Abstract
Cerebral edema is a devastating consequence of brain injury leading to cerebral blood flow compromise and worsening parenchyma damage. In the present study, we investigated the effects of arginine-vasopressin (AVP) V1a receptor inhibition following an intracerebral hemorrhagic (ICH) brain injury in mice and closely assessed the role it played in cerebral edema formation, neurobehavioral functioning, and blood-brain-barrier (BBB) disruption. To support our investigation, SR49059, an AVP V1a receptor competitive antagonist, and NC1900, an arginine-vasopressin analogue, were used. Male CD1 mice (n=205) were randomly assigned to the following groups: naïve, sham, ICH, ICH with SR49059 at 0.5 mg/kg, ICH with SR49059 at 2 mg/kg, ICH with NC1900 at 1 ng/kg, ICH with NC1900 at 10 ng/kg, and ICH with a combination of SR49059 at 2 mg/kg and NC1900 at 10 ng/kg. ICH was induced by using the collagenase injection model and treatment was given 1hr after surgery. Post assessment was conducted at 6, 12, 24, and 72hrs after surgery and included brain water content, neurobehavioral testing, Evans Blue assay, western blotting, and hemoglobin assay. The study found that inhibition of the AVP V1a receptor significantly reduced cerebral edema at 24 and 72hrs post-ICH injury and improved neurobehavioral function while reducing BBB disruption at 72 hours. Western blot analysis demonstrated increased protein expression of aquaporin 4 (AQP4) in vehicle, which was reduced with AVP V1a receptor inhibition. Our study suggests that blockage of the AVP V1a receptor, is a promising treatment target for improving ICH-induced brain injury. Further studies will be needed to confirm this relationship and determine future clinical direction.
Keywords: Arginine-Vasopressin (AVP), AVP V1a receptor, SR49059, Intracerebral hemorrhage (ICH), Aquaporin-4 (AQP4), Collagenase model
1.0 INTRODUCTON
Perihematomal cerebral edema is a serious life threatening consequence following an intracerebral hemorrhagic (ICH) brain injury (Xi et al., 1998). Two types of edema can present itself after such an event - namely vasogenic and cytotoxic edema. Vasogenic edema occurs predominately as a result of BBB disruption whereas, cytotoxic edema refers to the intracellular accumulation of water. Today, despite promising basic science research aimed at reducing and/or eliminating cerebral edema, bench work has not been able to translate into clinical improvements. This is partly due to the lack of clear understanding behind the pathophysiology of edema evolution.
Arginine vasopressin (AVP) is an antidiuretic non-peptide hormone responsible for regulating water and electrolyte homeostasis in the body (Shuaib et al., 2002). It is produced in the hypothalamus and released by the posterior pituitary into the blood stream, initiating a wide array of complex actions in the body. There is increasing evidence that AVP can be released directly into the brain by an alternate pathway, acting as a neurotransmitter regulating water permeability, ion homeostasis, and cerebrospinal fluid production (Vakili et al., 2005). Studies across a wide spectrum of brain injury paradigms including subarachnoid hemorrhage (Doczi et al., 1984), traumatic brain injury (Trabold et al., 2008), ischemic stroke (Liu et al, 2000), and global cerebral ischemia (Molnar et al., 2008) have speculated AVPs key role in brain edema formation. In an acute ischemic stroke model in gerbils, cerebral edema was exacerbated by intracerebroventricular administration of AVP while reduced by AVP antiserum injection (Liu et al., 1996). While in a middle cerebral artery occlusion model, AVP knock-out rats showed a reduction in cerebral edema four hours after injury (Dickinson et al., 1992). However, even with previously published work acknowledging the role of AVP in brain edema formation, the exact mechanism has not been elucidated.
One possible mechanism that has been speculated is the role of brain aquaporins (AQP) in brain water homeostasis. Specifically AQP-4, the most abundant water channel in the brain, has been implicated in a number of brain injury-induced cerebral edema cases (Liu et al., 2009). Located in the blood-brain interface, AQP-4 is an integral membrane protein that regulates the flow of water in and out of the membrane (Yukutake et al., 2009). Because there is an interaction between AVP and AQP in the kidneys where body water homeostasis is regulated, it begs the question if there is an interaction between these two proteins in the brain.
In the present study, we investigated the role of AVP V1a receptor inhibition and its effects on cerebral edema, neurobehavioral functioning, and BBB disruption. We hypothesize that AVP activation is responsible for cerebral edema accumulation after injury and may do so, through opening of AQP4 channels. In order to test our hypothesis, two pharmacologic interventions were made to manipulate the AVP V1a receptor – SR49059, a V1a receptor competitive antagonist and NC1900, an AVP analog. Both drugs were selected because of their high specificity to the V1a receptor and marked affinity, selectivity, and efficacy toward both animal and human receptors (Serradeil-Le Gal et al., 1993).
2.0 EXPERIMENTAL PROCEDURES
2.1 Animal Groups
This study was in accordance with the guidelines of the National Institute of Health for the treatment of animals and was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Male CD1 mice (weight 35–45 grams, Charles River, MA, USA) were housed in a 12-hour light/dark cycle at a controlled temperature and humidity with free access to food and water. Mice were divided into the following groups: sham (n=31), ICH (n=48), ICH treated with low-dose antagonist (SR49059 at 0.5 mg/kg; n=6), ICH treated with a high-dose antagonist (SR49059 at 2 mg/kg; n=50), ICH treated with NC1900 at 1 ng/kg (n=6), ICH treated with NC1900 at 10 ng/kg (n=30), and ICH treated with a combination SR49059 at 2 mg/kg and NC1900 at 10 ng/kg (n=24).
