Abstract
Anterior hip or groin pain is a common complaint for which people are referred for physical therapy. We have observed that people with anterior hip pain often walk in greater hip extension than people without anterior hip pain, and that the pain is reduced when they walk in less hip extension. Therefore, we investigated anterior hip joint forces which may contribute to anterior hip pain and examined the effect of end range hip extension on the anterior hip joint force during gait. To do this, we used a 6 degree of freedom, 3-dimensional musculoskeletal model to estimate hip joint forces during gait. Within subjects, the maximum anterior hip joint force for gait trials with the most hip extension was compared to the anterior hip joint force for gait trials with the least hip extension. The musculoskeletal model indicated that increasing the maximum end range hip extension when walking results in an increase in the anterior hip joint force when compared to walking in less hip extension. Walking in greater hip extension may result in an increase in the anterior hip joint force, and thereby contribute to anterior hip pain. The findings of this study provide some evidence supporting the use of gait modification to reduce anterior hip force when treating people with anterior hip pain.
Keywords: acetabular labral tear, groin pain, hip joint force, hip pain
1. Introduction
Hip or groin pain is a common complaint for which people are referred for physical therapy, with the hip region being involved in approximately 2% to 11% of running injuries[1]. The Physical Stress Theory, as presented by Mueller and Maluf[2], proposes that excessive stress, that is stress that exceeds a tissue's tolerance, results in tissue injury and pain. In the case of anterior hip pain, we propose that the excessive stress can be due to repetitive forces into the connective tissues of the anterior hip joint (e.g. acetabular labrum). Running may be one example of an activity where low magnitude forces are repeatedly applied, and may contribute to the development of a labral tear[3, 4] and anterior hip pain[5]. The exact mechanism by which running may lead to a tear in the acetabular labrum has not been specified; however, the pattern of repeated hip hyperextension has been implicated[4, 5]. Sahrmann proposes that long distance running is often associated with increased anterior glide of the femoral head relative to the acetabulum as a result of hip hyperextension[5]. The increased femoral anterior glide could lead to increased force on the anterior hip joint structures and tearing of the anterior acetabular labrum. This mechanism could explain the labral tears which Guanche and Sikka[4] found in 8 high-level runners with hip pain. None of the runners reported any associated trauma with the development of the hip pain yet all eight had a tear of the anterosuperior region of the acetabular labrum. Guance and Sikka[4] theorized that the hip hyperextension inherent in the stance phase of running leads to “subtle instability and increasing stress at the cartilage-labral junction” (p.584).
Clinically, we have noted that patients with anterior hip pain often walk with greater maximum hip extension than people without hip pain. The patients typically report pain at the end of the stance phase of gait when the hip is in hyperextension. Furthermore, we have noted that patients with anterior hip pain report an immediate reduction in their hip pain when instructed to walk with less hip extension. We theorize that this reduction in hip pain is due to a decrease in the anterior hip joint force subsequent to the decreased hip extension.
Prior musculoskeletal simulations of hip exercises have demonstrated a relationship between hip joint forces and hip angle[6, 7]. In these studies, hip joint force was estimated when performing hip flexion in supine and hip extension in prone. Anterior hip joint force was found to increase with increasing hip extension angle independent of the muscles activated. The models used in these studies, however, were quasi-static evaluations of simulated single plane movements. Only the torque due to gravity was included. It is unclear if a similar relationship between force and hip angle exists during more complex activities such as gait.
The purpose of this paper, therefore, was to investigate forces on the anterior hip joint tissues during gait which may contribute to hip pain on a daily basis. We used a musculoskeletal model to estimate hip joint forces during normal gait to investigate the effect of end range hip extension on the anterior hip joint force. We hypothesized that the anterior hip joint force would be higher in walking trials with greater hip extension range of motion than in trials with less hip extension.
2. Methods
We used a 6 degree of freedom (DOF), 3-dimensional musculoskeletal model of a leg to estimate joint forces in the hip, knee, and ankle. This model was a simplification of a bilateral model developed by Carhart[8] and has been used in other studies[6, 7]. The model consists of a pelvis, thigh, shank and foot of the right leg. The six DOF represent the primary motions at the hip, knee and ankle as follows: (i) 3 DOF at the hip to model abduction-adduction, internal-external rotation and flexion-extension, (ii) 1 DOF at the knee to model knee flexion-extension, and (iii) 2 DOF at the ankle to model inversion-eversion and dorsiflexion-plantar flexion[8].
