Summary
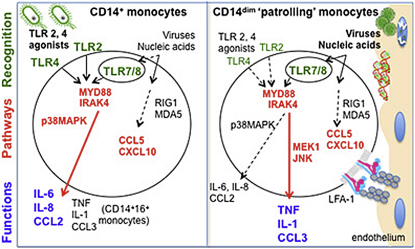
Monocytes are effectors of the inflammatory response to microbes. Human CD14+ monocytes specialize in phagocytosis and production of reactive oxygen species and secrete inflammatory cytokines in response to a broad range of microbial cues. Here, we have characterized the functions of human monocytes that lack CD14 (CD14dim) and express CD16. CD14dim monocytes were genetically distinct from natural killer cells. Gene expression analyses indicated similarities with murine patrolling Gr1dim monocytes, and they patrolled the endothelium of blood vessels after adoptive transfer, in a lymphocyte function-associated antigen-1-dependent manner. CD14dim monocytes were weak phagocytes and did not produce ROS or cytokines in response to cell-surface Toll-like receptors. Instead, they selectively produced TNF-α, IL-1β, and CCL3 in response to viruses and immune complexes containing nucleic acids, via a proinflammatory TLR7-TLR 8-MyD88-MEK pathway. Thus, CD14dim cells are bona fide monocytes involved in the innate local surveillance of tissues and the pathogenesis of autoimmune diseases.
Graphical Abstract
Highlights
► Human CD14dim monocytes patrol the vasculature ► CD14dim monocytes respond poorly to TLR1, TLR2, and TLR4 agonists ► CD14 dim monocytes respond to viruses and nucleic acids via TLR7-8-MyD88-MEK pathway
Introduction
Blood monocytes are bone marrow-derived phagocytes involved in the innate response to bacterial, fungal, parasitic, and viral infection (Barbalat et al., 2009; Gupta et al., 2001; Serbina et al., 2009; Serbina et al., 2008; Ströher et al., 2001). Nonetheless, the full spectrum of monocyte function, and in particular the specific functions of monocyte subsets, is not yet known. Recent work has revealed heterogeneity of monocytes, with two distinct subsets in rodents as well as in humans (Ancuta et al., 2009; Auffray et al., 2009; Geissmann et al., 2003; Ingersoll et al., 2010; Sunderkötter et al., 2004; Zhao et al., 2009; Ziegler-Heitbrock, 2007). Akin to other leucocytes, such as lymphocytes, evidence suggests that the diversity of functions ascribed to monocytes might reflect cellular heterogeneity, with distinct subsets endowed with specific functions.
On the basis of in vivo studies, murine monocytes have been separated into two subclasses. A major subset of “inflammatory” Gr1+ monocytes specializes in the production of tumor necrosis factor (TNF)-α, reactive oxygen species (ROS), and nitric oxide (NO) but little interleukin (IL)-10 upon in vivo infection with bacteria such as Listeria monocytogenes or parasites such as Toxoplasma gondii (Serbina et al., 2008). Gr1+ monocytes also produce type 1 interferon in response to viral ligands (Barbalat et al., 2009). In contrast, Gr1− monocytes patrol the blood vasculature, can differentiate into macrophages after extravasation into tissues, and have been suggested to be associated with tissue repair (Auffray et al., 2007; Nahrendorf et al., 2007). Their role in response to infection is not documented.
Human CD14+ CD16− monocytes resemble Gr1+ cells based on surface marker expression and gene expression arrays (Geissmann et al., 2003; Ingersoll et al., 2010; Strauss-Ayali et al., 2007) and are reportedly producers of IL-10 and weak producers of TNF-α in vitro. Human CD16+ monocytes, which resemble Gr1− cells, are described as the main producers of inflammatory cytokines such as TNF-α and IL-1β in response to bacterial lipopolysaccharides (LPSs) (Belge et al., 2002). Recent work demonstrates distinct responses of human monocyte subsets to Aspergillus fumigatus conidia (Serbina et al., 2009). Although both monocyte subsets efficiently phagocytose conidia, CD14+ CD16− monocytes inhibit conidial germination yet secrete little TNF-α, whereas CD14+ CD16+ monocytes do not inhibit conidial germination but secrete large amounts of TNF-α (Serbina et al., 2009). Of note, an additional degree of complexity was introduced by the work of Grage-Griebenow (Skrzeczyńska-Moncznik et al., 2008) and Schäkel (Schäkel et al., 2002), indicating that human CD16+ monocytes are not a homogeneous cell population. Thus, on the one hand it is increasingly clear that monocyte subsets exert critical specific functions in the response to microbes, whereas on the other hand the reported differences in the behavior of monocyte subsets in mice and human have hampered the description of potential evolutionary conserved functions (Auffray et al., 2009; Skrzeczyńska-Moncznik et al., 2008; Ziegler-Heitbrock, 2007).
The murine population of Gr1− monocytes patrol the blood vasculature and is therefore ideally located to survey local tissue damage and infection (Auffray et al., 2007). The present study was therefore designed to search for putative human functional homologs to murine Gr1− patrolling monocytes and to characterize the full spectrum of their functions.
Results
Human Homologs to Murine Monocytes
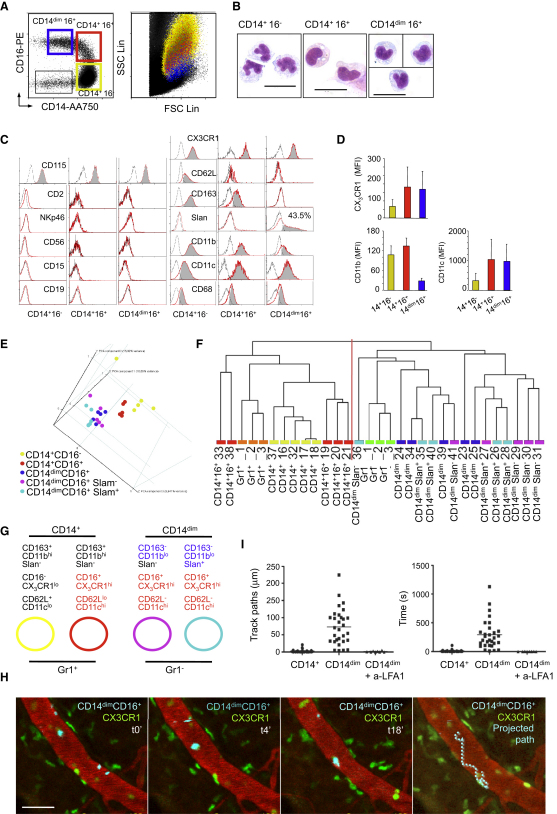
Human monocytes can be distinguished from other cell types in the peripheral blood by flow cytometry (Figure 1 and Figure S1 available online). Large CD14+ CD16− monocytes (85% of blood monocytes) can be distinguished from a population of large CD14+CD16+ monocytes (5%), on the basis of the level of expression of CD16, CD62L, CD11c, CD163, and CX3CR1 (Figures 1A–1D and Figure S1). Monocytes expressing low amounts of CD14 (CD14dim, 7% of monocytes) have a smaller size and granularity, express CD16, and have lower expression of CD11b and CD163 (Figures 1A–1D). These small CD14dimCD16+monocytes can be further subdivided into CD14dimCD16+ 6-sulfo LacNAc (Slan)+ and CD14dimCD16+Slan− (Figure 1C). Blood monocytes Gr1+ and Gr1−, from C57/Bl6 mice (n = 3, samples Gr1+1, 2, and 3 and Gr1− 1, 2, and 3), were therefore compared to the prospective human monocyte subsets from three healthy donors with whole-genome expression arrays (as described in the Experimental Procedures). For the three healthy donors, five samples were analyzed for each subset, including biological replicates from donor 1 (D1 samples 17–19, 19–21, 23–25, 26–28, and 29–31), and simplicates from donor 2 (32, 33, 34, 35, and 36) and donor 3 (37, 38, 39, 40, and 41) (see also Supplemental Experimental Procedures). Common expressed genes between human and mouse chips were selected with Ingenuity. Principal component analysis (PCA) on the human data set indicated that CD14+CD16−, CD14+CD16+, and CD14dimCD16+ subsets segregated in independent clusters (Figure 1E). Expression of Slan did not allow discrimination of subsets among CD14dimCD16+cells (Figure 1E). Human and mouse data sets were then merged and submitted to a hierarchical clustering with the Spearman correlation similarity measure with average linkage that used “all expressed genes” and “fold 2 expressed” genes (Figure 1F and Figure S1), and submitted independently to a PCA with a covariance matrix (Figure S1). These two unsupervised analyses showed comparable results, summarized in Figure 1G and Figure S1. Results from the hierarchical clustering analysis using D1, D2, and D3 with all individual D1 biological replicates (Figure 1F), with the mean of D1 replicates (Figure S1), or with individual replicates (not shown) were similar and led to an identical conclusion. In all cases the samples were split into two main groups, each of which included human and mouse samples. Gr1− murine samples always segregated with the samples of CD14dim CD16+ human monocytes samples, whereas Gr1+ murine monocyte samples segregated with all of the CD14+ human samples, independently of expression of CD16 (Figure 1F and Figure S1).