In order to test the potential systemic effects of SR49059, ten additional mice were used to evaluate blood pressure and blood gas analysis (5 naïve and 5 naïve animals treated with high-dose SR49059).
2.2 Operative Procedure
The collagenase-induced intracerebral hemorrhage model (Rosenberg et al., 1990) was adapted as previously described in mice (Choudhri et al., 1997). Briefly, mice were anesthetized with a ketamine (100 mg/kg)/xylazine (10 mg/kg) combination intraperitoneal injection and positioned prone in a stereotaxic head frame (Stoelting, Wood Dale, IL, USA). An electronic thermostat-controlled warming blanket was used to maintain core temperature at 37° C. The calvarium was exposed by a midline scalp incision from the nose to the superior nuchal line, and the skin was retracted laterally. With a variable speed drill (Fine Scientific Tools, Foster City, CA, USA) a 1.0 mm burr hole was made 0.9 mm posterior to the bregma and 1.45 mm right-lateral to the midline. A 26-G needle on a Hamilton syringe was inserted with stereotaxic guidance 4.0 mm into the right deep cortex/basal ganglia at a rate 1 mm/min. Collagenase (0.075 units in 0.5 μl saline; Sigma, St Louis, MO, USA) was then infused into the brain at a rate of 0.25 μl/min over 2 minutes using an automatic infusion pump (Stoelting, Wood Dale, IL, USA). The needle was left in place for an additional 10 minutes after injection to prevent the possible leakage of collagenase solution. After removal of the needle, the incision was sutured closed and mice were allowed to recover. Sham operation was performed with needle insertion only.
In order to effectively measure potential systemic effects of our drugs, blood pressure and blood gas analysis were conducted on 10 separate mice. Animals were anesthetized first by ketamine (100 mg/kg IP) and xylazine (10 mg/kg IP). The left femoral artery was then cannulated for blood pressure recordings and withdrawal of blood samples. A blood pressure analyzer (Digi-Med BPA-100, Micro Med Inc. Louisville, USA) was used to measure blood pressure while the blood gas analysis was conducted using the GEM4000 premier (Instrumentation Laboratory Bedford USA). Baseline pO2 and pCO2 values were evaluated immediately after induction of anesthesia. Afterwards, SR49059 or vehicle was injected as described in the ‘treatment method’ section. The effects of the drug were evaluated at 15, 30 and 60 minute intervals following administration.
2.3 Treatment Method
SR49059 (Tocris Bioscience, Ellisville, Missouri) was dissolved in 0.5% DMSO and administered one time intraperitoneally approximately 1 hour after ICH induction. The agonist, NC-1900 was dissolved in saline and administrated as a single dose subcutaneously 1 hour after ICH.
2.4 Brain Water Content
Brain water content was measured as previously described (Yang et al., 1994). Briefly, animals were sacrificed at 6hrs, 12hrs, 24hrs and 72hrs post ICH and brains were immediately removed and divided into five parts: ipsilateral and contralateral basal ganglia, ipsilateral and contralateral cortex, and cerebellum. The cerebellum was used as an internal control for brain water content. Tissue samples were then weighed on an electronic analytical balance (APX-60, Denver Instrument; Arvada, CO) to the nearest 0.1 mg to obtain the wet weight (WW). The tissue was then dried at 100°C for 48 hours to determine the dry weight (DW). The percent brain water content was calculated as [(WW - DW)/WW] × 100.
2.5 Assessment of Neurobehavioral Deficits
Neurological outcomes were assessed by a blind observer at 6hrs, 12hrs, 24hrs and 72hrs post ICH using the Modified Garcia Score (Garcia, J. H. et al. 1995). The Modified Garcia Score is an 21-point sensorimotor assessment system consisting of seven tests with scores of 0–3 for each test (max score = 21). These seven tests included: (i) spontaneous activity, (ii) side stroking, (iii) vibris touch, (iv) limb symmetry, (v) climbing (vi) lateral turning, and (vii) forelimb walking.
In the beam balance test, animals were placed at the middle of a beam, 590 cm in length by 51cm in width, and positioned between two platforms horizontally. The ability of the animals to reach one of the platforms was recorded. Animals who reached one of the two platforms within 25 second were given a 5 point rating. Animals that moved on the platform between 25 and 40 seconds were given a 4 point rating. Animals that moved more than half the distance to the platform and stayed on the beam for at least 25 seconds were given a 3 point rating. Animals that stayed on the beam at least 25 second but moved less than half a distance to the platform were given a 2 point rating. Animals that were able to stay on the beam for at least 40 seconds without movement were given a 1point rating while those who fell off before 40 seconds got a 0 rating.
Wire hanging test was carried out similarly to what was described previously (Gerlai et al., 2000). Briefly mice were placed on a horizontal wire between two platforms. The animals were given an opportunity to grasp the wire with both forepaws before the blinded tester let go. Animals that were able to reach one of the platforms in 45 second or less were given a 5 point rating. Those animals that were able to hang on for more than 30 second and use more than their forelimbs (hind limps or tail) but did not reach the platform in less than 45 second were given a 4 point rating. Animals that were able to hang on for more than 30 second and use their forelimbs in a symmetrical manner for at least 10 second were given a 3 point rating. Mice that were able to hang on for more than 30 seconds in any manner were given a 2 point rating. Finally, animals that were able to hang on between 15–30 seconds were given a 1 point rating while those animals that fell off the wire within 15 seconds were given a 0 point rating.
