Abstract
HER2 is an important predictive marker for response to trastuzumab and lapatinib in breast cancer. It is also a powerful prognostic marker in node-positive patients. Although standardized assays are used to help select patients for anti-HER2 therapy, there are no standardized criteria for assessing HER2 as a prognostic marker. Recent data using quantitative image analysis suggest that both high and low HER2 expression are associated with poor clinical outcome. Using the immunohistochemical scoring criteria currently recommended by the College of American Pathologists and American Society of Clinical Oncology to help select patients for trastuzumab, we evaluated HER2 protein expression in tumor tissue microarrays of 91 node-positive patients with invasive breast carcinoma treated with mastectomy and doxorubicin-based chemotherapy without trastuzumab and without irradiation with a median follow-up of 12.5 years. A wide range of HER2 expression (HER2 ≥ 1 +) in the primary tumor was significantly associated with decreased locoregional recurrence-free survival (P = 0.014), decreased disease-specific survival (P = 0.001), and decreased overall survival (P = 0.001). Even in the subset considered HER2 negative by current College of American Pathologists and American Society of Clinical Oncology guidelines, HER2 = 1 + was associated with worse outcome than HER2 = 0 in this patient cohort. The association between HER2 ≥ 1 + and worse outcome had the greatest statistical significance in the hormone receptor-positive subset of patients. These findings support the hypothesis that low-level HER2 expression may have significant clinical implications. Although the assessment of HER2 expression is most important for predicting response to anti-HER2 therapy, detection of low-level HER2 expression might also be useful in helping to select a more aggressive treatment regimen for patients ineligible for anti-HER2 therapy.
Keywords: node-positive, breast cancer, HER2, immunohistochemical staining, prognostic marker
HER2 overexpression in invasive breast carcinoma is associated with increased tumor recurrence and decreased patient survival in node-positive patients. 4,5,15,17,23,24,26–28,30,34 Overexpression of HER2 also predicts increased response to trastuzumab and lapatinib. 1,2,11,12,14,21,22,25,32 HER2 testing, therefore, is currently recommended on all invasive breast carcinomas, primarily to identify patients who may benefit from anti-HER2 therapy.3 All predictive and prognostic markers should have explicit scoring criteria for clinical use. College of American Pathologists and American Society of Clinical Oncology (CAP and ASCO) guidelines for HER2 testing have been updated recently in an attempt to standardize HER2 reporting and to improve the accuracy of HER2 testing and its utility as a predictive marker.33 There are no standardized criteria, however, for assessing HER2 as a prognostic marker.
Immunohistochemical (IHC) staining for HER2 remains the most widely used initial approach for evaluating HER2 as a predictor of response to anti-HER2 therapy. Current clinical guidelines provide specific criteria for positive (3 +), equivocal (2 +), and negative (0 or 1 +) IHC staining results.33 Despite low-level expression, tumors with weak, incomplete membranous staining (1 +) are considered negative for HER2 overexpression because such tumors are believed unlikely to respond to anti-HER2 therapy. However, many studies on the prognostic significance of HER2 in node-positive breast cancer were published before this clinical scoring system was used, and HER2 positivity in those studies was defined by gene amplification, protein expression above a given threshold by western blot, or variable degrees of membranous staining.5,15,17,23,24,26–28,30,34
Following Food and Drug Administration approval of a standardized IHC kit (HercepTest) for HER2 testing and its corresponding 0 to 3 + clinical scoring system, node-positive patients with HER2 = 3 + were reported to have decreased survival in univariate analysis.4 No independent prognostic significance was observed, although patients in that study received a variety of treatments, and those with HER2 = 0 and HER2 = 1 + were grouped together under the assumption that low-level HER2 expression is biologically equivalent to negative expression. Despite this assumption, it has not actually been shown that the level of HER2 expression that predicts response to trastuzumab is the same as that which confers an adverse clinical outcome in the absence of anti-HER2 therapy. Recent data using quantitative image analysis suggest that both high and low HER2 expression are associated with poor clinical outcome.6
In this study, we evaluated IHC staining for HER2 in a cohort of node-positive breast cancer patients treated with a doxorubicin-based chemotherapy regimen without trastuzumab, and we investigated patient outcome in each of the currently used clinical IHC scoring categories.
MATERIALS AND METHODS
Patients
This study was approved by the M.D. Anderson Institutional Review Board. Patients included in this retrospective study were selected from a clinical database at M.D. Anderson Cancer Center of patients treated on Protocol DM86-12, a randomized study comparing 6 cycles of 5-fluorouracil, doxorubicin (50 mg/m2), and cyclophosphamide (FAC) in the adjuvant setting to 6 cycles of FAC followed by 4 cycles of methotrexate and vinblastine (MV) in patients of below 50 years of age or 50 years or above with negative or unknown estrogen receptor (ER) status.1 Patients with the age of 50 years or above with ER-positive disease were randomized to tamoxifen or 6 cycles of FAC and 4 cycles of MV. The protocol failed to demonstrate any benefit from the addition of 4 cycles of MV to 6 cycles of FAC.
