Abstract
Population studies strive to determine the prevalence of Alzheimer dementia but prevalence estimates vary widely. The challenges faced by several noted population studies for Alzheimer dementia in operationalizing current clinical diagnostic criteria for Alzheimer’s disease (AD) are reviewed. Differences in case ascertainment, methodological biases, cultural and educational influences on test performance, inclusion of special populations such as underrepresented minorities and the oldest old, and detection of the earliest symptomatic stages of underlying AD are considered. Classification of Alzheimer dementia may be improved by the incorporation of biomarkers for AD if the sensitivity, specificity, and predictive value of the biomarkers are established and if they are appropriate for epidemiological studies as may occur should a plasma biomarker be developed. Biomarkers for AD also could facilitate studies of the interactions of various forms of neurodegenerative disorders with cerebrovascular disease, resulting in “mixed dementia”.
1. Introduction
In US population studies designed to ascertain the prevalence of Alzheimer’s disease (AD), investigators typically use established diagnostic criteria but may operationalize them in somewhat different ways. The problem is compounded by the fact that diagnostic determinations are often made on the basis of single encounters with the subject (without benefit of follow-up evaluations). What’s more, critical information about some subjects may only be available from informants who may be unreliable. In short, subtle and not-so-subtle differences in the way diagnostic determinations are made may contribute to differences in prevalence estimates between studies.
This article is devoted to some important issues in applying clinical diagnostic criteria to population studies of AD and other cognitive impairments. These relate to brain imaging, special populations, and early diagnosis. With the goal of relating vascular factors to AD, Mayeux, Reitz, and Brickman use data from MRI brain scans to assess the validity of clinical stroke histories. Haan presents the case ascertainment approach for dementia that was used in the Sacramento Area Latino Study on Aging (SALSA). Manly, Glymour, and Weiss focus on cultural and educational considerations as diagnostic issues, for a racially and ethnically diverse population. Yaffe and Middleton consider the challenge of diagnosing dementia in the oldest-old, where the vast majority of them are, to some extent, functionally impaired. Hendrie draws upon his experience in international studies to present a diagnostic process for cross-cultural epidemiologic investigations. Warren, Hayden, Welsh-Bohmer, and Breitner address the problem of identifying early symptomatic or prodromal AD in the population setting. In the Discussion, Morris considers this material and identifies come common themes.
2. Doctors with and without MRIs: The Washington Heights experience
The Washington Heights-Columbia Aging Project (WHICAP) began over 20 years ago as a registry of patients with late-onset neurological disorders. Subsequently, the study evolved into a population-based investigation of AD and related disorders in elderly Medicare recipients residing in northern Manhattan [1,2]. Specifically, the participants recruited and maintained are a representative sample of Medicare recipients living in northern Manhattan in a region extending from 145th Street as its southern boundary and the Hudson, East, and Harlem Rivers as the western, eastern and northern boundaries. Based on 2000 Census data among individuals age 65 years or older in this area, there were 37.5% African-Americans, 43.5% Hispanics, and 19% Whites. The WHICAP cohorts have been a combination of successive waves of recruitment of elderly participants from northern Manhattan. The original cohort was recruited in the years 1992 to 1994 (n=2,125), and a second cohort was recruited between 1999 and 2001 (n=2,174). The population has comprised individuals from several different countries of origin, representing three broadly defined ethnic categories (i.e., Caribbean Hispanic, African-American, and non-Hispanic White). Potential participants were excluded at the time of recruitment if they did not speak English or Spanish. In 1992, approximately 62% of eligible people who were contacted agreed to participate (38% refused); in 1999, participation rate was approximately 40% (60% refused). Prior analyses of the demographic characteristics of the participants recruited in 1992 and 1999 indicate that WHICAP study participants are matched to US Census data of older adults living in northern Manhattan with respect to age, years of education, race/ethnicity, and gender.
Participants have undergone an in-person interview of general health and functional ability at the time of entry into the study followed by a standardized assessment during each follow-up wave, including medical history, physical and neurological examination, and a neuropsychological battery [3]. Ethnic group was classified by participant’s self-report using the format of the 1990 US Census. Each participant is examined at 18 to 24 month intervals thereafter, with repeated interviews and examinations standardized to preserve consistency. Demographic information, including current social situation collected at initial assessment is updated at each follow-up wave. Each person completes a Disability and Functional Limitations Scale that includes items assessing personal self-maintenance activities, perceived difficulty with memory, and mobility. Comprehensive neuropsychological testing includes measures of memory, language, executive functioning, visuospatial skill, and orientation. Neuropsychological data are available as raw or adjusted (age, gender, and ethnic group) tests scores. All participants receive a medical and brief psychiatric evaluation, anthropometric measurement, and an assessment of functional capacity that is independent of neuropsychological test performance. Participants with history of stroke or Parkinson’s disease or those with functional or neuropsychological signs of dementia also receive an evaluation by a neurologist.
2.1. Assessment of stroke and cerebrovascular disease
Self-administered questionnaires are frequently used to obtain information on a previous history of stroke, but the validity of a self-reported stroke remains inconclusive. Stroke is associated with motor impairment but can also be accompanied by impairments in memory, sensation, and speech or language, diminishing the ability of an individual to accurately report a history of stroke. Moreover, stroke and antecedents of cardiovascular and cerebrovascular disease are also considered risk factors for AD. Most previous studies assessing validity of stroke self-report either used neurological examination or review of medical records as the standard or performed brain imaging only on persons reporting to have had a stroke [4,5]. This likely produces underreporting of patients with ambiguous symptoms or silent strokes, and consequently increased false-negatives and diminished sensitivity estimates.
During the 2005–2007 assessment wave, participants in WHICAP who were still alive and not demented at the previous assessment, had no contraindications, and were still residing in the community (1,176) were recruited for imaging (MRI) studies and 769 (65%) participants were ultimately scanned [6]. Based on the concern that the identification of cerebrovascular disease from clinical history alone might have missed lesions, MRI scans were obtained in a large sample of participants.
MRI scan acquisition was performed on a 1.5T Philips Intera scanner at Columbia University Medical Center, and the resulting images were transferred electronically to the University of California at Davis for morphometric analysis in the Imaging of Dementia and Aging Laboratory by Dr. Charles DeCarli. For measures of total brain volume, ventricular volume, and white matter hyperintensities (WMH) volume, fluid attenuated inverse recovery (FLAIR) weighted images (TR=11,000 ms, TE=144.0 ms, 2800 inversion time, FOV 25 cm, 2 nex, 256×192 matrix with 3 mm slice thickness) were acquired in the axial orientation. T1-weighted images acquired in the axial plane and re-sectioned coronally were used to quantify hippocampus and entorhinal cortex volumes (TR=20 ms, TE = 2.1 ms, FOV 240 cm, 256×160 matrix with 1.3 mm slice thickness). The presence or absence of brain infarction on MRI was determined according to previously published protocol from the size, location, and imaging characteristics of the lesion [7,8]. Signal void, best seen on the T2-weighted image, was interpreted to indicate a vessel. Only lesions 3 mm or larger qualifiedpfor consideration as brain infarcts. Other necessary imaging characteristics included cerebrospinal fluid (CSF) density on the subtraction image and distinct separation from the circle of Willis vessels and perivascular spaces if the infarct was in the basal ganglia area. Scans were further interpreted for number of infarcts, their locationp(brain side, cortical or subcortical, and specific region), and size of infarcts (small: <1 cm or large: >1 cm). Previously, kappa values for agreement among raters had been generally good, ranging from 0.73 to 0.90 [7].
Of the 769 participants with structural MRI data, 52 (6.8%) met diagnostic criteria for dementia at the clinical evaluation closest to image acquisition. In the total MRI sample were 484 (62.9%) women, the mean age was 80.1±5.5 years and 22.2% had a history of diabetes, 11.7% a history of myocardial infarction and 66.5% a history of hypertension [9]. Overall 238 persons (30.9%) had a stroke on MRI, out of these, 71 persons (9.2 %) had a large infarct (Fig. 1a) and 198 persons (25.7%) a small infarct (Fig. 1b). All but one participant in WHICAP imaging had measurable WMH. Dividing the total volume of WMH by the total brain volume (×100), approximately one third of the subjects had WMH that comprised at least 2% of the total brain volume (Fig. 1c).
Fig. 1.

In the Washington Heights Study (WHICAP), the MRIs were completed in 769 elderly individuals. The characteristics of the group were: 62.9% women and the mean age was 80.1 ± 5.5 years. Almost 9% had large infarcts (Fig. 1a), 24.2% had small infarcts (Fig. 1b), and ~30% had high density white matter hyperintensities (Fig. 1c).
Individuals with an infarct on MRI but who failed to report a history of stroke were more likely to be women, older, and less likely to be African-American than persons reporting stroke correctly. Using MRI as the “gold standard,” the sensitivity and specificity of stroke by history was low (for stroke in any vascular territory: sensitivity, 32.4%; specificity, 78.9%). The corresponding positive and negative predictive values were 41.5% and 71.6%. Among the 225 persons who had a brain infarct on MRI, 73 (32.4%) reported correctly to have had a stroke, while 152 persons (67.6%) falsely reported to never have had a stroke. When the analyses were stratified by stroke size, sensitivity of stroke self-report was better for large than small strokes (51.5 vs. 28.5%). When the analyses were repeated separately for the various brain territories, sensitivity of stroke self-report was highest for strokes in the middle cerebral artery territory (100.0%), and better for cortical than subcortical infarcts (40.0% vs. 33.1%). In analyses stratified by ethnic group, there was a slightly, but not significantly higher sensitivity of stroke self-report in African-Americans than Caucasians or Hispanics in all analyses performed, except cortical strokes that Caucasians reported slightly more accurate. In analyses stratified by the median age of 80 years, sensitivity and specificity of self-report were significantly better in the younger than the older age group (p-value for differences in sensitivity of stroke self-report for all vascular territories between genders = 0.02).
