Abstract
Background
Port systems are easy to implant on an in- or outpatient basis and provide reliable, long-lasting central venous access. They are used mainly for cancer patients.
Methods
This article is based on a selective literature review, the guidelines of the German Society for Nutrition Medicine and of the European Society for Clinical Nutrition and Metabolism, and the recommendations of the German Society for Pediatric Oncology and Hematology.
Results
In modern oncology, central venous port systems are increasingly replacing short-term and permanently tunneled central venous catheters. They are indicated for patients who need long-term intravenous treatment involving, e.g., the repeated administration of chemotherapeutic drugs, parenteral nutrition, transfusions, infusions, injections, and/or blood sample collection. Port systems can markedly alleviate the burden of intravenous therapy and thereby improve these patients’ quality of life. The planning, preparation, and performance of port system implantation require meticulous attention to detail. The rate of implantation-associated complications is less than 2% in experienced hands; overall complication rates have been reported from 4.3% to as high as 46%. The proper postoperative use and care of the port system are of decisive importance to the outcome. Reported infection rates during port system use range from 0.8% to 7.5% in current clinical studies.
Conclusion
The treatment, follow-up care, and rehabilitation of cancer patients are interdisciplinary tasks. Optimal treatment and complication avoidance require a collaborative effort of all of the involved specialists—not just the physician implanting the port system, but also the oncologists, nutritionists, visiting nurses, and other home health care providers. Continuing medical education, too, plays a role in improving outcomes.
The recent advent of more intensive methods of chemotherapy and parenteral treatment has heightened the need for implantable devices that afford _reliable central venous access over the long term. Central venous ports that are wholly implanted beneath the skin play a key role. In oncology, the patient’s quality of life is the prime consideration, yet economic aspects of the often expensive treatments for cancer must also be taken into account; this is mandated in Germany by the Law on Cost-Efficient Medical Treatment (Arzneimittelversorgungs-Wirtschaftlichkeitsgesetz, AVWG) (1). Central venous port systems, which cost well under 500 euros each and can be used for years, eminently satisfy the economic requirements of modern oncology and add no more than a marginal amount to the cost of chemotherapy.
Learning objectives
The learning objectives for readers of this article are
to become acquainted with the various uses of port systems,
to gain an overview of the indications and the inclusion and exclusion criteria for port implantation,
to know the special considerations in the medical and nursing care of patients with port systems.
In this review article, we discuss the function of, and indications for, port-catheter systems in the light of a selective review of the literature, including the main studies (mostly from the last 10 years) on complications and their management.
Definition.
A central venous port is a venous access system that is implanted entirely under the skin.
Underlying principles and function
Chemotherapeutic drugs can damage the wall of peripheral veins and thereby rapidly put an end to peripheral access. According to the current recommendations of the European Society for Parenteral and Enteral Nutrition (ESPEN), infusions of low osmolarity (<850 mOsm/L) may be given through indwelling peripheral venous catheters (2). Many chemotherapeutic drugs, however, are administered in solutions of substantially higher osmolarity. Complications such as infection, narrowing of the venous caliber, and thrombotic occlusion can make peripheral venous infusion difficult or impossible. If a catheter is to get past the narrow peripheral vasculature, its tip must be advanced all the way to the vena cava near the heart. When the catheter tip is in this position, a large volume of blood flows past it, so that medications given through the catheter are immediately diluted and can no longer damage the vessel wall. The first catheters of this type to be developed were percutaneous, non-tunneled catheters, which came into use in the 1950’s (3).
Determining the indication.
The indication for a port system is usually determined by oncologists, radiotherapists, and dieticians.
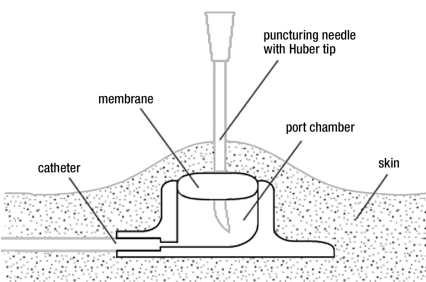
Niederhuber et al. introduced the currently used type of port system into clinical use in 1982 (4). Unlike other implants, these devices are used repeatedly by many physicians and nurses and require transcutaneous puncture whenever an infusion is given. Improper use can lead to complications such as infection, extravasation, necrosis, or material failure (5). All persons treating cancer patients with port systems must assume responsibility for the meticulous care of the system. Catheter systems without ports have a connecting device at their distal (extracorporeal) end; in contrast, access to a port system requires puncture with a needle through the skin and the silicone membrane of the port chamber (Figure 1). Ports are always punctured under sterile conditions, with skin prep and sterile gloves, to prevent infection (6, 7).
Figure 1.
A subcutaneously implanted central venous port system
Port puncture is easier when the port chamber is immobilized between the tips of two or three fingers of the puncturer’s nondominant hand (8). Special needles with a non-punching Huber tip are used to puncture the silicone membrane of the port system (9, 10).
Indications
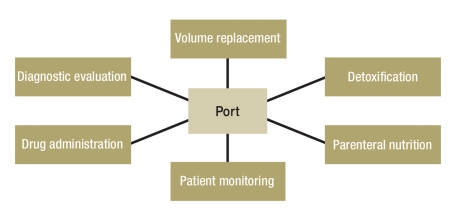
The determination that a port system should be implanted is usually made by oncologists of various subdisciplines, radiotherapists, or dieticians (Figure 2). The physician who is to implant the device reviews the indication and assesses the anatomical situation, which may be far from normal in patients who have previously undergone chemotherapy, radiotherapy, and/or surgery (11). There may be major changes of the skin, soft tissues, veins, or bones of the shoulder girdle. Ultrasonography of the central veins at the thoracic outlet is recommended to rule out anatomical variants and venous thromboses (12– 16).
Figure 2.
While chemotherapy is the most common indication, central venous ports also have other uses
Pre-implantation examination.
Ultrasonography of the central veins at the upper thoracic aperture is recommended to rule out anatomic variants and vascular thromboses.
Implantation
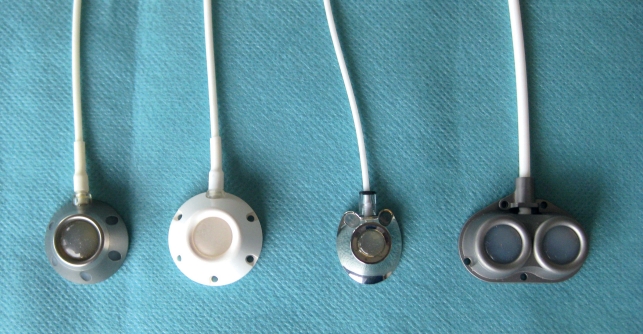
A port can be implanted in an inpatient or outpatient setting or in a day surgery unit. The implanting physician should perform a physical examination and, where indicated, venous ultrasonography at the intended implantation site, during the same preoperative visit in which the patient is informed about the procedure and asked to give consent (17). The findings of these examinations determine the approach for central venous access and the appropriate type of port system (Figure 3). Thin or cachectic patients are best served with a so-called low-profile port system with a flat chamber (18), while a higher-profile port is suitable for obese patients. By choosing appropriately, one can avoid skin necrosis due to large ports in thin patients, as well as difficulties in localizing and puncturing small ports within the abundant subcutaneous fat of obese patients. Double-chamber port systems are available for patients who need simultaneous treatment with chemotherapeutic drugs and parenteral nutrition (19, 20). Computed tomography with the intravenous administration of contrast medium is now used for staging in patients with many different types of cancer; to this end, port systems have recently become available through which contrast medium can be injected (21).
Figure 3.
The choice of port system depends on the indication. From left to right: titanium, synthetic, low-profile, and double-chamber port systems
Complications.
Interventional complications occur in less than 2% of cases in experienced hands.
Duration of the intervention.
