Abstract
Stretch is an essential mechanism for lung growth and development. Animal models in which fetal lungs have been chronically over- or under-distended demonstrate a disrupted mix of type II and type I cells, with static overdistention typically promoting a type I cell phenotype. The Rho GTPase family, key regulators of cytoskeletal signaling, are known to mediate cellular differentiation in response to stretch in other organs. Using a well-described model of alveolar epithelial cell differentiation and a validated stretch device, we investigated the effects of supraphysiologic stretch on human fetal lung (HFL) alveolar epithelial cell phenotype. Static stretch applied to epithelial cells suppressed type II cell markers (SP-B and Pepsinogen C, PGC), and induced type I cell markers (Caveolin-1, Claudin 7 and Plasminogen Activator Inhibitor-1, PAI-1) as predicted. Static stretch was also associated with Rho A activation. Furthermore, the Rho kinase (ROCK) inhibitor Y27632 decreased Rho A activation, and blunted the stretch-induced changes in alveolar epithelial cell marker expression. Together these data provide further evidence that mechanical stimulation of the cytoskeleton and Rho activation are key upstream events in mechanotransduction-associated alveolar epithelial cell differentiation.
Introduction
The alveolar epithelium is composed of two cell types: type II and type I cells (1). Type II cells are responsible for surfactant production and play a role in lung host defense. Type I cells, while less numerous, cover the majority of the gas exchange surface area of the lung. Development and maintenance of this mixed population of alveolar epithelial cells depends on both the biochemical milieu of growth factors, hormones, and extracellular matrix, and the interplay of physical forces mediated intrinsically by the cytoskeleton and extrinsically by cell-cell and cell-matrix interactions.
Stretch plays a critical role in lung development (2). Static stretch provided by fetal lung fluid provides a constant distending force of approximately 2.5 mmHg (3). Fetal breathing movements provide intermittent cyclic stretch (4) resulting in 3 to 5% change in alveolar surface area (3). By comparison, changes in surface area with tidal breathing in adults are minimal (5), while expansion to total lung capacity changes surface area by 40–45% (6). The importance of stretch as a mechanism for lung development has been shown in human pregnancy complicated by premature membrane rupture (7), in neonatal neuromuscular disorders (8), and in animal models (9).
By extension, enhanced stretch, generally from tracheal obstruction, promotes lung growth (10, 11), providing the rationale for the use of tracheal occlusion to reverse pulmonary hypoplasia in congenital diaphragmatic hernia. While tracheal occlusion increases lung growth through the retention of fetal lung fluid, the effects of this supraphysiologic stretch on differentiation of the alveolar epithelium are less clear (12). Animal studies suggest that static stretch favors the formation of type I cells (13) while cyclic stretch favors type II cells (14), but the mechanisms by which stretch is translated into molecular signals to modify gene expression in the alveolar epithelium are poorly understood.
The Rho-GTPase family of small messengers is an attractive candidate for mediating stretch-induced cell signaling, due to its tight coupling to the cytoskeleton. As the cytoskeleton is a global receiver and transmitter of mechanical forces (15), Rho-GTP activation could be an early, upstream intracellular event in response to stretch. Rho GTPases have been implicated in lung branching morphogenesis (16), alveolar epithelial permeability (17), migration (18), and recently, maturation of alveolar type 2 cells (19). We now show, using a validated, equibiaxial stretch device and human fetal lung epithelial cells, that changes in epithelial cell phenotype between type I and type II cells with static stretch are associated with activation of the Rho GTPase pathway.
Methods
Reagents
Dexamethasone, isobutyl methylxanthine, and 8-bromo-cAMP were purchased from Sigma Chemical Co. (St. Louis, MO). All other supplies were purchased from Fisher (Fair Lawn, NJ), Pierce (Rockford, IL) or Invitrogen (Carlsbad, CA). Antisera included SP-B (Chemicon, Temecula, CA), Pepsinogen C (Abcam, Cambridge, UK), Claudin 7 (Zymed, South San Francisco, CA), Plasminogen Activator Inhibitor-1 (BD Transduction Laboratories, Lexington, KY), Caveolin-1α (Santa Cruz Biotechnologies, Santa Cruz, CA), and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Chemicon).