Although stroke is mainly a clinical diagnosis, brain imaging validates the occurrence of infarct, particularly in those cases characterized by ambiguous or silent symptoms. Studies that do not include brain imaging may lead to a misclassification of patients with cerebrovascular disease who have subtle symptoms or silent strokes. This in turn can lead to the failure to estimate the effects of stroke and cerebrovascular disease on disease risk. Previous findings of an inverse association between cognitive ability, memory function, and language function suggest that impaired cognition contributes to a higher false-negative rate of self-reported stroke in the elderly compared with younger persons. Recall problems in the elderly when asked about prior events would lead to lower estimates of sensitivity and positive predictive value.
Cerebrovascular disease and its antecedents are important factors in cognitive impairment and dementia in the elderly. The entities implicated include not only various vascular risk factors but large cortical infarcts, single strategically placed or multiple small infarcts, cerebral hemorrhage, cortical changes due to hypoperfusion (e.g., hippocampal sclerosis, ischemic-anoxic damage, cortical laminar sclerosis), white matter changes, and a variety of other vasculopathies. While there is general agreement that vascular disease likely contributes to AD, the underlying mechanism by which vascular risk factors and cerebrovascular disease lead to dementia remains unclear. Thus, while the use of MRI scans in elderly cohorts provides new insight into the aging brain, these findings raise new questions: does cerebrovascular disease, including white matter lesions and silent infarcts, lead to a specific pattern of cognitive impairment? Does cerebrovascular disease lead to a dementia syndrome indistinguishable from AD? What are the molecular mechanisms underlying the associations between the various vascular risk factors and cognitive impairment? Do changes in the microvasculature lead to the development of AD?
3. Ascertaining dementia in a population-based study of older Mexican Americans: experience of the Sacramento Area Latino Study on Aging (SALSA)
There are major challenges to conducting population-based studies of dementia and AD in Hispanic communities. Such an enterprise requires validation and equivalency of cognitive tests in different languages and cultures, and effective unbiased recruitment of representative samples. Only a handful of well-designed cohort studies are large enough and include sufficient diversity to evaluate racial or ethnic differences in dementia risk [10–14]. For any group, accurate estimation of the prevalence of dementia requires recruitment of a representative sample. However, most clinical research on dementia and AD is accomplished in specialty referral settings, and generalizability of findings to community populations is limited at best. Similarly, participants recruited from other medical care systems or insurance records better represent middle class populations, as members of minority groups are more often uninsured than majority groups.
Over 47 million Hispanics reside in the United States and 66% are of Mexican descent [15]. Low education and poverty are frequent among Hispanic elderly, which is a group expected to increase nearly 3-fold by 2050. Type 2 diabetes, obesity, and hypertension are highly prevalent in Mexican Americans and other Hispanic groups [10,16,17]. These conditions may increase the risk of dementia and AD in older Hispanics [18–21].
This section focuses on the case-ascertainment approach implemented in the SALSA, a population-based cohort study conducted in 1998 through 2008. The overall goal of the SALSA was to identify and evaluate vascular, metabolic, genetic, and socio-cultural risk factors for dementia in persons of Hispanic descent [10]. Study participants were residents of six contiguous counties in California’s Sacramento Valley. To be eligible, individuals must have been age 60 years or older in 1998 and self-designated as ‘Latino.’
Within the six contiguous counties, Hispanics were highly concentrated in a relatively small number of census tracts (n=259). So, to maximize efficiency in recruitment, a census tract was included in the SALSA sampling frame only if its Hispanic residents were at least 5% of the total population. Because the US Census is believed to undercount Hispanics by about 5% [22], it was deemed prudent to employ multiple strategies to locate eligible participants—including use of motor vehicle lists and commercial mailing lists. Despite repeated attempts to reach potential subjects by mail and telephone, the most effective way to identify and enroll them was by means of door-to-door neighborhood enumeration.
Face-to-face contact was essential in overcoming the many barriers to recruitment [23], and only Hispanic bilingual interviewers were used for this purpose. At baseline (1998–99), 1,789 people were enrolled in the study. Compared to the 2000 US Census, the sample was generally similar to older Hispanics residing in the target area census tracts. The sample, however, had a greater proportion of people with no formal education. In 85% of households that were enumerated and eligible, at least one person participated in the study. The sample ultimately included people of Mexican (95.2% of sample), Central American (4.5%), or South American (0.3%) descent. The sample members were between 60 and 101 years old, 58% were women, and 51% were born in Mexico (45%) or another Latin American country (6%). All US-born participants were of Mexican ancestry. Mean education was 10.8 years among English speakers and 4.7 years among Spanish speakers; about 15% had no formal education. Participants were interviewed in the language of their choice. Nearly 63% of interviews and tests were conducted in Spanish. All interviews, clinical tests, and cognitive assessments were done in the participants’ homes. All cognitive tests were pilot tested, and language norms were developed during the pilot studies [24].
In SALSA a case-ascertainment process involving three phases was used to identify dementia cases, as shown in Fig. 2.
Fig. 2.

Case-ascertainment phases for baseline prevalence in Sacramento Area Latino Study on Aging (SALSA) (reprinted with permission from Haan [10]).
Phase 1
The entire sample was screened with the Modified Mini-Mental State Exam (3MSE) [25] and the Spanish English Verbal Learning Test (SEVLT) [26,27]. If the score on either test was at or below the 20th percentile of the age- and education-adjusted score on that same test, a participant was referred to the next assessment phase. In order to estimate the sensitivity and specificity of the screening procedure, a 20% random subsample of all participants was referred to the next phase. Among those individuals who met screening criteria, the sensitivity and specificity were 100% and 76%, respectively, and the positive predictive value was 14% [10].
Phase 2
A neuropsychological assessment was conducted using the Spanish English Neuropsychological Assessment Scales (SENAS) [24,28–30]. The SENAS were developed to be psychometrically equivalent in both languages and includes six tests: conceptual thinking, semantic memory, attention span, episodic memory, non-verbal spatial abilities and verbal comprehension. In addition, informant interviews were accomplished using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) [31,32]. Participants were referred to a clinical exam (phase 3) if they scored at or below the 10th percentile of the normative sample on at least one test and their IQCODE score was at or above 3.0. Alternatively, participants could be referred if they scored below the 10th percentile on four tests or the IQCODE score was above 4.0. Language fluency can influence scores. Consequently the Peabody Language Fluency Test was given to participants who engaged in SENAS neuropsychological testing [24]. Although general fluency was significantly lower in Spanish speakers than in English speakers (p<0.0001), the effect was explained entirely by adjustment for education. In general, scores on most cognitive tests were lower in Spanish speakers but this was again explained by adjustment for education.
Phase 3
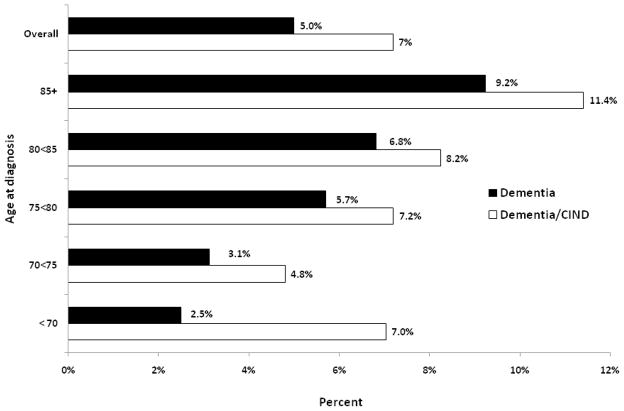
For those referred from phase 2, a standard clinical neurologic examination was conducted in the home by a project neurologist or geriatrician. A clinical dementia rating (CDR) was also completed. Information for all participants seen in phase 2 was reviewed by an adjudication team comprised of neurologists, neuropsychologist, a geriatrician and the study nurse project manager. Dementia was classified using DSM IV diagnostic criteria [33], and AD was classified using NINCDS-ADRDA criteria [34]. Cognitive Impairment not Dementia (CIND) was determined using criteria developed as part of the study [10]. Vascular dementia was defined using ADDTC criteria [35,36]. Five percent (5%) of dementia cases were defined as mixed AD or vascular. The CDR was rated for those classified as demented: 11% were 0, 63% were 0.5–1.0, 18.5% at 2, and 7.5% at 3.0. Fig. 3 shows overall and age-specific baseline prevalence. During the entire study, a total of 274 dementia/CIND cases were identified (prevalent and incident cases). The overall baseline prevalence of dementia was 5.0% and increased with advancing age to 9.2% in those participants age 85 years or older.
Fig. 3.
Estimation of prevalence of dementia and dementia/CIND by age at diagnosis in baseline years of 1998 and 1999: Sacramento Area Latino Study on Aging (SALSA).
The SALSA participants were younger (49% were age 60–69 years) than participants in other population-based studies of dementia, and they had lower prevalence and less severe dementia. For example, in African-Americans from Indianapolis, the prevalence of dementia was similar to SALSA at younger ages but higher at older ages [11].
