studied in this work, photoexcitation via 405 nm of (G•+:C) form produces the maximum amount of Cl2•−.In order to ascertain whether formation of additional Cl2•− occurred via photoexcitation of G•+ and not as a result of direct photooxidation of the matrix, we have performed control experiments. We employed samples of 7.5 M LiCl/D2O at pDs ca. 3, 5, and 9 in the presence of K2S2O8 handled, γ-irradiated, annealed, and then photoexcited by 405 nm laser identical to DNA samples. These results are presented in the supporting information Figure S2 and show that upon photoexcitation by 405 nm light, no formation of Cl2•− occurred. This establishes that the formation of Cl2•− is caused by photoexcitation of G•+ with 405 nm light through hole transfer to the matrix (one-electron oxidation of the matrix).As discussed in section 1, for TGT (ssDNA) and the nucleoside dGuo at pH ca. 5, one-electron oxidized guanine is an equilibrium mixture (ca. 50 % each) of the cation radical (G•+) and the deprotonated (G(N1-H)•) forms. For dGuo and for TGT, owing to the lack of base pairing, the G(N1-H)• form results from deprotonation from G•+ to the solvent. 13 - 15 However, for dsDNA oligomers at pH ca. 5, one-electron oxidized guanine is in the G(N1-H)•:C(+H+) form owing to intra-base pair proton transfer. In order to explain the Cl2•− formation via excited (G(N1-H)•:C(+H+)) form in dsDNA oligomers, we propose that within the lifetime of the excited state of (G(N1-H)•:C(+H+)), proton transfer should occur from C(+H+) to the N1 atom in G(N1-H)• is required (see scheme 4). Thus, we propose that in the excited state, the presence of the guanine N1-H proton is crucial for Cl2•− formation. We note here that using photoexcited ds DNA oligomers containing G:C sequence, the Kohler group 20, 21 have shown the proton transfer occurs within the lifetime of ion-radical exciplexes.There is no formation of Cl2•− via 640 nm light in all the compounds studied at all pHs (i.e., in all the protonation states). Further, one-electron oxidized ds oligomers which are deprotonated to solvent, i.e., one-electron oxidized oligomers in the G(N1-H)•:C and G(N2-H)•:C forms, also show no formation of Cl2•− via photoexcitation at 640 as well as at 405 nm.We conclude that excited states of the one-electron oxidized G produced by 640 nm light are less oxidative than those created by 405 nm light. Clearly, each wavelength is exciting a different band in the G•+ spectrum14 and only the higher energy band results in matrix oxidation.We note here that, although Cl2•− is a strong oxidant (E° (Cl2•− / 2Cl− = 2.0 V vs. normal hydrogen electrode [NHE] in aqueous solution at room temperature)19, it cannot oxidize thymidine (Thd) and 2′-deoxycytidine (dCyd) in our system (homogeneous LiCl solution (pH/pD ca. 5) around 77 – 150 K) via one-electron oxidation. From this observation, and from the reported values of reduction potentials of the DNA base cation radicals 3, 4, we predict that the reduction potential of excited G•+ (i.e., (G•+)* for dGuo and TGT, and ((G:C)•+)*, see scheme 4) for dsDNA oligomers) >1.8 V in our system (homogeneous aqueous solution at low temperature).