Port implantation takes 15 to 30 minutes and can be performed by one physician.
Surgical complications arise in less than 2% of cases in experienced hands (17). Potential approaches for insertion of the central venous catheter are by way of the cephalic and subclavian veins in the area of the shoulder girdle, the basilic vein on the medial side of the arm (22) or forearm (23), or the internal jugular vein on the anterolateral aspect of the neck (5, 24, 25). Alternatively, the external jugular vein can be used for surgical vascular access, particularly in children (e1, e2). In very rare cases, the great saphenous vein can be used for access, either directly in the thigh (e3) or by way of collateral vessels (e4, e5), if all of the approaches mentioned above are unavailable because of prior treatments, operations, and/or venous thromboses. Catheter placement by direct puncture of the subclavian or internal jugular vein has many advantages (e6– e8). In particular, it ordinarily does not require general anesthesia. The mode of local or regional anesthesia should be chosen so that pain does not arise after the procedure (e9). The use of small quantities of tumescence anesthesia has been found to be helpful. The side of central access is often determined by unilateral breast carcinoma (e10), ulcerations on the chest, previously implanted pacemakers, pre-existing unilateral venous thromboses, or other circumstances. Port implantation takes 15 to 30 minutes and can be performed by one physician. The appropriate catheter length is a function of the size of the patient and of the site of implantation of the port chamber. When the catheter is to be implanted by way of the right jugular or subclavian vein, the average intravascular catheter length from the site of vessel entry to the cavo-atrial junction is 12 cm; when access is from the left side, the average length is 18 cm (14). An ECG registered throughout the procedure reveals elevation of the heart rate as soon as the guide wire is introduced into the heart, indicating that central venous access has been successfully achieved. An intraoperative X-ray with a so-called C-arm is thus not always needed, but should nonetheless be available for potential use in every case. When the procedure is performed under fluoroscopic control, the catheter length can be adjusted as needed during port implantation. Whether or not this is done, a chest X-ray is obligatory after the procedure, both to document the catheter position and to rule out pneumothorax. This post-procedural X-ray must also be checked by the physician responsible for the patient’s further treatment (e11, e12).
Implantation techniques.
Classic surgical cut-down
Direct puncture based on anatomic landmarks
Ultrasound-guided puncture
In Germany, ports are implanted by surgeons and interventional radiologists (25, e13). The techniques used for implantation include the classic surgical cut-down method with exposure of the vessel to be punctured, direct puncture with the aid of anatomic landmarks, and ultrasound-guided puncture (e14, e15). Biffi et al., in a randomized, controlled trial published in 2009, found that the choice of implantation technique, approach, and side of implantation made no significant difference with respect to early or late complications (16). They did find, however, that punctures could generally be performed more successfully under ultrasound guidance, as was also found by other authors (15, e16– e18). In the eTable, we provide an overview of prospective and retrospective studies of port implantation at the chest wall that included more than 100 patients and that yielded information about complication rates and port survival times as a function of the approach used (16; e19–e34).
eTable. Prospective and retrospective studies on port implantation in the chest wall*1.
| Authors | No. ports | Discipline*2 | Technique*3 | Approach*4 | Catheter days | Complications | Infections | Venous thromboses | Pneumothorax | Catheter fracture | Explantation*5 |
| Ignatov et al.2009 (e19) | 561 | Surg. (7%) | Surg. | EJV (358) | 675 | 104 (19%) | 42 (7.5%) | 30 (5.3%) | 2 (0.36%) | NA | 46 (8.2%) |
| Rad. (3%) | Seld./lm | IJV (15) | |||||||||
| SclV (179) | |||||||||||
| CephV (9) | |||||||||||
| Hsieh et al. 2009 (e20) | 1348 | Surg. | Seld./lm | SclV (196) | 178 | 102 (7.5%) | 40 (2.96%) | 47 (3.48%) | NA | NA | 102 (7.6%) |
| Surg. | CephV (1100) | ||||||||||
| NA | Other (52) | ||||||||||
| Biffi et al.2009 (16) | 401 | Surg. | Seld./lm | IJV (132) | 356.5(0–1087) | 60 (15%) | 1 (0.8%) | 15 (12.8%) | 0 | 0 | 1 (0.9%) |
| Seld/US | SclV (136) | 3 (2.4%) | 8 (6.5%) | 0 | 0 | 1 (0.8%) | |||||
| Surg. | CephV (133) | 3 (2.5%) | 11 (9.2%) | 1 (0.7%) | 0 | 6 (5%) | |||||
| Rouzrokh et al. 2009 (e21) | 524 | Surg. | Surg. | IJV | 329.5(8–2028) | 120 (22.9%) | 29 (5.5%) | 7 (1.3%) | NA | 0 | 14 (2.7%) |
| Vandoni et al. 2009 (e22) | 228 | Surg. | Seld./lm | SclV | 441(6–3090) | 56 (24.6%) | 10 (4.3%) | NA | 10 (4.3%) | 13 (5.7%) | 24 (10.5%) |
| Samaras et al. 2008 (e24) | 201 | Surg. | Seld./lm | SclV (62) | 175(1–831) | 46 (22.9%) | 14 (7%) | 12 (6%) | 4 (2%) | 0 | 20 (10%) |
| Surg. | CephV (139) | ||||||||||
| Araujo et al.2008 (e23) | 1231 | Surg. | Seld./lm | SclV (617) | 363 (3–1132) | 118 (20.8%) | 19 (3.4%) | 11 (2%) | 6 (1%) | 2 (0.4%) | 43 (17.3%) |
| IJV (614) | 244 (3–853) | 48 (9.2%) | 11 (2%) | 3 (0.6%) | 2 (0.3%) | 0 | 16 (13.9%) | ||||
| Chen et al.2007 (e25) | 100 | Surg. | Surg. | CephV/SclV | 170(65–274) | 9 (9%) | 4 (8%) | 1 (2%) | 0 | 0 | 7 (14%) |
| An. | Seld./lm | IJV | 1 (2%) | 0 | 0 | 0 | 1 (2%) | ||||
| Marcy et al.2005 (e26) | 100 | Surg. | Surg. | CephV | 222(12–680) | 16 (16%) | 6 (6%) | 3 (3%) | 0 | 0 | 11 (11%) |
| Stein et al.2005 (24) | 2359 | Surg. | Surg. | CephV (2253) | NA | 147 (4.3%) | 57 (2.4%) | 49 (2%) | 5 (0.2%) | 0 | 15 (0.6%) |
| Seld./lm | SclV (106) | ||||||||||
| Caers et al.2005 (e27) | 448 | Surg. | Surg. | CephV | 366(3–1 206) | 91 (20.8%) | 19 (4.3%) | 37 (8.5%) | 3 (0.7%) | 1 (0.2%) | 42 (9.6%) |
| Vardy et al.2004 (e28) | 111 | Rad. | Seld./lm | SclV | 210(60–570) | 23 (20.7%) | 6 (5.4%) | 2 (2%) | 2 (2%) | NA | 8 (7.2%) |
| Biffi et al.2004 (e29) | 377 | Surg. | Seld./lm | SclV | 473(1–1419) | 19 (5%) | 5 (1.33%) | 4 (1.1%) | 7 (1.9%) | 0 | 11 (2.9%) |
| Wolosker et al. 2004 (e30) | 519 | Surg. | Surg. | EJV (383) | 353(5–1729) | 83 (16%) | 44 (8.5%) | 14 (2.7%) | 0 | 1 | 35 (6.7%) |
| Seld./lm | Other (40) | (0.2%) | |||||||||
| Seld./lm | IJV (73) | ||||||||||
| Other (23) | |||||||||||
| Moureau et al. 2002 (e31) | 8210 | Surg./Rad. | NA | NA | NA | 362 (4.4%) | 208 (2.5%) | 43 (0.5%) | NA | 16 (0.2%) | NA |
| Yip et al.2002 (e32) | 118 | Rad. | Seld/US | IJV | 342.8(21–813) | 10 (8.5%) | 5 (4.2%) | 2 (1.7%) | 0 | 0 | 8 (6.8%) |
| Biffi et al.2001 (e33) | 302 | Surg./Rad. | Seld./lm | SclV | 237 | 55 (18.2%) | 4 (1.3%) | 17 (5.6%) | 8 (2.6%) | 1 (0.3%) | 9 (3%) |
| Hartkamp et al. 2000 (e34) | 126 | Surg. | NA | NA | 192.5(2–1091) | 58 (46%) | 20 (16.3%) | 9 (7.3%) | 1 (0.8%) | 0 | 16 (13%) |
*1This table includes studies involving more than 100 port implantations in the period 2000 to 2010.