The tests were repeated three times, and an average score was taken.
2.6 Western Blotting of Aquaporin-4
Animals were perfused with cold 0.1M PBS at 72hrs post ICH. The peri-hematomal (right cortex) was isolated and snap-frozen in liquid nitrogen for analysis. Individual protein samples (50 ug each) were subjected to electrophoresis and then transferred to a nitrocellulose membrane for 80 minutes at 70 V (Bio-Rad). Blotting membranes were incubated for 2 hours with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 and then incubated overnight at 4°C with primary antibody (anti-aquaporin) (1:200; Santa Cruz Biotechnology, Santa Cruz, Calif). The membranes were incubated for 1 hour with secondary antibodies (1:1000; Santa Cruz Biotechnology) and processed with an enhanced chemiluminescent reagent kit (Amersham Bioscience, Arlington Heights, Ill) on X-ray film (Kodak, Rochester, NY).
2.7 Evans Blue Assay
BBB permeability was measured as previously reported (Saria and Lundberg, 1983). Under general anesthesia Evans Blue dye (2%; 4 mL/kg) was injected intravenously into the jugular vein and allowed to circulate for one hour. This was followed by perfusion with PBS (40 mL) via the aorta. The brains were subsequently removed and divided into right and left hemispheres frozen in liquid nitrogen and stored at 80 C. Brain samples were homogenized in 600 μL of PBS and centrifuged (30 min, 15000 rcf, 4°C). The supernatant was collected and equal amounts of 50% trichloroacetic acid was added followed by another centrifuge (30 min, 15000 rcf, 4°C). The amount of Evans Blue dye was measured by the spectrophotometer (Spectronix 3000, Milton-Roy, Rochester, NY, USA) and quantified according to a standard curve.
2.8 Hemoglobin Assay
Initially, a standard curve was obtained using a “virtual” model of hemorrhage. Hemispheric brain tissue was obtained from mice subjected to complete transcardial perfusion to remove intravascular blood. Incremental volumes of homologous blood (0, 2, 4, 8, 16, 32μl) were added to each brain tissue sample with phosphate buffered saline (PBS) to reach a total volume of 1000 ml, followed by homogenization for 30 sec, sonication on ice for 1 min, and centrifugation at 15,000 rpm for 30 min. Drabkin’s reagent (0.8 ml, Sigma) was added to 0.2 ml supernatant aliquots and allowed to stand for 15 min at room temperature. Optical density was measured and recorded at 540 nm with a spectrophotometer (Spectronix 3000, Milton-Roy, Rochester, NY, USA). These procedures yielded a linear relationship between measured hemoglobin concentrations in the perfused brain and the volume of added blood.
For hematoma evaluation brain and supernatant was collected as described above. Similar to the procedure used for standard curve generation, Drabkin’s reagent (0.8 ml, Sigma) was added to 0.2 ml supernatant aliquots and allowed to stand for 15 min at room temperature. Optical density was measured and recorded at 540 nm with a spectrophotometer (Spectronix 3000, Milton-Roy, Rochester, NY, USA).
2.9 Statistical Analysis
Quantitative data was expressed as the mean ± SEM. One-way ANOVA and Tukey test were used to determine significance in differences between the means. Neurological scores were evaluated using the Dunn Method. A p-value < 0.05 was considered statistically significant.
3.0 RESULTS
3.1 AVP V1a receptor inhibition has no affect on blood pressure and blood gas analysis
In order to properly evaluate the potential systemic effects of V1a receptor antagonization, both blood pressure and blood gas analysis were performed. The results showed that there were no systemic effects as a result of drug administration between naïve and naïve-treated mice (data not shown).
3.2 AVP V1a receptor inhibition decreases cerebral edema after ICH injury
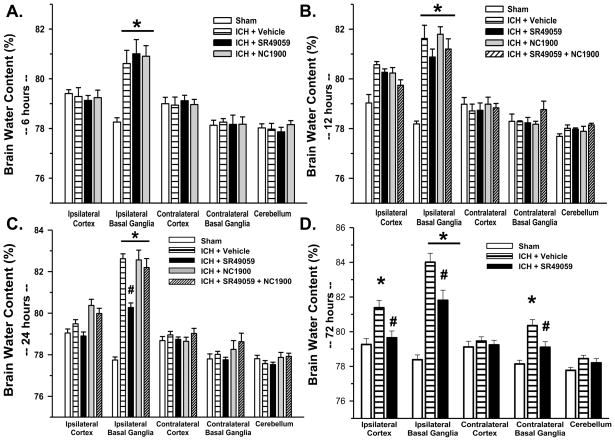
Vehicle groups demonstrated a consistently elevated level of cerebral edema in the ipsilateral basal ganglia (at 24 hours 82.62+/−0.24% (Figure 1C); at 72 hours 84.01 +/− 0.52% (Figure 1D)) compared to the sham-operated animals (at 24 hours 77.75 +/− 0.15%: at 72 hours 78.23+/−0.25%). Inhibition of the AVP V1a receptor using high-dose SR49059 was able to significantly reduce the cerebral edema at 24 (80.27+/−0.22%) and 72hrs post injury (81.81+/−0.58%) (p<0.05). At 12hrs, SR49059 showed a trend towards a reduction in cerebral edema, however it did not reach statistical significance (p-value 0.595) (Figure 1B). We did not observe some effect of treatment at 6 hours (Figure 1A). Low dose SR49059 did not reduce cerebral edema at any time point (data not shown).
