Abstract
Purpose
To investigate the incidence and local control of internal mammary lymph node metastases (IMN+) in patients with clinical N2 or N3 locally advanced breast cancer.
Methods and Materials
We retrospectively reviewed the records of 809 breast cancer patients diagnosed with advanced nodal disease (clinical N2–3) who received radiation treatment at our institution from January 2000 December 2006. Patients were considered IMN+ on the basis of imaging studies.
Results
We identified 112 of 809 patients who presented with IMN+ disease (13.8%) detected on ultrasound, computed tomography (CT), positron emission tomography/CT (PET/CT), and/or magnetic resonance imaging (MRI) studies. All 112 patients with IMN+ disease received anthracycline and taxane-based chemotherapy. Neoadjuvant chemotherapy (NCT) resulted in a complete response (CR) on imaging studies of IMN disease in 72.1% of patients. Excluding 16 patients with progressive disease, 96 patients received adjuvant radiation to the breast or the chest wall and the regional lymphatics including the IMN chain with a median dose of 60 Gy if the internal mammary lymph nodes normalized after chemotherapy and 66 Gy if they did not. The median follow-up of surviving patients was 41 months (8–118 months). For the 96 patients able to complete curative therapy, the actuarial 5-year IMN control rate, locoregional control, overall survival, and disease-free survival were 89%, 80%, 76%, and 56%.
Conclusion
Over ten percent of patients with advanced nodal disease will have IMN metastases on imaging studies. Multimodality therapy including IMN irradiation achieves excellent rates of control in the IMN region and a DFS of more than 50% after curative treatment.
Keywords: Breast cancer, Internal mammary lymph node, Radiation therapy
INTRODUCTION
In the management of breast cancer, although there is general consensus on how to detect and treat metastases to the axillary and the supraclavicular lymph nodes, local management of metastases to the internal mammary lymph nodes (IMN) remains controversial. Although surgical resection of internal mammary lymph nodes has not been demonstrated to improve overall survival in randomized trials (1–4) these trials predate the use of systemic chemotherapy and are criticized for being underpowered due to sample size (5). Similarly, randomized trials of postmastectomy radiation (PMRT) before the use of systemic chemotherapy failed to demonstrate an overall survival benefit; however, more recent trials including systemic therapy demonstrated a benefit (6–8). Importantly, these trials included radiation to the internal mammary lymph nodes. Although these data do not specifically address the added value of IMN radiation as a component of postmastectomy radiation therapy (PMRT), some physicians routinely target the internal mammary lymph nodes based on these data.
Although contemporary randomized trials to better assess the value of treating the internal mammary lymph nodes are currently accruing, these data will not be available for several years. Because the internal mammary lymph nodes are non-palpable on clinical exam, the rate of IMN recurrence may be underreported. Herein we present the incidence of IMN+ on routine staging studies and the rate of local control of IMN after systemic therapy, modified radical mastectomy, and postmastectomy radiation.
METHODS AND MATERIALS
We retrospectively reviewed the records of 809 patients diagnosed with clinical T1–4 N2–3 M0 breast cancer who received radiation treatment at the M. D. Anderson Cancer Center from January 2000 to December 2006. The first author, Dr. Zhang, spent 8 months as a visiting scientist from the Sun Yat-Sen University Cancer Center; however, all patients for this study were patients at the M. D. Anderson Cancer Center. All staging was converted to the 2002 American Joint Cancer Committee (AJCC) staging system. Patients were considered to have IMN-positive disease on the basis of imaging reports that noted suspicious nodes given the size and morphology on ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), or by fluorodeoxyglucose (FDG) avidity on positron emission tomography (PET) or PET/CT in the IMN region. Only 10 patients underwent ultrasound-guided fine-needle aspiration to obtain pathologic confirmation. One hundred percent of sampled lymph nodes were confirmed to contain malignant cells. The majority (96/112) of patients with IMN+ disease received trimodality treatment comprising of chemotherapy, surgery, and radiation therapy. Patients were treated with either breast conservation surgery (n = 25) or mastectomy (n = 80) with a Level I–II axillary lymph node dissection.
Radiation therapy
Radiation therapy was targeted to the intact breast or the chest wall, the ipsilateral axillary apex and supraclavicular fossa, and the IMN region. Radiation treatment planning used a CT-based, three-dimensional conformal technique. Routinely, 50 Gy was delivered in 25 fractions to the breast and/or chest wall and axillary apex/supraclavicular fossa using 6 MV ± 18 MV photons. The IMN typically was treated inclusive of the first to third intercostal spaces with electrons of various energies determined by the depth of the IMN vessels. For example, a low-energy electron would target the IMN region using an appositional technique angled 10° to be parallel to the matched tangents. This approach minimizes the cold triangle at the match between electron and photon beams. An alternate technique to include the IMN region is to move the posterior border of tangents deeper until the IMN target is included in the field (“deep tangents”). A 10-Gy boost was then given to the lumpectomy bed or the chest wall region and to initially positive lymph nodes in the supraclavicular fossa or the IMN region that normalized on imaging studies after neoadjuvant chemotherapy. For residual abnormal lymph nodes on imaging studies after chemotherapy (not removed at surgery) a 16-Gy boost was delivered. Initial pre-chemotherapy imaging studies provided the anatomical guides for final boost volumes when necessary.