Musculoskeletal parameters were adapted from Delp[9] for the 43 muscle units included in the model. Delp subdivided large or complex muscles such as the gluteus maximus muscle into multiple muscle units to more accurately represent their muscle paths and functions than would single muscle units. To check the validity of our model, we compared the muscle moment arms calculated by our model to the muscle moment arms calculated by SIMM (MusculoGraphics, Inc, Santa Rosa, CA) for a published model[9] and found them to be in agreement. Kane's Method[10] and AUTOLEV 3.1 (OnLine Dynamics, Inc., Sunnyvale, CA) were used to generate the dynamic equations of motion. The dynamic equations of motion quantitatively represent the interrelationships between internal (muscle) forces and external forces and the skeletal motions that result from the forces. The general form of the dynamic equations of motion is:
In this equation, 𝐌 is the mass matrix. , and are the column vectors of the joint angles, angular velocity and angular acceleration respectively. is the column vector of net joint torques generated by the muscles. is the column vector of the torques developed passively in the joints due to viscoelastic damping and passive joint structures. , , and are column vectors of the instantaneous segmental torques caused by the inertial, gravitational, and external forces, respectively. We determined all parameters of the model except the joint torques due to muscle ( ) using kinematic and kinetic data from gait trials. The model then calculated for each time point based on the kinematic and kinetic input data. We used a pseudoinverse optimization routine minimizing squared muscle stress to solve for the optimal set of muscle stresses[11] to generate the measured joint torques. Muscle stress is defined as the force per unit area that a muscle generates and is expressed in N/cm2. Once the optimized muscle stresses were solved simultaneously across all joints, the model calculated the resulting 3D joint forces in both the femoral and pelvic reference frames at the hip (Figure 1). As we were concerned with forces on the hip, particularly on the acetabular labrum, all forces are presented in the pelvic reference frame. For example, an “anterior force” indicates a force which is imparted from the femur onto the acetabulum, and is in the anterior direction without regard for the position of the femur.
FIGURE 1.
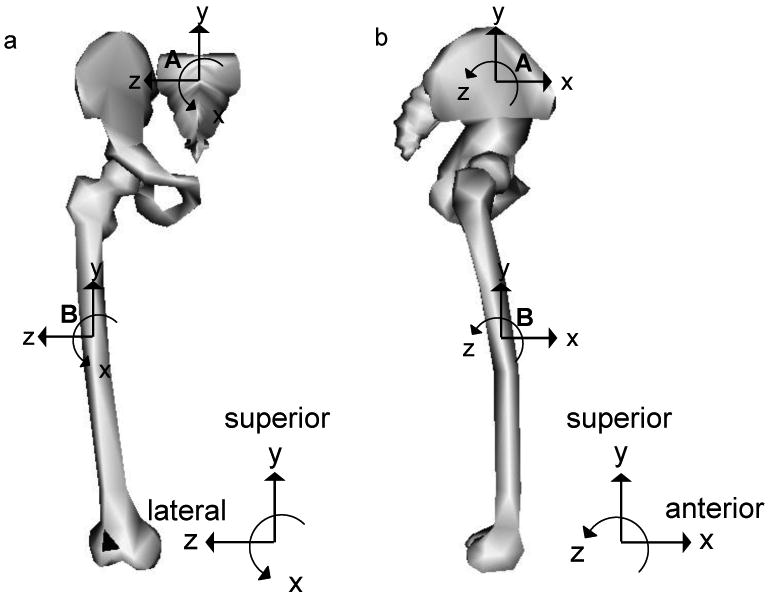
Illustration of the segmental reference frames in the frontal (a) and sagittal (b) plane for the pelvis (A), and thigh (B) with the subject standing in the anatomical position (adapted from Carhart, 2000). In the pelvic reference frame, the superior / inferior axis is in line with the trunk when in a standing posture. The anterior / posterior axis is perpendicular to the superior / inferior axis and in line with the progression of movement in the anterior direction. The medial / lateral axis is defined as the cross product of the other two axes. All forces presented here are in the pelvic reference frame. (Figure adapted from OpenSim 1.1)
As part of a previous study, 3-dimensional kinematic and kinetic data were collected from 5 healthy college-aged male subjects (height: 177.2 cm (range 168-188 cm); mass: 81.2 kg (range 75-91 kg)) who provided informed consent[8]. Each subject walked for 5 trials at a consistent self-selected speed. The data from these 25 trials were used as input for the musculoskeletal model. For each subject, the musculoskeletal model was scaled based on the subject's height and weight to adjust segment inertial parameters and muscle origin, insertion and via points.