Figure 1. Brain Water Content.
Brain water content increased significantly in the ipsilateral basal ganglia at 6, 12, 24 and 72hrs post ICH injury. This increase was attenuated by high dose SR49059 treatment at 24 and 72hrs post ICH injury. * Significant Difference vs. Sham (p<0.05); # Significant Difference vs. Vehicle (p<0.05);
(A) sham=6; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=6; (ICH+ NC1900 [10 ng/kg])=6
(B) sham=6; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=6; (ICH+ NC1900[10 ng/kg])=6; (ICH+SR49059[2 mg/kg]+NC1900 [10 ng/kg])=6
(C) sham=7; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=7; (ICH+ NC1900[10 ng/kg])=6: (ICH+SR49059 [2 mg/kg]+NC1900 [10 ng/kg])=6
(D) sham=6; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=7
3.3 Cerebral edema accumulation is mediated by AVP V1a receptor activation
At 24hrs post-injury, ICH-induced mice treated with high-dose NC1900 had a significantly larger amount of cerebral edema (82.56+/−0.47% at 24 hours) as compared to SR49059 treatment alone. Giving in combination with SR49059, NC 1900 reverses the edema lowering effects of SR49059 (82.20+/−0.43) (Figure 1C). Low dose NC1900 had no effect on cerebral edema (data not shown).
3.4 AVP V1a receptor inhibition improves neurobehavioral deficits
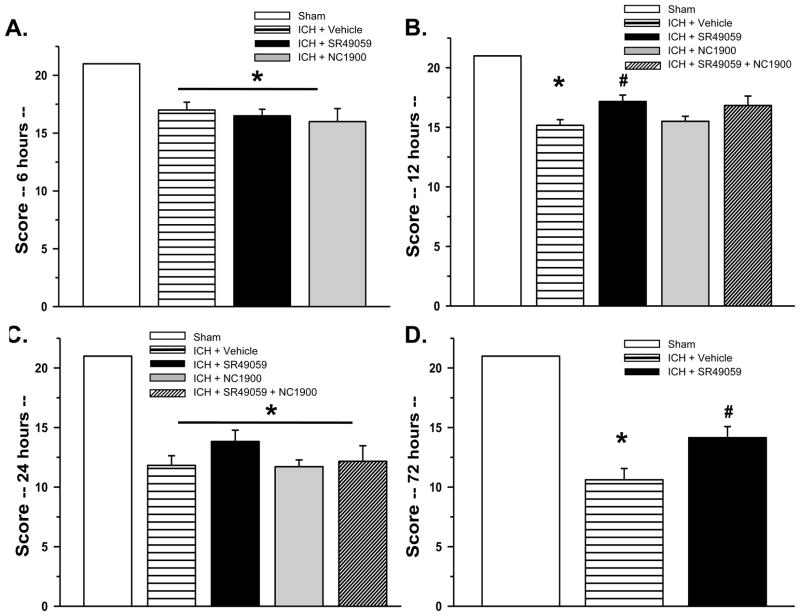
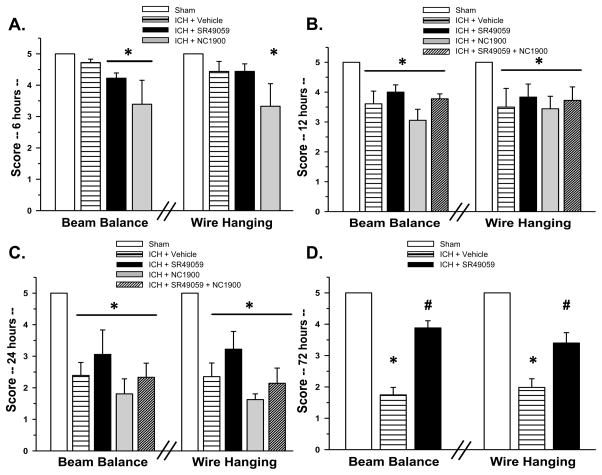
Neurological deficits were present in all animals after collagenase injection. High-dose SR49059 significantly improved behavior at 12 and 72hrs post injury according to the Modified Garcia test (Figure 2B and 2D) and at 72hrs with both the beam balance and wire hanging test (Figure 3D). There was no improvement seen with low-dose SR49059.
Figure 2. Modified Garcia Behavioral Test.
Neurobehavioral deficits were present in all vehicle compared to sham animals. With high dose SR49059 treatment, there was a significant improvement in scores at 12 and 72 hours post ICH. * Significant Difference vs. Sham (p<0.05); # Significant Difference vs. Vehicle (p<0.05); (A) sham=6; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=6; (ICH+ NC1900 [10 ng/kg])=6
(B) sham=6; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=6; (ICH+ NC1900 [10 ng/kg])=6; (ICH+SR49059 [2 mg/kg]+ NC1900 [10 ng/kg])=6
(C) sham=7; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=7; (ICH+ NC1900 [10 ng/kg])=6: (ICH+SR49059 [2 mg/kg]+ NC1900 [10 ng/kg])=6
(D) sham=6; (vehicle)=21; (ICH+SR49059 [2 mg/kg])=21
Figure 3. Beam Balance and Wire Hanging Tests.