Median dose to the IMN region was 60 Gy (50–72 Gy) delivered in conventional 2-Gy daily fractions (IMN− 50 Gy, IMN+ with complete imaging response 60 Gy, grossly abnormal IMN after chemotherapy 66 Gy). The most common technique used to target the IMN region was an appositional electron beam matched to the chest wall tangents after a mastectomy or with deep tangents to encompass both the breast and the IMN region when the breast was intact. Sixteen patients demonstrated progressive disease (PD) during neoadjuvant chemotherapy and did not receive a definitive dose to their IMN region. Of these 16 patients, 11 patients underwent chemoradiotherapy with preoperative intent followed by mastectomy (n = 4) or no surgery (n = 7), and 5 patients underwent a mastectomy followed by adjuvant radiation (n = 2) or chemoradiation (n = 3).
Chemotherapy
Chemotherapy consisted of weekly taxane-based chemotherapy followed by four cycles of antracycline-based chemotherapy given every 4 weeks. Postmastectomy radiation was delivered in all patients unless they had preoperative radiation for reasons of borderline resectability (n = 11). Patients with Her2/neu-positive tumors (n = 34) received trastuzumab (Herceptin) during and after the radiation therapy.
Study endpoints
Locoregional control (LRC) was analyzed both for the 112 patients with IMN+ and for the 96 of these patients (86%) in whom curative, trimodality treatment was completed. All but six of these patients had posttreatment imaging studies documenting response of their initial IMN disease. Six patients who received no posttreatment imaging studies that specifically commented on the IMN disease status; however, all 6 patients were alive and disease-free at the last date of follow-up and therefore were considered locally controlled for this analysis.
LRC was defined as freedom from disease recurrence in the ipsilateral breast or the chest wall region, in patients who underwent breast conservation or mastectomy, respectively, and the axillary, supraclavicular, infraclavicular, or IMN regions. Local IMN control was defined as freedom from disease progression or recurrence in the ipsilateral IMN region as detected on clinical examination and imaging studies and was included regardless of whether a regional failure was isolated or occurred simultaneously or after a distant metastases. Disease-free survival (DFS) was defined as freedom from locoregional recurrence or distant metastasis. The 5-year actuarial rates of LRC, DFS, and overall survival (OS) were calculated by the Kaplan–Meier method from the date of breast cancer diagnosis. Differences in outcome as a function of various prognostic factors were compared using the log–rank test. Multivariate analyses of LRC, DFS, and OS were performed using a Cox proportional hazards model on the 96 patients who completed curative multimodality treatment. All p values were two-sided, and p values ≤0.05 were considered significant. Statistical analyses were carried out using SPSS 12.0 software.
RESULTS
Patient and tumor characteristics
The median patient age at diagnosis was 48 years (range 25–79 years). Patient and tumor characteristics are presented in Table 1. Seventy-six percent (85 of 112) of patients with positive IMN disease presented with modified Black’s nuclear grade (MBNG) 3 tumors. Twenty-three patients had MBNG 2 tumors, and one patient had a MBNG 1 tumor. Three patients had no record of the tumor’s histological grade. Tumor location was classified into three groups. Forty-nine percent (55 of 112) of tumors were central or medial in location, 40% (45 of 112) of tumors were lateral, and 11% (12 of 112) spanned either inner and outer breast quadrants, was inflammatory breast cancer, or was at the 6 o’clock position). Stage at presentation was Stage IIIA in eight patients, Stage IIIB in two patients, and Stage IIIC made up the majority (91%) of patients. The histologic tumor type, defined according to the World Health Organization’s classification system, was infiltrating ductal carcinoma in 105 patients, infiltrating lobular carcinoma in 4 patients, mixed infiltrating ductal and lobular carcinoma in 1 patient, and not reported in 2 patients. Estrogen receptor was negative in 63 patients, positive in 46 patients, and unknown in 3 patients. The progesterone receptor was negative 67 patients, positive in 39 patients, and borderline or unknown in 6 patients. Immunohistochemistry and fluorescence in-situ hybridization (FISH) analyses determined Her-2/neu positive tumors in 30% (34 of 112) of patients.
Table 1.