*2Medical discipline: Surg. = surgery; Rad. = radiology; An. = anesthesia;
*3Technique: Surg. = open surgical; Seld./lm = Seldinger/landmark; Seld/US = Seldinger/ultrasound;
*4Approach: EJV = external jugular vein; IJV = internal jugular vein; SclV = subclavian vein; CephV = cephalic vein; NA = data not available;
*5Explantation due to complications
Infection rates in recent studies range from 0.8% (16) to 7.5% (e19); infection remains both the most common port complication and the most common cause for explantation. Pre- or post-procedural antimicrobial prophylaxis is generally unnecessary. Gebauer et al. found, however, that a single periprocedural intravenous short infusion of a broad-spectrum antibiotic lowererd the rate of port infections from 6.7% to 1.3 % (e35). Controversy surrounds both post-procedural thrombosis prophylaxis and regular flushing of the port catheter with heparin solution (e36– e40). The manufacturers of port systems recommend flushing the system after each use with heparin in normal saline in concentrations ranging from 10 to 100 IU/mL (5), yet there are no scientific data to justify doing this routinely. Bisseling et al., in a trial carried out on a small number of patients, found that flushing the catheter with taurolidine, rather than heparin, significantly lowered the rate of catheter infection (e41). A possible reduction of the infection rate by flushing with ethanol has also been published (e42– e47). Safdar et al. concluded in a meta-analysis that catheter flushing with vancomycin may reduce the frequency of catheter-related bacteremia in high-risk patients (e43).
Complication management
Once a port system has been implanted, it should be used in such a way as to minimize functional disturbances and complications. It is essential that the treating physicians and nurses be trained in the proper use of port systems. The implanting physician clearly plays a central role in user training. He or she should remain the first resort of all treating personnel for any questions or problems that might arise.
Infection rates.
The reported infection rates in recent studies range from 0.8% to 7.5%. Infection remains the most common complication and the most common reason for port explantation.
Port complications can be subdivided into procedural complications that arise during implantation, catheter-related complications, and vascular complications. Early complications are, by definition, those arising between 24 hours and 4 weeks after implantation, while late complications are those arising more than 4 weeks after implantation. Late complications are unlikely to be due to the port implantation procedure itself.
Grouping complications by the time at which they arise facilitates their classification as well as the determination of causes (Table) (18).
Table. Complications associated with port implantation.
| Compliications | Intervention*1 | Early | Late |
| Interventional/surgical complications | |||
| Inadvertent arterial puncture (e48) | + | – | – |
| Air embolism (e49) | + | + | + |
| Pneumothorax (e50) | ++ | + | – |
| Hematoma (e51) | ++ | + | – |
| Perforation (heart, major vessels) (e52– e55) | + | + | + |
| Cardiac arrhythmia (e56) | + | + | + |
| Plexus irritation (e57) | + | + | – |
| Catheter-related complications | |||
| Catheter dislocation (e58– e61) | + | + | + |
| Catheter entrapment (“pinch-off syndrome”) (e62– e65) | – | + | ++ |
| Catheter leakage and embolism (e66) | + | ++ | ++ |
| Fibrinous sheath (e67, e68) | – | + | ++ |
| Catheter thrombosis/occlusion (e60) | – | + | + |
| Migration or torsion of the port reservoir (e69) | + | + | + |
| Infection | – | ++ | ++ |
| Cutaneous necrosis (e70) | – | + | ++ |
| Vascular | |||
| Thrombosis | – | ++ | +++ |
| Arteriovenous fistula (e71) | + | + | + |
*1complications arising during impalntation; frequency: – rare; + occasional; ++ common +++ very common
Infection at the port site shows the typical clinical features of local infection. Bacterial colonization of the catheter or port chamber arises after the system is used and manifests itself as fever, shaking chills, and malaise (e72– e75). Extravasation is generally treated conservatively; extensive extravasation may necessitate the implantation of a subcutaneous drain or the explantation of the port (e76).
Pneumothorax manifests itself with coughing fits, shortness of breath, and anxiety (e50). Patients with pneumothorax must be hospitalized immediately and often need drainage through a chest tube. If hemorrhage is found to have occurred in connection with the implantation procedure, the site should be shown at once to the implanting physician (rather than to third parties). Post-procedural hemorrhage is often a complication of the underlying illness (e51). The port should be left in place if possible; a sucutaneous drain can be inserted if necessary. The port system can be used again in a few days (5).
Port complications.
Port complications are classified as procedural (arising during implantation), catheter-related, and vascular.
If the port system cannot be flushed and no blood can be aspirated from it after a properly performed chamber puncture, an obstruction is likely to be present somewhere in the system. The common causes of obstruction are bood clot, remnants of parenteral nutrition, and encrusted medications (e60). To determine the type of obstruction that is present, one should inquire specifically about the manner in which the system was last used. The following procedure can be followed to eliminate the obstruction (e77, e78): First, 100 IU of heparin in 5 mL of 0.9% saline are injected and aspirated without pressure through a 5 mL syringe. If the system is still blocked, the port needle should be removed, the skin-reprepped, and another attempt made to unblock the system with a fresh port needle. If the blockage persists, one should dissolve 10 000 IU of urokinase in 2 mL of 0.9% saline and inject 1 mL of this solution. 20 minutes later, this solution is aspirated out of the port and the port is flushed with 20 mL of 0.9% saline. The procedure can be repeated up to three times. According to the literature, unblocking with alteplase is comparably effective (e79– e81); here, an attempt is made to flush the system with 1 to 4 mg of alteplase (e82).
Obstruction within the system.
If the port chamber cannot be flushed and blood cannot be aspirated from it after a properly performed puncture, there is likely to be an obstruction somewhere within the system.
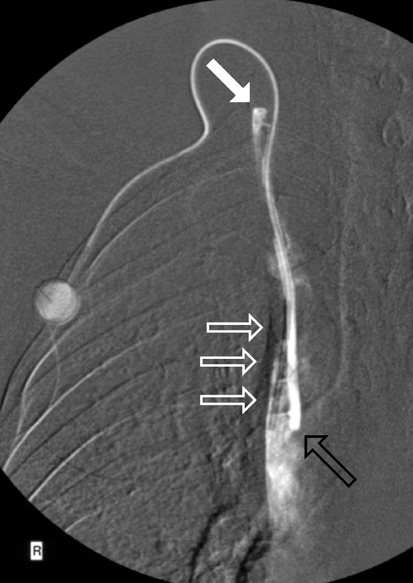
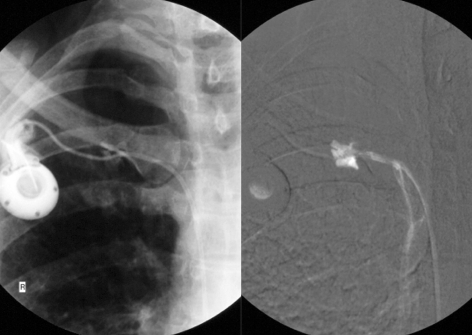
If the port system cannot be unblocked by flushing, it should be investigated with a radiographic contrast study. Contrast medium is injected through a port needle, and fluoroscopy is performed. Movement of the catheter, leading to kinking or to displacement of the catheter tip, is a possible cause of sudden loss of patency of the system (e61). Such catheter dislocations can usually be repositioned in an interventional radiological procedure performed by way of the femoral vein, obviating the need for explantation of the port and implantation of a new port. If a port functions well at first but then gradually becomes more difficult to use, this is often due to the formation of a fibrinous sheath around the catheter near its tip (Figure 4) (e67, e68). Catheter fractures and leaks can be caused by entrapment of the catheter between the first rib and the clavicle (“pinch-off syndrome”), if the catheter has been inserted through the subclavian vein; leakage is dangerous, as chemotherapeutic drugs leaking out into the surrounding tissue can cause extensive necrosis (e70, e83). A catheter leak can be demonstrated with a fluoroscopic constrast study of the port system (Figure 5). Catheter-tip dislocation can occur months after implantation of the system. Patients sometimes complain of pressure in a neck vein during infusion. Port-catheter-associated thromboses can lead to the occlusion of central veins and even to superior vena cava syndrome (e84). Malposition of the catheter tip in the mediastinum is a very serious complication that may lead to the entry of infused solutions into the mediastinum or pleural space (“infusion thorax”) (e53– e85).