Force Variables
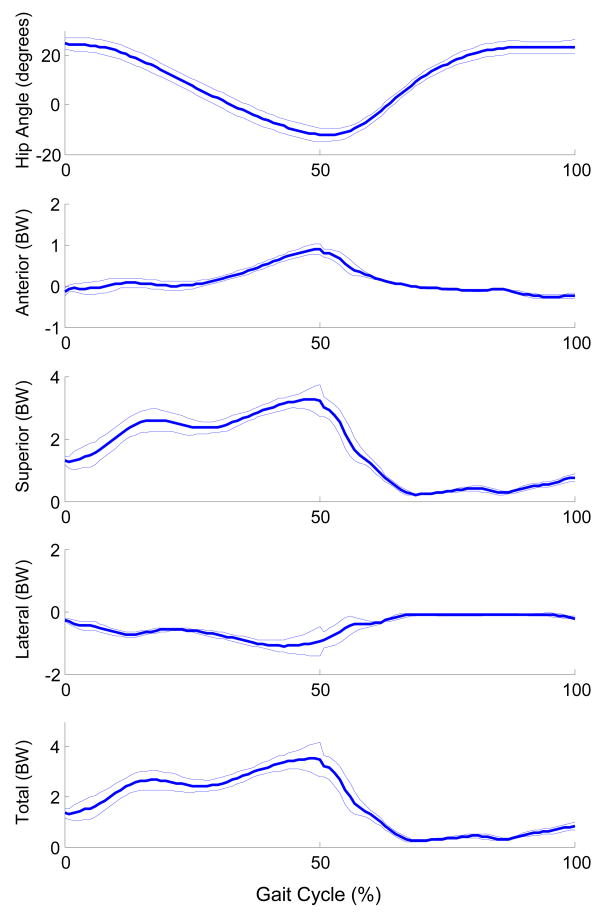
Using the musculoskeletal model, we estimated the hip joint forces during gait from each trial collected. The gait cycle was defined as heel strike to ipsilateral heel strike. For each trial, we normalized the data as a percentage of the gait cycle. The hip forces were interpolated at each 1% of the cycle (Figure 2).
FIGURE 2.
Average hip angle and hip joint forces (mean and standard deviation) due to muscle during gait. For hip angle (degrees), hip flexion is positive and hip extension is negative. Forces in the anterior, superior and lateral directions are represented as positive. All forces are expressed in terms of body weight (BW).
To examine the effect of hip extension on the anterior hip joint force, we separated each subject's walking trials into the two trials with the most hip extension (MHE) range of motion and the two trials with the least hip extension (LHE) range of motion. Within subjects, the maximum anterior force for the 2 MHE trials and the 2 LHE trials were each averaged, resulting in a mean MHE anterior hip force value and a mean LHE anterior hip force value for each subject.
All statistical analyses were performed in SYSTAT 10.2 (SYSTAT Software, Inc, Point Richmond, CA) with an alpha level of 0.05. Variables were first tested for normality, and then we used paired t-tests to compare the maximum anterior hip joint force within subjects to determine if trials with greater hip extension have higher hip force than trials with less hip extension. We also compared walking speed and hip flexion torque at the point of maximum anterior hip joint force to determine if there was a difference between the MHE and LHE trials.
3. Results
The musculoskeletal model indicated that the hip force in the transverse plane due to muscle alone is primarily in the anterior direction during the stance phase of gait (Figure 2). During the initial 8% of the gait cycle, the hip joint force is posterior. From approximately 9% to 67% of the gait cycle, the force is in the anterior direction. The anterior force peaks around 50% of the gait cycle, just prior to ipsilateral toe off. The maximum hip extension angle occurs at about 51% of the gait cycle.