Cell Culture
Human fetal lungs from 14–18-wk therapeutic abortions were obtained from Advance Bioscience Resources, Inc. (Alameda, CA) and used in protocols approved by the Committee for Human Research at The Children’s Hospital of Philadelphia. A stable population of alveolar type II cells (with an average 10% contaminating fibroblasts, <5% endothelial cells, and no inflammatory cells) were prepared as described previously (20), and plated at a density of 7 X 105 cells/cm2 on deformable Silastic membranes (Specialty Manufacturing, Saginaw, MI) coated with 50 μg/ml of fibronectin (BD Biosciences, Medford, MA) and mounted into custom-made wells. Waymouth’s media containing 10 nM dexamethasone, 0.1 mM 8-bromo-cAMP, and 0.1 mM isobutyl methylxanthine (DCI) was used to maintain the type II cell phenotype.
Equibiaxial Stretch
Cells on Silastic membranes were mounted onto individual cell-stretching devices capable of applying static equibiaxial strain, as previously described (6). 72 h after plating, cells were stretched continuously for 24 h at either 10% or 37% change in surface area. These equibiaxial deformations correspond to static stretches in isolated rat lungs at 55 and 100% of total lung capacity, respectively.
Cell viability and apoptosis
Ethidium homodimer-1 and calcein AM were added to the wells to assess cell viability (LIVE/DEAD, Molecular Probes, Eugene, OR). Apoptosis was assessed by immunoblotting for activated caspase 3 (R and D Systems, Minneapolis, MN). Cells treated with 1μM Staurosporine (Sigma) served as a positive control.
Western immunoblotting
Cells were harvested in lysis buffer (1% Triton X-100, 150 mM NaCl, 50mM Tris-HCl, 5 mM ethylenediamine tetraacetic acid, 5% glycerol, pH 8.0) with 1X Protease inhibitor (Roche, Indianapolis, IN), and samples immunoblotted using NuPAGE Bis-Tris gels (Invitrogen). Primary antibody concentrations were: SP-B 1:4000; PGC 1:5000; Claudin 7, PAI-1, and Caveolin 1 at 1:1000; GAPDH at 1:20,000. Secondary antibodies conjugated to Alexa Fluor 680 (Molecular Probes) or IRdye 800 (Rockland, Gilbertsville, PA) were used at a dilution of 1:10,000. Membranes were analyzed using the Odyssey infrared imaging system (Li-Cor, Lincoln, NE).
Real-time reverse transcriptase PCR (RT-PCR)
Total cellular RNA was isolated using RNA STAT-60 Reagent (Tel-Test, Friarswood, TX). Purity was verified by the OD 260:280 ratio and integrity assessed using the Agilent 2100 bioanalyzer system (Agilent, Palo Alto, CA). Real-time RT-PCR assays using a singleplex strategy were done using an ABI Prism 7900 system (ABI, Foster City, CA). Details of the two-step protocol have been described previously (21). The primer/probe sets (listed on the ABI website, http://www.allgenes.com) were: SP-B Hs00167036, PGC Hs00160052, claudin 7 Hs00600772, PAI-1 Hs00167155, Cav-1 Hs00184697, and 18s Hs99999901_S1. Standards for comparison of RT-PCR results were derived from RNA isolated from alveolar type II cells cultured from human fetal lung or from banked frozen adult human lung tissue.
Immunofluorescence Imaging
Experimental membranes with adherent cells were mounted on glass slides using Fluoromount™ (Sigma). Cells were fixed with 1% paraformaldehyde in PBS, and immunostained with Claudin 7 antibody (1:100). Nuclei were counterstained with DAPI. Fluorescence was examined at 20X with an Olympus IX81 microscope and Slidebook 4.2.0 digital microscopy software (Olympus, San Diego, CA).
Stress fiber analysis
Alexa Fluor 549 Phalloidin (Molecular Probes, Eugene, OR) was used to stain the actin cytoskeleton at the end of the stretching period. Stress fibers were counted in 7 random high power fields (60X) per treatment group using Image Pro-Plus software (Version 6.0, MediaCybernetics Inc, Bethesda, MD) to determine mean stress fiber intensity per cell.
Rho inhibition
Y27632, a selective ROCK inhibitor (Tocris, Ellisville, MO) was added to cells at a concentration of 20μM/mL, 1 h prior to stretch and maintained throughout the experiment. Direct activation of Rho was assessed using the G-Lisa Rho A Activation Assay (Cytoskeleton, Denver, CO), which measures activated Rho-GTP, per the manufacturer’s instructions.
Statistical Analysis
Results are expressed as mean ± standard error. Analysis of variance (for LIVE/DEAD) and t-tests (all other experiments) were performed with GraphPad Prism 5.0 for Macintosh (GraphPad, San Diego, CA). All protein and RNA studies were normalized with GAPDH and 18S, respectively.