-
- Sugar radical formation in DNA-model systems (monomers to ds DNA oligomers) – influence of wavelength, pH, and the length of the oligomers:
- Our work shows that the yield of individual sugar radical and the extent of photoconversion are crucially controlled by the wavelength of the incident light and the pH of the solution and the length and nature of the oligomer. This is illustrated in Table 1 and led to the following salient points:
-
Effect of pH: For one-electron oxidized dGuo, TGT, and the dsDNA oligomers at pD ca. 3, where G•+ or G•+:C is present, only 30 min of photoexcitation at 405 nm is required for a substantial conversion (ca. 50 to 90 % of the total radicals produced) to the sugar radicals. Even at pD ca. 5, for one-electron oxidized dGuo and TGT existing in the equilibrium mixture of G•+ and G(N1-H)•, and for ds DNA oligomers existing in the intra-base pair proton transferred form (G(N1-H)•:C(+H+)•), we find excellent conversion to sugar radicals via 30 min photo-excitation at 405 nm. The presence of the N1-H proton in G is crucial for the formation of sugar radicals (see scheme 4). We already reported formation of sugar radicals via photoexcitation of one-electron oxidized d[TGCGCGCA]2 using photoflood lamp at the following order of the pH of the solution: pH ca. 3 > pH ca.5 >> pH ca. 9.13aTherefore, this work using lasers of specific wavelengths (405 and 640 nm) and our previous observations using photoflood lamp 13a clearly suggest that excitation of (G(N1-H)•:C(+H+)) in dsDNA results in reverse proton transfer from N3-atom of cytosine C to N1-atom on G thereby leading to the excited cation radical ((G:C)•+)* formation (see scheme 4). This proton transfer process must occur within the lifetime of the excited state of this incipient excited cation radical. Subsequent deprotonation from the sugar moiety in the excited cation radical leads to the neutral sugar radical formation (see scheme 4). Such proton transfers in photoexcited ds DNA oligomers containing G:C sequence have been shown by the Kohler group 20, 21.We find that where deprotonation of one-electron oxidized guanine has occurred to the surrounding solvent, no sugar radicals are formed on photoexcitation. Thus, we conclude that the excitation of the G(N1-H)•, G(N1-H)•:C, and G(N2-H)•:C is ineffective in causing such oxidations either owing to the lifetime or redox properties of the excited states of these protonation states.
- Effect of wavelength: We find that the total conversion to sugar radicals decreases substantially with increasing wavelength for all DNA systems (i.e., from dGuo to ds DNA oligomers). Note that more extended time periods are needed to photo-convert one-electron oxidized G in dGuo and in TGT used at pD ca. 3 and at pD ca. 5 to the sugar radicals at 640 nm than at 405 nm. For G•+:C and G(N1-H)•:C(+H+) forms of the longer ds DNA oligomer d[TATAGCGGCCGCTATA]2, the sugar radical conversion becomes negligible at 640 nm. Moreover, 405 nm photoexcitation in dsDNA oligomers led to the predominant formation of C1′• along with little formation of C5′•. Sugar radical formation via photo-excitation of G•+ in γ-irradiated, hydrated (Γ = 12 D2O / nucleotide) DNA has been found to be wavelength dependent.9 In the 310-480 nm range, formation of C1′• in substantial yields have been observed in dsDNA via photo-excitation of G•+ from 77 K to 180 K but little conversion occurs at longer wavelengths.8, 9, 11 Therefore, the findings shown in Table 1 bridge our previous findings that a wavelength dependence exists for the formation of sugar radicals in ss DNA oligomers 11 and in d[TGCGCGA]2 13a and in highly polymerized DNA9. Results obtained by TD-DFT calculations for excited G•+ in TpdG and in other dinucleoside phosphates 10 show that base-to-base hole transfer takes place at wavelengths longer than 500 nm, in preference to base-to-sugar hole transfer. Only base-to-sugar hole transfer leads to the formation of sugar radicals (see scheme 4). Therefore, the substantially lower yields of sugar radicals in ds DNA found at 640 nm are likely explained by the excitation-induced base-to-base hole transfer which competes with the excited base-to-sugar hole transfer.