Patient and tumor characteristics
| Characteristics | No. of patients | % |
|---|---|---|
| Age, years | ||
| ≤45 | 47 | 42.0 |
| >45 | 65 | 58.0 |
| T stage | ||
| T1 | 15 | 13.4 |
| T2 | 27 | 24.1 |
| T3 | 15 | 13.4 |
| T4 | 55 | 49.1 |
| N stage | ||
| N2b (IMN+/ALN−) | 10 | 8.9 |
| N3b (IMN+/ALN+) | 66 | 58.9 |
| N3c (SCF+) | 36 | 32.1 |
| Pathological grade | ||
| 1–2 | 25 | 22.3 |
| 3 | 84 | 75.0 |
| Unknown | 3 | 2.7 |
| Estrogen receptor status | ||
| Positive | 46 | 41.1 |
| Negative | 63 | 56.3 |
| Unknown | 3 | 2.7 |
| Progesterone receptor status | ||
| Positive | 39 | 34.8 |
| Negative | 67 | 59.8 |
| Unknown | 6 | 5.4 |
| Her2/neu status | ||
| Positive | 34 | 30.4 |
| Negative | 71 | 63.4 |
| Unknown | 7 | 6.3 |
| Primary tumor location | ||
| Inner/central quadrant | 55 | 49 |
| Outer quadrant | 45 | 40 |
| Inner and outer quadrants | 7 | 6 |
| Unknown | 5 | 5 |
| IMN location by intercostal space | ||
| First | 54 | 55.1 |
| Second | 57 | 59.2 |
| Third | 22 | 22.4 |
| Fourth | 1 | 1.0 |
| Chemotherapy before IMN+ diagnosis | ||
| Yes | 15 | 13.4 |
| No | 97 | 86.6 |
| IMN response to NCT | ||
| CR | 75 | 67.0 |
| PR | 11 | 9.8 |
| SD | 13 | 11.6 |
| PD | 5 | 4.5 |
| Unknown | 8 | 7.1 |
| IMN response after radiotherapy | ||
| CR | 87 | 77.7 |
| PR | 5 | 4.5 |
| SD | 4 | 3.6 |
| Unknown | 16 | 14.3 |
| Surgical therapy before radiotherapy | ||
| Mastectomy | 76 | 67.9 |
| Breast conservative therapy | 24 | 21.4 |
| Biopsy only (inoperable) | 12 | 10.7 |
| Treatment intent | ||
| Curative | 96 | 85.7 |
| Palliative | 16 | 14.3 |
Abbreviations: ALN = axillary lymph node; CR = complete response; IMN = internal mammary node; NCT = neoadjuvant chemotherapy; PR = partial response; SCF = supraclavicular fossa; SD = stable disease.
Incidence, method of detection, and response to therapy
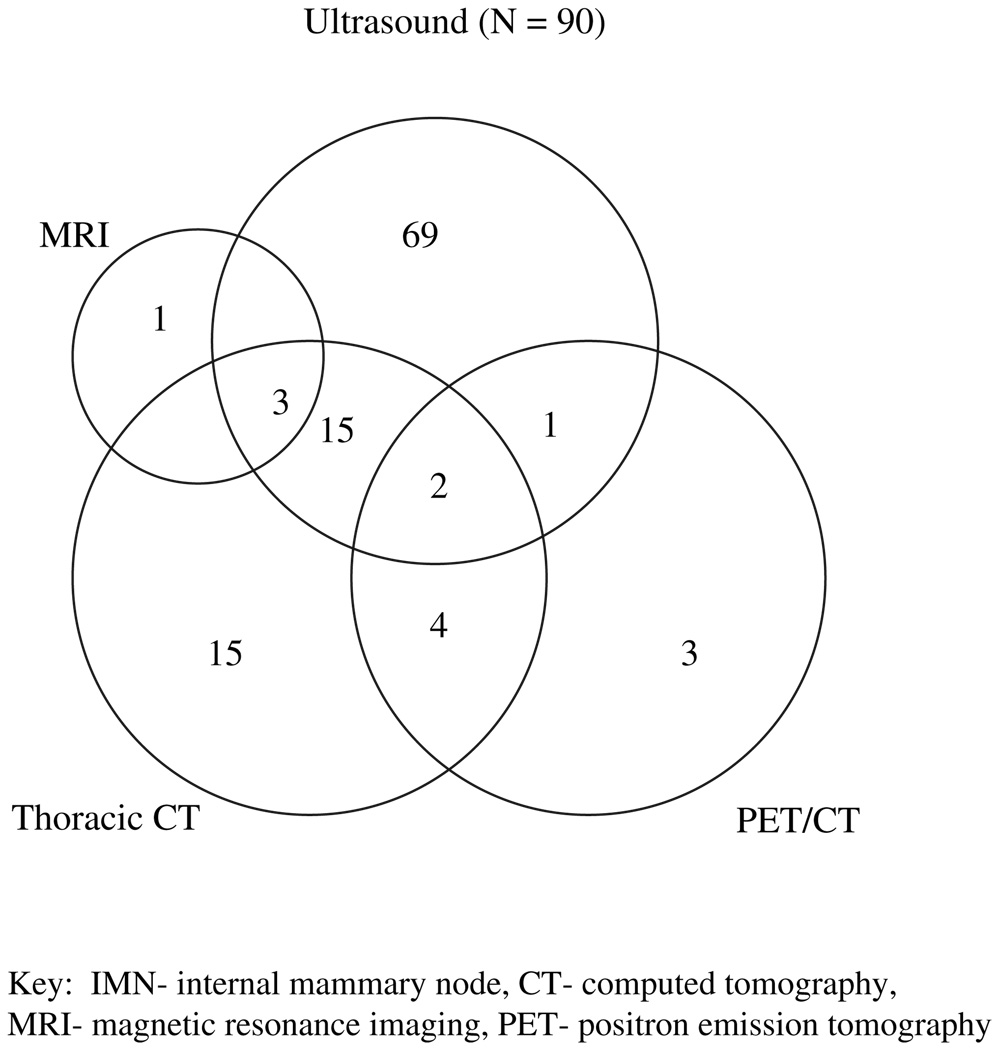
The overall incidence of IMN involvement was 13.8% (112 of 809). Among the 809 patients with clinical N2–N3 nodal disease, 90% underwent ultrasound as part of their initial staging studies, and of these, 12.3% (90/732) were found to have IMN+ disease. Additional imaging studies including chest CT and PET/CT were not as widely used. The rate of detecting IMN involvement was 17.9% (39 of 218) by chest CT and 31.3% (10 of 32) by PET/CT (Fig. 1). The number of breast MRI reports with any comment on the IMN was too few for analysis.