Results
Static stretch modified alveolar epithelial cell phenotype without cell toxicity
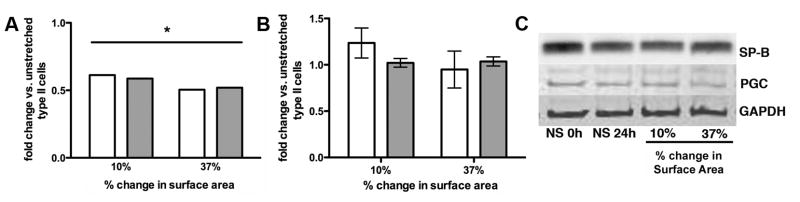
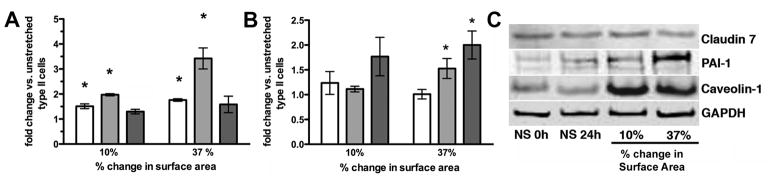
Random field counting (n=4 experiments) revealed no changes in cell viability due to culture or stretch (before stretch 92.8 ± 2.6% live cells; no stretch 24h 91.6 ± 1.2%; 10% stretch 24h 94.0 ± 1.1%; 37% stretch 24h 93.8 ± 2.4%; p>0.05), and there was no evidence of apoptosis after static stretch (Figure 1). RT-PCR revealed decreased expression of the type II cell markers SP-B and PGC (Figure 2A) at 10% (SP-B: 61 ± 8% and PGC: 50 ± 5% of unstretched control) and 37% change in surface area (SP-B: 50 ± 5% and PGC: 51 ± 7%; n=5–7, p<0.01). These changes were not evident at the protein level (Figures 2B and C). Induction of RNA for the type I cell markers Claudin 7 and PAI-1 (Figure 3A) occurred at both 10% (Claudin 7: 1.5 ± .09-fold and PAI-1: 2.0 ± .04-fold vs. unstretched) and 37% change in surface area (Claudin 7: 1.8 ± 0.1-fold and PAI-1: 3.4 ± 0.4-fold; n=3, p<0.05). There was a modest induction of Caveolin-1 RNA with 10% stretch that did not reach statistical significance (n=3, p=0.08). Immunoblotting demonstrated induction of PAI-1 and Caveolin-1 protein with 37% stretch (Figures 3B and C; n=4–5, p<0.05), and a modest increase in Claudin 7 protein expression with 10% stretch. We characterized Claudin 7 localization to the plasma membrane as a proxy for alveolar epithelial barrier changes since the silastic membranes precluded traditional permeability testing. Claudin 7 was distributed throughout the cytoplasm prior to stretch (Figure 4A) and in unstretched cells (not shown). With static stretch (Figures 4B and C), Claudin 7 immunostaining was more prominent at the plasma membrane.
Figure 1. Apoptosis during static stretch.

Representative immunoblot for activated caspase 3 in type II cells after 24 h of no stretch (NS), 10% or 37% stretch. Staurosporine-treated type II cells served as a positive control. Equal loading was confirmed with GAPDH.
Figure 2. Alveolar type II cell markers during with static stretch.
(A) Graphic representation of real time RT-PCR results from 5–7 experiments for SP-B (open bars) and PGC (shaded bars) mRNA at 10% and 37% static stretch for 24 h, normalized to 18S rRNA (*p<0.01 vs. no stretch). (B) Immunoblot densitometry, normalized to GAPDH, and (C) representative immunoblot.
Figure 3. Alveolar type I cell markers during with static stretch.
(A) Graphic representation of real time RT-PCR results from 3–4 experiments for Claudin 7 (open bars), PAI-1 (light bars), and Caveolin-1 (dark bars) at both 10 and 37% stretch for 24h, normalized to 18S rRNA (*p<0.05 vs. no stretch). (B) Immunoblot densitometry, normalized to GAPDH (*p<0.05 vs. no stretch), and (C) representative immunoblot.
Figure 4. Claudin 7 localization in response to static stretch.
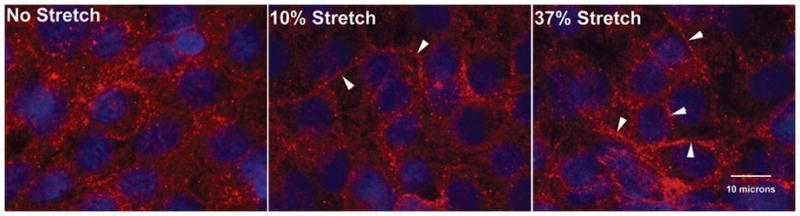
Representative images were taken at 60X of Claudin 7 immunostaining (red; DAPI staining of nuclei, blue) in cells after 24 h of no stretch (A), 10% (B), or 37% (C) stretch. Plasma membrane distribution of Claudin 7 is shown (arrowheads; n=2 experiments, duplicate slides, 4 fields each).