Figure 4.
The cause of progressively severe dysfunction of a port catheter system: a fibrinous sheath (transparent white arrows) forms around the proximal portion of the catheter near its tip in the superior vena cava. The tip lies in correct position (transparent black arrow) at the cavo-atrial junction. Contrast medium injected into the port is seen to run along the catheter beneath the fibrinous sheath and to enter the vascular lumen at an undesired position (white arrow)
Figure 5.
Catheter fractures due to entrapment of the catheter between the first rib and the clavicle (“pinch-off syndrome”) are especially dangerous, because chemotherapeutic drugs can leak out through the fracture into the surrounding tissue and cause extensive necrosis
Figures 4 and 5 from: Teichgräber U, Gebauer B, Benter T, Wagner H: Langfristige zentralvenöse Zugänge und deren Komplikationsmanagement.
Fortschr Röntgenstr 2004; 176(7): 944–952. With kind permission from Thieme-Verlag
If the rules of proper use are observed and the system is flushed with 20 mL of 0.9% saline before each infusion, the patient becomes aware of the problem through a sensation of pressure and burning at the level of the defect. Injections of less than 10 mL should not be given at the port, because the higher pressure under which such injections are delivered may lead to catheter disconnection or tearing. If this happens, the damaged system has to be explanted. Current studies do not support the notion that port systems need regular puncturing, flushing, and heparin flushing in the interval between treatments (e37, e86), even though some port manufacturers recommend that this be done, citing the requirements of the German Law on Medical Products. In any case, the manufacturer’s recommendations with respect to pressures should be followed, and contrast medium should only be administered through high-pressure port systems (21).
Overview
Frequent puncturing of peripheral veins and the local effects of chemotherapeutic drugs cause damage, thrombosis, and sclerosis of the vascular wall. Port systems for permanent central venous access therefore play an essential role in modern oncology. They have the advantage that the puncturing needle can be removed after each infusion and the skin covering the port reservoir serves as a natural protection against infection. Open, tunneled central venous catheter systems, such as Hickman or Borviak catheters, have a higher infection rate, because one end of the catheter remains outside the body (e31); they also produce a cosmetic deformity and markedly restrict the patient’s physical activity.
Serious complications.
Malposition of the catheter tip in the mediastinum is an especially serious complication because it may lead to the entry of infused solutions into the mediastinum or pleural space.
Port systems can now be implanted in a minimally invasive procedure by a surgeon or interventional radiologist. Venous access is gained by way of central veins at the upper thoracic aperture, or by way of the arm veins. The risk of complications is a function of the patient’s condition, the approach for central venous access, the techniques of implantation and puncture, and the implanting physician’s experience (e87). Patient satisfaction after port implantation ultimately depends not just on what happens in the hands of specialized oncologists, but on the collegial and patient-oriented collaboration of all physicians and nurses involved in the care of the patient. The proper use and care of port systems is very important to cancer patients. Potential complications must be promptly recognized and adequately treated, and prevented whenever possible, to ensure the continued availability of central venous access for infusion therapy.
Patient satisfaction after port implantation.
Patient satisfaction depends not just on what happens in specialized oncology practices, but also on the collegial and patient-oriented collaboration of all physicians and nurses participating in the care of the patient.
Further Information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under „meine Daten“ (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in issue 17/2011. The CME unit “The Treatment of Spinal Metastases” (issue 5/2011) can be accessed until 18 March 2011.
For Issue 13/2011, we plan to offer the topic “The Management of Psychiatric Emergencies.”
Solutions to the CME questions in issue 1–2/2011:
Harms E, Olgemöller B:
Neonatal Screening for Metabolic and Endocrine Disorders.
Solutions: 1c, 2a, 3a, 4b, 5d, 6b, 7e, 8d, 9b, 10a
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
According to the current recommendations of the European Society for Parenteral and Enteral Nutrition, which of the following types of infusion may be given through an indwelling peripheral venous catheter?
Infusions of low osmolarity
Infusions of high osmolarity
Infusions of high equivalent concentration
Infusions of low equivalent concentration
Hypertonic glucose infusions
Question 2
How do port systems differ from catheter systems without a port?
Port systems have a pumping chamber which can be manually compressed to facilitate infusion.
Access to a port system requires needle puncture through the skin and the silicone membrane of the port chamber.
Port systems have a connector at their external end.
Port systems enable sterile, needle-free vascular access for fluid administration.
The tip of the catheter of a port system is located in the inferior vena cava.
Question 3
A man whose body-mass index is 30 needs a suitable port system. He does not need parenteral nutrition. Which type of port system would be best for him?
A double-chamber port system
A titanium port system
A port system made of synthetic material
A low-profile port system
A high-profile port system
Question 4
What is the intervention-related complication rate of port system implantation, in experienced hands?
Up to 2%
Up to 4%
Up to 6%
Up to 8%
Up to 10%
Question 5
What surgical/interventional complication of port system implantation sometimes arises during the procedure itself?
Hypovolemic shock
Catheter dislocation
Inadvertent arterial puncture
Cutaneous necrosis
Catheter entrapment
Question 6
A 68-year-old woman underwent the implantation of a low-profile port system two weeks ago. Now she complains of coughing fits, shortness of breath, and anxiety. What is your provisional diagnosis?
Myocardial infarction
Clavicular fracture
Bronchitis
Pneumothorax
Pleurisy
Question 7
How can a catheter leak be detected fluoroscopically?
With the aid of a red lamp
With the aid of electrostimulation of the surrounding tissue
With the aid of positron emission tomography
With the aid of manual displacement of the port
With the aid of contrast medium injection into the port
Question 8
Having punctured a port chamber with proper technique, you find that you cannot flush it, nor can you aspirate any blood from it. You suspect obstruction of the port catheter. What should you do next?
Flush the obstruction away with a 2-mL syringe
Unblock the catheter with urokinase
Explant the port system
Attempt systemic lysis therapy with alteplase
Perform loop extraction via an inguinal approach to remove an obstructing fibrinous sheath
Question 9
Where should the catheter tip of a port system lie?
In the brachiocephalic vein
In the internal thoracic vein
In the azygous vein
At the cavo-atrial junction
In the right atrium
Question 10
You have a high suspicion of port catheter infection in a patient currently receiving chemotherapy. What should you do?
Flush the port system with antibiotics daily
Continue chemotherapy and observe the further course
Take blood cultures from the port system, await the result, then initiate specific treatment
Temporarily stop chemotherapy and observe until the port infection has resolved
Explant the port system at once, obtain alternative central venous access contralaterally, and continue chemotherapy if indicated
Key Clinical Messages.