-
Oligomer Effect: While C3′• is formed via photoexcitation of G•+ in dGuo, (see C3′• benchmark spectrum in Figure1C) the spectra obtained from photoexcited one-electron oxidized TGT and ds DNA oligomers clearly show that photoexcitation of one-electron oxidized G in these samples does not produce C3′• and instead forms mainly C1′• and some C5′•In case of dGuo, both 3′- and 5′-sites have free –OH groups, whereas for G in TGT and in ds DNA oligomers, the 3′- and the 5′-sites have phosphate groups. We have previously provided evidence that the presence of a phosphate group (instead of a hydroxyl group) at C3′- and C5′- sites deactivates the site to radical formation. 22 This finding was supported by previous theoretical calculations for the relative stability of sugar-phosphate radicals that indicated a destabilization of the resultant radical upon phosphate substitution. 23, 24 Thus, in comparison to the predominant C5′• formation and significant C3′• formation via 405 nm photoexcitation of one-electron oxidized guanine in dGuo, the corresponding 405 nm photoexcitation in TGT and in ds DNA oligomers in both at pD ca. 3 and ca. 5 show predominant formation of only C1′• along with no observable C3′• formation and a small yield of C5′•.
- Length of the dsDNA oligomer: It is evident from Table 1 with the increase of the length of the ds DNA oligomer (from 8-mer to 14-mer) both the overall yield of the sugar radical, mostly C1′, as well as the yield of the Cl2•− formed via 405 nm photoexcitation decrease. This decrease in the radical yield for the longer dsDNA oligomer may be a result of the increase in the number of deactivation modes such as base-to-base hole transfer (see section (b)).
- Photostability of C1′• in dGuo: In dGuo samples, 405 nm photoexcitation does not produce observable C1′• (Table 1). C1′• formation is observed only at 640 nm. This suggests that either the increased formation of C5′• at 405 nm is the result of a wavelength dependence on specific radical yields or the photoconversion of already produced C1′• to C5′•. Our preliminary work suggests photoconversion of C1′• to C5′• and this is being further investigated currently in our laboratory.
-
Scheme 4.
Schematic representation of the formation of the matrix radical (Cl2•−) and sugar radicals via photoexcited G•+ :C and G(N1-H)•:C(+H+) in DNA. The facile reverse proton transfer from cytosine N3H to guanine N1 via excitation of G(N1-H)•:C(+H+) form is also shown in this scheme.
Conclusion
(i) Mechanism involved in the formation of the matrix radical (Cl2•−) via photoexcited one-electron oxidized G
This work provides evidence for two excited state hole transfer processes induced by excitation of guanine cation radical, G•+ (i) transfer to the sugar moiety leading to neutral sugar radical formation which has also been shown in previous efforts 8 - 13a and (ii) hole transfer to matrix forming Cl2•− (see scheme 4).
We find that one-electron oxidized DNA systems in the cation radical form, G•+:C, are most active for either sugar radical or Cl2•− formation which demonstrate that the presence of N1-H proton in G is essential to these processes (see scheme 4). The G(N1-H)•:C(+H+) form is also found to be active in these processes. The data in Table 1 clearly demonstrate that deprotonation of one-electron oxidized G in DNA to the surrounding solvent leads to form G(N1-H)•:C or G(N2-H)•:C, via which no photooxidation results. Hence, proton transfer from one-electron oxidized G in DNA to the surrounding solvent prevents hole transfer from one-electron oxidized G to sugar or to the matrix. Thus, for the G(N1-H)•:C(+H+) form, formation of sugar radical or Cl2•− is very likely a result of facile reverse protonation in the excited state of G(N1-H)•:C(+H+) to form G•+ :C (see scheme 4).
The formation of sugar radicals and Cl2•− at 405 nm and the formation of sugar radicals and the complete lack of formation of Cl2•− at 640 nm (Table 1) is attributed to the fact that each wavelength is exciting a different band in the G•+ spectrum.14 Only the higher energy 405 nm band results in matrix oxidation. The yield of Cl2•− on photoexcitation at 405 nm is found to follow the order: dsDNA (8-mer) > dsDNA (14-mer) ≈TGT> dGuo. The lack of formation of either radical on excitation of G(N1-H)•, G(N1-H)•:C, and G(N2-H)•:C suggests their excited states, which are ionic in nature (scheme 4), have lower redox potentials and likely short lifetimes.