Rho-GTP function in response to static stretch
Stress fibers are longitudinal bundles of contractile actin-myosin filaments resulting from activation of the Rho-GTP/Rho kinase pathway (23). Stress fibers (as shown in Figure 5A) are present in most cultured cells, but are markedly increased by stretch (24). There was a 27% increase in the mean intensity of phalloidin-positive stress fibers per cell at 37% stretch compared to unstretched controls (Figure 5B; n=3, p<0.05), whereas no significant change was observed at 10% stretch. Because stress fibers are a late endpoint in Rho pathway activation, we measured direct Rho/Rho kinase pathway activation by ELISA in response to 37% stretch (Figure 6). Activated Rho-GTP increased by 21.4 ±1.5% 15 minutes after initiation of 37% stretch (n=3–4, p<0.01), followed by a decrease at 1 and 4 h (at 4 h: 46.7 ± 5.4%, n=4, p<0.01), with variable rebound to baseline by 24 h.
Figure 5. Stress fiber formation in response to static stretch.
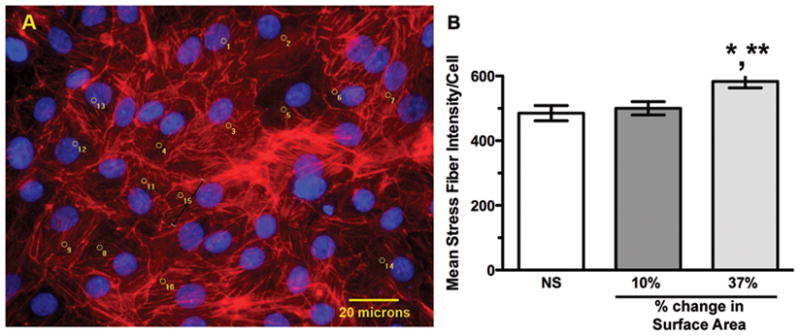
(A) Representative image taken at 20X of cells after 24 h of 37% stretch. Phalloidin-stained stress fibers are red, DAPI-stained nuclei are blue. Image analysis software markings on counted cells appear in yellow, and the black line (cell 15) illustrates a representative stress fiber fluorescent signal density measurement. (B) Graphic representation of mean fluorescent stress fiber intensity per cell (n=3 experiments, *p<0.05 vs. NS, **p<0.05 vs. 10%).
Figure 6. Rho activation in response to static stretch.
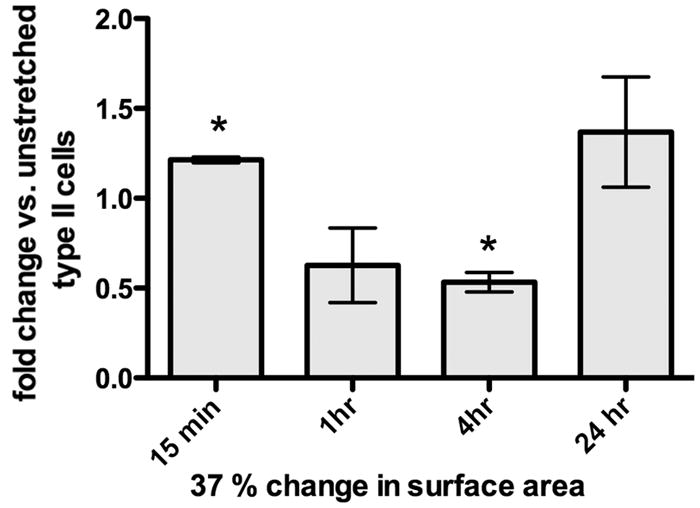
Time course of Rho-GTP by G-Lisa after initiation of 37% static stretch (n= 3–4 experiments, *p<0.01).
Inhibition of Rho diminishes stretch phenotype changes
To determine whether the stretch induced changes in gene expression could be due to activation of Rho, we used a selective Rho inhibitor, Y27632, during epithelial cell stretch (Figure 7). Y27632 partially restored SP-B RNA expression at 10% stretch but not at 37% stretch (n=4, p<0.05 vs. 10% stretch without inhibitor). By comparison, expression of PAI-1 was blunted at both 10% and 37% stretch in the presence of Y27632 (n=4–5, p<0.05 vs. no inhibitor).