There were significant differences between the sham and vehicles groups with beam balance and wire hanging testing at 72 hours. * Significant Difference vs. Sham (p<0.05); # Significant Difference vs. Vehicle (p<0.05);
(A) sham=6; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=6; (ICH+ NC1900 [10 ng/kg])=6
(B) sham=6; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=6; (ICH+ NC1900 [10 ng/kg])=6; (ICH+SR49059 [2 mg/kg]+ NC1900 [10 ng/kg])=6
(C) sham=7; (vehicle)=6; (ICH+SR49059 [2 mg/kg])=7; (ICH+ NC1900 [10 ng/kg])=6: (ICH+SR49059 [2 mg/kg]+ NC1900 [10 ng/kg])=6
(D) sham=6; (vehicle)=21; (ICH+SR49059 [2 mg/kg])=21
3.5 High-dose SR49059 decreases BBB disruption
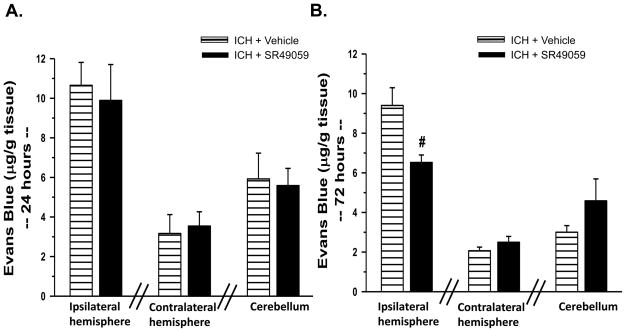
We observed extravasations of Evans Blue dye in the ipsilateral hemisphere of all animal groups subjected to collagenase injection (Figure 4). Specifically at 72hrs (Figure 4B), Evans Blue assay showed a reduction in BBB disruption with high-dose SR49059 (6.52+/−0.38 (μg/g tissue)) treatment compared to vehicle (9.40+/−0.89 (μg/g tissue)). This reduction in BBB disruption did not translate to other time points (Figure 4A).
Figure 4. Evans Blue Assay.
At 72hrs post injury, there was a reduction in Evans Blue staining in the brain tissue with high dose SR49059 [2 mg/kg] treatment compared to vehicle (n=6 for each group). # Significant Difference vs. Vehicle (p<0.05)
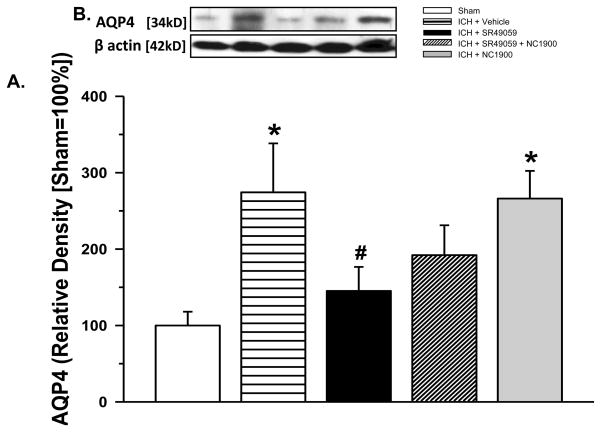
3.6 AVP V1a receptor inhibition modulates AQP4 expression
Western blot measurement of AQP4 protein expression showed a significant increase in the vehicle group (2.74 times compared to the sham), with a reduction seen with high-dose SR49059 treatment (1.45 times compared to the sham). NC1900 administration with SR49059 reversed the beneficial effects of the antagonist (2.66 times compared to the sham) (Figure 5A).
Figure 5. AQP4 Western Blot Protein Expression.
(A) Protein expression of AQP4 increased significantly with ICH brain injury. The increase was attenuated with high dose SR49059 [2 mg/kg] treatment. NC1900 [10 ng/kg], the AVP analogue reversed the protective effects of SR49059. (n=6 for each group)* Significant Difference vs. Sham (p<0.05); # Significant Difference vs. Vehicle (p<0.05); (B) Representative western blot
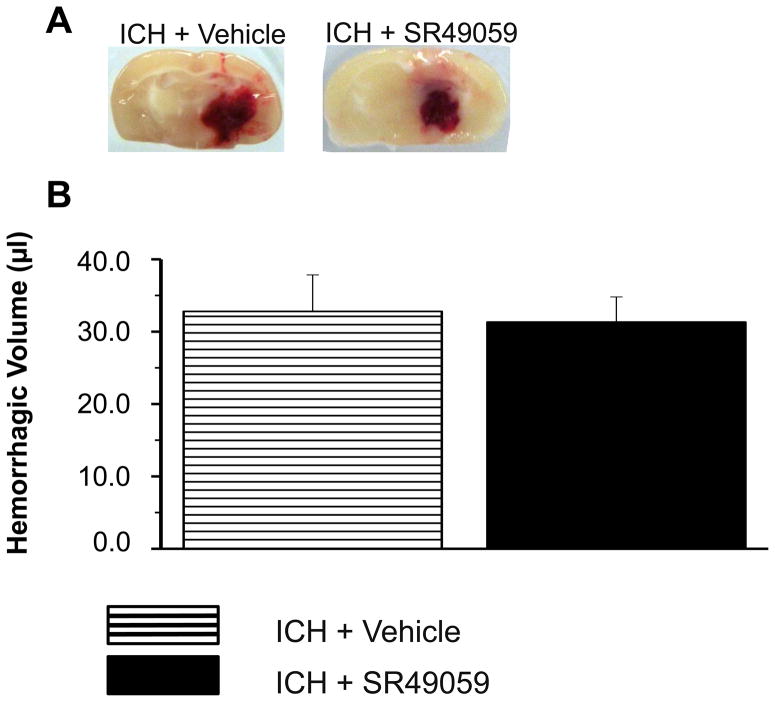
3.7 SR49059 had no effect on hemorrhagic volume
Hemoglobin assay revealed no difference between vehicle and SR49059 treated mice (Figure 6).