The musculoskeletal model predicted that increasing maximum hip extension during gait resulted in an increased maximum anterior hip joint force (Table 1). In the MHE trials, the normalized anterior hip joint force was 24% higher than in the LHE trials (Figure 3). The maximum anterior hip joint force always occurred during the late stance phase of gait while the hip was in extension. The mean difference in the maximum hip extension angle between the MHE and LHE trials was only 2 degrees. This 2 degree difference resulted in a difference in the anterior hip joint force of 156 N, approximately 20% of body weight. This increase in anterior hip force occurred despite no difference in gait speed (paired t-test: t = 1.162, p = 0.31) and no consistent change in hip flexion torque at the point of maximum anterior force (paired t-test: t = 1.638, p = 0.18). No variables violated the normality assumption.
Table 1.
Hip extension angle and joint force: Comparison of maximum hip extension angle and maximum anterior hip force in Newtons (N) as well as a percentage of body weight (BW) between gait trials with the most hip extension (MHE) and trials with the least hip extension (LHE).
| MHE | LHE | t-value | p-value | |
|---|---|---|---|---|
| Max Hip Extension Angle (°) | 13.5±3.2 | 11.5±2.8 | 4.64 | 0.01 |
| Anterior Joint Force (N) | 834±215 | 677±92 | 2.60 | 0.03 |
| Anterior Joint Force (% BW) | 105±27% | 85±12% |
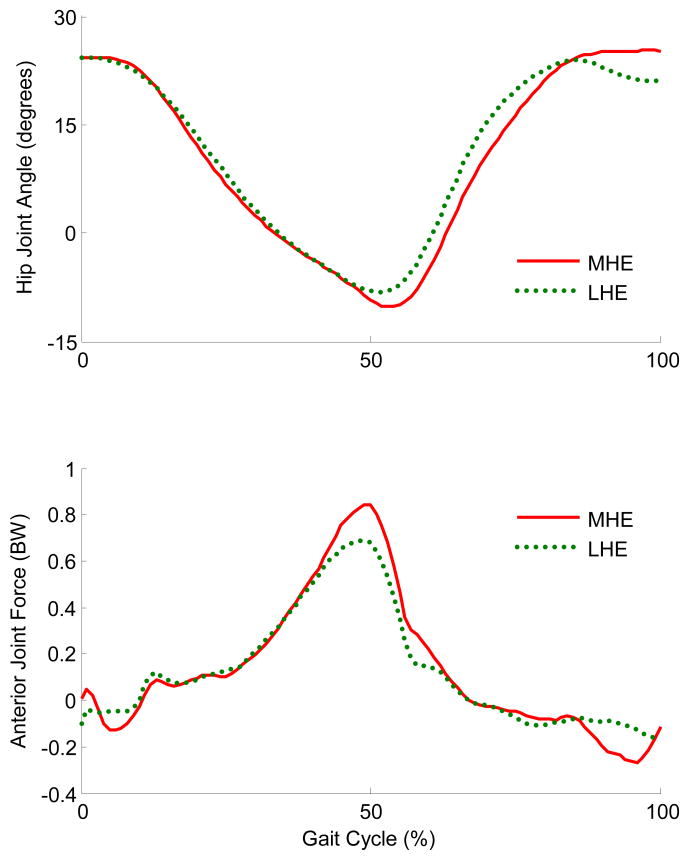
FIGURE 3.
Hip joint angle and anterior hip joint forces due to muscle during gait for a single representative subject. For hip angle (degrees), hip flexion is positive and hip extension is negative. Forces in the anterior direction are represented as positive, and expressed in body weight (BW). In the trial with the most hip extension (MHE, red), the peak anterior hip joint force is greater than in the least hip extension (LHE, green) trial.
The primary muscles contributing to anterior hip joint force at the time of peak force were the anterior portion of the gluteus medius, the iliacus and the psoas muscles. Other muscles which also contributed to anterior hip joint force at this point in the gait cycle included the rectus femoris, tensor fascia lata, and the middle portion of the gluteus medius muscles.
4. Discussion
The results of the musculoskeletal model indicate that the anteriorly directed hip joint forces are higher when walking in greater hip extension than when walking in less hip extension. The model predicted a 24% increase in the normalized anterior hip joint force due to muscle with only a 2 degree increase in the maximum hip extension angle. The maximum anterior hip joint force occurs just before the hip reaches its maximum hip extension angle, further implicating an interaction between anterior hip force and hip angle.