Fig. 1.
Number of patients diagnosed with internal mammary node (IMN) metastasis detected on ultrasound, thoracic computed tomograph (CT), breast magnetic resonance imaging (MRI), and/or positron emission tomography/CT.
The size and location of the enlarged IMNs was available in 98 of 112 patients. The median size of the enlarged IMN was 1.3 cm (range, 0.5–3.0 cm) and involved the first intercostal space in 55% of patients, the second intercostal space in 58% of patients, and the third intercostal space in 22% of patients. One patient had involvement in the fourth intercostal space. The IMN involvement was limited to a single intercostal space in 68 patients with the remaining 30 patients presenting with suspicious IMNs spanning the first through fourth intercostal spaces (Table 1). Among the 112 patients with IMN disease, IMN nodal involvement as the only involved nodal region was rare, involving 10 of 112 (9%) of patients (N2b). Sixty-six of 112 (59%) patients presented with simultaneous internal mammary and axillary or infraclavicular nodal disease (N3b) at presentation. Thirty-two percent of patients presented with simultaneous supraclavicular and IMN involvement, with or without axillary or infraclavicular nodal involvement (N3c). Biopsy of the IMN was not routine as most patients had simultaneous, confirmed metastases in the axillary or the supraclavicular lymph nodes that were sampled by fine-needle aspiration (FNA). At the physician’s discretion, 10 patients did undergo FNA of the suspected IMN, and cytology confirmed metastatic carcinoma in all these patients.
Ultrasound was routinely performed after neoadjuvant chemotherapy (NCT) to assess response. Detailed imaging reports describing the response of IMN disease to NCT were available for all 112 patients. Patients with initially positive IMN disease achieved a complete response (CR) to NCT in 81 cases (72.3%), partial response (PR) in 11 cases (10%), stable disease (SD) in 15 cases (13.4%), and progressive disease (PD) in 5 cases (4.5%). After NCT, 101 operable patients went on to have a mastectomy or breast-conservation surgery with axillary lymph node dissection. Eleven inoperable patients underwent chemoradiotherapy. After radiation therapy including the positive IMN region, all 11 patients achieved a complete response on ultrasound for a total of 92 patients achieving imaging CR after NCT and radiation (Table 2).
Table 2.
IMN local control according to response to chemotherapy and IMN radiation therapy dose
| Response to chemotherapy (N = patients) |
50–58 Gy | 60 Gy | 62–72 Gy |
|---|---|---|---|
| CR (71) | 18/18 | 40/43 | 9/9 |
| PR (10) | 2/3 | 4/4 | 3/3 |
| SD (10) | 1/2 | 3/4 | 3/4 |
| Unknown (5) | 3/3 | 1/1 | 1/1 |
Abbreviations: CR = complete response; IMN = internal mammary node; PR = partial response; SD = stable disease.
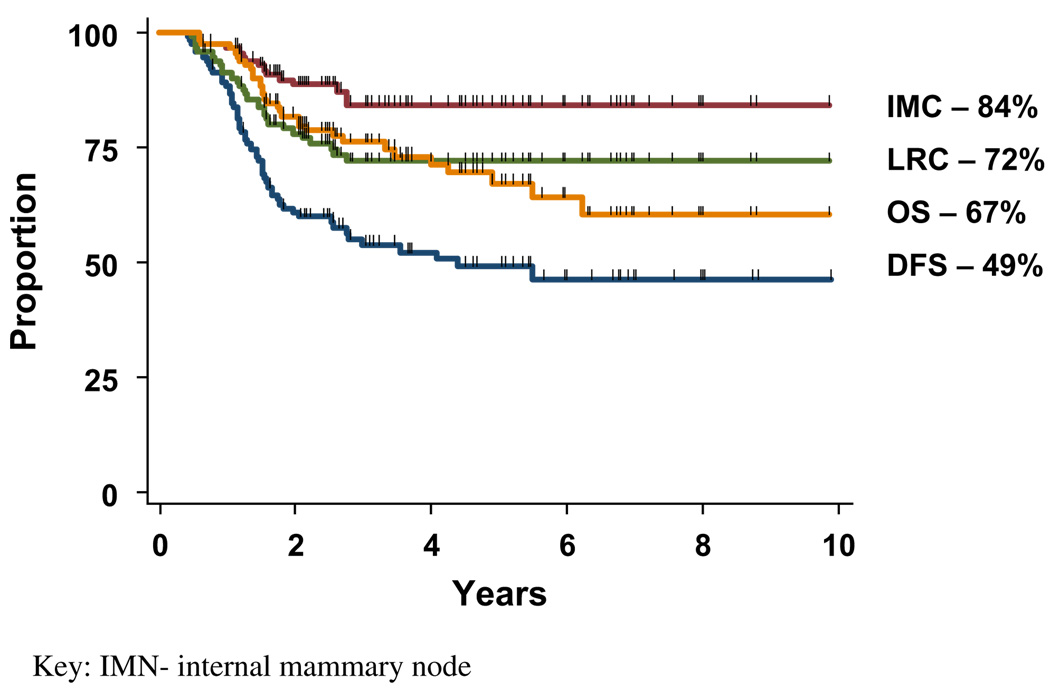
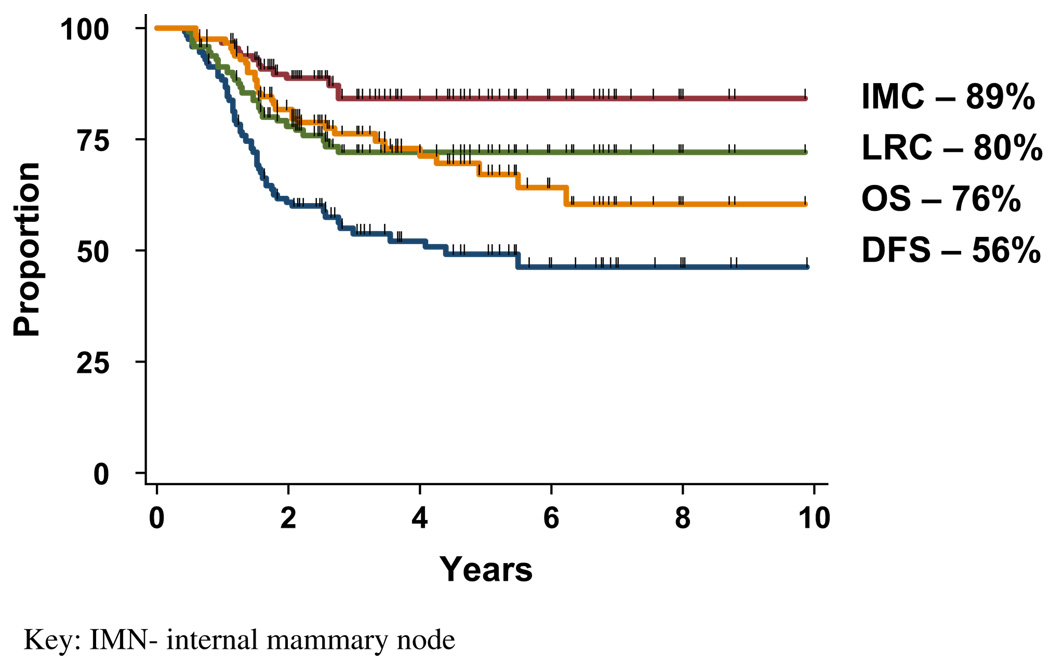
Locoregional and internal mammary node control