Central venous port systems are devices for long-term venous access that are implanted entirely under the skin. They have become increasingly important in recent years as more intensive types of chemotherapy and parenteral treatment have come into use.
A port system can be implanted on an in- or outpatient basis or in a day surgery unit.
Port infection is both the commonest complication and the commonest reason for explantation. Infection rates in current studies range from 0.8% to 7.5%.
Controversy surrounds the question whether port catheter systems should be regularly flushed with heparin to prevent thrombotic catheter occlusion. No available scientific evidence justifies doing this routinely.
If the patient complains of pain when the port system is used, or if the system cannot be flushed and blood cannot be aspirated from it after a correctly performed puncture, no infusion therapy should be given through it. A diagnostic evaluation should be performed, including fluoroscopy after the injection of contrast medium through the port.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
PD Dr. Teichgräber serves as a paid consultant to the Bard company and receives research support from med-Kom. The remaining authors state that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Domagk K. Therapiekostenberechnungen in der Onkologie im Zeichen des AVWG. Onkol Pharm. 2008;10:11–13. [Google Scholar]
- 2.Pittiruti M, Hamilton H, Biffi R, MacFie J, Pertkiewicz M. ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications) Clinical Nutrition. 2009;28:365–377. doi: 10.1016/j.clnu.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Aubaniac R. L’injection intraveneuse sons-infraclaviculaire. Avantages et technique. Presse Med. 1952;60 [PubMed] [Google Scholar]
- 4.Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;92:706–712. [PubMed] [Google Scholar]
- 5.Teichgräber UK, Gebauer B, Benter T, Wagner J. [Long-term central venous lines and their complications] RoFo. 2004;176:944–952. doi: 10.1055/s-2004-813258. [DOI] [PubMed] [Google Scholar]
- 6.Bansal A, Binkert CA, Robinson MK, Shulman LN, Pellerin L, Davison B. Impact of quality management monitoring and intervention on central venous catheter dysfunction in the outpatient chemotherapy infusion setting. J Vasc Interv Radiol. 2008;19:1171–1175. doi: 10.1016/j.jvir.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 8.Menyhay SZ, Maki DG. Disinfection of needleless catheter connectors and access ports with alcohol may not prevent microbial entry: the promise of a novel antiseptic-barrier cap. Infect Control Hosp Epidemiol. 2006;27:23–27. doi: 10.1086/500280. [DOI] [PubMed] [Google Scholar]
- 9.Haindl H, Müller H. [An atraumatic needle for the puncture of ports and pumps] Klin Wochenschr. 1988;66:1006–1009. doi: 10.1007/BF01733442. [DOI] [PubMed] [Google Scholar]
- 10.Goossens GA, Moons P, Jérôme M, Stas M. Prospective clinical evaluation of the Polyperf Safe, a safety Huber needle, in cancer patients. J Vasc Access. 2010 doi: 10.5301/JVA.2010.6075. DOI: 10.5301/JVA.2010.6097 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Grant JP. Anatomy and physiology of venous system vascular access: implications. J Parenter Enteral Nutr. 2006;30(1) suppl. 7 doi: 10.1177/01486071060300S1S7. [DOI] [PubMed] [Google Scholar]
- 12.Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19:1516–1519. doi: 10.1097/00003246-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Slama M, Novara A, Safavian A, Ossart M, Safar M, Fagon JY. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23:916–919. doi: 10.1007/s001340050432. [DOI] [PubMed] [Google Scholar]
- 14.Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22:23–26. doi: 10.1055/s-2001-11243. [DOI] [PubMed] [Google Scholar]
- 15.Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer. 2003;11:148–155. doi: 10.1007/s00520-002-0399-3. [DOI] [PubMed] [Google Scholar]
- 16.Biffi R, Orsi F, Pozzi S, et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol. 2009;20:935–940. doi: 10.1093/annonc/mdn701. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann HAF. Die Portimplantation. Chirurgische Praxis. 2008;69:695–708. [Google Scholar]
- 18.Teichgräber UK, Gebauer B, Benter T, Wagner HJ. Central venous access catheters: radiological management of complications. Cardiovasc Intervent Radiol. 2003;26:321–333. doi: 10.1007/s00270-003-0112-z. [DOI] [PubMed] [Google Scholar]
- 19.Platzbecker U, Illmer T, Schaich M, et al. Double lumen port access in patients receiving allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2001;28:1067–1072. doi: 10.1038/sj.bmt.1703285. [DOI] [PubMed] [Google Scholar]
- 20.Chaitowitz I, Heng R, Bell KW. Exchanging dual-lumen central venous catheters: how I do it. Australas Radiol. 2007;51:106–109. doi: 10.1111/j.1440-1673.2007.01678.x. [DOI] [PubMed] [Google Scholar]
- 21.Wieners G, Redlich U, Dudeck O, Schütte K, Ricke J, Pech M. [First experiences with intravenous port systems authorized for high pressure injection of contrast agent in multiphasic computed tomography] RoFo. 2009;181:664–668. doi: 10.1055/s-0028-1109345. [DOI] [PubMed] [Google Scholar]
- 22.Marcy PY, Chamorey E, Amoretti N, et al. A comparison between distal and proximal port device insertion in head and neck cancer. Eur J Surg Oncol. 2008;34:1262–1269. doi: 10.1016/j.ejso.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Lenhart M, Schätzler S, Manke C, et al. [Radiological placement of peripheral central venous access ports at the forearm. Technical results and long term outcome in 391 patients] RoFo. 2010;182:20–28. doi: 10.1055/s-0028-1109453. [DOI] [PubMed] [Google Scholar]
- 24.Stein M, Wagner RH. [Complications of central venous access devices: outcome analysis of 2359 implantations] Dtsch Med Wochenschr. 2005;130:1129–1132. doi: 10.1055/s-2005-866798. [DOI] [PubMed] [Google Scholar]
- 25.Kock HJ, Pietsch M, Krause U, Wilke H, Eigler FW. Implantable vascular access systems: experience in 1500 patients with totally implanted central venous port systems. World J Surg. 1998;22:12–16. doi: 10.1007/s002689900342. [DOI] [PubMed] [Google Scholar]
- e1.Zhang Q, Jiao L, Zhou H. Comparison of implantable central -venous ports with catheter insertion via external jugular cut down and subclavian puncture in children: single center experience. Pediatr Surg Int. 2009;25:499–501. doi: 10.1007/s00383-009-2376-0. [DOI] [PubMed] [Google Scholar]
- e2.Tsai HL, Liu CS, Chang JW, Wei CF, Chin TW. Totally implantable venous access ports via the external jugular vein: safety and -effectiveness for young pediatric patients. J Pediatr Hematol Oncol. 2008;30:366–368. doi: 10.1097/MPH.0b013e31816916bf. [DOI] [PubMed] [Google Scholar]
- e3.D’Angelo FA, Ramacciato G, Aurello P, et al. Alternative insertion sites for permanent central venous access devices. Eur J Surg Oncol. 1997;23:547–549. doi: 10.1016/s0748-7983(97)93205-4. [DOI] [PubMed] [Google Scholar]