Inclusion criteria for this retrospective study were resectable stages 2 and 3A breast cancer with axillary lymph node metastases, surgical treatment with mastectomy and axillary dissection without irradiation, age younger than 75 years at diagnosis, no evidence of distant disease at diagnosis, and no history of a previous or concurrent malignancy. Additional entry criteria included availability of sufficient archival paraffin-embedded tumor tissue from the primary breast tumor to obtain cores for tissue microarrays. The patients randomized to tamoxifen in Protocol DM86-12 were excluded from this study. There were 94 patients who met the study criteria. A subset of 75 patients also had tumor tissue available from a corresponding lymph node metastasis. All of these patients had surgery performed at M.D. Anderson Cancer Center between 1986 and 1994.
Tissue Preparation
Paraffin blocks containing tissue from the primary breast tumor were available for 94 patients. Tissue blocks from a lymph node metastasis were available for 75 of these patients. To facilitate the efficient use of patient specimens, tissue microarrays were constructed. The microarrays were prepared using a manual tissue puncher/array (Beecher Instruments, Sun Prairie, WI). Up to 6 cores, 0.6mm in diameter, were cut from each primary tumor and lymph node metastasis and aligned within the recipient block in a rectilinear array. All cores were placed 0.2mm apart in the recipient blocks. One set was prepared from the primary breast carcinomas, and a second set was prepared from the corresponding lymph node metastases.
Before IHC staining, deparaffinization of the samples was performed by rinsing first in xylene (3 × 5 min), followed 100% ethanol (2 × 5 min), 95% ethanol (1 × 5 min), 80% ethanol (1 × 5 min). Samples were then treated with 3% H2O2, diluted in methanol to reduce background due to red blood cell fragments left in the tissue. Sections were then rinsed in phosphate buffered saline (PBS) and incubated with 10% horse serum in PBS (1 h, room temperature) to reduce nonspecific background staining.
IHC Staining
IHC staining was performed using monoclonal antibodies against ER (clone 6F11, Novocastra, Newcastle upon Tyne, UK), progesterone receptor (PR, clone 1A6, Neomarkers, Fremont, CA), and HER2 (clone e2–4001, Neomarkers). After the pretreatment described above, tissue sections were washed with PBS and then incubated with primary antibody in PBS and 3% bovine serum albumin for 1 hour at room temperature at the dilutions indicated. Samples were then washed with PBS and incubated for 1 hour at room temperature with biotinylated, affinity-purified rabbit anti-goat antibody (Vector Labs) diluted at 1:200 in PBS and 3% bovine serum albumin. After washing with PBS, the samples were incubated for 1 hour at room temperature with reagents A and B from the ABC kit (Vector Labs) at 1:1 ratio in PBS. After washing with PBS, the samples were developed using peroxidase-based 3,3′-diaminobenzidine chromogen solution (DakoCytomation) according to the manufacturer’s protocol. The samples were then washed with PBS and counterstained with Mayer’s hematoxylin solution (Dako) for 2 to 5 minutes at room temperature. Samples were rinsed with warm water and dried, and then visualized using bright field microscopy.
The IHC stains were scored without knowledge of the clinical outcome. Stains for ER and PR were scored as positive or negative based on the presence or absence of any unequivocal nuclear staining. HER2 was scored using a modification of the HercepTest 0–3 + scoring system3 as currently recommended by CAP and ASCO.33 HER2 was scored as 0 (absence of staining), 1 + (any amount of partial membranous staining), 2 + (weak to moderate complete membranous staining in more than 10%, or strong complete membranous staining in 30% or less), or 3 + (strong complete membranous staining in more than 30%). The IHC staining results were correlated with multiple clinicopathologic variables.
Statistical Analyses
Fisher exact test was used to evaluate associations between IHC staining results for ER, PR, and HER2 in primary tumors versus lymph node metastases and between HER2 scores and multiple clinicopathologic variables. All P values were 2 sided. Survival estimates were calculated using the Kaplan-Meier product limit method and were expressed ± SE. The 2-sided log-rank test was used to test the association between particular factors and survival. Multivariate analysis was performed using the Cox proportional hazards regression model. All statistical analyses were carried out using SSPS 12.0 for Windows (SPSS Inc, Chicago, IL). Locoregional recurrence-free survival was defined as the interval from the date of surgery to the date of locoregional disease recurrence or to the last follow-up date. All locoregional recurrences were scored as events regardless of the presence of distant metastatic disease, and patients without recurrence were censored at the last follow-up. Disease-specific survival was defined as the interval from the date of surgery to the date of death from breast cancer or to the last follow-up date. Patients who died from causes other than breast cancer were censored when disease-specific survival was considered. Overall survival was defined as the interval from the date of surgery to the date of death from any cause or to the last follow-up date.
RESULTS
The patients in this study ranged in age from 28 to 74 years (mean 49 y). Thirty-eight of the 94 patients were ≥ 50 years of age. Thirty-nine patients were postmenopausal, 51 were premenopausal, and the menopausal status of 4 was unknown. Sixty-six of the patients were White, 8 were Black, 14 were Hispanic, and 6 were of other races. According to the Tumor, Nodes, Metastases (TNM) classification system, there were 25 T1, 57 T2, 7 T3, and 5 TX tumors. Most patients were staged as N1 (92 patients), but 2 patients were staged as N2. Clinical follow-up ranged from 3 to 226 months (mean 130 mo). The number of lymph nodes removed at axillary dissection ranged from 5 to 48 (mean 18). The number of positive axillary nodes ranged from 1 to 30 (mean 4). The primary breast carcinomas ranged in size from 0.5 to 10 cm (mean 3.0 cm). Six were grade 1, 40 were grade 2, and 48 were grade 3. Lymphovascular invasion was present in the primary tumor specimen in 39 cases and absent in 55.