Mortality in SALSA is higher than in studies of other Hispanic populations [37] (30% to date), and cognitive impairment or dementia are strong predictors of mortality in the study. In continuing mortality surveillance, 13 cases of dementia have been found so far that are recorded on death certificates and not in SALSA clinical evaluations. Cognitive impairment or impending dementia is related to mortality and contributes to an underestimation of the occurrence of dementia, if cases die before they can be diagnosed. At baseline, cognitive screening test cut-points for referral to phase 2 were adjusted for age and education. In retrospect, this is likely to have introduced bias and resulted in an underestimate of dementia as noted by Kraemer et al. [38] and O’Connell et al. [39], especially given the low education level in nearly the entire cohort.
Case-ascertainment challenges in SALSA related to lack of reading ability, level of bilingual ability, fluency in test language, and cultural equivalency of cognitive tests [26,40]. Of those diagnosed with dementia/CIND at any time during the study, none had previously been diagnosed by a physician. This was not directly related to access to care, as 91% had health insurance (73% on Medicare). Over 88% reported having a regular health-care provider. Of those without regular care, most (68%) said they could not afford to go to a doctor. SALSA physicians did contact the participants’ primary care doctor about dementia and other conditions identified in the project. A formal letter was sent to the provider, and that was followed up with a telephone call. Few primary care providers responded to the contacts about dementia. Follow-up for a few study subjects with dementia was initiated at the Alzheimer’s Center at the University of California at Davis. But the vast majority of study subjects with dementia remained without any specific care for that condition. By contrast, physician response to project notifications about type 2 diabetes and high blood pressure was much higher and contributed to a 25% increase in diabetes treatment and doubling of hypertension treatment over the study period.
An estimated 3.8 million Hispanics over age 60 years live in the United States in 2010, of whom two thirds (2.5 million) are Mexican American [15]. Applying the overall study estimate of 5% prevalence, there may be about 190,000 cases of dementia among Hispanic elderly, of whom few are diagnosed or treated for that condition.
4. Cultural and educational considerations for diagnosis of mild cognitive impairment and AD: the Washington Heights experience
Research among community-based cohorts of older adults has raised several challenges for research on the prevalence of mild cognitive impairment (MCI) and AD among ethnically and educationally diverse older adults. When participants are representative of the increasingly diverse population of older adults in the United States, the effects of race, culture, and education are observed on the measures used to diagnose MCI and AD. These measures must be valid and possess high diagnostic accuracy across a wide range of backgrounds that affect familiarity and comfort with the stimuli and procedures. Furthermore, ethnic/racial classifications are proxies for more meaningful, underlying variables representing cultural experiences and educational quality. Cultural effects on diagnostic tools will have increasing importance as the search for accurate markers of AD moves to detection in the preclinical stages of the disease.
The data are from a longitudinal study of aging, cognitive function, and dementia among Medicare-eligible older adults residing in the neighborhoods of Washington Heights – Hamilton Heights – Inwood in Northern Manhattan, New York (see Section 2). The sampling strategies and recruitment outcomes of these two cohorts are detailed in prior publications [1, 41]. Participants were visited in their homes and administered a comprehensive neuropsychological test battery, an interview of medical and functional status, and a neurological exam. Re-evaluations occurred during follow-up waves that were spaced approximately 18 to 30 months apart. Data from the in-home evaluation were discussed in a consensus conference of neuropsychologists and neurologists, during which research criteria for AD and other dementias were applied.
Prior studies in the cohort have reported higher prevalence and incidence of AD among Caribbean Hispanics and African-Americans as compared to non-Hispanic Whites [1,42]. However, since these diagnoses were in part based on neuropsychological test performance, the next step was to determine whether these findings reflect true differences in AD, or whether biased assessment measures contributed to these discrepancies. Table 1 shows baseline neuropsychological test data from 1,258 participants who, when asked about their daily functioning, did not report any cognition- or memory-related problems or decline in simple and complex activities of daily living over the course of three assessment waves, an average of about 5 years. Using a standardized neuropsychological algorithm for “cognitive impairment consistent with dementia” that was independent of the functional assessment [3], both African-American and Hispanic older adults were significantly more likely to meet cognitive criteria for dementia as compared to non-Hispanic Whites. This pattern was also present within specific cognitive domains, as is shown using the memory composite score (unadjusted and also accounting for years of school). These data demonstrate low specificity of cognitive measures among ethnic minorities: such African-Americans and Hispanics who were functioning normally at baseline and remained high functioning over the course of about five years were more likely than Whites to obtain impaired scores on cognitive measures, even after adjusting for years of schooling.
Table 1.
Baseline neuropsychological test performance among participants with no functional impairment at baseline and two subsequent follow-ups: Washington Heights Study.
| White | African American | Hispanic | Race effect (χ or F) | |
|---|---|---|---|---|
| N | 384 | 455 | 419 | |
| N (%) “below cuts” on neuropsychological battery | 4 (1%) | 32 (7%) | 45 (11%) | 39.1* |
| Memory composite z-score (mean) | 0.684 | 0.301 | 0.162 | 71.5* |
| Years of education-adjusted memory composite z-score (mean) | 0.545 | 0.261 | 0.333 | 21.7* |
Note:
p< .001
It is premature to examine discrepancies in diagnostic validity until it is demonstrated that the cognitive tests have construct validity among the groups tested. A confirmatory factor analysis was performed on the cognitive test battery, and revealed four factors for measures of memory, language, visual spatial skill and speed [43]. This factor structure was invariant across configural (the factor structure was the same across language groups) and metric (the loadings between the tests and the latent factors were the same across Spanish and English speakers) levels. The model did not have structural invariance across language groups (the relationships between the factors was slightly different across Spanish and English speakers), but this does not pose a major threat to the validity of the factor structure.
Robust, local norms [44] were generated for the neuropsychological test battery, and scores were adjusted for age, gender, years of education, and ethnicity. Using standard MCI criteria [45] and information about memory complaints and functional status from the in-home interview, there were no differences in prevalence of MCI (with and without memory impairment) across ethnic groups [41]. Over the course of follow-up, older adults with MCI (all subtypes) were about three times more likely to develop AD at follow up, and those with MCI with memory impairment were about 4 ½ times more likely to develop AD at follow up [46]. However, even using norms that took ethnicity into account, ethnic minorities with MCI were at higher risk than Whites with MCI for developing AD [46].
Another focus of this work is to determine the underlying reasons why race/ethnicity remains a persistent predictor of risk for cognitive impairment and AD, by deconstructing racial classifications into more direct measures of cultural and educational background. In the United States there is a great deal of discordance between years of education and quality of education; this is true especially among African-Americans. African-Americans educated in the South before the Supreme Court’s 1954 Brown v. Board of Education decision attended segregated schools, which received inferior funding as compared to White Southern schools and most integrated Northern schools [47,48]. Since the Coleman report [49] was released, the source of this discordance between years of education and achievement has been investigated through studies of school characteristics, such as pupil expenditures, teacher quality, pupil/teacher ratios, and length of school year/days attended, as well as achievement measures that reflect school quality, such as reading level.
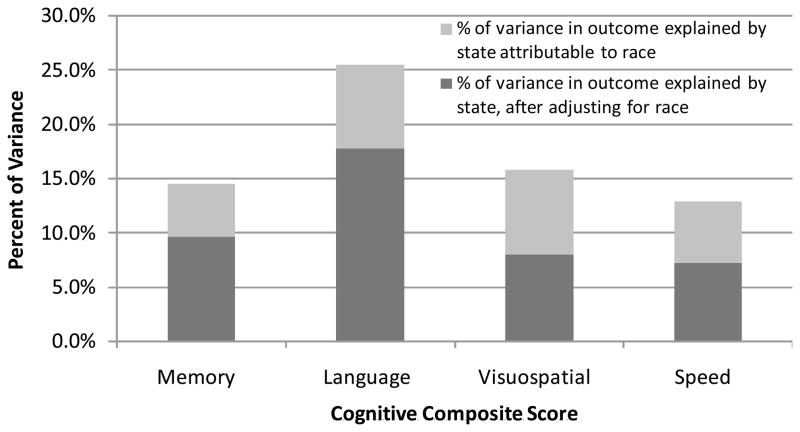
The effect of location of elementary school on cognitive test performance was examined among English-speaking, US-born, African-Americans and Whites in this Washington Heights cohort. Many of the African-Americans in the study were born and raised in the South, in states like North Carolina, South Carolina, and Virginia, and moved to the New York Area to find work as an adult. Fig. 4 shows that state location of education accounts for a significant proportion of variance in scores on cognitive composite measures both (1) unadjusted and (2) after adjusting for the effects of race. This work demonstrates that expectations for performance differ depending on the state in which education occurred. Further study revealed that among Spanish-speaking immigrants who were tested in Spanish, English fluency is a strong predictor of neuropsychological test performance, suggesting that level of acculturation predicts test performance, even when tested in one’s native language. Furthermore, among English speakers, older adults with low reading level had more rapid decline in memory as compared to those people with high reading level, and these results did not change if the sample was stratified by race [50]. In other words, reading level is a strong predictor of memory decline even among non-Hispanic Whites who were born and raised in the United States. Finally, among older adults who were not demented at baseline, reading level was shown to be the best predictor incident AD, and reading level accounted for the effect of race on risk of developing AD.
Fig. 4.
Proportion of variance in cognitive test scores explained by state of elementary education, with and without adjusting for race: Washington Heights Study.
These studies demonstrate that no cognitive measure is free of cultural or educational effects. Therefore, there is no cognitive measure for which one cut-off can be set to achieve equivalent diagnostic accuracy across racial, cultural, and language groups. However, psychometric techniques such as invariance analyses using structural equation models can demonstrate that cognitive tests are measuring the same psychological trait across groups. Specificity of neuropsychological tests improves when one takes into account, not just years of education, but also where schooling occurred and the quality of school, and fluency in English (among immigrant groups tested in their native language). These factors are associated with cognitive test scores and should be used to adjust expectations for performance and determining presence of impairment at one time point. Longitudinally, quality of education predicts future cognitive decline and risk of progression to AD, in both ethnic minorities and among non-Hispanic Whites. Therefore, while cultural and educational factors should be taken into account to reduce over-diagnosis of cognitive impairment, these variables are also strong predictors of cognitive decline. In cross-sectional and longitudinal studies, cultural and educational factors are better predictors of neuropsychological test performance than racial classification.