At a median follow-up of 40 months (range, 7–98 months), the 5-year actuarial rates of locoregional and IMN control for all 112 patients, including 16 patients who progressed during treatment and were unable to complete the intended therapy, were 72% and 84%, respectively. (Fig. 2) For comparison, the LRC and IMN control rates for the 96 patients able to complete curative trimodality therapy was 80% and 89%, respectively (Fig. 3). After initiation of chemotherapy, 15 patients had progressive or stable disease that prohibited them from completing definitive radiation or planned surgery, and 1 developed brain metastasis. Multivariate analyses on the 96 patients who completed curative trimodality therapy identified IMN response after NCT and radiation therapy to be a statistically significant predictor of improved LRC (p = 0.04; confidence interval [95% CI], 0.08–0.95), DFS (p = 0.02; 95% CI, 1.31–15.28), and OS (p = 0.04; 95% CI, 0.09–0.92). Pathologic tumor grade was also predictive of LRC with a relative risk of 0.28 for Grade 1–2 tumors vs. Grade 3 disease (p = 0.04, 95% CI, 0.08–0.95). The maximum size or number of intercostal spaces occupied by enlarged IMNs did not affect LRC. Earlier T stage (T1–2) was associated with superior DFS compared with T3–4 tumors (relative risk [RR], 2.07; p = .04, 95% CI, 1.02–4.18). Primary tumor location was not significant on univariate analysis for LRC (RR, 0.81; p = 0.70, 95% CI, 0.28–2.31), IMN control (RR, 2.5; p = 0.26, 95% CI, 0.50–12.39), DFS (RR, 1.01; p = 0.97, 95% CI, 0.51–2.01), or OS (RR, 1.17; p = 0.75, 95% CI, 0.44–3.08) with inner or central tumors vs. outer quadrant tumors.
Fig. 2.
Kaplan–Meier survivals for all patients with internal mammary node (IMN) metastases (N = 112). DFS = disease-free survival; LRC = locoregional control; OS = overall survival.
Fig. 3.
Kaplan–Meier survivals for patients with internal mammary node (IMN) metastases treated with curative aim (n = 96). DFS = disease-free survival; LRC = locoregional control; OS = overall survival.
The rate of IMN failure after trimodality treatment was 11% in curative-treated patients. All patients with local failure at the IMN chain had confirmation of recurrence by thoracic CT or PET/CT reevaluation (in one case precipitated by chest pain and parasternal prominence). Several patients developed multiple sites of locoregional failure simultaneously. Sites of recurrence included the ipsilateral chest wall in 6 patients, the breast in 2 patients, supraclavicular fossa in 6 patients, infraclavicular fossa in 2 patients, and the axilla in 2 patients. After the possibility of an independent, contralateral breast cancer was eliminated, there were several patients who recurred in the opposite chest wall or lymphatics. Namely, there were 5 observed failures in the contralateral IMN region, 10 in the contralateral axillary or infraclavicular lymph nodes, 7 in the contralateral supraclavicular fossa, and 1 in the contralateral chest wall where a prophylactic mastectomy had been performed.