Figure 1.
Phenotype, Expression Arrays, and In Vivo Behavior of Human Monocyte Subsets
(A–D) (A) Monocytes are contained within CD115+ cells, or DR+ cells, that do not express B cell (CD19−), T cell (CD2−), NK cell (NKp46−), or granulocyte markers (CD15−). Within this gate, large CD14+CD16− and CD14+CD16+ monocytes, small CD14dimCD16+ monocytes, and CD14−CD16− dendritic cells can be separated. (A and B) The size, morphology, and (C and D) phenotype of monocytes subsets are shown. The scale bar in (B) represents 20 μm.
(E) Principal component analysis (PCA), using probes with significant statistical variation between at least two groups.
(F) Hierarchical clustering with the Spearman correlation similarity measure and average linkage in clustering algorithm with probes corresponding to the homologenes and filtering by fold two.
(G) Hypothesis generated from biostatistical analysis. In (A) and (D)–(G), monocytes populations are color coded. CD14+CD16− are yellow, CD14+CD16+ are red, and CD14dim CD16− are dark blue. Within CD14dim CD16− cells, Slan+ cells are light blue and Slan− cells are magenta.
(H) Intravital microscopy of the ear dermis from Cx3cr gfp/+ Rag2−/−Il2rg−/− mice. Blood vessels are labeled by intravenous (i.v.) injection of fluorescent 70 kDa dextran (red), and murine monocytes express gfp (green). CD14dim human monocytes are purified by flow cytometry labeled with DiD (cyan) and injected i.v.; images are stills from Movie S1. The scale bar in (H) represents 50 μm.
(I) Dot plots represent the length and duration of crawling CD14+ monocytes (circles) and CD14dimCD16+ monocytes before (squares) and after blocking human LFA-1 (triangles) from nine independent experiments.
See also Figure S1 and Movies S1 and S2.
These results predicted that the absence of expression of the CD14 and CD163 antigens distinguishes the putative human homologs of murine Gr1− “patrolling” monocytes, whereas both CD14+CD16− and CD14+CD16+ cells would resemble mouse Gr1+ “inflammatory” monocytes, in contrast to previous hypotheses (Geissmann et al., 2003; Ingersoll et al., 2010; Ziegler-Heitbrock, 2007).
“Patrolling” CD14dim Monocytes
To test this prediction, we purified human monocyte subsets from peripheral blood by flow cytometry (as described in Figure S1), labeled them with fluorescent probes, transferred them intravenously into Rag2−/− Il2rg−/− Cx3cr1gfp mice, and examined them by intravital microscopy (Figure 1H and Movies S1 and S2). Most human CD14dim monocytes attached immediately to the endothelium after intravenous transfer and crawled for an extended period of time, whereas crawling was not observed after intravenous transfer of CD14+ monocytes (Figure 1I), further suggesting that CD14dim monocytes are the functional homologs of murine “patrolling” Gr1− monocytes. The ability of human monocytes to attach and crawl on murine endothelium was consistent with interspecies conservation of integrins, in particular lymphocyte function-associated antigen-1 (LFA-1) (Vidovic et al., 2003), which is critical for murine monocyte crawling (Auffray et al., 2007). To test this hypothesis, we therefore performed intravenous injection of human LFA1 blocking antibody (clone 38[Dransfield et al., 1992]), and observed that it abolished crawling by human monocytes inside mouse blood vessels (Figure 1I). These data indicated that human CD14dim monocytes can patrol blood vessels in vivo, in a LFA1-dependent manner.
“Inflammatory” CD14+CD16− and CD14+CD16+ Monocytes Respond to Bacteria-Associated Signals
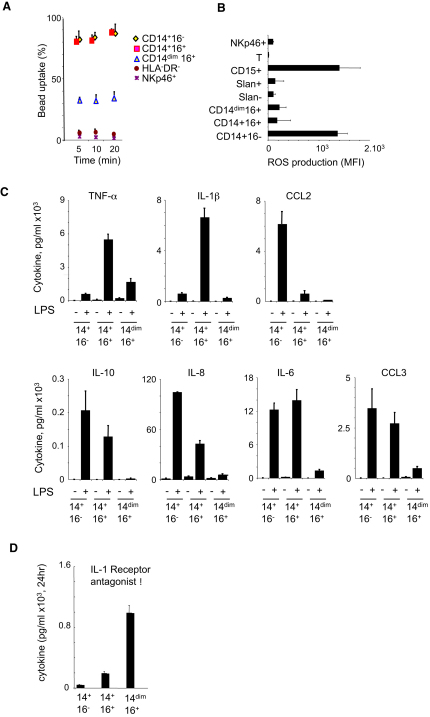
As expected, CD14+ monocytes were very efficient at phagocytosing latex beads (Figure 2A). CD14+CD16− monocytes produced high level of ROS (Figure 2B) and also expressed more mRNA for myeloperoxidase and lysozyme (Figure S2). In the presence of LPS, CD14+CD16− monocytes produced very high amounts of a distinct and limited set of chemokines and cytokines, including IL-6, IL-8, CCL2, and CCL3 (Figure 2C).
Figure 2.
Functional Analysis of Monocyte Subsets
Human monocytes were obtained from whole blood from healthy donors and purified by flow cytometry as indicated in the Experimental Procedures (see Figure S1).