Figure 7. Alveolar epithelial marker expression in response to ROCK inhibition during stretch.

Graphic representation of real time RT-PCR results from 4–5 experiments for (A) SP-B and (B) PAI1 mRNA after 24h of 37% static stretch in the presence or absence of the ROCK inhibitor Y27632, normalized to 18S rRNA (*p<0.05). Cells were pre-treated with Y27632 for 1 h prior to initiating stretch.
Discussion
Mechanical forces are important regulators of organogenesis and differentiation during fetal development. Although reduced stretch results in lung hypoplasia while overdistention stimulates lung growth (3), the effects of stretch on differentiation have been less clear. Animal models suggest that static stretch promotes type I cell phenotype (13), and cyclic stretch promotes type II cell phenotype (25). More recent studies of animal models of congenital diaphragmatic hernia (CDH) and post-mortem human studies suggest that despite improved lung growth, supraphysiologic stretch in utero does not result in a mature alveolar epithelium with an appropriate mixed population of type I and type II cells (26, 27). This is the first study to assess the impact of supraphysiologic stretch on alveolar epithelial cell differentiation using a well-characterized model of type II cells derived from human fetal lung in which both the local effects of static stretch and alterations in intracellular pathways can be easily monitored.
Mechanistic studies of stretch-induced epithelial differentiation have been hampered by controversy surrounding alveolar epithelial cell lineage and alveolar epithelial marker expression. The classic dogma—alveolar type II cells as the progenitor for terminally differentiated alveolar type I cells—came from interpretations of older studies using electron microscopy to understand the resolution of lung injury (28). These early studies were supported by later evidence that type II cells served as progenitors for injured type I cells in mature lung (29), and that isolated type II cells in culture for over 24 h to lose characteristics of type II cells and adopt features of type I cells in the absence of serum (30). Another obstacle to understanding the impact of stretch on differentiation is the paucity of markers that clearly differentiate type I from type II cells across species. Markers such as T1α (31) and RTI40 (32), now recognized as podoplanin, have not been useful in human lung. Markers that clearly distinguish type I cells from type II cells, such as Caveolin 1, are also found in other cells locally in the lung (33). Antibodies to markers in rodent models have poor cross-reactivity to human cells. The present study attempts to solve these problems by using a relevant human model and choosing markers that have been reproducible in this and other human models. Our well-characterized cell culture model has been used to examine the cell biology and biochemistry of alveolar type 1 and 2 cells (20, 34–36). Cells are derived from human fetal lung making this model particularly relevant to lung development. The primary cultures have been consistently ~90% pure, with contamination chiefly from fibroblasts. However, the use of a human cell culture model restricts the choice of available type I cell markers. We have previously shown that PAI-1, Caveolin 1, and Claudin 7 are robust, reproducible markers of type I cells in this system (35). While not exclusively expressed by type I cells in the lung, they are clearly not expressed by human type 2 cells or by contaminating fibroblasts. Therefore, our choice of cell culture system and markers provide a robust model to study the effects of stretch on human fetal alveolar epithelial cell phenotype.
Equibiaxial stretch of type II cells had very predictable effects on the epithelial cell markers. We demonstrated a decline in SP-B and PGC RNA within 24 h of initiating both 10% and 37% stretch, concomitant with an increase in PAI-1 and Caveolin 1 RNA. Compared to our prior report of epithelial cell marker expression in transdifferentiation experiments (35), the decline in type II cell markers with stretch despite the presence of hormones was blunted yet significant. In the prior study, DCI withdrawal effectively eliminated SP-B and PGC RNA expression by 96h, with persistence of some SP-B protein through 120h and PGC protein through 96h. Persistence of type II cell proteins after cessation of RNA expression has been commonly reported in rodent models of alveolar epithelial cell transdifferentiation (37, 38). Importantly, despite culture conditions that should sustain type II cell marker expression, specifically the presence of glucocorticoid and cAMP, stretch significantly impaired SP-B and PGC expression.