(ii) Relevance of these studies to the reported hole transfer processes in DNA
A variety of experimental techniques (e.g., (a) UV-vis photoexcitation of specific sequences of dsDNA oligomers having donors and acceptors at fixed distances at ambient temperatures, (b) ultrafast laser flash photolysis of specific sequences of dsDNA oligomers having donors and acceptors, (c) ESR spectral studies of irradiated DNA systems at low temperatures have been used to study the extent and rate of hole transfer processes in DNA. 5 - 7, 13, 14, 25 - 31 Our previous work as well as other reported experimental and theoretical studies have provided evidence for the prototropic equilibria of the hole (i.e., the guanine cation radical) in dsDNA 3 - 7, 23 - 31 (shown in scheme 1) and that these reversible proton transfer processes (intra-base pair proton transfer and duplex-to-solvent deprotonation) crucially control the rate and extent of the hole transfer processes in DNA in the ground state. 5 - 7, 13, 14, 25 - 31 The work reported here shows that two excitation events induced hole transfer processes via the ((G:C)•+)* state (scheme 4) resulting in sugar radical or matrix radical (Cl2•−) would clearly terminate such long range hole transfer processes in DNA.
In the work reported here annealing of one-electron oxidized d[TGCGCGCA]2 and d[TATAGCGGCCGCTATA]2 up to 175 K resulted in an equilibrium mixture of G(N1-H)•:C and G(N2-H)•:C but little G•+:C is present.17 Previous work employing γ-irradiated hydrated (Γ = (12 ± 2) H2O molecules per nucleotide) DNA samples, clearly established that formation of •GOH and 8-oxo-G•+ occurs via multiple one-electron oxidations via thermally activated hole hopping at ca. ≥ 200 K 17. At these elevated temperatures (≥ 200 K), the prototropic equilibria between various forms of one-electron guanine in DNA (scheme 1) are established as theory shows the energy needed is relatively small.13, 14(b)-(e) Thus as significant amounts of G•+:C become available on annealing above 200 K, nucleophilic addition of water takes place at C-8 atom in the guanine moiety leading to the formation of •GOH which by subsequent two one-electron oxidations forms 8-oxo-G•+. 17 Thermally activated hole transfer from one site to another is activated at temperatures above 200K. Thus, in our system the hole localizes at a guanine site and only moves along the strand by further annealing or photoexcitation.
(iii) Implications of matrix radical (Cl2•−) formation from ((G:C)•+)*
In our system, the high concentration of Cl− (318 mg LiCl/ml) facilitated the transfer of holes from ((G:C)•+)* to the matrix via 405 nm photoexcitation. The cell nucleus has a very different environment but is not a dilute aqueous solution owing to the high global concentration of macromolecules (DNA, RNA, proteins etc.) which range from 65 to 220 mg/ml.32a The concentration of Cl−, ca. 4 mM, is of course much lower than in our system.32 In cellular systems, dsDNA is packaged in nucleosomes with the DNA wrapped around a histone protein core which forms the basic unit of the chromatin structure.32 Consequently, hole transfer from the ((G:C)•+)* state in dsDNA to the surrounding nucleoproteins or other oxidizable species, would lower the extent of the sugar damage as illustrated in scheme 4. Our mechanism of formation of the matrix radical (Cl2•−) via ((G:C)•+)* may extend to systems in which DNA is closely associated with oxidizable structures. This would, of course, act as a protective mechanism.
Supplementary Material
Acknowledgements
This work was supported by the NIH NCI under grant no. R01CA045424. AA is grateful to Prof. R. F. Anderson (Auckland Cancer Society Research Center, the University of Auckland, New Zealand) for his suggestion to characterize the protonation states of one-electron oxidized guanine in a longer ds DNA oligomer.