DISCUSSION
Our study found that approximately 14% of patients with N2–3 nodal disease had clinically apparent metastases to the internal mammary lymph nodes detected on imaging studies. Multimodality treatment including targeted radiation therapy aimed at the involved IMN metastasis resulted in acceptable local control and a DFS >50%. The use of ultrasound to examine the IMN region at the time of presentation was the most common modality for diagnosing internal mammary lymph node metastases. This is the first report on the incidence of clinically apparent internal mammary nodal disease on imaging studies.
Lymph node metastases are critical in predicting prognosis in cancer patients, yet according to the Patterns of Care study (9) from 1998 to 1999, only 23% of patients treated with postmastectomy radiation therapy received IMN radiation. However, randomized data demonstrating a survival benefit of postmastectomy radiation included irradiation of the IMN chain. Among the randomized trials summarized in the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) analysis were the landmark trials conducted by British Columbia and the Danish Breast Cancer Cooperative Group (DBCG). Both of these trials included irradiation of the IMN chain in women receiving postmastectomy radiation. The British Columbia study (6) randomized 318 pre-menopausal women with node-positive breast cancer treated with modified radical mastectomy. Its results at 20-year follow-up published in 2005 demonstrated that compared with chemotherapy alone, chemotherapy and radiation therapy achieved a statistically significant improvement in all endpoints including survival free of isolated LRC (90% vs. 74%), systemic relapse-free survival (48% vs. 31%), breast cancer-free survival (48% vs. 30%), event-free survival (35% vs. 25%), breast cancer-specific survival (53% vs. 38%), and OS (47% vs. 37%).
The DBCG 82b and 82c studies (7) included 3,083 high-risk breast cancer patients after total mastectomy and axillary sampling, and its updated results published in 2006 indicate that the 18-year probability of any first breast cancer event was 73% vs. 59% (p = 0.001) in patients after no postmastectomy irradiation vs. those with postmastectomy irradiation, respectively. The EBCTCG analysis by irradiated volume failed to identify the component of the target volume that favorably influenced survival. Furthermore, IMN irradiation has been deemed controversial, largely because of the increased technical complexity in treatment planning and increased potential for cardiac toxicity (8). Regarding the potential increased risk of cardiac toxicity in the British Columbia trial, long-term cardiac deaths rates were reportedly minimal for both arms (1.8% vs. 0.6%) with modern irradiation techniques (6). These results were supported by the Danish trial as well when analyzed for cardiac toxicity. At 10-year follow-up, the study revealed similar rates of death from ischemic heart disease between patients in the radiotherapy group and no-radiotherapy group (0.8% vs. 0.9%) (10).
Metastases to the IMN have been described in the published literature as occurring in 4% and 65% of cases, increasing with medial tumor location and greater axillary lymph node involvement (6, 11–15). A recently published study of 1,679 Chinese breast cancer patients treated with extended radical mastectomy between 1956 and 2003 identified subgroups of breast cancer patients at high risk of internal mammary node metastases. Huang and colleagues (15) found the incidence of IMN metastasis to be 20% to 40% in patients with risk factors such as (1) N2 disease, (2) medial tumors and positive axillary disease, (3) T3 tumors and age <35 years, (4) T2 tumors and positive axillary lymph nodes, and (5) T2 tumors of medial location. This large surgical series highlights the relevance of IMN involvement, particularly for patients with one or more high-risk feature.
Other investigators have proposed occult IMN metastases as an explanation to inferior outcome seen with medially located tumors. Between 1978 and 1999, 8,422 patients were enrolled in International Breast Cancer Study Group clinical trials in which tumors were classified as medial (1,622 patients) and lateral, central, and other sites (6,800) within the breast. With a median follow-up of 11 years, a statistically significant difference was observed for patients with medial tumors vs. those with nonmedial tumors in DFS (10-year DFS, 46% vs. 48%; hazard ratio [HR], 1.10; 95% CI, 1.02–1.18; p = .01) and OS (10-year OS 59% vs. 61%; HR, 1.09; 1.01–1.19; p = .04) (12). In the subgroup of 2,931 patients with negative axillary lymph nodes, 10-year DFS was 61% vs. 67%, and OS was 73% vs. 80% for medial vs. nonmedial sites, respectively (HR, 1.33; 95% CI, 1.15–1.54; p = .0001 for DFS; and HR, 1.40; 95% CI, 1.17–1.67; p = .0003 for OS) (11). The authors concluded that tumor site has a significant prognostic influence, especially for axillary lymph node–negative disease, in which there is less suspicion of occult internal mammary nodal metastases, and subsequently, these patients receive less aggressive treatment. To address the inferior outcome observed in patients with medial tumors, the authors recommended that staging procedures such as biopsy of the sentinel internal mammary nodes or that novel imaging methods should be further studied in patients with medial tumors (12).