(A) Percentage of cells that have phagocytosed 1 μm beads after the indicated time. The mean ± SD of triplicates is shown. A representative of five independent experiments is shown.
(B) ROS production.
(C) Cytokine production after overnight incubation with (+) or without (−) LPS (100 ng/ml).
(D) Production of IL1-RA after 18 hr incubation. Bar graphs in (B)–(D) show mean ± SD of triplicates, from experiments representative of three to five donors. See also Figure S2.
The double-positive CD14+CD16+ monocyte population did not produce ROS (Figure 2B), expressed mRNA for myeloperoxidase and lysozyme at low levels (Figure S2), and produced cytokines distinct from those produced by CD14+CD16− monocytes (Figure 2C). They were the main producers of IL-1β and TNF-α in response to LPS and additionally produced IL-6 and CCL3 (Figure 2C). CD14+CD16+ also accounted for the bulk of cytokine production in response to the Toll-like receptor (TLR) 2 agonist Pam3ck4 (data not shown and Figure S2). Both CD14+CD16− and CD14+CD16+ monocytes produced moderate amount of the anti-inflammatory cytokine IL-10, with slightly different kinetics (Figure S2) that may explain previous contradictory reports (Belge et al., 2002; Skrzeczyńska-Moncznik et al., 2008).
Therefore, CD14+ monocytes as a population exhibited the basic characteristics of Gr1+ “inflammatory” TNF-α and ROS-producing murine monocytes (Serbina et al., 2008). Distinct cell types within this population, i.e., CD14+CD16− and CD14+CD16+, are responsible for the bulk production of ROS, IL-6, IL-8, and CCL2 and of TNF-α and IL-1, respectively, in the presence of LPS. These observations are in line with the published evidence that LPS induces expression of different cytokines by CD14+ and CD16+ monocytes (Belge et al., 2002; Skrzeczyńska-Moncznik et al., 2008). The secretion of type I IFN, IL-2, IL-4, IL-5, IL-7, IL-9, IL-12, IL-13, IL-15, IL-17, CCL11, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) was not detected in monocytes in these experiments (data not shown).
“Patrolling” CD14dim Monocytes Respond Poorly to Bacterial Cues
CD14dim cells exhibited a limited ability to uptake latex beads in comparison to CD14+ monocytes (Figure 2A), did not produce ROS (Figure 2B), expressed little mRNA for myeloperoxidase (MPO) and lysozyme (Figure S2), and gave little response to LPS or to PAM3CK4 TLR1 and two agonists (Figure 2C and Figure S2). Thus, the production of IL-1β and TNF-α in response to LPS, previously attributed to the CD16+ fraction of monocytes, is restricted to the double-positive CD14+ CD16+ cells in our experiments.
As expected from PCA and clustering analysis, CD14dim monocytes expressed a distinct set of genes in comparison to both CD14+CD16+ and CD14+CD16− monocytes, including chemokine receptors, as well as different sets of apolipoproteins, scavenger receptors for lipids and dying cells, and cytokine receptors (Figure S2). CD14dim monocytes did not express inflammatory chemokine receptors such as CCR2 and expressed lower levels of CCR1, CCR5, IL-17RA, apolipoprotein (Apo) B48, CD36, MPO, and lysozyme. In contrast, they expressed higher amounts of IL-10 receptor and CXCL16 scavenger receptor ApoA and ApoE (Figure S2) and produced high amounts of IL-1 receptor antagonist during overnight culture (Figure 2D). Overall, the monocyte proinflammatory response, including phagocytosis and the production of ROS, MPO, lysozyme, and cytokines in response to well-characterized TLR1, TLR2, and TLR4 signals, appeared to be mediated by CD14+ monocytes, whereas the profile of CD14dim monocytes was, if anything, anti-inflammatory in this context.
Are CD14dim Cells Monocytes?
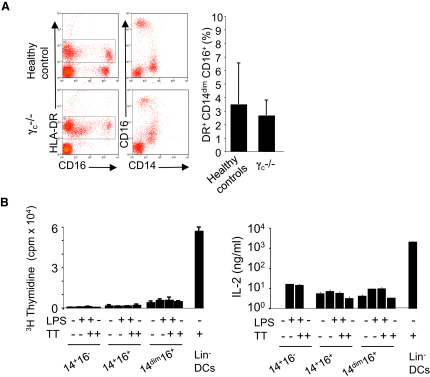
We thus considered the possibility that CD14dim cells might not be monocytes. Cytologic examination indicated that CD14dim cells had a morphology compatible with monocytes, and flow cytometric profiles indicated that they expressed the macrophage colony-stimulating factor (MCSF) receptor and CD68, but low CD11b and CD163 (see Figures 1B–1D). A subset of human natural killer (NK) lymphocytes express CD16 and HLA-DR, and recent studies have revealed that NK cells can be confused for myeloid cells (Blasius et al., 2007; Caminschi et al., 2007; Vosshenrich et al., 2007). Therefore, we tested the hypothesis that CD14dim cells might actually represent major histocompatibility complex (MHC) class II-positive NK cells by examining cells from patients deficient in the common cytokine receptor gamma chain (γc); these patients lack all lymphoid lineages. We found normal numbers of CD14dim CD16+ cells in three γc-deficient patients, whereas as expected CD16+ NK cells were absent from these patients (Figure 3A), indicating that CD14dim cells are not lymphoid.
Figure 3.
Development of Monocytes in γc-Deficient Patient and Antigen Presentation
(A) Phenotype of DR+ cells is analyzed by flow cytometry analysis of blood from γc-deficient patient (lacking T, B, and NK cells) versus controls. The bar graph represents the percentage (mean ± SD) of CD14dim monocytes among DR+ cells in three patients and ten controls.
(B) Antigen presentation assay. Monocyte subsets and DCs, from a tetanus toxoid-immunized donor were incubated with autologous T cells and tetanus toxoid. Thymidine incorporation measured during the last 18 hr. IL-2 is measured after 4 days. Data in (B) represent the mean ± SD of triplicates, from experiments representative of three donors.
Cells expressing HLA-DR, CD16, and Slan and negative for CD14 have also been described as antigen-presenting dendritic cells (DCs) (Schäkel et al., 2006). Both CD14+ and CD14dim monocytes stimulate allogenous mixed lymphocyte reaction at high effector/target ratio (Figures S2D and S2E). However, they do not process and present antigen (Figure 3B). For investigating the ability of CD14dim CD16+ cells to present a recall protein antigen to T cells in comparison to other monocyte subsets and blood DCs, tetanus toxoid was added to coculture of monocytes or DCs and T cells from autologous vaccinated donors (Sallusto and Lanzavecchia, 1994). Freshly isolated monocytes from any subset did not promote any significant antigen-dependent T cell proliferation or IL-2 production ,whereas CD14−CD16− HLA-DR+ DCs are able to efficiently present tetanus toxoid to T cells and to trigger strong IL-2 production (Figure 3B). Therefore, CD14dim cells appeared to be unable to process antigen, and genetic and functional evidence indicated that they are a bona fide monocyte subset. Of note, in our PCA, CD14dimCD16+Slan+ cells clustered together with CD14dimCD16+Slan− cells and total CD14dimCD16+ cells (see Figure 1).