Few studies of the effects of stretch on alveolar epithelial cell differentiation have examined type I cell markers (32). In the present study, type I cell markers behaved as predicted, increasing in response to 24h of static stretch as type II marker expression waned. The magnitude of changes in type I markers was less than we observed by transdifferentiation previously. Claudin 7 was helpful in the present study because of its type I cell specificity specific in human fetal lung (34, 35), and its role in alveolar epithelial barrier function (22). While Claudin 7 behaved similarly to our prior study, demonstrating only a modest induction of RNA and no significant change in protein content over 24h, it was remarkable that localization of Claudin 7 became directed to the plasma membrane with stretch. Studies of rat type I cell transdifferentiation also observed an increase in Claudin 7 that was maximal after 7d in culture, with a more plasma membrane distribution in type I cells that correlated with increased barrier function (39). The silastic membranes we used for the static stretch in our system precluded a more functional approach to assessing barrier function. However, redistribution of Claudin 7 is indirect but consistent evidence of enhanced barrier function with stretch. Taken together, changes in type I and II cell marker expression clearly show that despite culture conditions that support maintenance of type II cell phenotype, stretch fosters change toward a type I cell phenotype in human fetal lung epithelial cells.
Several signal transduction pathways have been implicated in stretch-mediated lung maturation, including extracellular signal related kinases (25), Protein Kinase A and C (2), heparin-binding epidermal growth-like factor and transforming growth factor alpha (40). The Rho/Rho kinase (ROCK) pathway is an excellent candidate for transmitting alveolar epithelial cell stretch into gene expression due to its pivotal role in regulating the actin cytoskeleton, and in mechanotransduction-mediated differentiation, most notably in smooth muscle cells (41). We provide compelling evidence that Rho pathway activation plays an important role in static stretch-induced differentiation of human fetal lung epithelium: indirectly by the increase in stress fibers with static stretch, and directly by detecting GTP-bound Rho within 15 minutes of applying static stretch. More importantly, stretch-induced changes in epithelial cell marker expression were blunted in the presence of a specific Rho inhibitor, Y27632. Our data echo similar observations in rodent type II cells, with the ROCK inhibitor H-1152 blunting the effect of cyclic stretch on expression of the type II cell marker SP-C (19). Like their studies, our data show that Rho A is a negative regulator of type II cell markers, especially at reduced levels of stretch—5% in their study, and 10% in ours. What is more impressive is that Rho A is a positive regulator of type I cell markers at both levels of stretch in the present study, placing the Rho pathway as a central regulator of alveolar epithelial differentiation in response to stretch. The differences in response between type I and type II markers, and between 10% and 37% stretch may reflect events downstream of Rho A. Y27632 is a ROCK inhibitor which will account for only one limb of the pathway after Rho A activation leading to inhibition of cofilin and promoting actin polymerization (24). Because an intact cytoskeleton is a critical factor in mediating mechanotransduction, the role of profilin actions could be important but are not regulated by ROCK and thus not susceptible to Y27632. The inability of ROCK inhibition to restore type 2 cell marker expression to unstretched levels illustrates that there are additional mechanotransduction pathways involved, such as the mitogen-activated protein kinase pathway (25). The resultant intermediate type I/II cell phenotype may simply reflect the short duration of these experiments. However, they suggest a reasonable explanation for the presence of such intermediate cells observed by others in response to lung injury as repopulating type 2 cells begin to transdifferentiate (27, 42, 43). While there is increasing evidence of type I to type II cell plasticity (44), it remains unclear whether this is stretch-responsive in vivo and which pathways might be involved.
In summary, we have shown that static stretch is an important determinant of alveolar epithelial cell plasticity, and that mechanotransduction is partially mediated by the Rho pathway. The Rho GTPase pathway may provide an important early indicator of alveolar epithelial cell well-being in studies designed to evaluate lung-protective ventilation strategies, and may offer a targeted pathway for the design of novel pharmacologic interventions, due to the accessibility of the alveolar epithelium, to prevent lung injury during mechanical ventilation.
Acknowledgments
Financial Support: This study was supported by grants HL-077266 [C.D.F.] and HL059959 [S.H.G.] from the National Institutes of Health.
We thank Ping Wang for cell preparation and James Hayden and Frederick Keeney, from the Wistar Institute Microscopy Facility for assistance with the stress fiber imaging and image quantitation studies.