Footnotes
Supporting Information Available:
Supporting information contains the following Figures: (1) Figure S1 shows that employing dG(N1-H)• spectrum from dGuo and G(N2-H)• spectrum from 1-Me-Guo as benchmarks, the spectrum of one-electron oxidized d[TATAGCGGCCGCTATA]2 at pD ca. 9 was found to be a mixture of its duplex-to-solvent deprotonated forms G(N1-H)•:C (ca. 70%) and G(N2-H)•:C (ca. 30%) (2) Figure S2 showing no formation of Cl2•− in homogeneous glassy solution of LiCl (7.5 M LiCL/D2O) via direct photoexcitation by 405 nm laser for 30 min at 143 K. This information is available free of charge via the internet at http://pubs.acs.org/.
Reference
- 1.Balzani V, Bolletta F, Gandolf MT, Maestri M. Top. Curr. Chem. 1978;75:1. [Google Scholar]
- 2.Lewis FD, Compton EM. In: CRC Handbook of Organic Photochemistry and Photobiology. Horspool WM, Lenci F, editors. CRC Press, Taylor & Francis; Boca Raton: 2003. pp. 7–1. [Google Scholar]
- 3.The reduction potential at pH 7 (i.e., midpoint potential (E7)) values of the DNA components at room temperature in aqueous solutions are: E7 (dG(−H)•/dG) = 1.29 V, E7 (dA(−H)•/dA) = 1.42 V, E7 (dT•/dT) = 1.7 V, E7 (dC•/dC) =1.6 V, and E7 (dR•/dR) >> 1.8 V (dR = 2′-deoxyribose). The pKa of the guanine cation radical (G•+) being 3.9, the reduction potential of G•+ is reported as (E° =1.58 V) –see Steenken S, Jovanovic SV. J. Am. Chem. Soc. 1997;119:617–618.
- 4.(a) Candeias LP, Steenken S. J. Am. Chem. Soc. 1989;111:1094–1099. [Google Scholar]; (b) Shinde SS, Maroz A, Hay MP, Anderson RF. J. Am. Chem. Soc. 2009;131:5203–5207. doi: 10.1021/ja8087339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Becker D, Adhikary A, Sevilla MD. In: Charge Migration in DNA: Physics, Chemistry and Biology Perspectives. Chakraborty T, editor. Springer-Verlag; Berlin, Heidelberg, New York: 2007. pp. 139–175. [Google Scholar]; (b) Dedon PC. Chem. Res. Toxicol. 2008;21:206–219. doi: 10.1021/tx700283c. [DOI] [PubMed] [Google Scholar]
- 6.Becker D, Adhikary A, Sevilla MD. In: Recent Trends in Radiation Chemistry. Rao BSM, Wishart J, editors. World Scientific Publishing Co.; Singapore, New Jersey, London: 2010. pp. 509–542. [Google Scholar]
- 7.Becker D, Adhikary A, Sevilla MD. In: Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces. Hatano Y, Katsumura Y, Mozumder A, editors. CRC Press, Taylor & Francis Group; Boca Raton, London, New York: 2010. pp. 503–541. [Google Scholar]
- 8.Khanduri D, Collins S, Kumar A, Adhikary A, Sevilla MD. J. Phys. Chem. B. 2008;112:2168–2178. doi: 10.1021/jp077429y. [DOI] [PubMed] [Google Scholar]
- 9.Adhikary A, Malkhasian AYS, Collins S, Koppen J, Becker D, Sevilla MD. Nucleic Acids Res. 2005;33:5553–5564. doi: 10.1093/nar/gki857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Adhikary A, Kumar A, Sevilla MD. Radiat. Res. 2006;165:479–484. doi: 10.1667/rr3563.1. [DOI] [PubMed] [Google Scholar]; (b) Kumar A, Sevilla MD. J. Phys. Chem. B. 2006;110:24181–24188. doi: 10.1021/jp064524i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kumar A, Sevilla MD. In: Radiation Induced Molecular