CD14dim Monocytes Produce a Selected Set of Proinflammatory Cytokines in Response to Viruses
The poor response via TLR1 and TLR 2 and via TLR4 does not preclude the involvement of monocytes in the inflammatory responses to other classes of pathogens. P. Ancuta and others have proposed a role for CD16+ monocytes in the immune response to HIV (Ancuta et al., 2006) and monocytic cells have been shown to produce inflammatory cytokines in the presence of viruses (Gupta et al., 2001; Ströher et al., 2001). A separate subset of white blood cells, plasmacytoid DCs, are known to be the main blood producers of type 1 IFN in response to viruses (Liu, 2005), although murine Gr1+ monocytes can contribute to production of type 1 IFN in response to viruses (Barbalat et al., 2009). Among TLRs that recognize nucleic acids and are involved in the immune response to viruses (TLR3, TLR7, TLR8, and TLR9), monocytes weakly express TLR3 and TLR9 and do not respond to TLR3 and TLR9 agonists (Jarrossay et al., 2001; Kadowaki et al., 2001) (Figure S3). However, human monocytes express TLR7 and TLR8 (Supplemental figure S3), the two other endosomal TLRs involved in the immune response to viruses (Diebold et al., 2004; Heil et al., 2004; Lund et al., 2004). We thus reasoned that human monocytes subsets may be involved in the pro-inflammatory response to viruses.
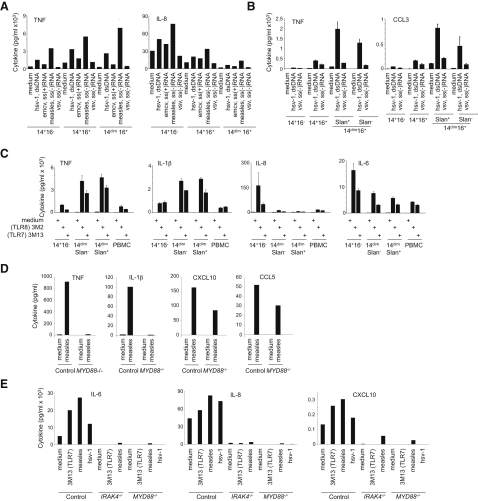
CD14+ monocytes produced very high amounts of the granulocyte, B cell, and T cell “helper” chemokines and cytokines IL-6, IL-8, and CCL2, in response to measles (ssRNA [−]) and herpes simplex virus 1 (HSV-1) (dsDNA) at a multiplicity of infection (MOI) of 1, similar to what was observed for LPS. In contrast, freshly purified CD16+ monocytes, especially CD14dim CD16+ monocytes, whether Slan+ or Slan−, selectively produced very high amounts of the proinflammatory cytokines TNF-α, IL-1β, and of CCL3 in response to measles and HSV-1 (Figures 4A and 4B). Of note, secretion of type I IFN by human monocytes was very low (not shown).
Figure 4.
Monocyte Subset-Specific Response to Viruses
(A and B) Cytokine production from two different donors after overnight incubation with viruses (A and B) (herpes simplex virus-1 (dsDNA, HSV-1), vesicular stomatitis virus [ss(−)RNA, VSV], measles virus [ss(−)RNA, MV], and Encephalomyocarditis virus [ss(+)RNA, EMCV'.
(C) Cytokine production after overnight incubation with TLR agonists.
(D and E) Cytokine production by CD14dim monocytes (D) and CD14+ monocytes (E) from controls, MYD88-deficient patients and IRAK-4-deficient patients after exposure to measles virus (MV) or herpes simplex virus-1 (HSV-1). Data are representative of at least three donors. Bar graphs in (A)–(C) show mean (±SD) of triplicates from experiments representative of five healthy donors. Bar graphs in (D) and (E) show results representative of two IRAK−/− patients, three MYD88−/− patients, and ten healthy donors.
See also Figure S3.
CD14dim Monocytes Respond to Viruses via a Unique TLR7-TLR 8-MYD88-MEK-Dependent Pathway
To investigate the mechanisms of cytokine production by CD14dim and CD14+ monocytes, we examined their response to TLR7 and TLR8 agonists. The response of monocyte subsets to stimulation with TLR8 agonists (3M2 and R848) and to a lesser extent to a TLR7 agonist (3M13) was similar to their response to intact viruses. CD14dim monocytes produced very high amounts of proinflammatory cytokines, but little IL-6, IL-8, and CCL2, whereas CD14+ CD16− monocytes produced little proinflammatory cytokines, but high IL-6 and IL-8 (Figure 4C). As was true for responses to viruses, the population of CD14+ CD16+ monocytes displayed an intermediate phenotype (Figure S3).
To investigate whether cytokine production by monocytes required infectious virus, we incubated monocytes with either live, ultraviolet light (UV)-inactivated, or heat-inactivated HSV1-green fluorescent protein (GFP) expressing virus. Control fibroblasts exhibited GFP fluorescence only when infected with live virus (Figure S3). In contrast, monocytes exhibited similar GFP fluorescence whether they were exposed to live, UV-inactivated, or heat-treated viruses (Figure S3), probably reflecting uptake rather than infection. Monocytes produced cytokines in response to live virus and UV-inactivated virus, but not in response to denatured virus particles obtained by heat-inactivation (Figure S3). These data indicate that monocytes are activated by HSV-1 particles, in line with a TLR-mediated response, but active HSV-1 replication may not be required for cytokine production at a MOI of 1. Similarly, replication of measles virus was not observed at a MOI of 1 (Figure S3).
To confirm the role of TLRs and specifically of TLR7 and TLR8 for cytokine production, we purified CD14dim and CD14+ CD16− monocytes from the peripheral blood of patients deficient in myeloid differentiation primary response gene 88 (MYD88) and in some cases in interleukin-1 receptor-associated kinase 4 (IRAK-4) (Picard et al., 2003; von Bernuth et al., 2008) and from controls and were stimulated with measles virus and/or HSV1. Production of TNF-α and IL-1β by CD14dim monocytes was totally dependent on MyD88, whereas production of chemokines CCL5 and CXCL10 was maintained, likely due to intracytoplasmic sensors and interferon-inducible RNA helicase MDA5 and RIG-I, (Figure 4D). Production of IL-6 and IL-8 by CD14+ CD16- monocytes also required MYD88 (Figure 4E). Similar results were obtained with IRAK4-deficient monocytes (Figure 4E). Altogether, these data indicated that cytokine production by monocytes in response to viruses (measles and HSV-1) is mediated via a TLR- MYD88 pathway, most likely via TLR8 and TLR7. Notably, TLR3 signaling is largely MYD88 independent and thus data from Figures 4D and 4E confirm that TLR3 is not involved in monocyte activation by viruses.
We then investigated whether selective monocyte subset activation via TLRs was also a feature of mouse monocytes. Murine blood monocytes were incubated with LPS or the TLR7 agonist 3M13. Gr1+ monocytes responded both to LPS and TLR7 agonist, whereas Gr1− monocytes responded better to TLR7 agonist than to LPS (Figures S1E and S1F), suggesting a similar response to TLR agonist by murine Gr1− and human CD14dim monocytes.