5. Dementia in the oldest old: diagnostic challenges and research directions
People age 85 years or older, referred to as the “oldest old,” are the fastest growing segment of the United States population. That segment is expected to increase by 40% in the next decade and quadruple in size over the next four decades [51]. The expansion of this sub-population will increase the number and proportion of dementia cases among the oldest old [52]. Consequently, characterization of dementia in the oldest old is of the utmost importance. However, the diagnosis and investigation of cognitive impairment in this population is challenging. This section will review current studies of dementia among the oldest old and describe methodological challenges to diagnosis and study of dementia in this group.
Much of the information regarding cognitive impairment among the oldest old have come from three studies: the 90+ Study, the Leiden 85-Plus Study, and the Vantaa 85+ Study (Table 2) [53–55]. However, many more studies will emerge given the importance of the oldest old. Among the newer studies of the oldest old is the Women Cognitive Impairment Study of Exceptional Aging (WISE), a population-based study of 1,534 oldest old women who have been well-characterized by demographics, co-morbidities, and lifestyle over 20 years.
Table 2.
Characteristics of major studies examining oldest old populations
| Study | Age (yrs) | N | Recruitment chronic | Clinical cognitive assessment |
|---|---|---|---|---|
| 90+ Study | ≥90 | 945 | Survivors of Leisure World Cohort Study | Cognitive screening Functional assessment Neurological examination |
| Leiden 85 Plus Study | ≥85 | 599 | Inhabitants of Leiden, Sweden | Cognitive screening Neurological Exam |
| Vantaa 85+ Study | ≥85 | 553 | Inhabitants of Vantaa, Finland who were aged 85+ | Cognitive screening Functional assessment Neurological Exam |
| WISE | ≥85 | 1534 | Ancillary study to Study of Osteoporotic Fractures | Cognitive screening Functional assessment Cognitive clinical adjudication |
Each of the major studies of the oldest old included at least 500 participants who were recruited either from an existing longitudinal study (90+ Study, WISE) or from age cohorts (Leiden 85-Plus and Vantaa 85+ Studies). There are advantages and disadvantages to each of these approaches. People who are recruited from a longitudinal study are likely to be well-characterized over years or decades. However, the population prevalence estimates of dementia may be biased due to differential retention over the study period. In contrast, recruitment from an age cohort may be more representative of dementia in the population but the group may be more poorly characterized over time and biased by cohort effects.
Most oldest old studies are not representative of the global oldest old population, and all large population studies originate from Western Europe or from the United States [53–58]. In particular, the vast majority of findings involving dementia in ≥ 90 year olds are from the 90+ Study, which is comprised of mostly white elders of high socio-economic status that were part of the Leisure World Cohort Study [59]. It is important that additional oldest old studies include different race and ethnic groups and geographical areas in order to represent the diverse oldest old population.
Most oldest old studies indicate that the prevalence of dementia is high, approximately 40% among women and 25% among men [53,60]. Early studies were inconclusive whether the prevalence increases after age 85 or 90 years [56,61–65]. The rising prevalence among women could be attributable either to higher incidence of dementia or increased survival with dementia relative to men. Evidence from incidence studies is conflicting. The 90+ Study found that the incidence of dementia among people age 90 years or older was similar in men and women [66], suggesting that differential survival likely caused gender differences in prevalence. However, other studies have suggested that oldest old women have up to a twofold higher incidence of dementia than men [54,67]. Because of competing mortality, it is likely that reports underestimate the prevalence of dementia in the oldest old [68], and more recent studies show an exponential increase in dementia incidence. For example, the 90+ Study reported an incidence of approximately 13% for people 90–94 years old and 22% for people 95 to 99 years old [66].
Many characteristics and subtypes of cognitive impairment among the oldest old are understudied. One meta-analysis of European dementia studies that stratified by age indicated that AD and vascular dementia composed most cases of dementia amongst the oldest old [69]. However, recent reports from the WISE indicated that AD and mixed dementia were the most common types of dementia in an oldest old [58]. Mixed dementia was not considered by many studies in the meta-analysis, which may account for the difference in findings. The prevalence of MCI and subtypes among the oldest old has also been poorly studied. However, recent findings from the WISE indicated that MCI was common, with an overall prevalence of 23% among oldest old women. Amnestic multiple domain and non-amnestic single domain were the most common MCI types, a pattern similar to younger groups [58].
The prevalence and incidence of dementia among the oldest old is, however, influenced by diagnostic challenges (Fig. 5). According to DSM-IV criteria [33], the diagnosis of dementia requires the presence of multiple cognitive deficits that include memory impairment and impairment in at least one other cognitive domain that is a decline from previous level of functioning and is sufficiently severe to cause impairment in function. However, the criteria by which to define cognitive and functional impairment have not been standardized in this age group. For example, few neuropsychological test norms have been determined for the oldest old and, thus, cut-points for impairment are less clear, particularly for those individuals over 90 years old. This, along with the fact that cognitive deficits are common among the oldest old [53,60,70,71], means that traditional methods of defining impairment relative to what is expected for age may not be suitable. Furthermore, the vast majority of oldest old people have some level of functional impairment, and it is often difficult to isolate the cause of impairment. For example, cognitive impairment, sensory impairment, arthritis, and other co-morbidities may all contribute to functional impairment. Thus, it is challenging to ascribe causation to one etiology. More research is needed to establish both neuropsychological norms and to better define functional impairment in this age group.
Fig. 5.
Challenges to diagnosing and studying dementia in the oldest old.
Classic neuropathologic features of AD such as neurofibrillary tangles and neuritic plaques are less predictive of dementia severity in the oldest old [72]. One study found that cerebral atrophy was the only feature strongly associated with dementia diagnosis in both the young-old and the oldest old [73]. The oldest old may have a greater number of co-existing neuropathologic features that contribute to cognitive impairment, making any one feature less predictive in isolation. Indeed, a recent study indicated that neuropathologic features consistent with mixed dementia were more common among the oldest old [74], confirming previous reports that the frequency of AD with vascular pathology is extremely common among very old men [75].
If underlying dementia pathology among the oldest old is more heterogeneous, then different diagnostic techniques or even dementia classifications may be needed. Furthermore, pharmaceuticals targeted to one dementia neuropathologic feature—for example, AD—may not be as well-suited to treat dementia in the oldest old.
Preliminary studies have identified risk factors for cognitive impairment among the oldest old, though few results have been replicated and most work stems from only a handful of cohorts. A number of classical risk factors for cognitive impairment in the young-old have also been associated with augmented rates of dementia or cognitive impairment in studies of people 85 years or older—for example, diabetes, dyslipidemia, and physical activity [76–78]. In contrast, elevated levels of inflammatory markers were associated with reduced cognitive decline [79], suggesting that the effect of some risk factors may be in the reverse direction among the very old.
Fewer risk factors for dementia have been identified among populations 90 years or older. In the 90+ Study, anti-oxidant intake, physical activity, and body mass index were not associated with the likelihood of dementia [80], despite being risk factors for dementia among the young-old [81]. Results from the 90+ Study also indicate that oxygen saturation was a novel risk factor in dementia [80]. Why fewer factors are associated with dementia among the ≥ 90 year old population than among the ≥ 85 year old population is unclear but may be due to differential bias or differences in cohort composition.
Studies regarding risk factors for dementia among the oldest old may also be confounded by the competing risks of dementia and death, which may lead to survival bias. Many risk factors for dementia in the young old such as diabetes and physical inactivity also increase risk of death [82,83]. As a result, ‘risk factors’ for dementia among the oldest old may be characteristics that protect against dementia or death at an earlier age. The importance of factors that enhance survival and, thus, increase the risk of dementia among the oldest old must be carefully evaluated.
Although a number of publications have examined cognitive impairment in the oldest old, the characterization of dementia and risk factors in the oldest old is incomplete. More studies are needed to address this topic, which will become increasingly important with the expansion of the oldest old population. Most importantly, norms for cognition and function need to be developed for this age group. Future studies should carefully investigate risk factors and dementia etiology among the oldest old and include racially and ethnically diverse populations.
6. Diagnosing dementia in culturally diverse populations
Over the past two decades, clinical epidemiologists at Indiana University, together with international colleagues, have had the opportunity to conduct a series of longitudinal community-based epidemiological studies of dementia, cognitive decline and potential risk factors, in a number of countries and with different cultural groups [84]. In most of these studies, an essential part of the methodology was the development of a process to ensure that the diagnoses of dementia across countries and cultural groups were comparable.
This section will address, briefly, five major issues that arose during the evolution of this diagnostic process. These include developing culture-fair instruments, the value of informant data, the development of a semi-structured home-based interview to allow clinicians to measure everyday function, and the diagnostic process which includes reconciling conflicting information and involving culturally knowledgeable health professionals. Although this diagnostic process was developed for use in epidemiological studies, it is believed that resolution of these five issues also has relevance for the diagnosis of dementia in clinical settings as well as research settings involving ethnically diverse populations.
In the absence as yet of easily administered biological tests to measure individual dementing disorders, the diagnosis of the dementia syndrome rests, to a large extent, on the interpretation of measurements of cognition and activities of daily living that themselves are subject to educational and cultural effects. One of the most difficult and intriguing tasks, therefore, in international and cross-cultural studies of dementia, particularly involving under-studied minority or educationally deprived groups, is the development of instruments that are culture-fair and comparable across educational levels.