Figure 6. Hemorrhagic Volume Assessment Using Hemoglobin Assay.
(A) Representative macrophotograph of hemorrhage
(B) There was no change noted in hemorrhagic volume between vehicle and SR49059 [2 mg/kg] treated mice (n=6 for each group).
4.0 DISCUSSION
The aim of this study was to determine the effects of AVP V1a receptor inhibition on ICH-induced brain injury. By blocking the AVP V1a receptor with high-dose SR49059, three key points were established: First, there was a significant reduction in cerebral edema on direct brain water content measurement and Evans Blue Assay of BBB integrity. This suggests that AVP may play a key role in water homeostasis in the brain. Second, AVP receptor inhibition significantly reduced the neurobehavioral deficits after ICH injury. And third, AQP4 expression was upregulated in the perihematomal tissue after ICH injury and reduced with receptor inhibition - suggesting AVP may be mediated by AQP4 modulation. To the best of our knowledge, this is the first study to elucidate the role of the AVP V1a receptor in ICH-induced brain injured mice.
Brain injury following ICH involves cerebral edema, mass effect of the growing hematoma, and secondary changes such as hemoglobin breakdown products and inflammation. Of those, cerebral edema is one of the most important consequences of brain injury as it can lead to elevated intracranial pressure, subsequent clinical deterioration, and life threatening herniation (Dringer et al., 1993, Adeoye et al., 2010).
Two types of edema can present itself following an ICH – cytotoxic edema and vasogenic edema. Cytotoxic edema, characterized by reversible cell swelling (neurons, glial and endothelial cells) in the brain, is commonly considered an early event that occurs following injury -specifically evolving over minutes to hours (Fishmann et al 1992). Cytotoxicity can lead to ion gradient disruption between intra- and extra-cerebral compartments and impairment of energy metabolisms (Kurita et al., 2003, Schubert et al., 2008). This can accelerate numerous pathophysiological processes including oxygen free radical (ROS) production. Overproduction of ROS leads to disruption of blood-brain barrier and consequentially, to development of vasogenic edema which is considered an irreversible process and believed to be delayed in onset. Accordingly, we suggest that in our study counterplay between cytotoxic and vasogenic edema could explain why by 24hrs we witnessed only a reduction in brain water content, possibly through restoration of AQP-4 regulated water homeostasis, but did not see an improvement in BBB integrity until later i.e. 72hrs.
To date, many studies have attempted to elucidate the role of AVP in maintenance of water and ion homeostasis in the brain. Although no formal mechanism has been determined, countless studies have demonstrated AVPs role in the water and ion homeostasis in the brain through corresponding G-coupled protein receptors. Studies on various brain injury models i.e. TBI, MCAO, have shown AVP injections into the brain to increase brain edema and reduce with receptor blockage. Additionally, studies on mice undergoing a focal cerebral ischemia found that receptor inhibition cerebral edema decreased substantially (Vakili et al., 2005). These studies are in line with our current findings that showed a reduction in BBB permeability with AVP V1a receptor blockage.
The question is how AVP carries out such a process? Various studies over the year have speculated that a relationship exist in the brain between AVP and AQP4. Similar to what we may see in the kidneys, AVP’s regulation of brain water homeostasis may be facilitated through AQP4 channels on the astrocytes lining the BBB. In one study, an upregulation of AQP4 was observed at both the injury and peri-injury sites with permanent middle cerebral artery occlusion (Yang et al., 2009). The same results were found in transient focal cerebral ischemic rat models (Zheng et al., 2008). Other studies have found a reduction in brain edema and improvement in neurobehavioral function with AQP4-null mice exposed to focal cerebral ischemia (Manley et al., 2000). In our study, we found an increase in AQP4 protein expression after ICH injury with a subsequent reduction in expression after AVP V1a receptor inhibition. This suggests that AQP4 channels could be involved with mediating the actions of AVP during injury. Although speculative, AVP binding to the V1a G-protein receptor could activate opening of the AQP4 channel through phosphorylation or other activating mechanisms.