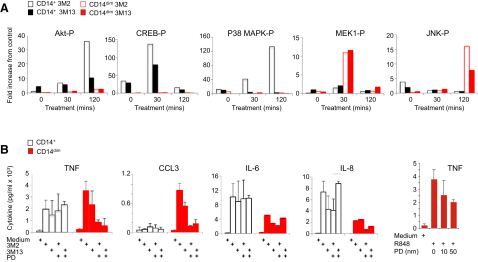
We reasoned that monocyte subsets might display distinct effector responses (i.e., the production of inflammatory cytokines versus “helper” cytokines) in response to viruses and TLR7 and TLR8 agonists because different downstream signaling pathways might be engaged in different cell types. To test this possibility, we examined activation of various signaling pathways in different subsets after TLR7 and/or TLR8 activation. As has been previously observed (Levy et al., 2006), p38 mitogen-activated protein kinase (MAPK) was rapidly phosphorylated in CD14+ monocytes in response to TLR8 agonists. In contrast, p42 mitogen-activated protein kinase kinase 1 (MEK1) phosphorylation, but not p38 MAPK, was observed 30 min after stimulation in CD14dim monocytes in response to TLR7 and TLR8 agonists (Figure 5A), followed 90 min later by Jun N-terminal kinases (JNK) phosphorylation (Figure 5A).
Figure 5.
Monocyte Subset-Specific Activation Pathways
(A) Phosphorylated Akt, p38-MAPK, MEK1, and JNK in CD14+(black bars) and CD14lo monocytes (red bars) following treatment with TLR8 agonist (3M2, open bars) or TLR7 agonist (3M13, closed bars) for 0, 30, and 120 min at 37°C. Data are presented as mean fold increase from vehicle control treated cells from three independent experiments.
(B) Cytokine production by CD14+ (open black bars) and CD14dim (closed red bars) monocytes treated with vehicle, 3M2, or 3M13 with or without MEK inhibitor PD98059. Data are presented as mean ± SD in expression (pg/ml) from three independent experiments.
See also Figure S4.
This suggested that different signaling pathways are activated in monocyte subsets. We therefore investigated whether pharmacological inhibitors of MEK1 or p38 affect cytokine production by monocyte subsets in response to TLR7 and TLR8 agonists. Production of proinflammatory cytokines in response to TLR7 and/or TLR8 agonists by CD14dim monocytes was strongly inhibited by the MEK1 inhibitor in a dose-dependent manner (Figure 5B), but not by a p38 inhibitor (Figure S4). In contrast, production of cytokines by CD14+ monocytes was partially inhibited by a p38 inhibitor (30% inhibition, Figure S4) but not by the MEK1 inhibitor (Figure 5B). Although pharmacological inhibitors can have off-target effects, subset-specific responses to these inhibitors indicate differential involvement of MAPK pathways.
Altogether, these data indicate that distinct subset-specific pathways control cytokine production by monocytes in responses to viruses and nucleic acids. We propose a model in which production of IL-6 and IL-8 by CD14+ monocytes involves activation of p38 in addition to a classical NF-κB pathway, whereas production of TNF-α, IL-1, and CCL3 by CD14dim monocytes is controlled by an alternative p42 MEK MAP kinase pathway (Figures S4B–S4F).
CD14dim Monocyte-Specific Inflammatory Response to Self-Nucleic Acids in Patients with Lupus
Intracellular TLRs activated by nucleic acids, and particularly RNA, have evolved under strong selection, suggesting an essential role in host survival (Barreiro et al., 2009). However, as a possible tradeoff mechanism, TLR7- and TLR9-dependent recognition of endogenous nucleic acids is also a feature of autoimmune diseases such as systemic lupus erythematosus (SLE), a disease associated with autoantibodies to nucleosome and ribonucleoproteins, and immune complex deposition in several organs (Rahman and Isenberg, 2008). Ribonucleoprotein-containing immune complexes stimulate immune cell such as plasmacytoid DCs and B cells via TLRs (Lau et al., 2005; Lövgren et al., 2006).
Although the mechanisms of Lupus-induced tissue damage, and in particular glomerulonephritis, are poorly understood, recent work in the mouse has proposed that activation of myeloid cells by glomerular immune complex, which requires both FcRγ and TLRs (Bergtold et al., 2006; Clynes et al., 1998), initiates an inflammatory response that results in glomerulonephritis. Murine Gr1− monocytes have recently been shown to be selectively expanded in nephritis, to accumulate inside the capillary vessels in the nephritic kidney, and to be activated by immune complexes in an FcRγ-dependent manner (Bergtold et al., 2006). In human, genetic studies studies have associated CD16 with lupus (Rhodes and Vyse, 2008). It was further shown that CD16+ monocytes adhere in vitro to endothelial cells better than CD14+ monocytes, a phenomenon in part dependent on the chemokine receptor CX3CR1 (Ancuta et al., 2003), and human CD14dimCD16+ monocytes patrol capillaries in vivo as shown in this report (see Figures 1H and 1I). Finally, elevated amounts of fractalkine (the CX3CR1 ligand) expression and accumulation of CD16+ monocytes inside glomerular blood vessels has been documented in human SLE (Hill et al., 2005; Yoshimoto et al., 2007). In accordance with the literature (Hill et al., 2005; Yoshimoto et al., 2007), we confirmed and observed a glomerular IgG deposition and monocytic infiltrate (CD68+ CD16+ CD14dim and CD15− CD3ɛ−) in glomerular vessels from patients with class IV-G lupus nephritis (Figure 6A).
Figure 6.
CD14dim Monocytes Detect Nucleic Acids and Mediate Inflammation in Lupus Patients
(A) Immunostaining for IgG, CD68, CD16, CD14, CD15, and CD3ɛ, on tissue sections from kidney biopsy with IV-G lupus glomerulonephritis (left panels) and postinfectious glomerulonephritis (right panels).
(B) Cytokine production by CD14dim or CD14+ monocytes from healthy controls (n = 3) incubated with serum from Lupus patients with autoantibodies to RNPs (n = 3) and controls (n = 2).
(C and D) Monocyte cytokine production after incubation with serum from patients without treatment (open bar), RNase+DNase treatment (red bar), addition of RNP (light yellow bar), depletion of immunoglobulins (green bar), depletion of immunoglobulins+RNP (blue bar), or depletion of immunoglobulins+RNase+DNase (orange bar). Two representative figures for each cytokine from at least three experiments are shown. ∗p < 0.01 for comparison RNP versus other treatments (one-way ANOVA).
We thus hypothesized that nucleic acid-containing immune complexes present in glomerular capillaries activate CD14dim monocytes to produce TNF-α, IL-1, and CCL3 cytokines that may damage the endothelium. We compared the effects of serum from individuals with SLE and anti-ribonucleoprotein (RNP) antibodies (see Experimental Procedures) on monocyte activation. Patient sera induced strong production of TNF-α and CCL3 by CD14dim monocytes isolated from healthy donors, as well as the production of IL-6 and IL-8 by CD14+ monocytes, in comparison with sera from controls (Figure 6B). Sera were then pretreated with RNase and DNase, and/or Ig depletion, and/or addition of ribonucleoprotein, before incubation with monocytes (Figures 6C and 6D). Both nucleic acids and Ig were observed to contribute 30%–50% of the production of CCL3 and TNF-α by CD14dim monocytes; the depletion of nucleic acids and Ig together did not have an additive effect on inhibition. These results indicated that in addition to TLR7-TLR8 agonists, and viruses, ribonucleoprotein-containing immune complexes induce CCL3 and TNF-α production by CD14dim monocytes.