A relationship between hip angle and hip pain during gait has previously been reported. Murray et al. studied 26 males with unilateral hip pain[12]. These authors noted that the hip extension excursion on the painful side was decreased compared both to the uninvolved side and to men without hip pain. They suggested that the reduced hip extension during gait was a pain-avoidance technique used to reduce the force on the femoral head. Our model confirms that the hip forces on the femoral head are higher in hip extension.
In previous studies, we have noted a relationship between hip angle and hip force during hip exercises[6, 7]. One exercise was supine hip flexion, which requires hip flexor muscle activity while in a hip extended position similar to the stance phase of gait. Based on the data from those studies, the increase in hip joint force with a two degree change in hip extension angle (from 8 degrees to 10 degrees of extension), the anterior hip joint force increased by approximately 13%. During gait, the anterior hip joint force increased by 24%. This finding may indicate that the increase in joint force during gait is not due to angle alone. While we did not find a significant change in the hip flexion torque at the time of peak anterior hip joint force between the MHE and LHE trials, there is a decrease in the hip flexion moment arm of the primary muscles with increasing hip extension. Therefore, the increase in hip force may be partially due to the increasing muscle activation to produce the same hip flexion torque.
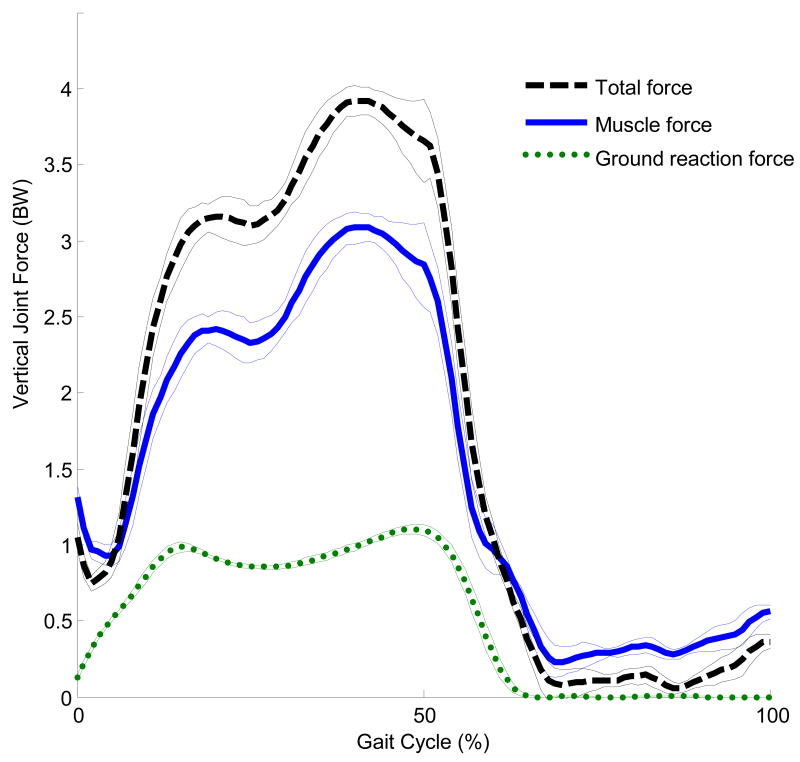
The current study focused only on the hip joint force due to muscle contraction. Prior studies have indicated that the muscular component of the hip joint force is greater than the component due to ground contact[13-15]. Typical vertical ground reaction forces peak at just over body weight while the vertical hip joint force calculated using a musculoskeletal model can exceed 4 times body weight[14]. Lu et al.[16] also found that the compressive force measured within the shaft of an instrumented femoral prosthesis was up to 3.5 times the ground reaction force. Our calculated joint forces are within these ranges. The average maximum vertical hip force was 3.3 times body weight for muscle alone and 4.1 times body weight when including forces due to inertia, gravity, and muscle (Figure 4). Thus, approximately 80% of the total vertical force at the hip is due to muscle contraction. The finding that a large proportion of joint force comes from muscle is consistent with the assertion that muscle imbalance or weakness may significantly increase the joint forces[17], and has been previously supported by musculoskeletal modeling[7].