Hormone receptor expression and HER2 status of the primary breast tumors were evaluated by IHC staining of the tumor tissue microarrays. Although primary tumor tissue from 94 patients and corresponding lymph node metastases from 75 patients were included in the tissue microarrays, a few cores had insufficient tumor and/or were technically unsuitable for evaluation. Satisfactory IHC scores for HER2 from the primary tumors and lymph node metastases were obtained in 91 and 74 patients, respectively. Of these, satisfactory stains for ER were obtained in 91 and 72 patients, respectively, and satisfactory stains for PR were obtained in 89 and 72 patients, respectively.
Fifty-six (62%) of the primary breast tumors were ER positive and 42 (47%) were PR positive. Forty-six (64%) of the corresponding lymph node metastases were ER positive and 37 (50%) were PR positive. There was a very strong correlation between ER positivity in the primary breast tumors and corresponding lymph node metastases and between PR positivity in the primary breast tumors and corresponding lymph node metastases. Thirty-nine patients (54%) had ER positivity in both the primary tumor and a corresponding lymph node metastasis, and 22 patients (31%) were ER negative in both the primary tumor and corresponding lymph node metastasis (P < 0.001). Twenty-eight patients (40%) had PR positivity in both the primary tumor and a corresponding lymph node metastasis, and 27 patients (39%) were PR negative in both the primary tumor and corresponding lymph node metastasis (P < 0.001).
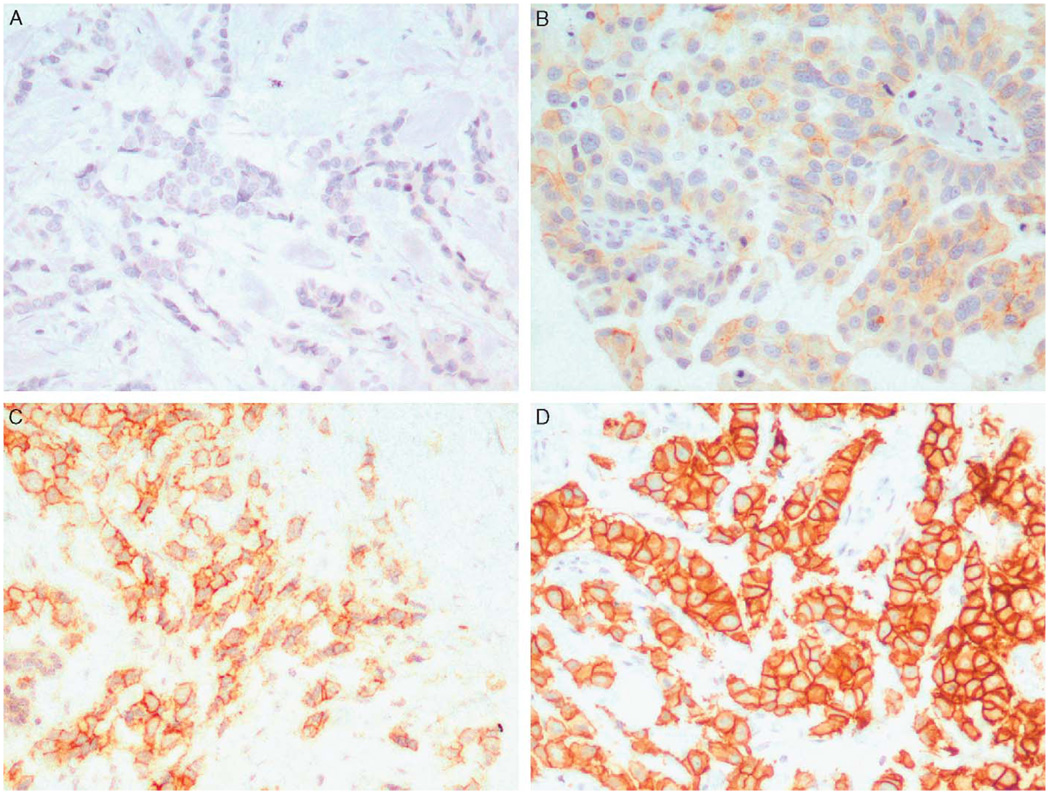
Fifty-three (58%) of the primary breast tumors had a HER2 score of 0, 13 (14%) were 1 +, 5 (5%) were 2 +, and 20 (22%) were 3 + (Fig. 1). Fifty (68%) of the lymph node metastases had a HER2 score of 0, 7 (9%) were 1 +, 0 (0%) were 2 +, and 17 (23%) were 3 +. There was a very strong association between HER2 expression in the primary tumors and corresponding lymph node metastases. Twenty-one patients (29%) had HER2 ≥ 1 + in both the primary tumor and corresponding lymph node metastasis, and 41 patients (57%) had HER2 = 0 in both the primary tumor and corresponding lymph node metastasis (P < 0.001).
FIGURE 1.
Photomicrographs of representative invasive breast carcinomas with immunohistochemical staining for HER2. A, HER2 = 0; B, HER2 = 1 +; C, HER2 = 2 +; D, HER2 = 3 + (immunoperoxidase; × 200 magnification).