Phenomena in Nucleic Acid: A Comprehensive Theoretical and Experimental Analysis. Shukla MK, Leszczynski J, editors. Springer-Verlag; Berlin, Heidelberg, New York: 2008. pp. 577–617. [Google Scholar]; (d) Kumar A, Sevilla MD. In: Radical and Radical Ion Reactivity in Nucleic Acid Chemistry. Greenberg MM, editor. John Wiley & Sons, Inc.; New Jersey: 2009. pp. 1–40. [Google Scholar]; (e) Kumar A, Sevilla MD. J. Phys. Chem. B. 2009;113:13374–13380. doi: 10.1021/jp9058593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikary A, Collins S, Khanduri D, Sevilla MD. J. Phys. Chem. B. 2007;111:7415–7421. doi: 10.1021/jp071107c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adhikary A, Khanduri D, Kumar A, Sevilla MD. J. Phys. Chem. B. 2008;112:15844–15855. doi: 10.1021/jp808139e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Adhikary A, Kumar A, Munafo SA, Khanduri D, Sevilla MD. Phys. Chem. Chem. Phys. 2010;12:5353–5368. doi: 10.1039/b925496j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) O'Neill M, Barton JK. J. Am. Chem. Soc. 2004;126:13234–13235. doi: 10.1021/ja0455897. [DOI] [PubMed] [Google Scholar]
- 14.(a) Adhikary A, Khanduri D, Sevilla MD. J. Am. Chem. Soc. 2009;131:8614–8619. doi: 10.1021/ja9014869. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cerón-Carrasco JP, Requena A, Perpe`te EA, Michaux C, Jacquemin D. J. Phys Chem. B. 2010;110:13439–13445. doi: 10.1021/jp101711z. [DOI] [PubMed] [Google Scholar]; (c) Reynisson J, Steenken S. Phys. Chem. Chem. Phys. 2002;4:5346–5352. [Google Scholar]; (d) Steenken S, Reynisson J. Phys. Chem. Chem. Phys. 2010;12:9089–9094. doi: 10.1039/c002528c. [DOI] [PubMed] [Google Scholar]; (e) Bera PP, Schaefer HF., III Proc. Natl. Acad. Sci. USA. 2005;102:6698–6703. doi: 10.1073/pnas.0408644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Adhikary A, Kumar A, Becker D, Sevilla MD. J. Phys Chem. B. 2006;110:24170–24180. doi: 10.1021/jp064361y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jena NR, Mishra PC, Suhai S. J. Phys Chem. B. 2009;113:5633–5644. doi: 10.1021/jp810468m. [DOI] [PubMed] [Google Scholar]; (c) Chatgilialoglu C, Caminal C, Altieri A, Vougioukalakis GC, Mulazzani QG, Gimisis T, Guerra M. J. Am. Chem. Soc. 2006;128:13796–13805. doi: 10.1021/ja062636h. [DOI] [PubMed] [Google Scholar]
- 16.Adhikary A, Kumar A, Khanduri D, Sevilla MD. J. Am. Chem. Soc. 2008;130:10282–10292. doi: 10.1021/ja802122s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Shukla LI, Adhikary A, Pazdro R, Becker D, Sevilla MD. Nucleic Acids Res. 2004;32:6565–6574. doi: 10.1093/nar/gkh989. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shukla LI, Adhikary A, Pazdro R, Becker D, Sevilla MD. Nucleic Acids Res. 2007;35:2460–2461. doi: 10.1093/nar/gkh989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevilla MD, Summerfield S, Eliezer I, Rak J, Symons MCR. J. Phys Chem. A. 1997;101:2910–2915. [Google Scholar]
- 19.Schwarz HA, Dodson RW. J. Phys. Chem. 1984;88:3643–3647. [Google Scholar]