Two approaches have been employed to accomplish this task, either trying to modify existing tests or designing new tests. The advantage of new tests is that they can be designed specifically for the educational and cultural groups being studied; the disadvantage is that it is sometimes difficult to compare results with other studies that use more standard tests without providing some cross-linking between the measurements. Comparability of results is an obvious advantage of modifying existing tests. However, such changes that might make the modified test culturally and educationally harmonious can also alter its psychometric properties. In various studies, Hendrie and colleagues have used both methods. In particular, they designed a new screening instrument, the Community Screening Interview for Dementia (CSID) [85], which they have used successfully in several populations including Yoruba, African-American, Jamaican, and Chinese groups, as well as the original target populations of Cree and English-speaking Canadians [86]. The CSID has also been incorporated into instruments used in the international 10/66 studies [87]. The complex process used in constructing the CSID (Table 3) involved not just translation and back-translation but also harmonization. For much of the diagnostic assessment process, the instruments developed by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [88] as well as the informant portion of the Cambridge Mental Disorders of the Elderly Examination (CAMDEX) [89] were used but they too underwent the same process of harmonization as did the CSID. Sometimes this resulted in the development of new tests, for example, the “Stick Design Test” which is quite structurally different from the tests designed to measure praxis in CERAD [90].
Table 3.
Process of cultural harmonization of cognitive and behavioral tests
| Steps to construct a culturally harmonious test |
|---|
|
Throughout the process, the necessity for developing local normative values representative of the educational and cultural groups being studied cannot be emphasized enough. For example, in the Indianapolis-Ibadan Study, a community-based comparative study of the prevalence and incidence of dementia in two populations, African-American and Yoruba, the normative values for the CERAD neuropsychological tests in the African-American sample are consistently lower than those reported for the original CERAD sample [91].
In the deliberations regarding the construct of the CSID, it became evident that the instrument would not be entirely free from educational or cultural effects/influence. Therefore, Hendrie and colleagues included in the instrument a section on performance on everyday activities based upon informant interview and used a combined discriminant score to identify participants for detailed clinical assessments. The inclusion of these informant data did add significantly to the performance characteristics of the CSID as a dementia screening tool [86]. The value of informant data in the diagnostic process will be discussed later. Unfortunately, particularly in Western countries where significant numbers of older people, even those who are demented, live alone, it is not always possible to identify a reliable informant. In order to overcome this problem, a 10-item semi-structured home based interview (the CHIF or Clinician Home-based Instrument to assess Function) was developed to allow skilled clinicians to collect information on every day function more directly by taking advantage of the fact that the interview is conducted at home and the home environment can also be assessed [92]. The CHIF is brief and easy to administer and score. In the Indianapolis Ibadan Study, it performed well in both sites, correlating significantly with both informant-based assessments and MMSE scores.
Despite the increasing precision of the clinical instruments available, the diagnosis of dementia and AD still relies to a considerable extent on the acumen of the experienced clinician. Part of this skill lies in the ability to interpret correctly information that is derived from different sources: clinical assessment, laboratory tests, information from a relative, and direct cognitive testing with the patient. This process is rendered more difficult when these sources provide apparently conflicting information. That this information conflicts is not uncommon, as illustrated in data derived from the Indianapolis-Ibadan Study which has a two-stage design, a screening stage followed by a clinical assessment of a subsample based upon the screening scores (Table 4) [93]. In this study a consensus process is utilized for the clinical assessment phase involving clinicians from both sites. The major sources of information for dementia diagnosis derive from the neuropsychological measurements, assessment of functional status from the key informant and similar direct assessment of function by the clinician (CHIF). For each of these assessment procedures, cutoff points have been derived from previous data indicating the likely diagnostic status: normal, CIND, and dementia. Table 4 shows the % of agreement and kappa values for these measurements in 228 African-American participants who had complete information from all three sources in a recent diagnostic assessment wave.
Table 4.
Arriving at a diagnostic consensus: data from the Indianapolis-Ibadan Dementia Project
| A comparison of the agreement for likely diagnoses between the 3 assessment procedures | |||
|---|---|---|---|
| Informant interview vs. CHIF | Informant interview vs. neuropsychological exam | CHIF vs. neuropsychological exam | |
| % Agreement | 60.4% | 64.2% | 75.7% |
| Kappa (95% confidence Interval) | 0.42 (0.34–0.50) | 0.47 (0.39–0.55) | 0.63 (0.55–0.70) |
N = 288; Overall Kappa=0.50 (95% Confidence interval: 0.45–0.55)
Possible diagnoses are dementia, cognitive impairment not demented, normal cognition
Likely diagnoses based upon algorithmically derived cut points.
This retrospective analysis of the diagnostic assessment data can also be used to indicate the relative weight apparently given to the information sources in arriving at a final consensus diagnosis (Table 5). The diagnosis of dementia appears to be driven largely by the information provided by the informant who agrees 94% of the time with the consensus diagnosis (as compared to 79% for the neuropsychological assessment and 83% for CHIF). The consensus diagnosis of CIND is primarily driven by the neuropsychological test data with agreement of 81% (as compared to 63% for informant data and 71% for CHIF scores). For the final diagnosis of normal cognitive function, the primary driver is the CHIF which has 96% agreement; and neuropsychological data and informant data had 92% and 58% agreement with this diagnosis.
Table 5.
Arriving at a diagnostic consensus: data from the Indianapolis-Ibadan Dementia Project
| A comparison of the agreement between the individual assessments likely diagnoses and the final consensus diagnosis | |||
|---|---|---|---|
| Demented (n=82) | CIND (n=98) | Normal (n=108) | |
| Informant Interview | 94% | 63% | 58% |
| CHIF | 83% | 71% | 96% |
| Neuropsychological exam | 79% | 81% | 92% |
Despite serious attempts at developing algorithmic diagnoses, it was impossible to entirely eliminate clinical judgment. With this regard, the input of a skilled clinician who also has an intimate knowledge of the culture of the target population was often crucial. It is important to partner with strong academic groups in the other countries that could provide this input.
In closing, diagnosis of the dementia syndrome in ethnically and culturally diverse populations poses many challenges but consideration of the five issues discussed above should help to improve its accuracy.
7. Identification of prodromal AD in population settings: Results from the Cache County Memory Study
Several different terms may describe the earliest symptoms of AD expression. Notable among these are several variations of the commonly used term, MCI [94,95]. MCI, and particularly its more recently described amnestic subtypes (single domain amnestic MCI, or aMCI; or its multiple domain analogue, amMCI). The latter appear to be strong predictors of subsequent AD in clinical samples [96]. The utility of the MCI construct as a predictor of AD in populations is less clear but of importance. As new diagnostic criteria for AD are advanced [97], reliable identification of the pre-dementia stage of disease is critical. Relevant questions are not only the predictive value of an MCI diagnosis (e.g., what proportion of identified MCI cases develop AD within a defined period?) but also the sensitivity of the MCI construct (e.g., what proportion of incident AD cases can be identified beforehand as having MCI?).
In the early 1990s the problem of identification of early symptomatic stages of AD was recognized by investigators working in a national twin cohort [98]. Researchers in this population-based study identified a diagnostic construct that they considered to be suggestive of early AD because it included “mild/ambiguous” AD symptoms including episodic memory impairment with subtle difficulties in language, praxis, executive functioning or geographic orientation [99]—most of the latter having been identified as typical or confirmatory diagnostic features of AD by the NINCDS-ADRDA criteria [34]. When detailed information was not available, the research group also accepted subtle functional changes in instrumental activities of daily living (IADLs) as a surrogate indicator for the latter non-memory disturbances. The key concept was that these disturbances could not be readily ascribed either to memory difficulty alone or to other identified medical or neurological disorders.
The same investigator group then undertook a longitudinal observational study of AD risk factors in a long-lived cohort in Utah (Cache County Memory Study, CCMS). In this context, they were able to examine the relationship of variously defined mild cognitive syndromes to later dementia outcomes [100]. More recently, they tested the performance characteristics of various operationally defined constructs of cognitive disorders as predictors of expressed dementia three years later. By determining the predictive characteristics of these various constructs over a relatively short interval (3 years), they reasoned that they might evaluate the key diagnostic features for the identification of true prodromal AD. By this was meant the early symptomatic stage of AD where the clinical features were not yet sufficiently severe for the diagnosis of AD by conventional criteria [34]. They applied operationalized criteria retrospectively, relying on standardized test measures of cognition and functional decline (Table 6) to arrive at diagnoses for each of the following:
Table 6.
Criteria and variables used to derive common constructs of cognitive impairment in aging
| Construct | Criteria | Variables used |
|---|---|---|
| Mild Ambiguous M/A AD [99] |
|
|
| MCI [94] |
|
|
| CDR=0.5 [95] |
|
|
| AAMI [101] |
|
|
| AACD [102] |
|
|
| CIND [103] |
|
|
Age-associated Memory Impairment (AAMI [101])
Mild/Ambiguous AD (M/A AD [99]),
Amnestic MCI (aMCI [94]),
Questionable dementia—functional decline determined by a Clinical Dementia Rating Scale of 0.5 (CDR=0.5 [95]),
Age-associated Cognitive Decline (AACD [102]), and
Cognitive Impairment – not Demented (CIND [103])
They then contrasted these six retrospectively defined groups as predictors of dementia outcomes 3 years later. Among the groups, M/A AD appeared to have the best overall performance (Table 7), with the highest positive predictive value (48.2 versus 19.1–34.8) and negative predictive value (95.7 versus 85–92.5) for dementia. Additionally, the M/A AD group had a low reversion rate to normality (24% versus 38–67%) although not as low as MCI defined as CDR–0.5 (12%).