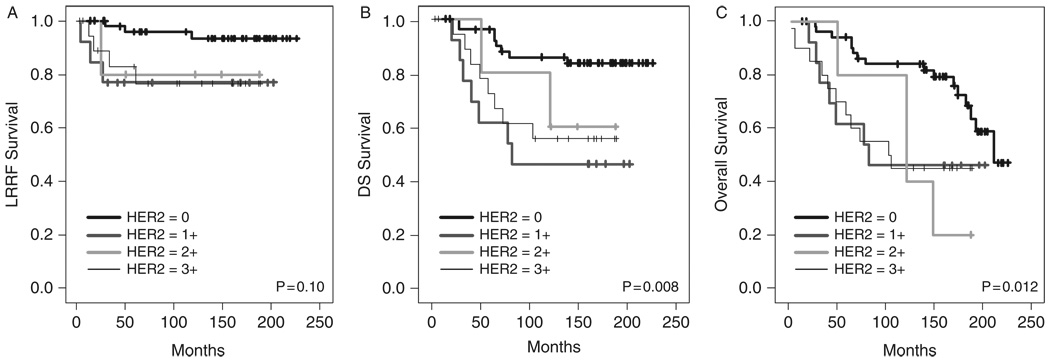
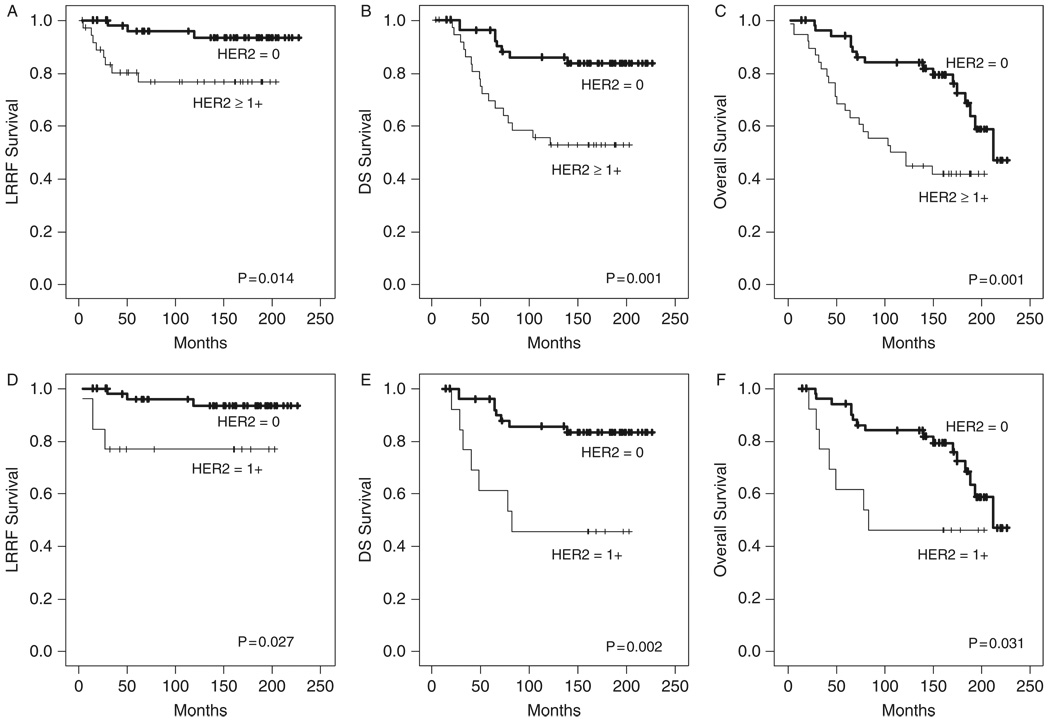
Univariate analysis (log-rank test) was performed to evaluate the association between HER2 expression in the primary tumor and clinical outcome (N = 91). When all of the individual HER2 scores were included in the analysis, HER2 expression was found to be associated with decreased disease-specific and overall survival (P = 0.008 and 0.012, respectively) but not with locoregional recurrence (P = 0.10) (Fig. 2). Although high-level HER2 expression was associated with decreased overall survival when dichotomized to HER2 ≤ 2 + and HER2 = 3 + (P = 0.032), the association between HER2 expression and clinical outcome had greater significance when HER2 status was dichotomized to HER2 = 0 and HER2 ≥ 1 +. Patients with HER2 ≥ 1 + in the primary tumor had decreased locoregional recurrence-free survival (P = 0.014), decreased disease-specific survival (P = 0.001), and decreased overall survival (P = 0.001) (Figs. 3A–C). The 10-year actuarial locoregional recurrence- free survival was 93 ± 4% versus 77 ± 7% for HER2 = 0 versus HER2 ≥ 1 +, respectively. The 10-year actuarial disease-specific survival was 86 ± 5% versus 55 ± 8%, and the 10-year actuarial overall survival was 84 ± 5% versus 50 ± 8%.
FIGURE 2.
Kaplan-Meier curves depicting locoregional recurrence-free (LRRF) (A), disease-specific (DS) (B), and overall (C) survival according to HER2 score (n = 91).
FIGURE 3.
Kaplan-Meier curves depicting locoregional recurrence-free (LRRF), disease-specific (DS), and overall survival for all patients dichotomized to HER2 = 0 versus HER2 ≥ 1 + (A– C, n = 91) and for the subgroup of patients clinically negative for HER2 overexpression dichotomized to HER2 = 0 versus HER2 = 1 + (D– F, n = 66).