- e4.Teichgräber UK, Streitparth F, Gebauer B, Benter T. Placement of a port catheter through collateral veins in a patient with central venous occlusion. Cardiovasc Intervent Radiol. 2010;33:417–420. doi: 10.1007/s00270-009-9613-8. [DOI] [PubMed] [Google Scholar]
- e5.Forauer AR, Brenner B, Haddad LF, Bocchini TP. Placement of -hemodialysis catheters through dilated external jugular and collateral veins in patients with internal jugular vein occlusions. Am J Roentgenol. 2000;174:361–362. doi: 10.2214/ajr.174.2.1740361. [DOI] [PubMed] [Google Scholar]
- e6.Lorenz JM, Funaki B, Van Ha T, Leef JA. Radiologic placement of implantable chest ports in pediatric patients. Am J Roentgenol. 2001;176:991–994. doi: 10.2214/ajr.176.4.1760991. [DOI] [PubMed] [Google Scholar]
- e7.Schwarz RE, Groeger JS, Coit DG. Subcutaneously implanted -central venous access devices in cancer patients: a prospective analysis. Cancer. 1997;79:1635–1640. [PubMed] [Google Scholar]
- e8.Shetty PC, Mody MK, Kastan DJ, et al. Outcome of 350 implanted chest ports placed by interventional radiologists. J Vasc Interv Radiol. 1997;8:991–995. doi: 10.1016/s1051-0443(97)70699-7. [DOI] [PubMed] [Google Scholar]
- e9.Meissner W, Mescha S, Rothaug J, et al. Quality improvement in postoperative pain management: Results from the QUIPS project. Dtsch Arztebl Int. 2008;105:865–870. doi: 10.3238/arztebl.2008.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Gandhi RT, Getrajdman GI, Brown KT, et al. Placement of subcutaneous chest wall ports ipsilateral to axillary lymph node dissection. J Vasc Interv Radiol. 2003;14:1063–1065. doi: 10.1097/01.rvi.0000082863.05622.2a. [DOI] [PubMed] [Google Scholar]
- e11.Lucey B, Varghese JC, Haslam P, Lee MJ. Routine chest radiographs after central line insertion: mandatory postprocedural evaluation or unnecessary waste of resources? Cardiovasc Intervent Radiol. 1999;22:381–384. doi: 10.1007/s002709900411. [DOI] [PubMed] [Google Scholar]
- e12.Giacomini M, Iapichino G, Armani S, Cozzolino M, Brancaccio D, Gallieni M. How to avoid and manage a pneumothorax. J Vasc Access. 2006;7:7–14. doi: 10.1177/112972980600700103. [DOI] [PubMed] [Google Scholar]
- e13.Wagner HJ, Teichgräber U, Gebauer B, Kalinowski M. [Transjugular implantation of venous port catheter systems] RoFo. 2003;175:1539–1544. doi: 10.1055/s-2003-43406. [DOI] [PubMed] [Google Scholar]
- e14.Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10 doi: 10.1186/cc5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Denys BG, Uretsky BF, Reddy PS. Ultrasound-assisted cannulation of the internal jugular vein. A prospective comparison to the external landmark-guided technique. Circulation. 1993;87:1557–1562. doi: 10.1161/01.cir.87.5.1557. [DOI] [PubMed] [Google Scholar]
- e16.Martin MJ, Husain FA, Piesman M, et al. Is routine ultrasound guidance for central line placement beneficial? A prospective analysis. Curr Surg. 2004;61:71–74. doi: 10.1016/j.cursur.2003.07.010. [DOI] [PubMed] [Google Scholar]
- e17.Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327 doi: 10.1136/bmj.327.7411.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e18.Hayashi H, Amano M. Does ultrasound imaging before puncture facilitate internal jugular vein cannulation? Prospective random-ized comparison with landmark-guided puncture in ventilated -patients. Journal of cardiothoracic and vascular anesthesia. 2002;16:572–575. doi: 10.1053/jcan.2002.126950. [DOI] [PubMed] [Google Scholar]
- e19.Ignatov A, Hoffman O, Smith B, Fahlke J, Peters B, Bischoff J, Costa SD. An 11-year retrospective study of totally implanted central venous access ports: complications and patient satisfac-tion. Eur J Surg Oncol. 2009;35:241–246. doi: 10.1016/j.ejso.2008.01.020. [DOI] [PubMed] [Google Scholar]
- e20.Hsieh CC, Weng HH, Huang WS, et al. Analysis of risk factors -for central venous port failure in cancer patients. World J Gastro-enterol. 2009;15:4709–4714. doi: 10.3748/wjg.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e21.Rouzrokh M, Shamsian BS, Khalegh Nejad Tabari A, et al. Totally implantable subpectoral vs. subcutaneous port systems in children with malignant diseases. Arch Iran Med. 2009;12:389–394. [PubMed] [Google Scholar]
- e22.Vandoni RE, Guerra A, Sanna P, Bogen M, Cavalli F, Gertsch P. Randomised comparison of complications from three different permanent central venous access systems. Swiss Med Wkly. 2009;139:313–316. doi: 10.4414/smw.2009.12523. [DOI] [PubMed] [Google Scholar]
- e23.Araujo C, Silva JP, Antunes P, et al. A comparative study between two central veins for the introduction of totally implantable venous access devices in 1201 cancer patients. Eur J Surg Oncol. 2008;34:222–226. doi: 10.1016/j.ejso.2007.04.003. [DOI] [PubMed] [Google Scholar]
- e24.Samaras P, Dold S, Braun J, et al. Infectious port complications are more frequent in younger patients with hematologic malignancies than in solid tumor patients. Oncology. 2008;74:237–244. doi: 10.1159/000151393. [DOI] [PubMed] [Google Scholar]
- e25.Chen PT, Sung CS, Wang CC, Chan KH, Chang WK, Hsu WH. Experience of anesthesiologists with percutaneous nonangiographic venous access. J Clin Anesth. 2007;19:609–615. doi: 10.1016/j.jclinane.2007.06.016. [DOI] [PubMed] [Google Scholar]
- e26.Marcy PY, Magné N, Castadot P, Bailet C, Macchiavello JC, Namer M, Gallard JC. Radiological and surgical placement of port devices: a 4-year institutional analysis of procedure performance, quality of life and cost in breast cancer patients. Breast Cancer Res Treat. 2005;92:61–67. doi: 10.1007/s10549-005-1711-y. [DOI] [PubMed] [Google Scholar]
- e27.Caers J, Fontaine C, Vinh-Hung V, et al. Catheter tip position as a risk factor for thrombosis associated with the use of subcutaneous infusion ports. Support Care Cancer. 2005;13:325–331. doi: 10.1007/s00520-004-0723-1. [DOI] [PubMed] [Google Scholar]
- e28.Vardy J, Engelhardt K, Cox K, et al. Long-term outcome of radiological-guided insertion of implanted central venous access port devices (CVAPD) for the delivery of chemotherapy in cancer patients: institutional experience and review of the literature. Br J Cancer. 2004;91:1045–1049. doi: 10.1038/sj.bjc.6602082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e29.Biffi R, Pozzi S, Agazzi A, Pace U, Floridi A, Cenciarelli S, et al. Use of totally implantable central venous access ports for high-dose chemotherapy and peripheral blood stem cell transplantation: results of a monocentre series of 376 patients. Ann Oncol. 2004;15 doi: 10.1093/annonc/mdh049. [DOI] [PubMed] [Google Scholar]
- e30.Wolosker N, Yazbek G, Nishinari K, et al. Totally implantable venous catheters for chemotherapy: experience in 500 patients. Sao Paulo Med J. 2004;122:147–151. doi: 10.1590/S1516-31802004000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e31.Moureau N, Poole S, Murdock MA, Gray SM, Semba CP. Central venous catheters in home infusion care: outcomes analysis in 50,470 patients. J Vasc Interv Radiol. 2002;13:1009–1016. doi: 10.1016/s1051-0443(07)61865-x. [DOI] [PubMed] [Google Scholar]