FIGURE 4.
Average and standard deviation of the vertical forces for a representative subject. The ground reaction force (green) is significantly lower than the joint force due to muscle alone (blue) and the total joint force (black). The joint force due to muscle is the largest component of the total hip joint force, providing approximately 80% of the maximum total force.
The primary muscles contributing to anterior hip joint force were the anterior portion of the gluteus medius, the iliacus and the psoas muscles. The gluteus medius at this point in the gait cycle primarily contributes hip abduction torque, while the iliacus and psoas contribute hip flexion torque. Reducing the activation of these muscles would, in theory, reduce the anterior hip joint force. However, our previous study demonstrated the unique role that the iliacus and psoas in particular play in generating hip flexion torque[7][18]. Reduction in the iliacus and psoas muscle activation would lead to a substantial increase in synergist muscle groups and a concomitant increase in joint force. Therefore, it may be more advantageous to decrease the needed hip flexion torque, by increasing ankle pushoff[19] for example.
Correa et al.[15] also found that the primary contributors to hip joint contact forces were the gluteus medius and the iliopsoas; however, they report that the anterior component of the contact force peaks at contralateral toe-off. This difference could be due to the reference frame used. Correa et al. use a femoral reference frame while our study uses a pelvis reference frame. We specifically selected the pelvic reference frame as we were interested in how the forces affected the acetabular structures.
As with all musculoskeletal models, there are limitations inherent in attempting to model complex human movement with simplified lines of action for muscles and computerized optimization routines for motor control. One major limitation is the determination of muscle stress. We utilized an optimization routine based on minimizing the sum of the squared stress over the system of muscles. Theoretically, the optimization function captures the physiological properties of muscle (muscle moment arm and maximum muscle strength) as well as the goal of maximum muscle endurance as proposed by Crowninshield and Brand[20]. Similar optimization routines have been used in the prediction of muscle stresses during gait and have resulted in “intuitively reasonable” solutions with muscle activation timing similar to electromyographic data[8]. Another limitation is that the results of the model are limited in their ability to be generalized to the population of people with hip pain. The model utilized gait data from 5 males, while anterior acetabular labral tears and hip pain are more common in females[21-25]. We also do not separate out the effect of hip angle from the effect of hip moment. It is possible that an increase in one variable, either angle or moment, would not result in a concomitant increase in anterior joint force. However, in human gait, the two variables are often closely related to each other.
The results of this study help to support the interventions we propose for patients with anterior hip pain and acetabular labral tears. We recommend correction of posture and avoiding hip and knee hyperextension during gait[5, 26], and have clinically noted that the modifications are effective in reducing a patient's hip pain. Based on the musculoskeletal model, the modifications would reduce the forces on the anterior hip joint structures. This reduction of force could reduce the stress on the anterior hip joint, and thereby reduce anterior hip pain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Mechelen W. Running injuries. A review of the epidemiological literature. Sports Med. 1992;14:320–335. doi: 10.2165/00007256-199214050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Mueller MJ, Maluf KS. Tissue adaptation to physical stress: a proposed “Physical Stress Theory” to guide physical therapist practice, education, and research. Phys Ther. 2002;82:383–403. [PubMed] [Google Scholar]
- 3.Fitzgerald RH., Jr Acetabular labrum tears. Diagnosis and treatment. Clin Orthop. 1995;311:60–68. [PubMed] [Google Scholar]
- 4.Guanche CA, Sikka RS. Acetabular labral tears with underlying chondromalacia: a possible association with high-level running. Arthroscopy. 2005;21:580–585. doi: 10.1016/j.arthro.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Sahrmann SA. Diagnosis and treatment of movement impairment syndromes. St. Louis, MO: Mosby, Inc.; 2002. [Google Scholar]