When analysis was limited to the subset of patients considered negative for HER2 overexpression (HER2 = 0 or 1 +) using the current CAP and ASCO guidelines (N = 66), patients with HER2 = 1 + had decreased locoregional recurrence-free survival (P = 0.027), decreased disease-specific survival (P = 0.002), and decreased overall survival (P = 0.031) compared with patients with HER2 = 0 (Figs. 3D–F). The 10-year actuarial locoregional recurrence-free survival was 93 ± 4% versus 77 ± 12% for HER2 = 0 versus HER2 = 1 +, respectively. The 10-year actuarial disease-specific survival was 86 ± 5% versus 46 ± 14%, and the 10-year actuarial overall survival was 84 ± 5% versus 46 ± 14%.
The statistical significance was even greater when only hormone receptor-positive patients were considered (N = 61). In hormone receptor-positive patients, HER2 ≥ 1 + in the primary tumor was associated with decreased locoregional recurrence-free survival (P = 0.018), decreased disease-specific survival (P = 0.001), and decreased overall survival (P < 0.001). The 10-year actuarial locoregional recurrence-free survival was 91 ± 5% versus 71 ± 10% for HER2 = 0 versus HER2 ≥ 1 +, respectively. The 10-year actuarial disease-specific survival was 83 ± 6% versus 47 ± 11%, and the 10-year actuarial overall survival was 83 ± 6% versus 41 ± 10%.
When analysis was limited to hormone receptor-positive patients considered negative for HER2 overexpression (HER2 = 0 or 1 +) using the current CAP and ASCO guidelines (N = 50), patients with HER2 = 1 + had decreased locoregional recurrence-free survival (P = 0.037), decreased disease-specific survival (P < 0.001), and decreased overall survival (P = 0.007) compared with patients with HER2 = 0. The 10-year actuarial locoregional recurrence-free survival was 91 ± 5% versus 72 ± 14% for HER2 = 0 versus HER2 = 1 +, respectively. The 10-year actuarial disease-specific survival was 83 ± 6% versus 36 ± 15%, and the 10-year actuarial overall survival was 83 ± 6% versus 36 ± 15%.
To determine whether HER2 expression was significantly associated with other factors known to be associated with increased risk, HER2 scores were evaluated in relation to multiple clinicopathologic characteristics (Table 1). A significant inverse relationship was noted between the HER2 score in the primary tumor and ER positivity (P = 0.011) and PR positivity (P = 0.025). No other significant association was observed.
TABLE 1.
IHC Scores for HER2 in Primary Breast Carcinomas According to Clinicopathologic Variables
| Variable | No. Patients (n = 91) | Primary HER2 = 0 | Primary HER2 = 1 + | Primary HER2 = 2 + | Primary HER2 = 3 + | |
|---|---|---|---|---|---|---|
| Age (y) | P = 0.16 | |||||
| 20–35 | 7 | 3 | 3 | 1 | 0 | |
| 36–50 | 49 | 32 | 5 | 3 | 9 | |
| 51–70 | 34 | 17 | 5 | 1 | 11 | |
| >70 | 1 | 1 | 0 | 0 | 0 | |
| Race | P = 0.56 | |||||
| White | 64 | 36 | 10 | 2 | 16 | |
| Black | 8 | 5 | 0 | 1 | 2 | |
| Hispanic | 14 | 8 | 2 | 2 | 2 | |
| Other | 5 | 4 | 1 | 0 | 0 | |
| Menopausal Status | P = 0.53 | |||||
| Pre | 48 | 29 | 8 | 4 | 7 | |
| Post | 39 | 21 | 5 | 1 | 12 | |
| Unknown | 4 | 3 | 0 | 0 | 1 | |
| Tumor Size (cm) | P = 0.85 | |||||
| ≤1 | 6 | 4 | 1 | 0 | 1 | |
| 1.1–2.0 | 18 | 11 | 3 | 0 | 4 | |
| 2.1–3.0 | 28 | 18 | 3 | 1 | 6 | |
| 3.1–4.0 | 19 | 9 | 3 | 2 | 5 | |
| 4.1–5.0 | 8 | 3 | 1 | 1 | 3 | |
| >5.0 | 7 | 3 | 2 | 1 | 1 | |
| Unknown | 5 | 5 | 0 | 0 | 0 | |
| Tumor Grade | P = 0.11 | |||||
| 1 | 6 | 5 | 1 | 0 | 0 | |
| 2 | 39 | 25 | 2 | 4 | 8 | |
| 3 | 46 | 23 | 10 | 1 | 12 | |
| No. Positive Lymph | P = 0.87 | |||||
| Nodes | ||||||
| 1–3 | 63 | 38 | 8 | 3 | 14 | |
| 4–9 | 19 | 11 | 3 | 1 | 4 | |
| >9 | 9 | 4 | 2 | 1 | 2 | |
| Lymphovascular | P = 0.33 | |||||
| Invasion | ||||||
| Present | 38 | 20 | 8 | 3 | 7 | |
| Absent | 53 | 33 | 5 | 2 | 13 | |
| ER | P = 0.011 | |||||
| Positive | 56 | 36 | 10 | 4 | 6 | |
| Negative | 35 | 17 | 3 | 1 | 14 | |
| PR* | P = 0.025 | |||||
| Positive | 42 | 28 | 8 | 2 | 4 | |
| Negative | 47 | 24 | 4 | 3 | 16 |
Of the 91 patients, satisfactory IHC stains for PR were obtained in 89.