- e32.Yip D, Funaki B. Subcutaneous chest ports via the internal jugular vein. A retrospective study of 117 oncology patients. Acta Radiol. 2002;43:371–375. doi: 10.1080/j.1600-0455.2002.430405.x. [DOI] [PubMed] [Google Scholar]
- e33.Biffi R, De Braud F, Orsi F, et al. A randomized, prospective trial of central venous ports connected to standard open-ended or -Groshong catheters in adult oncology patients. Cancer. 2001;92:1204–1212. doi: 10.1002/1097-0142(20010901)92:5<1204::aid-cncr1439>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- e34.Hartkamp A, van Boxtel AJ, Zonnenberg BA, Witteveen PO. Totally implantable venous access devices: evaluation of complications and a prospective comparative study of two different port systems. Neth J Med. 2000;57:215–223. doi: 10.1016/s0300-2977(00)00083-8. [DOI] [PubMed] [Google Scholar]
- e35.Gebauer B, Teichgräber U, Werk M, Wagner HJ. [Periinterventional prophylactic antibiotics in radiological port catheter implantation] RoFo. 2007;179:804–810. doi: 10.1055/s-2007-963276. [DOI] [PubMed] [Google Scholar]
- e36.Stephens LC, Haire WD, Tarantolo S, et al. Normal saline versus heparin flush for maintaining central venous catheter patency during apheresis collection of peripheral blood stem cells (PBSC) Transfus Sci. 1997;18:187–193. doi: 10.1016/s0955-3886(97)00008-8. [DOI] [PubMed] [Google Scholar]
- e37.Kuo YS, Schwartz B, Santiago J, Anderson PS, Fields AL, Goldberg GL. How often should a port-A-cath be flushed? Cancer Invest. 2005;23:582–585. doi: 10.1080/07357900500276923. [DOI] [PubMed] [Google Scholar]
- e38.Mayo DJ, Horne MK, Summers BL, Pearson DC, Helsabeck CB. The effects of heparin flush on patency of the Groshong catheter: a pilot study. Oncol Nurs Forum. 1996;23:1401–1405. [PubMed] [Google Scholar]
- e39.Kannan A. Heparinised saline or normal saline? J Perioper Pract. 2008;18:440–441. doi: 10.1177/175045890801801003. [DOI] [PubMed] [Google Scholar]
- e40.Cesaro S, Tridello G, Cavaliere M, et al. Prospective, randomized trial of two different modalities of flushing central venous catheters in pediatric patients with cancer. J Clin Oncol. 2009;27:2059–2065. doi: 10.1200/JCO.2008.19.4860. [DOI] [PubMed] [Google Scholar]
- e41.Bisseling TM, Willems MC, Versleijen MW, Hendriks JC, Vissers RK, Wanten GJ. Taurolidine lock is highly effective in preventing catheter-related bloodstream infections in patients on home -parenteral nutrition: A heparin-controlled prospective trial. Clin Nutr. 2010;29:464–468. doi: 10.1016/j.clnu.2009.12.005. [DOI] [PubMed] [Google Scholar]
- e42.Johnston DA, Walker K, Richards J, Pennington CR. Ethanol flush for the prevention of catheter occlusion. Clin Nutr. 1992;11:97–100. doi: 10.1016/0261-5614(92)90018-l. [DOI] [PubMed] [Google Scholar]
- e43.Safdar N, Maki DG. Use of vancomycin-containing lock or flush solutions for prevention of bloodstream infection associated with central venous access devices: a meta-analysis of prospective, randomized trials. Clin Infect Dis. 2006;43:474–484. doi: 10.1086/505976. [DOI] [PubMed] [Google Scholar]
- e44.Werlin SL, Lausten T, Jessen S, et al. Treatment of central venous catheter occlusions with ethanol and hydrochloric acid. J Parenter Enteral Nutr. 1995;19:416–418. doi: 10.1177/0148607195019005416. [DOI] [PubMed] [Google Scholar]
- e45.Opilla MT, Kirby DF, Edmond MB. Use of ethanol lock therapy to reduce the incidence of catheter-related bloodstream infections in home parenteral nutrition patients. J Parenter Enteral Nutr. 2007;31:302–305. doi: 10.1177/0148607107031004302. [DOI] [PubMed] [Google Scholar]
- e46.Metcalf SC, Chambers ST, Pithie AD. Use of ethanol locks to prevent recurrent central line sepsis. J Infect. 2004;49:20–22. doi: 10.1016/j.jinf.2003.08.010. [DOI] [PubMed] [Google Scholar]
- e47.Maiefski M, Rupp ME, Hermsen ED. Ethanol lock technique: review of the literature. Infect Control Hosp Epidemiol. 2009;30:1096–1108. doi: 10.1086/606162. [DOI] [PubMed] [Google Scholar]
- e48.Benter T, Teichgräber UK, Klühs L, Dörken B. Percutaneous central venous catheterization with a lethal complication. Intensive Care Med. 1999;25:1180–1182. doi: 10.1007/s001340051034. [DOI] [PubMed] [Google Scholar]
- e49.Teichgräber UK, Benter T. Images in clinical medicine. Air embolism after the insertion of a central venous catheter. N Engl J Med. 2004;350 doi: 10.1056/ENEJMicm020866. [DOI] [PubMed] [Google Scholar]
- e50.Kincaid EH, Davis PW, Chang MC, Fenstermaker JM, Pennell TC. „Blind“ placement of long-term central venous access devices: report of 589 consecutive procedures. Am Surg. 1999;65:520–523. discussion 523-4. [PubMed] [Google Scholar]
- e51.Ahmed Z, Mohyuddin Z. Complications associated with different insertion techniques for Hickman catheters. Postgrad Med J. 1998;74:104–107. doi: 10.1136/pgmj.74.868.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e52.Robinson JF, Robinson WA, Cohn A, Garg K, Armstrong JD. Perforation of the great vessels during central venous line placement. Arch Intern Med. 1995;155:1225–1228. [PubMed] [Google Scholar]
- e53.Rodier JM, Malbec L, Lauraine EP, Batel-Copel L, Bernadou A. Mediastinal infusion of epirubicin and 5-fluorouracil. A complication of totally implantable central venous systems. Report of a case. J Cancer Res Clin Oncol. 1996;122:566–567. doi: 10.1007/BF01213554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e54.Lee YM, Kim HJ, Lee JE, et al. Cardiac tamponade following insertion of an internal jugular vein catheter for hemodialysis. Clin Nephrol. 2009;72:220–223. doi: 10.5414/cnp72220. [DOI] [PubMed] [Google Scholar]
- e55.Ervo R, Angeletti S, Turrini Dertenois L, Cavatorta F. Dialysis -catheter associated cardiac tamponade: quick diagnosis by extemporaneous echocardiography. J Vasc Access. 2008;9:69–71. [PubMed] [Google Scholar]
- e56.Yilmazlar A, Bilgin H, Korfali G, Eren A, Ozkan U. Complications of 1303 central venous cannulations. J R Soc Med. 1997;90:319–321. doi: 10.1177/014107689709000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e57.Karakaya D, Baris S, Güldogus F, Incesu L, Sarihasan B, Tür A. Brachial plexus injury during subclavian vein catheterization for hemodialysis. J Clin Anesth. 2000;12:220–223. doi: 10.1016/s0952-8180(00)00144-6. [DOI] [PubMed] [Google Scholar]
- e58.Park HS, Choo IW, Do YS, Choo SW. Migrated Hickman catheters: a simple repositioning method using a stiff hydrophilic guidewire. Cardiovasc Intervent Radiol. 2000;23:70–72. doi: 10.1007/s002709910013. [DOI] [PubMed] [Google Scholar]
- e59.Schmitz-Rode T, Kilbinger M, Günther RW. [Repositioning of a central venous catheter with a guide wire with movable core] RoFo. 1999;170:222–224. [PubMed] [Google Scholar]