In order to evaluate the effects of AVP V1a receptor antagonization – SR49059 was used. In previous studies, the neuroprotective effect of this drug was described in a rat model of a middle cerebral artery occlusion (Shuaib et al., 2002). The drug concentration used in our study was similar to effective concentration dosages described by the author. Additionally, it was noted by the same study that further increases in drug concentrations had no additional neuroprotective effects – hence it was our decision to use a similar concentration dose (Shuaib et al., 2002). We also chose to use a selective AVP V1a receptor agonist i.e. NC-1900, based on appropriate chemical properties, concentration and a feasible application route as was previously described (Matsuoka et al., 2005; Mishima et al., 2003)
The clinical relevance of this study is profound, considering the lack of proper therapeutic options for ICH. If the potential to reduce one of the most devastating consequences of injury, brain edema, could be achieved, this would lead to a substantial decrease in mortality, not mention chronic motor-sensory disabilities. Our experimental study (i.e., use of a clinically relevant animal model, multiple treatment regimens, and neurobehavioral assessment) provides a foundation for exploring clinical translation. However, we anticipate the need for a dose response study, as well as a more comprehensive evaluation of safety before moving a V1A receptor inhibitor to the clinical setting as treatment against intracerebral hemorrhage induced brain damage.
4.1 Conclusion
We conclude that AVP V1a receptor inhibition ameliorates ICH-induced brain damage in adult male mice and may in fact reduce cerebral edema. We observed that this effect is associated with modulation of AQP4 activity in the brain and which may in fact be the potential mechanism behind its protection. Further studies will be needed to confirm this relationship and determine future clinical direction.
Acknowledgments
This study is partially supported by NIH 5R01NS60936-2 to J.T. and NIH NS53407 to J.H. Z. AVP V1a receptor agonist (NC1900) was kindly provided by Nippon Chemiphar Co., Ltd., Japan. We would also like to thank Suzzanne Marcantonio (the Departments of Anesthesiology, Loma Linda University) for her excellent technical assistance.
ABBREVIATIONS
- ICH
Intracerebral hemorrhage
- BBB
Blood-brain-barrier
- ICU
Intensive care unit
- AVP V1a
Arginine-Vasopressin V1a receptor
- AQP
Aquaporin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Adeoye O, Clark JF, Khatri P, Wagner KR, Zuccarello M, Pyne-Geithman GJ. Do Current Animal Models of Intracerebral Hemorrhage Mirror the Human Pathology? Transl Stroke Res. 2010 doi: 10.1007/s12975-010-0037-1. [DOI] [PubMed] [Google Scholar]
- Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, Peng B, Li Q, Su S, Tao QL, Li YC, Jiang JD, Tai LW, Zhang JL, Xu E, Cheng Y, Morgenstern LB, Chalmers J, Anderson CS. Significance of perihematomal edema in acute intracerebral hemorrhage: the INTERACT trial. Neurology. 2009;23:1963–8. doi: 10.1212/WNL.0b013e3181c55ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch. 2008;456:1005–1024. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Chen TY, Chen CH, Crain BJ, Toung TJ, Bhardwaj A. Plasma arginine-vasopressin following experimental stroke: effect of osmotherapy. J Appl Physiol. 2006;100:1445–1451. doi: 10.1152/japplphysiol.00763.2005. [DOI] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Pinsky D. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- Cintra EA, Araújo S, Quagliato EM, Castro M, Falcão AL, Dragosavac D, Terzi RG. Vasopressin serum levels and disorders of sodium and water balance in patients with severe brain injury. Arq Neuropsiquiatr. 2007;65:1158–1165. doi: 10.1590/s0004-282x2007000700013. [DOI] [PubMed] [Google Scholar]
- Dickinson LD, Betz AL. Attenuated development of ischemic brain edema in vasopressin-deficient rats. JCBM. 1992;12:681–90. doi: 10.1038/jcbfm.1992.93. [DOI] [PubMed] [Google Scholar]
- Diringer MN. Intracerebral hemorrhage: pathophysiology and management. Crit Care Med. 1993;2:1591–1603. doi: 10.1097/00003246-199310000-00032. [DOI] [PubMed] [Google Scholar]
- Fishman RA. Cerebrospinal Fluid in Diseases in the Nervous System. 2. Philadelphia, PA: W.B. Saunders Co; 1992. pp. 103–155. [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation Stroke. 1995;26:627–34. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Thibodeaux H, Palmer JT, Van Lookeren Campagne M, Van Bruggen N. Transient focal cerebral ischemia induces sensorimotor deficits in mice. Behavioural Brain Research. 2002;108:63–71. doi: 10.1016/s0166-4328(99)00130-8. [DOI] [PubMed] [Google Scholar]
- James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 2007;9:139–152. doi: 10.1007/s12028-007-9030-2. [DOI] [PubMed] [Google Scholar]
- Keep RF, XIG, Hua Y, Hoff JT. The deleterious or beneficial effects of different agents in intracerebral hemorrhage: think big, think small, or is hematoma size important? Stroke. 2005;36:1594–1596. doi: 10.1161/01.STR.0000170701.41507.e1. [DOI] [PubMed] [Google Scholar]
- Kurita D, Haida M, Shinohara Y. Energy metabolism and cerebral blood flow during cytotoxic brain edema induced by 6-aminonicotinamide. Acta Neurochir Suppl. 2003;86:41–44. doi: 10.1007/978-3-7091-0651-8_9. [DOI] [PubMed] [Google Scholar]