ER indicates estrogen receptor; IHC, immunohistochemical; PR, progesterone receptor.
Univariate analyses were performed to determine whether any clinicopathologic factor other than HER2 score was associated with clinical outcome. Factors evaluated included patient’s age, race, tumor size, tumor grade, percent of positive lymph nodes (positive nodes expressed as percent of nodes removed), lymphovascular invasion, and hormone receptor status. Greater than 20% positive lymph nodes was found to be associated with decreased locoregional recurrence-free survival (P = 0.013), decreased disease-specific survival (P = 0.021), and decreased overall survival (P = 0.021). Tumor size > 2 cm and tumor grade 2 or 3 (when dichotomized to grade 1 vs. grade 2 or 3) were found to be associated with decreased overall survival (P = 0.016 and P = 0.047, respectively). Hispanic race was associated with improved disease-specific survival and improved overall survival (P = 0.019 and P = 0.037, respectively).
To determine whether the HER2 score had independent prognostic significance, multivariate analysis using the Cox proportional hazards regression model was performed. Factors included in the analysis were patient age, race, tumor size, grade, hormone receptor status, HER2 score, and lymphovascular invasion. HER2 ≥ 1 + was the only factor that maintained independent adverse prognostic significance for locoregional recurrence-free survival (HR, 12.22; P = 0.018) and disease-specific survival (HR, 3.69; P = 0.004). HER2 ≥ 1 + and tumor size > 2 cm each maintained independent prognostic significance for overall survival (HR = 2.95, P = 0.003; and HR = 2.60, P = 0.049, respectively) (Table 2).
TABLE 2.
Cox Multivariate Analysis of Locoregional Recurrence-free, Disease-specific, and Overall Survival
| Outcome Measure | Variable | HR (95% CI) | P |
|---|---|---|---|
| Locoregional recurrence-free survival | HER2 ≥ 1 + | 12.22 (1.53–97.81) | 0.018 |
| Disease-specific survival | HER2 ≥ 1 + | 3.69 (1.52–8.98) | 0.004 |
| Overall survival | HER2 ≥ 1 + | 2.95 (1.46–5.97) | 0.003 |
| Tumor size >2 cm | 2.60 (1.00–6.73) | 0.049 |
CI indicates confidence interval; HR, hazard ratio.
DISCUSSION
The association between high-level HER2 expression and adverse clinical outcome in node-positive breast cancer is well known. Our finding that even 1 + and 2 + HER2 expression were associated with decreased survival in the node-positive patient cohort we evaluated may seem surprising, but the prognostic significance of low-level HER2 expression has not been sufficiently evaluated in previous studies. Most studies on the prognostic significance of HER2, and even most studies evaluating the association between HER2 expression and response to anti-HER2 therapy, have not adequately compared HER2 negativity to low-level HER2 expression. Instead, most investigators have grouped these categories together, assuming a priori that low-level expression is not clinically meaningful.
In the initial report on HER2 expression in breast cancer by Slamon et al,23 a strong correlation between HER2 gene amplification and nodal status was observed. In multivariate analysis of the 86 node-positive patients with sufficient DNA for evaluation and available clinical follow-up data, HER2 gene amplification was found to be an independent prognostic factor, second only to the number of positive lymph nodes. The study was subsequently expanded to include 345 node-positive patients, and multivariate analysis continued to show HER2 gene amplification to be an independent predictor of disease recurrence and overall survival.24
In the expanded study published in 1989, a comprehensive analysis of HER2 protein, RNA, and DNA was performed on tumors from a subset of the patients, and a reasonably good association between HER2 gene amplification and overexpression at the RNA and protein level was observed.24 Although there were insufficient clinical follow-up data available to evaluate the prognostic significance of HER2 protein expression in this subset of patients, a number of subsequent studies have evaluated the prognostic significance of HER2 protein expression in invasive breast cancer.
Most studies involving lymph node-negative patients have found no relationship between HER2 protein expression and clinical outcome, but the majority of studies on lymph node-positive patients have found that HER2 protein expression (detected either by western blotting or IHC) is associated with decreased patient survival.4,15,17,26,28,34 In fact, HER2 expression is reported to be one of the strongest adverse prognostic factors known for node-positive breast cancer patients.17,26,34 However, many of these studies were published before the 0–3 + IHC scoring system was used clinically, and HER2 positivity was defined by gene amplification, protein expression above a given threshold by western blot, or variable degrees of membranous staining. 5,15,17,23,24,26–28,30,34
HER2 overexpression was subsequently found to predict response to anti-HER2 therapy, first in the metastatic setting and then in the adjuvant setting.1,10,12,14,21,22,25,32 Routine HER2 testing, therefore, became the standard of care for its predictive utility in selecting patients for trastuzumab therapy. Nevertheless, even though the primary impetus for routine HER2 testing is to predict response to anti-HER2 therapy, our study suggests that levels of HER2 expression currently below those used to select patients for anti-HER2 therapy may have important clinical significance. In addition to observing a worse outcome for patients with HER2 = 3 +, we found that HER2 = 1 + or 2 + (regarded by current CAP and ASCO guidelines as negative and equivocal for HER2, respectively) was associated with increased locoregional tumor recurrence and decreased survival in node-positive patients after mastectomy and chemotherapy without irradiation.