- e60.Colón-Casasnovas NE, Lugo-Vicente H. Distal fragmented port catheter: case report and review of literature. Bol Asoc Med P R. 2008;100:70–75. [PubMed] [Google Scholar]
- e61.Elkhoury MI, Boeckx WD, Chahine EG, Feghali MA. Retrieval of port-A catheter fragment from the main and right pulmonary arteries 3 years after dislodgment. J Vasc Access. 2008;9:296–298. [PubMed] [Google Scholar]
- e62.Hofer S, Schnabel K, Vogelbach P, Herrmann R. [The „pinch off“ syndrome: a complication of implantable catheter systems in the subclavian vein] Schweiz Med Wochenschr. 1997;127:1247–1250. [PubMed] [Google Scholar]
- e63.Lenglinger FX, Hartl P, Kirchgatterer A, Lenglinger GM, Baldinger C. [Fracture and embolization of a central venous port catheter without prior compression between the clavicle and the 1st rib] Wien Klin Wochenschr. 2001;113:134–137. [PubMed] [Google Scholar]
- e64.Schlüter A, Stock K, von Poblozki A, Behrmann C, Jassoy A, Spielmann RP. [Radiological evaluation of complications of implantable venous access port systems] RoFo. 1999;171:324–328. doi: 10.1055/s-1999-11092. [DOI] [PubMed] [Google Scholar]
- e65.Biffi R, Orsi F, Grasso F, De Braud F, Cenciarelli S, Andreoni B. Catheter rupture and distal embolisation: a rare complication of central venous ports. J Vasc Access. 2000;1:19–22. doi: 10.1177/112972980000100106. [DOI] [PubMed] [Google Scholar]
- e66.Inaba K, Sakurai Y, Furuta S, Sunagawa R, Isogaki J, Komori Y, Uyama I. Delayed vascular injury and severe respiratory distress as a rare complication of a central venous catheter and total parenteral nutrition. Nutrition. 2009;25:479–481. doi: 10.1016/j.nut.2008.10.021. [DOI] [PubMed] [Google Scholar]
- e67.Faintuch S, Salazar GM. Malfunction of dialysis catheters: management of fibrin sheath and related problems. Tech Vasc Interv Radiol. 2008;11:195–200. doi: 10.1053/j.tvir.2008.09.008. [DOI] [PubMed] [Google Scholar]
- e68.Reddy AS, Lang EV, Cutts J, Loh S, Rosen MP. Fibrin sheath -removal from central venous catheters: an internal snare manoeuvre. Nephrol Dial Transplant. 2007;22:1762–1765. doi: 10.1093/ndt/gfm154. [DOI] [PubMed] [Google Scholar]
- e69.Gebauer B, Teichgräber UK, Podrabsky P, Werk M, Hänninen EL, Felix R. Radiological interventions for correction of central venous port catheter migrations. Cardiovasc Intervent Radiol. 2007;30:668–674. doi: 10.1007/s00270-007-9073-y. [DOI] [PubMed] [Google Scholar]
- e70.Teichgräber UK, Streitparth F, Cho CH, Benter T, Gebauer B. A comparison of clinical outcomes with regular- and low-profile -totally implanted central venous port systems. Cardiovasc Intervent Radiol. 2009;32:975–979. doi: 10.1007/s00270-008-9477-3. [DOI] [PubMed] [Google Scholar]
- e71.Venta LA, Feldman L. A subclavian line complication: embolisation of an arteriovenous fistula. Clin Radiol. 1988;39:210–211. doi: 10.1016/s0009-9260(88)80035-7. [DOI] [PubMed] [Google Scholar]
- e72.Brouns F, Schuermans A, Verhaegen J, De Wever I, Stas M. Infection assessment of totally implanted long-term venous access -devices. J Vasc Access. 2006;7:24–28. doi: 10.1177/112972980600700105. [DOI] [PubMed] [Google Scholar]
- e73.Wolf HH, Leithäuser M, Maschmeyer G, et al. Central venous catheter-related infections in hematology and oncology : guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2008;87:863–876. doi: 10.1007/s00277-008-0509-5. [DOI] [PubMed] [Google Scholar]
- e74.Elishoov H, Or R, Strauss N, Engelhard D. Nosocomial coloniza-tion, septicemia, and Hickman/Broviac catheter-related infections in bone marrow transplant recipients. A 5-year prospective study. Medicine (Baltimore) 1998;77:83–101. doi: 10.1097/00005792-199803000-00002. [DOI] [PubMed] [Google Scholar]
- e75.Worth LJ, Seymour JF, Slavin MA. Infective and thrombotic complications of central venous catheters in patients with hematological malignancy: prospective evaluation of nontunneled devices. Support Care Cancer. 2009;17:811–818. doi: 10.1007/s00520-008-0561-7. [DOI] [PubMed] [Google Scholar]
- e76.Fischer L, Knebel P, Schröder S, et al. Reasons for explantation of totally implantable access ports: a multivariate analysis of 385 consecutive patients. Ann Surg Oncol. 2008;15:1124–1129. doi: 10.1245/s10434-007-9783-z. [DOI] [PubMed] [Google Scholar]
- e77.Fraschini G, Jadeja J, Lawson M, Holmes FA, Carrasco HC, Wallace S. Local infusion of urokinase for the lysis of thrombosis -associated with permanent central venous catheters in cancer patients. J Clin Oncol. 1987;5:672–678. doi: 10.1200/JCO.1987.5.4.672. [DOI] [PubMed] [Google Scholar]
- e78.Monturo CA, Dickerson RN, Mullen JL. Efficacy of thrombolytic therapy for occlusion of long-term catheters. J Parenter Enteral Nutr. 1990;14:312–314. doi: 10.1177/0148607190014003312. [DOI] [PubMed] [Google Scholar]
- e79.Sugimoto K, Hofmann LV, Razavi MK, et al. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and -venous occlusions. J Vasc Surg. 2003;37:512–517. doi: 10.1067/mva.2003.41. [DOI] [PubMed] [Google Scholar]
- e80.Eyrich H, Walton T, Macon EJ, Howe A. Alteplase versus urokinase in restoring blood flow in hemodialysis-catheter thrombosis. Am J Health Syst Pharm. 2002;59:1437–1440. doi: 10.1093/ajhp/59.15.1437. [DOI] [PubMed] [Google Scholar]
- e81.Semba CP, Deitcher SR, Li X, Resnansky L, Tu T, McCluskey ER. Cardiovascular thrombolytic to Open Occluded Lines Investigators: Treatment of occluded central venous catheters with -alte-plase: results in 1,064 patients. J Vasc Interv Radiol. 2002;13:1199–1205. doi: 10.1016/s1051-0443(07)61965-4. [DOI] [PubMed] [Google Scholar]
- e82.Whigham CJ, Lindsey JI, Goodman CJ, Fisher RG. Venous port salvage utilizing low dose tPA. Cardiovasc Intervent Radiol. 2002;25:513–516. doi: 10.1007/s00270-002-2615-4. [DOI] [PubMed] [Google Scholar]
- e83.Demircioðlu F, Ylmaz S, Oren H, Ozgüven AA, Irken G. Skin and soft tissue complications in pediatric leukemia patients with and without central venous catheters. J Pediatr Hematol Oncol. 2008;30:32–35. doi: 10.1097/MPH.0b013e31815cc429. [DOI] [PubMed] [Google Scholar]
- e84.Hasskarl J, Illerhaus G, Waller CF, Köberich S, Frydrychowicz A. Complete caval thrombosis secondary to an implanted venous port - a case study. Dtsch Arztebl Int. 2008;105:18–21. doi: 10.3238/arztebl.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e85.Dougherty L. IV therapy: recognizing the differences between infiltration and extravasation. Br J Nurs. 2008;17:898–901. doi: 10.12968/bjon.2008.17.14.30656. [DOI] [PubMed] [Google Scholar]
- e86.Beutel K, Simon A. Diagnostics and management of central -venous line infections in pediatric cancer patients. Klin Padiatr. 2005;217(Suppl. 1):91–100. doi: 10.1055/s-2005-872503. [DOI] [PubMed] [Google Scholar]
- e87.Jordan K, Behlendorf T, Surov A, Kegel T, Maher G, Wolf HH. -Venous access ports: frequency and management of complications in oncology patients. Onkologie. 2008;31:404–410. doi: 10.1159/000140451. [DOI] [PubMed] [Google Scholar]