- Liu X, Jin Y, Zheng H, Chen G, Tan B, Wu B. Arginine vasopressin gene expression in supraoptic nucleus and paraventricular nucleus of hypothalamus following cerebral ischemia and reperfusion. Chin Med Sci J. 1996;15:157–61. [PubMed] [Google Scholar]
- Liu X, Nakayama S, Ottersen OP, Bhardwaj A. Arginine Vasopressin V1 but not V2 receptor antagonism modulates infarct volume, brain water content, and aquaporin-4 expression following experimental stroke. Neurocrit Care. 2000 doi: 10.1007/s12028-009-9277-x. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Sumiyoshi T, Tanaka K, Tsunoda M, Uehara T, Itoh H, Kurachia M. NC-1900, an arginine–vasopressin analogue, ameliorates social behavior deficits and hyperlocomotion in MK-801-treated rats. Brain Research. 2005;1053:131–136. doi: 10.1016/j.brainres.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Mishima K, Tsukikawa H, Miura I, Inada K, Abe K, Matsumoto Y, Egashira N, Iwasaki K, Fujiwara M. Ameliorative effect of NC-1900, a new AVP4–9 analog, through vasopressin V1A receptor on scopolamine-induced impairments of spatial memory in the eight-arm radial maze. Neuropharmacology. 2003;44:541–552. doi: 10.1016/s0028-3908(02)00408-2. [DOI] [PubMed] [Google Scholar]
- Molnar AH, Varga C, Berko A. Prevention of hypoxic brain edema by the administration of vasopressin receptor antagonist OPC31260. Prog Brain Research. 2008;170:519–25. doi: 10.1016/S0079-6123(08)00439-1. [DOI] [PubMed] [Google Scholar]
- Okuno K, Taya K, Marmarou C, Ozisik P, Fazzina G, Kleindienst A, Gulsen S, Marmarou A. The modulation of aquaporin-4 by using PKC-activator (phorbol myristate acetate) and V1a receptor antagonist (SR49059) following middle cerebral artery occlusion/reperfusion in the rat. Acta Neurochir Suppl. 2008;102:431–436. doi: 10.1007/978-3-211-85578-2_84. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Saria A, Lundberg JM. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neuroscience Methods. 1983;8:41–9. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Wang CX, Yang T, Noor R. Effects of nonpeptide V1 vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke. 2002;33:3033–3037. doi: 10.1161/01.str.0000039405.31526.06. [DOI] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–96. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Strbian D, Durukan A, Tatlisumak T. Rodent Models of Hemorrhagic Stroke. Current Pharmaceutical Design. 2008;14:352–358. doi: 10.2174/138161208783497723. [DOI] [PubMed] [Google Scholar]
- Schubert GA, Poli S, Schilling L, Heiland S, Thome C. Hypothermia Reduces Cytotoxic Edema and Metabolic Alterations during the Acute Phase of Massive SAH: A Diffusion-Weighted Imaging and Spectroscopy Study in Rats. J Neurotrauma. 2008;25(7):841–52. doi: 10.1089/neu.2007.0443. [DOI] [PubMed] [Google Scholar]
- Taya K, Salih G, Okuno K, Prieto R, Marmarou C, Marmarou A. Modulation of AQP4 expression by the selective V1a receptor antagonist, SR49059, decreases trauma-induced brain edema. Acta Neurochir Suppl. 2008;102:425–429. doi: 10.1007/978-3-211-85578-2_83. [DOI] [PubMed] [Google Scholar]
- Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH. MMP-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. JCBM. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- Trabold R, Krieg S, Scholler K. Role of vasopressin v1a and v2 receptors for the development of secondary brain damage after traumatic brain injury in mice. J Neurotrauma. 2008;25:1459–65. doi: 10.1089/neu.2008.0597. [DOI] [PubMed] [Google Scholar]
- Vakili A, Hiroharu K, Plesnila N. Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. JCBM. 2005;25:1012–1019. doi: 10.1038/sj.jcbfm.9600097. [DOI] [PubMed] [Google Scholar]
- Westermann I, Dünser MW, Haas T, Jochberger S, Luckner G, Mayr VD, Wenzel V, Stadlbauer KH, Innerhofer P, Morgenthaler N, Hasibeder WR, Voelckel WG. Endogenous vasopressin and copeptin response in multiple trauma patients. Shock. 2007;28:644–649. doi: 10.1097/shk.0b013e3180cab33f. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, Brott TG, Hoff JT. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29(12):2580–6. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- Xu M, Su W, Huang WD, Lu YQ, Xu QP, Chen ZJ. Effect of AVP on brain edema following traumatic brain injury. Chin J Traumatol. 2007;10:90–93. [PubMed] [Google Scholar]
- Yang M, Gao F, Liu H, Yu WH, Sun SQ. Temporal changes in expression of aquaporin -3, -4, -5 and -8 in rat brains after permanent focal cerebral ischemia. Brain Res. 2009;1290:121–132. doi: 10.1016/j.brainres.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- Yukutake Y, Yasui M. Regulation of water permeability through aquaporin-4. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Zausinger S, Hungerhuber E, Baethmann A, Reulen H-J, Schmid-Elsaesser R. Neurological impairment in rats after transient middle cerebral artery occlusion: a comparative study under various treatment paradigms. Brain Research. 2000;863:94–105. doi: 10.1016/s0006-8993(00)02100-4. [DOI] [PubMed] [Google Scholar]
- Zheng YY, Lan YP, Tang HF, Zhu SM. Propofol pretreatment attenuates aquaporin-4 over-expression and alleviates cerebral edema after transient focal brain ischemia reperfusion in rats. Anesth Analg. 2008;107:2009–2016. doi: 10.1213/ane.0b013e318187c313. [DOI] [PubMed] [Google Scholar]