Discussion
This study establishes CD14dim human monocytes as a bona fide myeloid immune cell population that patrol blood vessels and exert specific effector functions in the inflammatory response to viruses and nucleic acids. Genome-wide expression analysis (Figure 1) predicted that these monocytes, which lack CD14 expression (CD14dim) and represent a minority (∼7%) of human blood monocytes, resemble mouse “patrolling” Gr1− monocytes and can be distinguished from lymphoid cells, dendritic cells, and CD14+ CD16− and CD14+ CD16+ monocytes. CD14dim monocytes patrol capillaries after adoptive transfer in vivo, do not produce ROS, exhibit low or absent myeloperoxidase and lysozyme production in a steady state, constitutively produce IL-1RA, and are weak producers of inflammatory cytokines after exposure to LPS or a TLR1 and TLR2 agonist. Nevertheless CD14dim cells belong to the monocytic lineage, given that they develop in individuals lacking lymphoid precursors due to loss of function mutations in X-linked common γ chain, implying that they are developmentally distinct from NK CD16+ lymphocytes. CD14dim monocytes exhibited an anti-inflammatory phenotype in a steady state and respond poorly to surface-associated TLR stimulation, but specialize in the production of the proinflammatory cytokines TNF-α, IL-1β, and of CCL3 in response to viruses and nucleic acids, via a unique TLR7-8, MyD88 and MEK-dependent pathway. Of note, murine “patrolling” Gr1− monocytes also responded better to TLR7 agonist than to LPS (Figure S1), suggesting a degree of functional similarity between human and murine patrolling monocytes.
Although they can stimulate the proliferation of allogenic T cells at high effector-to-target ratio (Sallusto and Lanzavecchia, 1994), blood monocytes, including the CD14dim subset, do not process and present a recall antigen to T cells, in contrast to blood DCs. This is in contrast to some previous reports that identified DCs as a population expressing CD16 and Slan (Schäkel et al., 2002). However, we observed that monocytes, in particular the CD14dim subset, can promote autologous T cell proliferation, in an antigen-independent manner, and without detectable increase in IL-2 production. Therefore, although monocytes are clearly distinct from DCs, further studies will be needed to clarify the functional outcomes of their interaction with T cells (Evans et al., 2009).
Reported differences in the behavior of monocyte subsets in mice and human (Auffray et al., 2009; Skrzeczyńska-Moncznik et al., 2008; Ziegler-Heitbrock, 2007) have stimulated efforts to identify human homologs to monocyte subsets (Ingersoll et al., 2010). The PCA and hierachical clustering analysis reported here suggested that monocytes expressing CD14 (CD14+), which represent more than 90% of blood monocytes, resemble mouse “inflammatory” Gr1+ monocytes, whether or not they express CD16. Indeed, they share their key functional characteristics; they are responsible for phagocytosis, ROS production, and cytokine production in response to LPS via a classical MyD88-, p38-, and NF-κB dependent pathway. Within this subset, distinct cells, i.e., CD14+CD16− and CD14+CD16+ cells, are responsible for the bulk of ROS, IL-8, and IL-6 production and for TNF-α and IL-1 production respectively in response to LPS. This observation may appear different from a recent work that concluded that CD14+ CD16− monocytes were homologous to mouse Gr1+ monocytes, whereas CD14+ CD16+ monocytes were homologous to mouse Gr1− monocytes (Ingersoll et al., 2010). However, this discrepancy can be accounted for by the fact that the Ingersoll study compared only two human subsets (CD14+ CD16− monocytes and human CD16+ monocytes) with two murine subsets (Gr1+ and Gr1− monocytes). Their CD16+ monocyte population may have contained both the CD14dim subset and the CD14+ CD16− subset.
We therefore propose that CD14dim CD16+ monocytes represent a monocyte subset that patrol blood vessels and selectively detect virally infected and damaged cells to produce proinflammatory cytokines. This role of CD14dim CD16+ monocytes is reminiscent of the main function of plasmacytoid DCs (PDCs), which are also specialized in response to viral stimulation via TLR7 and TLR9 (Liu, 2005). However, PDCs secrete type I interferons, whereas CD14+ monocytes produce IL-6 and IL-8, and patrolling monocytes produce TNF-α, IL-1β, and CCL3, thus exhibiting complementary functions in the TLR-dependent antiviral response. Of note, we did not detect significant secretion of type I interferon by either CD14+ or CD14dim monocytes in this study. In the murine system Gr1+ monocytes (which resemble CD14+ human monocytes) secrete IFN-β after activation by viruses (Barbalat et al., 2009).
During antiviral responses, a systemic type 1 interferon response is triggered by PDCs and Gr1+ monocytes and a local type 1 interferon response by infected fibroblasts and other tissue cells. Our data suggest that in addition, monocytes and in particular patrolling CD14dim monocytes, participate in a regional antiviral response characterized by the production of proinflammatory cytokines such as TNF-α and IL-1β and of CCL3 and are potentially involved in virus-induced immunopathology.
PCA and functional experiments indicated that CD14+CD16−, CD14dim and CD14+CD16+ monocytes represent distinct cell populations. The expression of CD16 by CD14+ monocytes may correspond to an activation and/or differentiation state of CD14+ monocytes, as suggested by several groups (Ziegler-Heitbrock, 2000). They both respond to extracellular TLR such as TLR4 and TLR2 and share a high phagocytic index.
In some donors the CD14+CD16+ population displayed an intermediate cytokine response to viruses and TLR7- TLR8 agonists between that of CD14dim and CD14+CD16− monocytes, and future studies will need to investigate potential heterogeneity of this “double positive” population. Although it is conceivable that CD14dim may also derive from CD14+ monocytes, there is no experimental evidence to support this claim, and results from this study argue that, whether CD14dim arise from CD14+ monocytes or directly from progenitors, their distinct morphology, behavior in vivo, and signal transduction apparatus make them two independent functional cell types.
Results presented here indicate that a TLR7-8, MyD88, and MEK pathway is critical for the secretion of a distinct set of cytokines by CD14dim monocytes, whereas p38 is important in the MyD88-dependent response of CD14+ monocytes. Activation of MAPK ERK1 and ERK2 has been previously shown to play an important role in regulating cytokine production by murine myeloid cells (Agrawal et al., 2006), and the regulation of distinct cytokine genes by different members of the MAPK family (ERK versus p38 MAPK) has been previously reported in DCs (Dillon et al., 2004). We used computational modeling to examine the potential relevance of this model. Computational modeling of this forward biochemical cascades for TLR7 and TLR8 signaling in monocyte subsets simulates in silico TNF-α, IL-1β, IL-8, and IL-6 expression profiles that are consistent with our experimental data. However, more investigations will be needed to define the consequences of distinct signaling pathways being involved in the effector functions of monocyte subsets.