Abbreviations
- DCI
Dexamethasone, isobutyl methylxanthine, and 8-bromo-cAMP
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- PGC
Pepsinogen C
- PAI-1
Plasminogen Activator Inhibitor-1
- ROCK
Rho kinase
- SP-B
Surfactant Protein B
References
- 1.Guttentag SH, Ballard PL. Lung Development: Embryology, Growth, Maturation, and Developmental Biology. In: Taeusch HW, Ballard RA, Gleason CA, editors. Avery’s Diseases of the Newborn. Elsevier Saunders; Philadelphia, PA: 2005. pp. 601–615. [Google Scholar]
- 2.Liu M, Post M. Invited review: mechanochemical signal transduction in the fetal lung. J Appl Physiol. 2000;89:2078–2084. doi: 10.1152/jappl.2000.89.5.2078. [DOI] [PubMed] [Google Scholar]
- 3.Kitterman JA. The effects of mechanical forces on fetal lung growth. Clin Perinatol. 1996;23:727–740. [PubMed] [Google Scholar]
- 4.Harding R, Liggins GC. Changes in thoracic dimensions induced by breathing movements in fetal sheep. Reprod Fertil Dev. 1996;8:117–124. doi: 10.1071/rd9960117. [DOI] [PubMed] [Google Scholar]
- 5.Martin TR. Interactions between mechanical and biological processes in acute lung injury. Proc Am Thorac Soc. 2008;5:291–296. doi: 10.1513/pats.200801-005DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol. 1999;86:2026–2033. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- 7.Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990;162:46–52. doi: 10.1016/0002-9378(90)90818-r. [DOI] [PubMed] [Google Scholar]
- 8.Page DV, Stocker JT. Anomalies associated with pulmonary hypoplasia. Am Rev Respir Dis. 1982;125:216–221. doi: 10.1164/arrd.1982.125.2.216. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa J, Chapin CJ, Sbragia L, Ertsey R, Gutierrez JA, Albanese CT, Kitterman JA. Tracheal occlusion stimulates cell cycle progression and type I cell differentiation in lungs of fetal rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L344–L353. doi: 10.1152/ajplung.00281.2002. [DOI] [PubMed] [Google Scholar]
- 10.Adzick NS, Harrison MR, Glick PL, Villa RL, Finkbeiner W. Experimental pulmonary hypoplasia and oligohydramnios: relative contributions of lung fluid and fetal breathing movements. J Pediatr Surg. 1984;19:658–665. doi: 10.1016/s0022-3468(84)80349-8. [DOI] [PubMed] [Google Scholar]
- 11.Kitterman JA, Chapin CJ, Vanderbilt JN, Porta NF, Scavo LM, Dobbs LG, Ertsey R, Goerke J. Effects of oligohydramnios on lung growth and maturation in the fetal rat. Am J Physiol Lung Cell Mol Physiol. 2002;282:L431–L439. doi: 10.1152/ajplung.00161.2001. [DOI] [PubMed] [Google Scholar]
- 12.Khan PA, Cloutier M, Piedboeuf B. Tracheal occlusion: a review of obstructing fetal lungs to make them grow and mature. Am J Med Genet C Semin Med Genet. 2007;145C:125–138. doi: 10.1002/ajmg.c.30127. [DOI] [PubMed] [Google Scholar]
- 13.Dobbs LG, Gutierrez JA. Mechanical forces modulate alveolar epithelial phenotypic expression. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:261–266. doi: 10.1016/s1095-6433(01)00322-1. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Esteban J, Wang Y, Filardo EJ, Rubin LP, Ingber DE. Integrins beta1, alpha6, and alpha3 contribute to mechanical strain-induced differentiation of fetal lung type II epithelial cells via distinct mechanisms. Am J Physiol Lung Cell Mol Physiol. 2006;290:L343–L350. doi: 10.1152/ajplung.00189.2005. [DOI] [PubMed] [Google Scholar]
- 15.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 16.Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 17.Olivera DS, Boggs SE, Beenhouwer C, Aden J, Knall C. Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro. Inhal Toxicol. 2007;19:13–22. doi: 10.1080/08958370600985768. [DOI] [PubMed] [Google Scholar]
- 18.Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L958–L965. doi: 10.1152/ajplung.90218.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silbert O, Wang Y, Maciejewski BS, Lee HS, Shaw SK, Sanchez-Esteban J. Roles of RhoA and Rac1 on actin remodeling and cell alignment and differentiation in fetal type II epithelial cells exposed to cyclic mechanical stretch. Exp Lung Res. 2008;34:663–680. doi: 10.1080/01902140802339615. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales LW, Angampalli S, Guttentag SH, Beers MF, Feinstein SI, Matlapudi A, Ballard PL. Maintenance of differentiated function of the surfactant system in human fetal lung type II epithelial cells cultured on plastic. Pediatr Pathol Mol Med. 2001;20:387–412. doi: 10.1080/15513810109168622. [DOI] [PubMed] [Google Scholar]
- 21.Foster C, Aktar A, Kopf D, Zhang P, Guttentag S. Pepsinogen C: a type 2 cell-specific protease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L382–L387. doi: 10.1152/ajplung.00310.2003. [DOI] [PubMed] [Google Scholar]