According to the current American Joint Cancer Committee staging system, lymph node Stage N2b and N3b indicates clinically apparent IMN metastasis found by physical examination or imaging studies excluding lymphoscintigraphy in the absence or presence of clinically evident axillary lymph node metastases, respectively (16, 17). Overall, positive IMN disease has often been regarded as a clinically irrelevant phenomenon; however, our study demonstrates the actual rate is as high as 14% in patients with Stage N2–3 breast cancer. Furthermore, patients who achieved a complete radiographic response with normalization of the internal mammary lymph nodes after neoadjuvant chemotherapy demonstrated superior LRC compared with patients with stable or progressive IMN disease. At some institutions, ultrasound of the ipsilateral IMN region is performed before treatment for staging purposes. Without directed radiographic imaging targeted the IMN chain, patients with N2–3 nodal disease at presentation are at risk of being understaged and therefore undertreated.
Limitations of our study include the fact that only 9% (10 of 112) of our patients had biopsy confirmation of their IMN metastasis, and they represent those rare patients who had suspicious IMN metastasis as the only regional node involved. The remaining majority had simultaneous findings of involved supraclavicular, infraclavicular, and/or axillary nodes, and these three nodal basins were routinely confirmed by FNA. Our study had too few IMN metastases detected on chest CT or PET/CT to compare their sensitivity to that of ultrasound; however, ultrasound has proven to be sensitive and cost-effective in many published studies (18–32).
Prospective, nonrandomized data of IMN irradiation vs. no irradiation in 100 patients (67 received IMN radiation and 33 did not) with high-risk breast cancer is available and reported a benefit in terms of LRC and DFS (73% vs. 52%, p = 0.02) with a trend for improved overall survival (78% vs. 64%, p = 0.08) at 6 years (10). However, the earliest Level I evidence of the specific contribution of IMN irradiation can be expected from the European Organization for Research and Treatment of Cancer (EORTC) 22922/10925 trial (16), which aims to evaluate the impact of upper IMN and medial supraclavicular fossa (IM-MS) irradiation on survival and treatment toxicity. Before it closed in December 2003, this trial had randomized 4,004 patients with node-positive or medial or centrally located tumors to receive additional irradiation to regional lymphatics—namely, the IM-MS region or not. Patients who had a mastectomy or breast-conserving surgery were eligible, as were patients who did or did not receive adjuvant chemotherapy. Its conclusion, however, will not be available for several years pending data analysis.
In conclusion, our study demonstrated a significant risk of radiographically detected IMN metastases in patients with N2–3 breast cancer. With multimodality therapy, these patients have more than 50% DFS with acceptable rates of local control at the IMN region. To date, recent National Comprehensive Cancer Network Clinical Practice Guidelines for breast cancer recommended radiation therapy to the IMN that are clinically involved or pathologically positive. If the IMN chain is not involved, treatment to the IMN is at the discretion of the treating radiation oncologist. Although this is reasonable, the important intervention before treating the IMN metastases is to include a reliable radiographic test to evaluate for clinically involved disease. To avoid locoregional recurrence, physicians should evaluate the IMN chain just as they would the axillary and supraclavicular nodes to tailor radiation fields accordingly.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Lacour J, Bucalossi P, Cacers E, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection: Five-year results of an international cooperative study. Cancer. 1976;37:206–214. doi: 10.1002/1097-0142(197601)37:1<206::aid-cncr2820370130>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Lacour J, Le MG, Hill C, et al. Is it useful to remove internal mammary nodes in operable breast cancer? Eur J Surg Oncol. 1987;13:309–314. [PubMed] [Google Scholar]
- 3.Lacour J, Le M, Caceres E, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection: Ten year results of an international cooperative trial in breast cancer. Cancer. 1983;51:1941–1943. doi: 10.1002/1097-0142(19830515)51:10<1941::aid-cncr2820511032>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Valagussa P. Inefficacy of internal mammary nodes dissection in breast cancer surgery. Cancer. 1981;47:170–175. doi: 10.1002/1097-0142(19810101)47:1<170::aid-cncr2820470128>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Chen RC, Lin NU, Golshan M, et al. Internal mammary nodes in breast cancer: Diagnosis and implications for patient management—a systematic review. J Clin Oncol. 2008;26:4981–4989. doi: 10.1200/JCO.2008.17.4862. [DOI] [PubMed] [Google Scholar]