Table 7.
Frequency, reversion rate per 3 years, and positive and negative predictive values & relative risk for dementia using multiple constructs of cognitive impairment
| Frequency | Reversion rate/3 yrs (%) | PPV (%)CI | NPV (%)CI | RR CI | |
|---|---|---|---|---|---|
| AAMI | Total (n=82) | 37.90 (26.18–49.58) | 15.15 (6.50–23.80) | 85.24 (81.74–88.75) | 2.74 (1.41–5.35) |
| AAMI (full criteria; n=21) | 42.11 (19.90–64.31) | 5.26 (−4.78–15.30) | 84.77 (81.42–88.13) | 1.07 (0.16–7.09) | |
| AAMI (w/exclusionary features; n=61) | 36.17 (22.43–49.91) | 19.15 (7.90–30.40) | 85.68 (82.30–89.06) | 3.32 (1.70–6.51) | |
| AACD | Total (N=263) | 41.08 (33.99–48.17) | 24.86 (18.64–31.09) | 91.97 (88.75–95.19) | 4.06 (2.44–6.74) |
| AACD (full criteria; N=118) | 46.07 (35.71–56.42) | 15.73 (8.17–23.30) | 85.41 (81.81–89.00) | 2.74 (1.44–5.20) | |
| AACD (w/exclusionary features; N=145) | 36.46 (26.83–46.09) | 33.33 (23.90–42.76) | 90.08 (87.01–93.16) | 5.14 (3.06–8.63) | |
| CIND | Total (N=249) | 41.82 (34.29–49.34) | 27.88 (21.04–34.72) | 92.52 (89.51–95.52) | 4.08 (2.56–6.48) |
| MCI | Total (N=12) | 66.67 (35.87–97.47) | 11.11 (−9.42–31.64) | 85.11 (81.82–88.40) | 2.08 (0.32–13.38) |
| aMCI (full criteria; N=10) | 75.0 (44.99–105.01) | 12.5 (−10.42–35.42) | 85.14 (81.86–88.43) | 2.08 (0.32–13.38) | |
| aMCI (w/exclusionary features; N=2) | 0 | 0 | 85.15 (81.90–88.41) | - | |
| CDR=0.5 | Total (N=440) | 11.75 (8.09–15.40) | 18.46 (14.05–22.86) | 91.93 (87.72–96.13) | 9.47 (3.62–24.79) |
| fMCI (full criteria; N=324) | 12.66 (8.36–16.97) | 13.54 (9.11–17.97) | 83.91 (79.16–88.66) | 8.01 (3.01–21.32) | |
| fMCI (w/exclusionary features; N=116) | 8.70 (2.05–15.34) | 34.78 (23.54–46.02) | 88.72 (85.58–91.86) | 12.40 (4.73–32.53) | |
| M/A AD | Total (N=312) | 24.14 (18.25–30.02) | 28.08 (21.90–34.26) | 95.70 (93.22–98.19) | 13.14 (6.23–27.72) |
| M/A AD (full criteria; N=209) | 27.59 (20.31–34.86) | 20.0 (13.49–26.51) | 87.58 (83.93–91.23) | 10.27 (4.72–22.32) | |
| M/A AD (w/exclusionary features; N=103) | 15.52 (6.20–24.84) | 48.28 (35.42–61.14) | 90.02 (87.09–92.96) | 18.49 (8.75–39.06) |
Ns reflect number of participants meeting full criteria at baseline for each construct and are non-mutually exclusive. Total for each group includes those meeting full criteria and those meeting inclusion criteria only (e.g., generally with an exclusionary medical condition). Remaining participants include those not classifiable.
A common feature in both of the best performing criteria (CDR=0.5 & M/A AD) was evidence of functional decline in IADLs. Although not a requisite for the M/A AD criteria, nearly 97% of the participants so-characterized had a history of such functional decline or had psychometric evidence of deficits in non-memory cognitive functions that typically provoke such decline. Other constructs (AACD, CIND) that rely on decline in non-memory cognitive functions or difficulties with ADLs also performed better than the remaining entities.
Reviewing these results, the Cache County investigators now suggest that impairment in non-memory areas such as language, praxis or executive functioning, or impairment in IADLs (presumably in consequence of such difficulties) is important for the identification of progressive dementias and very early AD. This idea is consistent with findings emerging from other population-based studies [104] and other clinical cohorts (e.g., [105,106]) suggesting that subtle deficits across many cognitive domains or the presence of mild functional impairment represent a very early but recognizable stage of AD dementia, i.e., prodromal AD.
A somewhat surprising finding in the Cache County population work was the relative rarity of cases with the pure amnestic form of MCI as diagnosed using strict adherence to its defining criteria. Similar findings have been reported in other population-based investigations that have adhered strictly to the original published criteria [107]. The requisite of a memory complaint in the aMCI criteria along with the requirement of no functional impairment are two factors likely to contribute to the discrepancies seen between aMCI diagnostic frequencies in clinical and population studies. Recent revisions in the MCI criteria clarify these important features [96,108] and recognize a broader range of mild cognitive disorders occurring in advance of dementia. Thus, investigators who have used expanded MCI criteria allowing for multiple cognitive complaints or mild functional losses in addition to episodic memory disorder have reported higher prevalence of MCI in the population and a higher predictive value for later dementia outcomes [109,110].
The reliable identification of cognitive impairment that represents true prodromal AD is a critical need with importance both for scientific discovery and drug development. As shown in the Cache County experience, the application of MCI criteria as a surrogate measure of early prodromal AD expression has limitations in diagnostic sensitivity and specificity. This problem has also been seen in secondary prevention studies designed to slow progression to AD dementia [97]. Such imprecision in diagnosis suggests that much larger sample sizes would be needed in clinical trials to detect treatment effects. With efforts now underway to revise the NINCDS-ADRDA diagnostic criteria for AD [34], the work reported here suggests that a framework for the diagnosis of the prodromal stage of the disorder should consider not only impairment in objective measures of episodic memory function but also more global impairments across specific cognitive domains affected by the disease (i.e., language expression, praxis, and executive function). In the absence of documented changes across these specific non-memory domains, a quantifiable change in IADLs would probably suffice. The utility of this approach, as detailed in the original M/A AD construct, is now under investigation in more diverse samples. If confirmed, this diagnostic approach may provide an important rationale for the identification of individuals at the highest risk of fully expressed AD dementia within a short time window. This approach to the diagnosis of prodromal AD could then be a valuable tool for clinical trials of treatments intended to slow symptom progression or conversion to dementia. This information could also be used to support recent suggestions of revising the diagnostic criteria for AD [97]. Complemented by reliable and predictive biomarkers [111], precision in the clinical criteria for the pre-dementia expression of AD, as suggested here, should ultimately lead to more secure diagnoses during early symptomatic disease.
8. Discussion
The clinical diagnosis of dementia caused by AD can approach an accuracy rate of 95%, but only in circumstances involving highly experienced clinicians working with selected individuals who generally are followed comprehensively over time. High diagnostic accuracy rates thus are the exception rather than the rule. Outside of specialized centers, AD dementia is accurately detected only in about 50% of affected individuals [112,113]. Although the sensitive and accurate recognition of Alzheimer dementia in population studies is critical for determinations of the prevalence of AD, in practice it often is difficult to achieve owing to differences in how dementia criteria are operationalized across studies, the use of varying methods to obtain the information needed for diagnosis, and intrinsic differences in the populations under investigation. Diagnosis of Alzheimer dementia is further complicated by the frequent presence in older adults of comorbidities that also can contribute to cognitive dysfunction. Given all these variables, it is not surprising that estimates of the prevalence of AD vary widely.
Current criteria for dementia of the Alzheimer type (DAT) require that an individual has experienced decline in cognitive ability from his or her previously attained level (i.e., the principle of intra-individual decline); also, the decline must affect memory and at least one other cognitive domain and must interfere with the individual’s function in accustomed everyday activities [33]. Operationalizing these criteria to population studies can be problematic. Brief performance-based cognitive screening tests lack specificity and often are insensitive to milder stages of Alzheimer dementia [114]. Even multi-stage case ascertainment methods (e.g., initial screening with cognitive tests, then more detailed evaluation of individuals who fall below a predetermined cutpoint on test performance) may not identify the earliest impairment that is associated with Alzheimer pathology. Moreover, it is difficult to ascertain with a single assessment whether a person has experienced decline in cognitive and functional abilities because the prior baseline is unknown. Self-reported estimates of health and function may be unreliable [115]. Use of informants with knowledge of the individual’s past abilities relative to current status often improves dementia diagnosis, but time and expense is required to obtain such information; also, an observant informant may not always be available. Cognitive and functional assessments provide objective measures of performance, but establishing what is “normal” versus “abnormal” as determined by those assessments presents many challenges, especially for the earliest symptomatic stages of AD that are encompassed by MCI and similar constructs.