- 20.de La Harpe K, Crespo-Hernández CE, Kohler B. J. Am. Chem. Soc. 2009;131:17557–17559. doi: 10.1021/ja9076364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de La Harpe K, Crespo-Hernández CE, Kohler B. ChemPhysChem. 2009;10:1421–1425. doi: 10.1002/cphc.200900004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla LI, Pazdro R, Huang J, DeVreugd C, Becker D, Sevilla MD. Radiat. Res. 2004;161:582–590. doi: 10.1667/rr3167. [DOI] [PubMed] [Google Scholar]
- 23.Colson A-O, Sevilla MD. J. Phys. Chem. 1995;99:3867–3874. [Google Scholar]
- 24.Colson A-O, Sevilla MD. Int. J. Radiat. Biol. 1995;67:627–645. doi: 10.1080/09553009514550751. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi K, Tagawa S. J. Am. Chem. Soc. 2003;125:10213–10218. doi: 10.1021/ja036211w. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Yamagami R, Tagawa S. J. Phys. Chem. B. 2008;112:10752–10757. doi: 10.1021/jp804005t. [DOI] [PubMed] [Google Scholar]
- 27.(a) O'Neill MA, Barton JK. In: Charge Transfer in DNA: From Mechanism to Application. Wagenknecht H-A, editor. Wiley-VCH Verlag GmbH & Co. KGaA; Weiheim: 2005. pp. 27–75. [Google Scholar]; (b) Genereux JC, Barton JK. Chem. Rev. 2010;110:1642–1662. doi: 10.1021/cr900228f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Genereux JC, Boal AK, Barton JK. J. Am. Chem. Soc. 2010;132:891–905. doi: 10.1021/ja907669c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster GB, editor. Topics in Current Chemistry. Springer-Verlag; Berlin, Heidelberg: 2004. Long Range Charge Transfer in DNA. I and II. [Google Scholar]
- 29.Giese B. Ann. Rev. Biochem. 2002;71:51–70. doi: 10.1146/annurev.biochem.71.083101.134037. [DOI] [PubMed] [Google Scholar]
- 30.(a) Spalletta RA, Bernhard WA. Radiat. Res. 1992;130:7–14. [PubMed] [Google Scholar]; (b) Weiland B, Hüttermann J. Int. J. Radiat. Biol. 1998;74:341–358. doi: 10.1080/095530098141483. [DOI] [PubMed] [Google Scholar]; (c) Weiland B, Hüttermann J. Int. J. Radiat. Biol. 1999;75:1169–1175. doi: 10.1080/095530099139647. [DOI] [PubMed] [Google Scholar]; (d) Debije MG, Bernhard WA. J. Phys. Chem. B. 2000;104:7845–7851. [Google Scholar]
- 31.(a) Bernhard WA, Close DM. In: Charged Particle and Photon Interactions with Matter Chemical, Physicochemical and Biological Consequences with Applications. Mozumdar A, Hatano Y, editors. Marcel Dekkar, Inc.; New York, Basel: 2004. pp. 431–470. [Google Scholar]; (b) Close DM. In: Radiation Induced Molecular Phenomena in Nucleic Acid: A Comprehensive Theoretical and Experimental Analysis. Shukla MK, Leszczynski J, editors. Springer-Verlag; Berlin, Heidelberg, New York: 2008. pp. 493–529. [Google Scholar]
- 32.(a) Hancock R, editor. Methods in Molecular Biology 463. Humana Press; New Jersey: 2008. The Nucleus. Volume 1: Nuclei and Subnuclear components. [Google Scholar]; (b) Clegg JS. Am. J. Physiol. 1984;246:R133–R145. doi: 10.1152/ajpregu.1984.246.2.R133. [DOI] [PubMed] [Google Scholar]; (c) Lett JT. Prog. Nucleic Acid Res. Mol. Biol. 1990;39:318. doi: 10.1016/s0079-6603(08)60630-3. [DOI] [PubMed] [Google Scholar]; (d) Cadet J, Douki T, Ravanat JL. Acc. Chem. Res. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.