Our findings support a recent study that found using quantitative image analysis that both high and low HER2 expression were associated with poor clinical outcome in patients not treated with trastuzumab.6 In that study, HER2 expression was evaluated using an automated image analysis system for quantitating IHC expression. The investigators observed that invasive breast cancers with a low but detectable amount of HER2 expression appeared to be as aggressive as those with high HER2 expression. Although low levels of HER2 expression detected with their automated system were associated with poor clinical outcome, low levels (1 + or 2 +) detected by manual scoring were not associated with poor outcome. Complete treatment information was not available for all patients, however, and the patients received different treatment. Only 15% received chemotherapy, 27% received tamoxifen, and most but not all received local radiation.6 In contrast, all the patients in our study received doxorubin-based chemotherapy without tamoxifen after total mastectomy, and none of the patients received radiation therapy. The uniform treatment and long clinical follow-up of our patient cohort was more ideally suited for assessing the prognostic significance of HER2 expression.
A number of studies suggest that HER2 positivity predicts increased benefit from anthracycline-containing chemotherapy regimens.8,9,16,18,20,29 Our retrospective study does not specifically address whether HER2 ≥ 1 + predicts a worse clinical outcome with and without doxorubicin, as all patients received the anthracycline. Moreover, the dosage of doxorubicin used (50 mg/m2) was different from that of most reported studies. 8,9,16,18,20,29 Nevertheless, in patients treated with this particular doxorubicin-based regimen, the outcome of patients with HER2 = 1 + was significantly different from those with HER2 = 0, indicating that low-level HER2 expression seems to be biologically active. This raises the intriguing possibility that low-level HER2 expression may impart other biologic effects, such as response to anti-HER2 therapy.
It has been assumed that patients with low-level HER2 expression are unlikely to benefit from trastuzumab, but the existing data that address response to trastuzumab in patients with low-level HER2 expression are actually limited and contradictory. In a retrospective evaluation of National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31, 161 patients were found upon repeat testing to be HER2 negative by both IHC and fluorescence in suit hybridization. In this subgroup of patients, the rate of relapse for those treated with chemotherapy alone versus chemotherapy and trastuzumab was 21% and 8%, respectively, and this difference was statistically significant.19 Likewise, of 103 patients in intergroup 9831 found upon repeat testing to be negative for HER2 by both IHC and fluorescence in suit hybridization, the rate of relapse for patients treated with chemotherapy alone versus chemotherapy and trastuzumab was 32% and 15%, respectively. This difference was not statistically significant, but the trend resembled that of the overall study population.31
The only trial originally designed to evaluate HER2-negative patients, CALGB 9840, did not show a significantly increased response rate to paclitaxel and trastuzumab compared with paclitaxel alone (35% vs. 29%, P = 0.34). A retrospective analysis of these patients, however, did find that patients negative for HER2 amplification but with chromosome 17 polysomy (most of which were HER2 = 2 +) had a greater response to paclitaxel and trastuzumab compared with paclitaxel alone (63% vs. 25%, P = 0.043).13 This suggests that the level of HER2 protein useful in selecting patients for anti-HER2 therapy may be lower than generally recognized.
It should be noted that our study involved node-positive patients treated with doxorubicin-based chemotherapy without tamoxifen, despite ER positivity in a large proportion of the patients. Future studies are needed to determine whether the adverse clinical outcome of patients with even low-level HER2 expression is maintained in the setting of hormonal therapy. High expression of HER2 is known to confer relative endocrine resistance.7,11 If lower levels confer any resistance as well, the difference in clinical outcome between patients with low-level HER2 expression treated with endocrine therapy and those with truly negative expression (HER2 = 0) might be even greater than observed in this study.
Prognostic assays are generally considered most useful in the node-negative subgroup, because results might help to decide whether patients will receive systemic therapy. For node-positive patients, however, the evaluation of HER2 as a prognostic factor could be useful in helping to select patients who will benefit from adjuvant radiation therapy after total mastectomy. If the decreased locoregional recurrence-free survival we observed for patients with HER2 ≥ 1 + is confirmed by other investigators, some patients with HER2 ≥ 1 + might benefit from adjuvant radiation after total mastectomy, particularly those with low-level HER2 expression who do not receive anti-HER2 therapy. For patients ineligible for anti-HER2 therapy, low-level HER2 expression might also be useful in helping to select a more aggressive chemotherapy regimen or alternate therapies in the future.
It should be emphasized that our study is best regarded as hypothesis generating. It was a retrospective study involving a relatively small patient cohort at a single institution. Nevertheless, if confirmed by other investigators, the findings could lead to a paradigm shift in how we define HER2 positivity. We observed that any degree of HER2 expression (1 +, 2 +, or 3 +) was associated with increased tumor recurrence and decreased patient survival in a node-positive cohort of breast cancer patients treated with doxorubicin-based chemotherapy without trastuzumab. These results suggest that even low-level HER2 expression may confer important biologic potential. This finding might be particularly relevant to patients with low-level HER2 expression who are ineligible for anti-HER2 therapy.
Acknowledgments
Supported by grants from the Susan G. Komen Breast Cancer Foundation to MZG (BCTR022043) and by Cancer Center Support Grant #CA16672 from the NCI.