TLR7 and TLR8 have evolved under strong selection, indicating an essential role in host survival (Barreiro et al., 2009). Our present identification of a specific human monocyte subset relying on TLR7 and TLR8 to detect viruses and nucleic acids for the production of the proinflammatory cytokines TNF-α and IL-1β further suggests that TLR7 and TLR8 are endowed with an essential physiological role. However, there have been no reports of susceptibility to viral infections in human patients with impaired MYD88 and IRAK-4 signaling, who are unresponsive to TLR7, TLR8, and TLR9 (Ku et al., 2007; von Bernuth et al., 2008; Yang et al., 2005). Patients unresponsive to TLR3, TLR7, TLR8, and TLR9 (UNC-93B deficiency) share the narrow viral phenotype of TLR3-deficient patients, with herpes simplex encephalitis (Casrouge et al., 2006; Zhang et al., 2007). Thus, TLR7 and TLR8 are apparently redundant for host defense against most common viruses, although it is possible that TLR7 and TLR8 are required for the defense against yet unidentified virus(es). Alternatively, they may have been selected by virtue of their role in recognition of endogenous nucleic acids.
In support of a role in the sensing of endogenous nucleic acids for TLR7-TLR8 expressing monocytes, our results indicate that activation of CD14dim monocytes by endogenous nucleic acids can contribute to the pathogenesis of inflammatory autoimmune diseases such as SLE, during which immune complexes containing nucleoproteins accumulate in tissues and in particular the glomeruli. Because glomerular damage associated with SLE leads to kidney failure, requiring dialysis or transplantation, CD14dim monocytes may therefore represent a potentially useful cellular therapeutic target in selected inflammatory diseases.
Experimental Procedures
Monocyte Phenotyping and Purification
Anticoagulated whole blood was collected from healthy volunteers, MYD88-deficient, IRAK-4-deficient, and γc-deficient patients after informed consent. Red blood cell lysis was used to avoid activation of monocytes by ficoll density centrifugation. Monocytes (CD14+CD16−, CD14dimCD16+ and CD14+CD16+ cells) were analyzed and purified as described in Figure S1 and the Supplemental Experimental Procedures.
Intravital Microscopy and Adoptive Transfer
Intravital microscopy and adoptive transfer were performed as described in the Supplemental Experimental Procedures.
Array Analysis
Samples
The human samples come from three different healthy donors (D1, D2, and D3) but the samples from donor D1 are in triplicate. Twenty-five “Whole Human Genome” chips were therefore obtained from five prospective subsets and three different donors, including triplicate samples from one donor (see Supplemental Experimental Procedures). Six “Whole Mouse Genome” chips from Gr1+ and Gr1− mouse monocyte subsets were generated as described (Auffray et al., 2007). MIAME data are available at www.ebi.ac.uk/arrayexpress: Geissman_Miltenyi_Human, ArrayExpress accession: E-MEXP-2544 and Geissman_Miltenyi_Mouse: ArrayExpress accession: E-MEXP-2545.
We submitted this series of expression arrays to an unsupervised analysis in order to unveil possible correspondences between samples from the two species. The two sets of data were obtained and processed independently as described in the Supplemental Experimental Procedures.
Stimulation of Monocytes
CD14hiCD16lo, CD14loCD16hi, and CD14hiCD16hi cells were sorted from the blood of healthy controls, and MyD88 and IRAK deficient patients when indicated, as described in the Supplemental Experimental Procedures.
TLR Agonist Stimulations. A total of 104 cells/well were laid in a 96-well plate in 100 μL and stimulated with PBS control or TLR agonists. Optimal concentration of TLR agonists were determined in preliminary experiments. In the experiments reported in this paper, the following concentration were used: LPS (TLR4 agonist) 1 ng/ml, Pam3ck4 (TLR2) 100 μg/mL, 3M2 (TLR8) 3 μg/ml, R838 (TLR8) 3 μg/ml, and 3M13 (TLR7) 3 μg/ml, in 10% FCS, 1% P/S, and incubated for 6, 24, or 48 hr at 37°C and 5% CO2. When indicated, monocytes were preincubated (30 min) with the MEK inhibitor PD98059 (10–100 μM), the P38 inhibitor SB-203580 (50 μM), or DMSO vehicle control before exposure to TLR agonists.
Viral Stimulations. We stimulated 2 × 104 monocytes or peripheral blood mononuclear cells (PBMCs) per well from controls and, when indicated, from MYD88-deficient and IRAK-4-deficient patients, with the indicated intact viruses (Yang et al., 2005) at a multiplicity of infection of 1 and placed on ice for 30 min to obtain a synchronous infection. Cell supernatant was collected and analyzed for cytokine production with the Biorad multiplex assay or ELISA tests.
Human Serum. Human serum were obtained and treated as described in the Supplemental Experimental Procedures. ROS production, phagocytosis assay, antigen presentation assay, cytokine measurements, protein phosphorylation, and histological studies were performed as described in the Supplemental Experimental Procedures.
Acknowledgments
Authors are indebted to members of the Geissmann Lab for support and critical discussions, to M. Bertolaccini for preparation of patient serums, C. Wirrig for help with preparation of arrays and Facs analysis, to F. Rosenberg for HSV-1 GFP, to M. Dionne, A. Fischer, M. Marber, E. Zorn, and R. Medzhitov for helpful discussions and critical reading of the manuscript. Work was supported by Arthritis Research UK, and EURYI and Medical Research Council (G0900867) Grants to FG. K.W. is a British Heart Foundation fellow. F.G., J.C., and K.W. designed the study and wrote the manuscript. N.C. and J.P.J. performed the bioinformatical analysis. J.C., K.W., C.T., N.P., S.Y.Z., A.P., C.P., D.M., S.B., and D.D.'C. performed experiments and contributed to the analysis of results.
Published online: September 9, 2010
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and two movies and can be found with this article online at doi:10.1016/j.immuni.2010.08.012.
Accession Numbers
The microarray data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under the accession numbers E-MEXP-2544 and E-MEXP-2545.
Supplemental Information
CD14dimCD16+ human monocytes purified from whole blood (see Experimental Procedures) were labeled and injected intravenously into Cx3cr gfp/+ Rag2−/− γc−/− mice. Movie show patrolling mouse monocytes (gfp, green) and human CD14dim monocytes (Vybrant-DiD, Cyan). The time lapse is 23 min, the scale bar represents 100 μm, an initial magnification ×20 is shown, and the field is ∼250 μm/200 μm.
Same experimental setting as in Movie S1, with a time lapse of 45min; the scale bar represents 100 μm and an initial magnification ×20 is shown. The field is ∼500 μm/500 μm.