- 6.Lewis CL, Sahrmann SA, Moran DW. Anterior hip joint force increases with hip extension, decreased gluteal force, or decreased iliopsoas force. J Biomech. 2007;40:3725–3731. doi: 10.1016/j.jbiomech.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis CL, Sahrmann SA, Moran DW. Effect of position and alteration in synergist muscle force contribution on hip forces when performing hip strengthening exercises. Clin Biomech (Bristol, Avon) 2009;24:35–42. doi: 10.1016/j.clinbiomech.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carhart MR. Biomechanical analysis of compensatory stepping: implications for paraplegics standing via FNS. Arizona State University; 2000. [Google Scholar]
- 9.Delp SL. Parameters for a model of the lower limb. [12/1/2005]; http://www.isbweb.org/data/delp/
- 10.Kane TR, Levinson DA. Dynamics: theory and application. New York, NY: McGraw-Hill; 1985. [Google Scholar]
- 11.Yamaguchi GT, Moran DW, Si J. A computationally efficient method for solving the redundant problem in biomechanics. J Biomech. 1995;28:999–1005. doi: 10.1016/0021-9290(94)00145-t. [DOI] [PubMed] [Google Scholar]
- 12.Murray MP, Gore DR, Clarkson BH. Walking patterns of patients with unilateral hip pain due to osteo-arthritis and avascular necrosis. J Bone Joint Surg Am. 1971;53:259–274. [PubMed] [Google Scholar]
- 13.Bassey EJ, Littlewood JJ, Taylor SJ. Relations between compressive axial forces in an instrumented massive femoral implant, ground reaction forces, and integrated electromyographs from vastus lateralis during various ‘osteogenic’ exercises. J Biomech. 1997;30:213–223. doi: 10.1016/s0021-9290(96)00043-7. [DOI] [PubMed] [Google Scholar]
- 14.Stansfield BW, Nicol AC. Hip joint contact forces in normal subjects and subjects with total hip prostheses: walking and stair and ramp negotiation. Clin Biomech (BristoAvon) 2002;17:130–139. doi: 10.1016/s0268-0033(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 15.Correa TA, Crossley KM, Kim HJ, Pandy MG. Contributions of individual muscles to hip joint contact force in normal walking. J Biomech. 2010;43:1618–1622. doi: 10.1016/j.jbiomech.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Lu TW, Taylor SJ, O'Connor JJ, Walker PS. Influence of muscle activity on the forces in the femur: an in vivo study. J Biomech. 1997;30:1101–1106. doi: 10.1016/s0021-9290(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann G, Graichen F, Rohlmann A. Hip joint contact forces during stumbling. Langenbecks Arch Surg. 2004;389:53–59. doi: 10.1007/s00423-003-0434-y. [DOI] [PubMed] [Google Scholar]
- 18.Lewis CL, Sahrmann SA. Muscle activation and movement patterns during prone hip extension exercise in women. J Athl Train. 2009;44:238–248. doi: 10.4085/1062-6050-44.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis CL, Ferris DP. Walking with increased ankle pushoff decreases hip muscle moments. J Biomech. 2008;41:2082–2089. doi: 10.1016/j.jbiomech.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowninshield RD, Brand RA. A physiologically based criterion of muscle force prediction in locomotion. J Biomech. 1981;14:793–801. doi: 10.1016/0021-9290(81)90035-x. [DOI] [PubMed] [Google Scholar]
- 21.Santori N, Villar RN. Acetabular labral tears: result of arthroscopic partial limbectomy. Arthroscopy. 2000;16:11–15. doi: 10.1016/s0749-8063(00)90121-x. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda T, Awaya G, Suzuki S, Okada Y, Tada H. Torn acetabular labrum in young patients. Arthroscopic diagnosis and management. J Bone Joint Surg Br. 1988;70:13–16. doi: 10.1302/0301-620X.70B1.3339044. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy JC, Noble PC, Schuck MR, Wright J, Lee J. The Otto E. Aufranc Award: The role of labral lesions to development of early degenerative hip disease. Clin Orthop. 2001 Dec;:25–37. doi: 10.1097/00003086-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Dorrell JH, Catterall A. The torn acetabular labrum. J Bone Joint Surg Br. 1986;68:400–403. doi: 10.1302/0301-620X.68B3.3733805. [DOI] [PubMed] [Google Scholar]
- 25.Hase T, Ueo T. Acetabular labral tear: arthroscopic diagnosis and treatment. Arthroscopy. 1999;15:138–141. doi: 10.1053/ar.1999.v15.0150131. [DOI] [PubMed] [Google Scholar]
- 26.Lewis CL, Sahrmann SA. Acetabular labral tears. Phys Ther. 2006;86:110–121. doi: 10.1093/ptj/86.1.110. [DOI] [PubMed] [Google Scholar]