REFERENCES
- 1.Assikis V, Buzdar A, Yang Y, et al. A phase III trial of sequential adjuvant chemotherapy for operable breast carcinoma: final analysis with 10-year follow-up. Cancer. 2003;97:2716–2723. doi: 10.1002/cncr.11396. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J, Carbonell X, Castaneda-Soto NJ, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–2171. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Ravdin P, Hayes DF, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–1878. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [PubMed] [Google Scholar]
- 4.Birner P, Oberhuber G, Stani J, et al. Evaluation of the United States food and drug administration-approved scoring and test system of HER-2 protein expression in breast cancer. Clin Cancer Res. 2001;7:1669–1675. [PubMed] [Google Scholar]
- 5.Borg A, Tandon AK, Sigurdsson H, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990;50:4332–4337. [PubMed] [Google Scholar]
- 6.Camp RL, Dolled-Filhart M, King BL, et al. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res. 2003;63:1445–1448. [PubMed] [Google Scholar]
- 7.Carlomagno C, Perrone F, Gallo C, et al. c-erb B2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J Clin Oncol. 1996;14:2702–2708. doi: 10.1200/JCO.1996.14.10.2702. [DOI] [PubMed] [Google Scholar]
- 8.Del Mastro L, Bruzzi P, Nicolo G, et al. HER2 expression and efficacy of dose-dense anthracycline-containing adjuvant chemotherapy in breast cancer patients. Br J Cancer. 2005;93:7–14. doi: 10.1038/sj.bjc.6602660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dressler LG, Berry DA, Broadwater G, et al. Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients. J Clin Oncol. 2005;23:4287–4297. doi: 10.1200/JCO.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 11.Houston SJ, Plunkett TA, Barnes DM, et al. Overexpression of c-erbB2 is an independent marker of resistance to endocrine therapy in advanced breast cancer. Br J Cancer. 1999;79:1220–1226. doi: 10.1038/sj.bjc.6690196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman PA, Broadwater G, Lezon-Geyda K, et al. CALGB 150002: Correlation of HER2 and chromosome 17 (ch17) copy number with trastuzumab (T) efficacy in CALGB 9840, paclitaxel (P) with or without T in HER2+ and HER2− metastatic breast cancer (MBC) J Clin Oncol. 2007: ASCO Annual Meeting Proceedings Part I. 2007;25(18S):1009. [Google Scholar]
- 14.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 15.McCann AH, Dervan PA, O’Regan M, et al. Prognostic significance of c-erbB-2 and estrogen receptor status in human breast cancer. Cancer Res. 1991;51:3296–3303. [PubMed] [Google Scholar]
- 16.Moliterni A, Menard S, Valagussa P, et al. HER2 overexpression and doxorubicin in adjuvant chemotherapy for resectable breast cancer. J Clin Oncol. 2003;21:458–462. doi: 10.1200/JCO.2003.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Paik S, Hazan R, Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 18.Paik S, Bryant J, Park C, et al. erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptornegative breast cancer. J Natl Cancer Inst. 1998;90:1361–1370. doi: 10.1093/jnci/90.18.1361. [DOI] [PubMed] [Google Scholar]
- 19.Paik S, Kim C, Jeong J, et al. Benefit from adjuvant trastuzumab may not be confined to patients with IHC 3 + and/or FISH-positive tumors: Central testing results from NSABP B-31. J Clin Oncol. 2007: ASCO Annual Meeting Proceedings Part I. 2007;25(18S):511. [Google Scholar]
- 20.Petit T, Borel C, Ghnassia JP, et al. Chemotherapy response of breast cancer depends on HER-2 status and anthracycline dose intensity in the neoadjuvant setting. Clin Cancer Res. 2001;7:1577–1581. [PubMed] [Google Scholar]
- 21.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 22.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 23.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 24.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 25.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 26.Tandon AK, Clark GM, Chamness GC, et al. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989;7:1120–1128. doi: 10.1200/JCO.1989.7.8.1120. [DOI] [PubMed] [Google Scholar]
- 27.Tetu B, Brisson J. Prognostic significance of HER-2/neu oncoprotein expression in node-positive breast cancer. The influence of the pattern of immunostaining and adjuvant therapy. Cancer. 1994;73:2359–2365. doi: 10.1002/1097-0142(19940501)73:9<2359::aid-cncr2820730919>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Thor AD, Schwartz LH, Koerner FC, et al. Analysis of c-erbB-2 expression in breast carcinomas with clinical follow-up. Cancer Res. 1989;49:7147–7152. [PubMed] [Google Scholar]
- 29.Thor AD, Berry DA, Budman DR, et al. erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst. 1998;90:1346–1360. doi: 10.1093/jnci/90.18.1346. [DOI] [PubMed] [Google Scholar]
- 30.Thor AD, Liu S, Edgerton S, et al. Activation (tyrosine phosphorylation) of ErbB-2 (HER-2/neu): a study of incidence and correlation with outcome in breast cancer. J Clin Oncol. 2000;18:3230–3239. doi: 10.1200/JCO.2000.18.18.3230. [DOI] [PubMed] [Google Scholar]
- 31.Tuma RS. Inconsistency of HER2 test raises questions. J Natl Cancer Inst. 2007;99:1064–1065. doi: 10.1093/jnci/djm075. [DOI] [PubMed] [Google Scholar]
- 32.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 33.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 34.Wright C, Angus B, Nicholson S, et al. Expression of c-erbB-2 oncoprotein: a prognostic indicator in human breast cancer. Cancer Res. 1989;49:2087–2090. [PubMed] [Google Scholar]