References
- Agrawal A., Dillon S., Denning T.L., Pulendran B. ERK1-/- mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:5788–5796. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- Ancuta P., Rao R., Moses A., Mehle A., Shaw S.K., Luscinskas F.W., Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancuta P., Kunstman K.J., Autissier P., Zaman T., Stone D., Wolinsky S.M., Gabuzda D. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344:267–276. doi: 10.1016/j.virol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Ancuta P., Liu K.Y., Misra V., Wacleche V.S., Gosselin A., Zhou X., Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genomics. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Auffray C., Sieweke M.H., Geissmann F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Barbalat R., Lau L., Locksley R.M., Barton G.M. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro L.B., Ben-Ali M., Quach H., Laval G., Patin E., Pickrell J.K., Bouchier C., Tichit M., Neyrolles O., Gicquel B. Evolutionary dynamics of human Toll-like receptors and their different contributions to host defense. PLoS Genet. 2009;5:e1000562. doi: 10.1371/journal.pgen.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belge K.U., Dayyani F., Horelt A., Siedlar M., Frankenberger M., Frankenberger B., Espevik T., Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J. Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- Bergtold A., Gavhane A., D'Agati V., Madaio M., Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J. Immunol. 2006;177:7287–7295. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- Blasius A.L., Barchet W., Cella M., Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J. Exp. Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I., Ahmet F., Heger K., Brady J., Nutt S.L., Vremec D., Pietersz S., Lahoud M.H., Schofield L., Hansen D.S. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J. Exp. Med. 2007;204:2579–2590. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A., Zhang S.Y., Eidenschenk C., Jouanguy E., Puel A., Yang K., Alcais A., Picard C., Mahfoufi N., Nicolas N. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- Clynes R., Dumitru C., Ravetch J.V. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- Diebold S.S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Dillon S., Agrawal A., Van Dyke T., Landreth G., McCauley L., Koh A., Maliszewski C., Akira S., Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- Dransfield I., Cabañas C., Craig A., Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H.G., Gullick N.J., Kelly S., Pitzalis C., Lord G.M., Kirkham B.W., Taams L.S. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc. Natl. Acad. Sci. USA. 2009;106:6232–6237. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F., Jung S., Littman D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Gupta M., Mahanty S., Ahmed R., Rollin P.E. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology. 2001;284:20–25. doi: 10.1006/viro.2001.0836. [DOI] [PubMed] [Google Scholar]
- Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hill G.S., Delahousse M., Nochy D., Bariéty J. Class IV-S versus class IV-G lupus nephritis: Clinical and morphologic differences suggesting different pathogenesis. Kidney Int. 2005;68:2288–2297. doi: 10.1111/j.1523-1755.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- Ingersoll M.A., Spanbroek R., Lottaz C., Gautier E.L., Frankenberger M., Hoffmann R., Lang R., Haniffa M., Collin M., Tacke F. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrossay D., Napolitani G., Colonna M., Sallusto F., Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kadowaki N., Ho S., Antonenko S., Malefyt R.W., Kastelein R.A., Bazan F., Liu Y.J. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C.L., von Bernuth H., Picard C., Zhang S.Y., Chang H.H., Yang K., Chrabieh M., Issekutz A.C., Cunningham C.K., Gallin J. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C.M., Broughton C., Tabor A.S., Akira S., Flavell R.A., Mamula M.J., Christensen S.R., Shlomchik M.J., Viglianti G.A., Rifkin I.R., Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O., Suter E.E., Miller R.L., Wessels M.R. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Lövgren T., Eloranta M.L., Kastner B., Wahren-Herlenius M., Alm G.V., Rönnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjögren's syndrome autoantigen-associated RNA. Arthritis Rheum. 2006;54:1917–1927. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W., Iwasaki A., Flavell R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M., Swirski F.K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J.L., Libby P., Weissleder R., Pittet M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Puel A., Bonnet M., Ku C.L., Bustamante J., Yang K., Soudais C., Dupuis S., Feinberg J., Fieschi C. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- Rahman A., Isenberg D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- Rhodes B., Vyse T.J. The genetics of SLE: An update in the light of genome-wide association studies. Rheumatology (Oxford) 2008;47:1603–1611. doi: 10.1093/rheumatology/ken247. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäkel K., Kannagi R., Kniep B., Goto Y., Mitsuoka C., Zwirner J., Soruri A., von Kietzell M., Rieber E. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi: 10.1016/s1074-7613(02)00393-x. [DOI] [PubMed] [Google Scholar]
- Schäkel K., von Kietzell M., Hänsel A., Ebling A., Schulze L., Haase M., Semmler C., Sarfati M., Barclay A.N., Randolph G.J. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006;24:767–777. doi: 10.1016/j.immuni.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Serbina N.V., Jia T., Hohl T.M., Pamer E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina N.V., Cherny M., Shi C., Bleau S.A., Collins N.H., Young J.W., Pamer E.G. Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J. Immunol. 2009;183:2678–2687. doi: 10.4049/jimmunol.0803398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzeczyńska-Moncznik J., Bzowska M., Loseke S., Grage-Griebenow E., Zembala M., Pryjma J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand. J. Immunol. 2008;67:152–159. doi: 10.1111/j.1365-3083.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali D., Conrad S.M., Mosser D.M. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- Ströher U., West E., Bugany H., Klenk H.D., Schnittler H.J., Feldmann H. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 2001;75:11025–11033. doi: 10.1128/JVI.75.22.11025-11033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkötter C., Nikolic T., Dillon M.J., Van Rooijen N., Stehling M., Drevets D.A., Leenen P.J. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Vidovic D., Graddis T.J., Stepan L.P., Zaller D.M., Laus R. Specific stimulation of MHC-transgenic mouse T-cell hybridomas with xenogeneic APC. Hum. Immunol. 2003;64:238–244. doi: 10.1016/s0198-8859(02)00780-2. [DOI] [PubMed] [Google Scholar]
- von Bernuth H., Picard C., Jin Z., Pankla R., Xiao H., Ku C.L., Chrabieh M., Mustapha I.B., Ghandil P., Camcioglu Y. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich C.A., Lesjean-Pottier S., Hasan M., Richard-Le Goff O., Corcuff E., Mandelboim O., Di Santo J.P. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J. Exp. Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Puel A., Zhang S., Eidenschenk C., Ku C.L., Casrouge A., Picard C., von Bernuth H., Senechal B., Plancoulaine S. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S., Nakatani K., Iwano M., Asai O., Samejima K., Sakan H., Terada M., Harada K., Akai Y., Shiiki H. Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis. Am. J. Kidney Dis. 2007;50:47–58. doi: 10.1053/j.ajkd.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Zhang S.Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P., Segal D., Sancho-Shimizu V., Lorenzo L., Puel A. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhao C., Zhang H., Wong W.C., Sem X., Han H., Ong S.M., Tan Y.C., Yeap W.H., Gan C.S., Ng K.Q. Identification of novel functional differences in monocyte subsets using proteomic and transcriptomic methods. J. Proteome Res. 2009;8:4028–4038. doi: 10.1021/pr900364p. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H.W. Definition of human blood monocytes. J. Leukoc. Biol. 2000;67:603–606. doi: 10.1002/jlb.67.5.603. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J. Leukoc. Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD14dimCD16+ human monocytes purified from whole blood (see Experimental Procedures) were labeled and injected intravenously into Cx3cr gfp/+ Rag2−/− γc−/− mice. Movie show patrolling mouse monocytes (gfp, green) and human CD14dim monocytes (Vybrant-DiD, Cyan). The time lapse is 23 min, the scale bar represents 100 μm, an initial magnification ×20 is shown, and the field is ∼250 μm/200 μm.
Same experimental setting as in Movie S1, with a time lapse of 45min; the scale bar represents 100 μm and an initial magnification ×20 is shown. The field is ∼500 μm/500 μm.