- 6.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen HM, Overgaard M, Grau C, et al. Loco-regional recurrence after mastectomy in high-risk breast cancer—risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol. 2006;79:147–155. doi: 10.1016/j.radonc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 9.White J, Moughan J, Pierce LJ, et al. Status of postmastectomy radiotherapy in the United States: A patterns of care study. Int J Radiat Oncol Biol Phys. 2004;60:77–85. doi: 10.1016/j.ijrobp.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Hojris I, Overgaard M, Christensen JJ, et al. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: Analysis of DBCG 82b and 82c randomized trials. Radiotherapy Committee of the Danish Breast Cancer Cooperative Group. Lancet. 1999;354:1425–1430. doi: 10.1016/s0140-6736(99)02245-x. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, Marubini E, Mariani L, et al. Risk of internal mammary lymph node metastases and its relevance on prognosis of breast cancer patients. Ann Surg. 1983;198:681–684. doi: 10.1097/00000658-198312000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colleoni M, Zahrieh D, Gelber RD, et al. Site of primary tumor has a prognostic role in operable breast cancer: the international breast cancer study group experience. J Clin Oncol. 2005;23:1390–1400. doi: 10.1200/JCO.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 13.Buchholz TA. Internal mammary lymph nodes: To treat or not to treat? Int J Radiat Oncol Biol Phys. 2000;46:801–803. doi: 10.1016/s0360-3016(99)00482-4. [DOI] [PubMed] [Google Scholar]
- 14.Freedman GM, Fowble BL, Nicolaou N, et al. Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist? Int J Radiat Oncol Biol Phys. 2000;46:805–814. doi: 10.1016/s0360-3016(99)00481-2. [DOI] [PubMed] [Google Scholar]
- 15.Huang O, Wang L, Shen K, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008;107:379–387. doi: 10.1007/s10549-007-9561-4. [DOI] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Handbook. 6th ed. New York: Springer-Verlag; 2002. pp. 257–281. [Google Scholar]
- 17.Singletary SE, Alled C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Uren RF, Howman-Giles RB, Chung D, Thompson JF. Nuclear medicine and diagnostic ultrasound and discipline of medicine, the University of Sydney, Sydney, NSW, Australia. Cancer Treat Res. 2005;127:15–38. doi: 10.1007/0-387-23604-x_2. [DOI] [PubMed] [Google Scholar]
- 19.Bruneton JN, Dalfin FY, Caramella E, et al. Value of ultrasound in localizing the internal mammary vessels. Eur J Radiol. 1986;6:142–144. [PubMed] [Google Scholar]
- 20.Scararige JC, Hamper UM, Sheth S, et al. Parasternal Sonography of the Internal Mammary Vessels: Technique, Normal Anatomy, and Lymphadenopathy. Radiology. 1989;172:453–457. doi: 10.1148/radiology.172.2.2664869. [DOI] [PubMed] [Google Scholar]
- 21.Scatarige JC, Boxen I, Smathers RL. Internal mammary lymphadenopathy: Imaging of a vital lymphatic pathway in breast cancer. Radiographics. 1990;10:857–870. doi: 10.1148/radiographics.10.5.2217975. [DOI] [PubMed] [Google Scholar]
- 22.Esen G. Ultrasound of superficial lymph nodes. Eur J Radiol. 2006;58:345–359. doi: 10.1016/j.ejrad.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Unlü M, Ercan MT, Alanyali H, et al. Internal mammarial lymphoscintigraphy with PECT after ultrasound-guided injection of 99 m Tc-dextran. Nuklearmedizin. 1990;29:35–39. [PubMed] [Google Scholar]
- 24.Noguhci M, Micigishi T, Nakajima K, et al. The diagnosis of internal mammary node metastases of breast cancer. Int Surg. 1993;78:171–175. [PubMed] [Google Scholar]
- 25.Ozdemir H, Atilla S, Ilgit ET, et al. Parasternal sonography of the internal mammary lymphatics in breast cancer: CT correlation. Eur J Radiol. 1995;19:114–117. doi: 10.1016/0720-048x(94)00575-w. [DOI] [PubMed] [Google Scholar]
- 26.Kuzo RS, Ben-Ami TE, Yousefzadeh DK, et al. Internal mammary compartment window to the mediastinum. Radiology. 1995;195:187–192. doi: 10.1148/radiology.195.1.7892466. [DOI] [PubMed] [Google Scholar]
- 27.Johnson N, Soot L, Nelson J, et al. Sentinel node biopsy and internal mammary lymphatic mapping in breast cancer. Am J Surg. 2000;179:386–388. doi: 10.1016/s0002-9610(00)00365-2. [DOI] [PubMed] [Google Scholar]
- 28.Uren RF, Howman-Giles R, Renwick SB, et al. Lymphatic mapping of the breast: Locating the sentinel lymph nodes. World J Surg. 2001;25:789–793. doi: 10.1007/s00268-001-0006-7. [DOI] [PubMed] [Google Scholar]
- 29.Struikmans H, Scheihmans LJ, van Dalen A, et al. Lateralisation and depth of the internal mammary chain determined by scintigraphy and by ultrasonography: A comparative study in 124 primary breast cancer patients. Radiother Oncol. 2002;62:159–162. doi: 10.1016/s0167-8140(02)00021-x. [DOI] [PubMed] [Google Scholar]
- 30.Saarnak AE, Hurkmans CW, Pieters BR, et al. Accuracy of internal mammary lymph node localization using lymphoscintigraphy, sonography and CT. Radiother Oncol. 2002;65:79–88. doi: 10.1016/s0167-8140(02)00190-1. [DOI] [PubMed] [Google Scholar]
- 31.Scatarige JC, Hamper UM, Sheth S, Allen HA., 3rd Parasternal sonography of the internal mammary vessels: Technique, normal anatomy, and lymphadenopathy. Radiology. 1989;172:453–457. doi: 10.1148/radiology.172.2.2664869. [DOI] [PubMed] [Google Scholar]
- 32.Ozdemir H, Atilla S, Ilgit ET, Isik S. Parasternal sonography of the internal mammary lymphatics in breast cancer: CT correlation. Eur J Radiol. 1995;19:114–117. doi: 10.1016/0720-048x(94)00575-w. [DOI] [PubMed] [Google Scholar]