Several themes regarding the ascertainment of Alzheimer dementia, including its prodromal stages, are commonly identified in epidemiological investigations of AD dementia and are illustrated in the experiences reported above from several well-established population studies. Representative samples are mandatory for true prevalence studies to determine whether there are groups of individuals who may be at greater or lower risk of AD dementia. Hence, there is a great need for culture-fair diagnostic instruments and normative values that are representative of each age, educational, and cultural group. Prevalence studies often fail to validate the diagnosis of dementia by monitoring continuing cognitive and functional decline. When longitudinal studies are conducted, they may be biased by attrition, differential retention, and missing data. The influence of competing risk can lead to an underestimation of dementia when an affected individual dies prior to diagnosis. Diagnostic tools are influenced by cultural and language effects [116] and by educational level (more important than years of education is quality of education) [117–119]. Interpreting the collected information to arrive at a diagnosis of cognitive impairment or Alzheimer dementia requires clinical judgment which may introduce another source of variability, as clinicians may differ in their threshold for “cognitive impairment” or “dementia.” Risk and protective factors may operate differentially at different times during the lifespan, such that a factor in midlife that confers risk for later dementia (e.g., hypertension) may serve in late life to protect against dementia.
Addressing these themes and confronting the challenges of accurate detection of Alzheimer dementia across population studies may be considerably aided by the development of reliable and validated biomarkers for AD. Molecular biomarkers for AD (e.g., cerebrospinal fluid [CSF] assays of amyloid-beta [Aβ42] and tau proteins; amyloid imaging) currently show great promise for the in vivo detection of AD pathology [120,121]. An informant-based, brief (<3 minutes) screening test, the AD8 [122], that is derived from the Clinical Dementia Rating [123] had superior sensitivity in detecting early-stage Alzheimer dementia than the Mini Mental State Examination [124] and also correlated more strongly than performance-based screening tests with indicators of Aβ pathology, including CSF Aβ42 levels and cerebral amyloid burden as measured with Pittsburgh Compound-B [125]. Much work yet is needed, however, to determine the sensitivity, specificity, and predictive value of AD biomarkers for a diagnosis of Alzheimer dementia in clinical samples. Moreover, CSF and sophisticated imaging biomarkers are impractical for almost all epidemiological investigations, but less costly and less cumbersome biomarkers with greater applicability (e.g., plasma proteins) may become available to provide enhanced diagnostic confidence for the diagnosis of Alzheimer dementia.
The advent of AD biomarkers also permits the detection of AD pathology in living individuals who have no clinically discernible cognitive impairment. Such individuals are considered to have presymptomatic or preclinical AD, with the assumption (as yet unproven) that many or even all of these individuals eventually will develop symptomatic AD if they continue to live. Although persons with presymptomatic AD have no clinically apparent deficits, their longitudinal cognitive performance is marked by clear decline years prior to a clinical diagnosis of MCI or Alzheimer dementia [126,127]. Putatively normal samples of older adults thus are contaminated with individuals with presymptomatic AD, such that there is an overestimation of the decline in cognitive ability that is attributable solely to healthy aging (i.e., in the absence of presymptomatic AD) [128]. This leads to group norms that are too lenient to diagnose individuals in the earliest symptomatic stages of AD [129–131]. To accurately identify individuals with MCI and early stage Alzheimer dementia, therefore, future population studies should explore strategies to assess intra-individual cognitive decline and seek supporting evidence of underlying AD pathology with biomarkers.
Acknowledgments
This combined effort was supported by National Institute on Aging grants P01AG007232, R01AG012975, R01AG016206, R01AG009956, R01AG011380, P30AG028377, P50AG05681, and K01AG029336.
The work on prodromal Alzheimer’s disease was part of the dissertation of Dr. Lauren Warren at the University of North Carolina Chapel Hill (UNC-CH) with statistical assistance of Kathleen M. Hayden. Dissertation advisors: Dr Kathleen Welsh-Bohmer (Duke) and Dr. Marilyn Hartmann (UNC-CH). Lead investigators in the analyses presented include Cache collaborators: Lauren Warren, Kathleen Hayden, Maria Norton, JoAnn Tschanz, Linda Sanders, Truls Ostbye, Carl Pieper, Kathleen Welsh-Bohmer, and John C. S. Breitner, as well as neuropsychologist Marilyn Hartman on the faculty at University of North Carolina Chapel Hill. Other Cache County Study Investigators include: James Anthony, Erin Bigler, Ron Brookmeyer, James Burke, Eric Christopher, Jane Gagliardi, Robert Green, Michael Helms, Christine Hulette, Ara Khachaturian, Liz Klein, Carol Leslie, Constantine Lyketsos, Lawrence Mayer, John Morris, Ronald Munger, Chiadi Onyike, Ronald Petersen, Kathy Piercy, Brenda Plassman, Peter Rabins, Pritham Raj, Russell Ray, Ingmar Skoog, David Steffens, Martin Steinberg, Marty Toohill, Leslie Toone, Jeannette Townsend, Heidi Wengreen, Michael Williams, and Bonita Wyse. Special thanks to: Cara Brewer, Tony Calvert, Michelle McCart, Tiffany Newman, Roxane Pfister, Nancy Sassano, Sarah Schwartz, and Joslin Werstak.
Footnotes
Disclosure statement for authors
The authors have no conflicts to disclose. The sponsors had neither a role in the analysis or interpretation of these data, nor in the content of the paper. Appropriate approval procedures were used concerning human subjects.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, et al. The APOE-ε4 allele and the risk of Alzheimer disease among African Americans, Whites, and Hispanics. JAMA. 1998;279:751–5. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 3.Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, et al. Diagnosis of dementia in a heterogeneous population: Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–60. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: A comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147:969–77. doi: 10.1093/oxfordjournals.aje.a009387. [DOI] [PubMed] [Google Scholar]
- 5.Bots ML, Looman SJ, Koudstaal PJ, Hofman A, Hoes AW, Grobbee DE. Prevalence of stroke in the general population: The Rotterdam Study. Stroke. 1996;27:1499–501. doi: 10.1161/01.str.27.9.1499. [DOI] [PubMed] [Google Scholar]
- 6.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–61. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the Framingham Heart Study: Establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, et al. Predictors of brain morphology for the men of the NHLBI Twin Study. Stroke. 1999;30:529–36. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 9.Reitz C, Schupf N, Luchsinger JA, Brickman AM, Manly JJ, Andrews H, et al. Validity of self-reported stroke in elderly African Americans, Caribbean Hispanics, and Whites. Arch Neurol. 2009;66:834–40. doi: 10.1001/archneurol.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: The influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–77. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 11.Hall KS, Gao S, Baiyewu O, Lane KA, Gureje O, Shen J, et al. Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimers Dement. 2009;5:227–33. doi: 10.1016/j.jalz.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 13.Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14:481–93. [PubMed] [Google Scholar]
- 14.Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, et al. Incidence of Alzheimer’s disease in a biracial urban community: Relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–9. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 15.Pew Hispanic Center. Statistical Portrait of Hispanics in the United States, 2008. Pew Hispanic Center; 2010. Available online: http://pewhispanic.org/factsheets/factsheet.php?FactsheetID=58. [Google Scholar]
- 16.Lorenzo C, Serrano-Rios M, Martinez-Larrad MT, Gabriel R, Williams K, Gonzalez-Villalpando C, et al. Prevalence of hypertension in Hispanic and non-Hispanic White populations. Hypertension. 2002;39:203–8. doi: 10.1161/hy0202.103439. [DOI] [PubMed] [Google Scholar]
- 17.Meigs JB, Wilson PW, Nathan DM, D’Agostino RB, Sr, Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52:2160–7. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 18.Haan MN. Therapy insight: Type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pract Neurol. 2006;2:159–66. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 19.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 20.Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Impact of diabetes on cognitive function among older Latinos: A population-based cohort study. J Clin Epidemiol. 2003;56:686–93. doi: 10.1016/s0895-4356(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 21.Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Impact of antidiabetic medications on physical and cognitive functioning of older Mexican Americans with diabetes mellitus: A population-based cohort study. Ann Epidemiol. 2003;13:369–76. doi: 10.1016/s1047-2797(02)00464-7. [DOI] [PubMed] [Google Scholar]
- 22.US Census Bureau. Net Undercount and Undercount Rate for US and States. US Census Bureau; 1990. Available online: http://www.census.gov/dmd/www/techdoc1.html. [Google Scholar]
- 23.Sweeney C, Edwards SL, Baumgartner KB, Herrick JS, Palmer LE, Murtaugh MA, et al. Recruiting Hispanic women for a population-based study: Validity of surname search and characteristics of nonparticipants. Am J Epidemiol. 2007;166:1210–9. doi: 10.1093/aje/kwm192. [DOI] [PubMed] [Google Scholar]
- 24.Mungas D, Reed BR, Marshall SC, Gonzalez HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14:209–23. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 26.Gonzalez HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc. 2001;7:544–55. doi: 10.1017/s1355617701755026. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez HM, Mungas D, Haan MN. A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. Clin Neuropsychol. 2002;16:439–51. doi: 10.1076/clin.16.4.439.13908. [DOI] [PubMed] [Google Scholar]
- 28.Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): Further development and psychometric characteristics. Psychol Assess. 2004;16:347–59. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- 29.Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19:466–75. doi: 10.1037/0894-4105.19.4.466. [DOI] [PubMed] [Google Scholar]