- 22.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 23.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- 24.Hellstrand P, Albinsson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Can J Physiol Pharmacol. 2005;83:869–875. doi: 10.1139/y05-061. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Esteban J, Wang Y, Gruppuso PA, Rubin LP. Mechanical stretch induces fetal type II cell differentiation via an epidermal growth factor receptor-extracellular-regulated protein kinase signaling pathway. Am J Respir Cell Mol Biol. 2004;30:76–83. doi: 10.1165/rcmb.2003-0121OC. [DOI] [PubMed] [Google Scholar]
- 26.Danzer E, Davey MG, Kreiger PA, Ruchelli ED, Johnson MP, Adzick NS, Flake AW, Hedrick HL. Fetal tracheal occlusion for severe congenital diaphragmatic hernia in humans: a morphometric study of lung parenchyma and muscularization of pulmonary arterioles. J Pediatr Surg. 2008;43:1767–1775. doi: 10.1016/j.jpedsurg.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 27.Flecknoe S, Harding R, Maritz G, Hooper SB. Increased lung expansion alters the proportions of type I and type II alveolar epithelial cells in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1180–L1185. doi: 10.1152/ajplung.2000.278.6.L1180. [DOI] [PubMed] [Google Scholar]
- 28.Adamson IY, Bowden DH. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest. 1975;32:736–745. [PubMed] [Google Scholar]
- 29.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 30.Borok Z, Hami A, Danto SI, Zabski SM, Crandall ED. Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am J Respir Cell Mol Biol. 1995;12:50–55. doi: 10.1165/ajrcmb.12.1.7811470. [DOI] [PubMed] [Google Scholar]
- 31.Williams MC, Cao Y, Hinds A, Rishi AK, Wetterwald A. T1a protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus, and ciliary epithelia of adult rats. Am J Respir Cell Mol Biol. 1996;14:577–585. doi: 10.1165/ajrcmb.14.6.8652186. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez JA, Gonzalez RF, Dobbs LG. Mechanical distension modulates pulmonary alveolar epithelial phenotypic expression in vitro. Am J Physiol. 1998;274:L196–L202. doi: 10.1152/ajplung.1998.274.2.L196. [DOI] [PubMed] [Google Scholar]
- 33.Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- 34.Daugherty BL, Mateescu M, Patel AS, Wade K, Kimura S, Gonzales LW, Guttentag S, Ballard PL, Koval M. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1266–L1273. doi: 10.1152/ajplung.00423.2003. [DOI] [PubMed] [Google Scholar]
- 35.Foster CD, Varghese LS, Skalina RB, Gonzales LW, Guttentag SH. In vitro transdifferentiation of human fetal type II cells toward a type I-like cell. Pediatr Res. 2007;61:404–409. doi: 10.1203/pdr.0b013e3180332c6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cyclic AMP. Am J Physiol Lung Cell Mol Physiol. 2002;283:L940–L951. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- 37.Bates SR, Gonzales LW, Tao JQ, Rueckert P, Ballard PL, Fisher AB. Recovery of rat type II cell surfactant components during primary cell culture. Am J Physiol Lung Cell Mol Physiol. 2002;282:L267–L276. doi: 10.1152/ajplung.00227.2001. [DOI] [PubMed] [Google Scholar]
- 38.Dobbs LG, Pian MS, Maglio M, Dumars S, Allen L. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am J Physiol. 1997;273:L347–L354. doi: 10.1152/ajplung.1997.273.2.L347. [DOI] [PubMed] [Google Scholar]
- 39.Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, Ann DK, Crandall ED, Borok Z. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol. 2005;98:322–328. doi: 10.1152/japplphysiol.00681.2004. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Maciejewski BS, Soto-Reyes D, Lee HS, Warburton D, Sanchez-Esteban J. Mechanical stretch promotes fetal type II epithelial cell differentiation via shedding of HB-EGF and TGF-alpha. J Physiol. 2009;587:1739–1753. doi: 10.1113/jphysiol.2008.163899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 42.Clegg GR, Tyrrell C, McKechnie SR, Beers MF, Harrison D, McElroy MC. Coexpression of RTI40 with alveolar epithelial type II cell proteins in lungs following injury: identification of alveolar intermediate cell types. Am J Physiol Lung Cell Mol Physiol. 2005;289:L382–L390. doi: 10.1152/ajplung.00476.2004. [DOI] [PubMed] [Google Scholar]
- 43.Olsen CO, Isakson BE, Seedorf GJ, Lubman RL, Boitano S. Extracellular matrix-driven alveolar epithelial cell differentiation in vitro. Exp Lung Res. 2005;31:461–482. doi: 10.1080/01902140590918830. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1045–L1055. doi: 10.1152/ajplung.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]