- 30.Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic Whites. J Int Neuropsychol Soc. 2005;11:620–30. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–22. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 32.Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21:785–90. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV) Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- 34.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 35.Tierney MC, Black SE, Szalai JP, Snow WG, Fisher RH, Nadon G, et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Arch Neurol. 2001;58:1654–9. doi: 10.1001/archneur.58.10.1654. [DOI] [PubMed] [Google Scholar]
- 36.Chui HC, Mack W, Jackson JE, Mungas D, Reed BR, Tinklenberg J, et al. Clinical criteria for the diagnosis of vascular dementia: A multicenter study of comparability and interrater reliability. Arch Neurol. 2000;57:191–6. doi: 10.1001/archneur.57.2.191. [DOI] [PubMed] [Google Scholar]
- 37.Patel KV, Eschbach K, Ray LA, Markides KS. Evaluation of mortality data for older Mexican Americans: Implications for the Hispanic paradox. Am J Epidemiol. 2004;159:707–15. doi: 10.1093/aje/kwh089. [DOI] [PubMed] [Google Scholar]
- 38.Kraemer HC, Moritz DJ, Yesavage J. Adjusting Mini-Mental State Examination scores for age and educational level to screen for dementia: Correcting bias or reducing validity? Int Psychogeriatr. 1998;10:43–51. doi: 10.1017/s1041610298005134. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell ME, Tuokko H, Graves RE, Kadlec H. Correcting the 3MS for bias does not improve accuracy when screening for cognitive impairment or dementia. J Clin Exp Neuropsychol. 2004;26:970–80. doi: 10.1080/113803390490510998. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez HM, Mungas D, Haan MN. A semantic verbal fluency test for English- and Spanish-speaking older Mexican-Americans. Arch Clin Neuropsychol. 2005;20:199–208. doi: 10.1016/j.acn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62:1739–46. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 42.Tang MX, Maestre G, Tsai WY, Liu XH, Feng L, Chung WY, et al. Relative risk of Alzheimer’s disease and age-at-onset distributions, based on APOE genotypes among elderly African-Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet. 1996;58:574–84. [PMC free article] [PubMed] [Google Scholar]
- 43.Siedlecki KL, Manly JJ, Brickman AM, Schupf N, Tang MX, Stern Y. Do neuropsychological tests have the same meaning in Spanish speakers as they do in English speakers? Neuropsychology. 2010;24:402–11. doi: 10.1037/a0017515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sliwinski M, Lipton RB, Buschke H, Stewart W. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J Gerontol B Psychol Sci Soc Sci. 1996;51:P217–25. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 46.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JPG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson JD. The education of Blacks in the South, 1860–1935. Chapel Hill: University of North Carolina Press; 1988. [Google Scholar]
- 48.Margo RA. Race and schooling in the South, 1880–1950: An economic history. Chicago: University of Chicago Press; 1990. [Google Scholar]
- 49.Coleman J. Equality of Educational Opportunity. Washington, DC: Government Printing Office; 1966. p. 32676. [Google Scholar]
- 50.Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and Memory Decline Among Ethnically Diverse Elders. J Clin Exp Neuropsychol. 2003;25:680–90. doi: 10.1076/jcen.25.5.680.14579. [DOI] [PubMed] [Google Scholar]
- 51.Moore A. Older people: We can work it out. Health Serv J. 2007;117(6038):24–6. [PubMed] [Google Scholar]
- 52.Schneider EL, Guralnik JM. The aging of America: Impact on health care costs. JAMA. 1990;263:2335–40. [PubMed] [Google Scholar]
- 53.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: Results from the 90+ Study. Neurology. 2008;71:337–43. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 54.Gussekloo J, Heeren TJ, Izaks GJ, Ligthart GJ, Rooijmans HG. A community-based study of the incidence of dementia in subjects aged 85 years and over. J Neurol Neurosurg Psychiatry. 1995;59:507–10. doi: 10.1136/jnnp.59.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rastas S, Pirttila T, Mattila K, Verkkoniemi A, Juva K, Niinisto L, et al. Vascular risk factors and dementia in the general population aged > 85 years: Prospective population-based study. Neurobiol aging. 2010;31:1–7. doi: 10.1016/j.neurobiolaging.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Fratiglioni L, Viitanen M, Backman L, Sandman PO, Winblad B. Occurrence of dementia in advanced age: The study design of the Kungsholmen Project. Neuroepidemiology. 1992;11 (Suppl 1):29–36. doi: 10.1159/000110958. [DOI] [PubMed] [Google Scholar]
- 57.Gatz M, Svedberg P, Pedersen NL, Mortimer JA, Berg S, Johansson B. Education and the risk of Alzheimer’s disease: Findings from the study of dementia in Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2001;56:292–300. doi: 10.1093/geronb/56.5.p292. [DOI] [PubMed] [Google Scholar]
- 58.Yaffe K, Middleton L, Lui L, Spira A, Stone K, Racine C, et al. Prevalence and characterization of mild cognitive impairment and dementia in the oldest old: The SOF-WISE Study. Poster session presented at the International Conference on Alzheimer’s Disease; Honolulu, HI. 10-7-2010. [Google Scholar]
- 59.Paganini-Hill A, Ross RK, Henderson BE. Prevalence of chronic disease and health practices in a retirement community. J Chronic Dis. 1986;39:699–707. doi: 10.1016/0021-9681(86)90153-0. [DOI] [PubMed] [Google Scholar]
- 60.Canadian Study of Health and Aging. Study methods and prevalence of dementia. CMAJ. 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- 61.Ebly EM, Parhad IM, Hogan DB, Fung TS. Prevalence and types of dementia in the very old: Results from the Canadian Study of Health and Aging. Neurology. 1994;44:1593–600. doi: 10.1212/wnl.44.9.1593. [DOI] [PubMed] [Google Scholar]
- 62.Riedel-Heller SG, Busse A, Aurich C, Matschinger H, Angermeyer MC. Prevalence of dementia according to DSM-III-R and ICD-10: Results of the Leipzig Longitudinal Study of the Aged (LEILA75+) Part 1. Br J Psychiatry. 2001;179:250–4. doi: 10.1192/bjp.179.3.250. [DOI] [PubMed] [Google Scholar]
- 63.Ritchie K, Kildea D. Is senile dementia "age-related" or "ageing-related"?. --evidence from meta-analysis of dementia prevalence in the oldest old. Lancet. 1995;346(8980):931–4. doi: 10.1016/s0140-6736(95)91556-7. [DOI] [PubMed] [Google Scholar]
- 64.von Strauss E, Viitanen M, De Ronchi D, Winblad B, Fratiglioni L. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol. 1999;56:587–92. doi: 10.1001/archneur.56.5.587. [DOI] [PubMed] [Google Scholar]
- 65.Wernicke TF, Reischies FM. Prevalence of dementia in old age: clinical diagnoses in subjects aged 95 years and older. Neurology. 1994;44:250–3. doi: 10.1212/wnl.44.2.250. [DOI] [PubMed] [Google Scholar]
- 66.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67:114–21. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer’s disease: Incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997;48:132–8. doi: 10.1212/wnl.48.1.132. [DOI] [PubMed] [Google Scholar]
- 68.McGee MA, Brayne C. Exploring the impact of prevalence and mortality on incidence of dementia in the oldest old: The sensitivity of a deterministic approach. Neuroepidemiology. 2001;20:221–4. doi: 10.1159/000054793. [DOI] [PubMed] [Google Scholar]
- 69.Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di CA, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S4–S9. [PubMed] [Google Scholar]
- 70.Cognitive function and dementia in six areas of England and Wales: the distribution of MMSE and prevalence of GMS organicity level in the MRC CFA Study. The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Psychol Med. 1998;28:319–35. doi: 10.1017/s0033291797006272. [DOI] [PubMed] [Google Scholar]
- 71.Dufouil C, Clayton D, Brayne C, Chi LY, Dening TR, Paykel ES, et al. Population norms for the MMSE in the very old: Estimates based on longitudinal data. Mini-Mental State Examination. Neurology. 2000;55:1609–13. doi: 10.1212/wnl.55.11.1609. [DOI] [PubMed] [Google Scholar]
- 72.Imhof A, Kovari E, von GA, Gold G, Rivara CB, Herrmann FR, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci. 2007;257:72–9. doi: 10.1016/j.jns.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 73.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 74.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–33. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 75.Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29:1587–90. doi: 10.1016/j.neurobiolaging.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sumic A, Michael YL, Carlson NE, Howieson DB, Kaye JA. Physical activity and the risk of dementia in oldest old. J Aging Health. 2007;19:242–59. doi: 10.1177/0898264307299299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia. 2006;49:2015–23. doi: 10.1007/s00125-006-0333-1. [DOI] [PubMed] [Google Scholar]
- 78.van Exel E, de Craen AJ, Gussekloo J, Houx P, Bootsma-van der Wiel A, Macfarlane PW, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann Neurol. 2002;51:716–21. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 79.Silverman JM, Beeri MS, Schmeidler J, Rosendorff C, Angelo G, Mavris RS, et al. C-reactive protein and memory function suggest antagonistic pleiotropy in very old nondemented subjects. Age Ageing. 2009;38:237–41. doi: 10.1093/ageing/afn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawas CH. The oldest old and the 90+ Study. Alzheimers Dement. 2008;4(1 Suppl 1):S56–9. doi: 10.1016/j.jalz.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–5. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes care. 1998;21:1138–45. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 83.Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–13. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 84.Hendrie HC. Lessons learned from international comparative cross-cultural studies on dementia. Am J Geriatr Psychiatry. 2006;14:480–8. doi: 10.1097/01.JGP.0000192497.81296.fb. [DOI] [PubMed] [Google Scholar]
- 85.Hall KS, Hendrie HC. CSI”D” and culture fair cognitive testing. In: Copeland J, Abou-Saleh M, Blazer D, editors. Principles and Practice of Geriatric Psychiatry. 2. John Wiley & Sons, Ltd; Sussex, England: Apr, 2002. [Google Scholar]
- 86.Hall KS, Gao SJ, Emsley CL, Ogunniyi AO, Morgan O, Hendrie HC. Community screening interview for dementia (CSI”D”): Performance in five disparate study sites. Int J Geriatr Psychiatry. 2000;15:521–31. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 87.Prince M, Acosta D, Chiu H, Scazufca M, Varghese M 10/66 Dementia Research Group. Dementia diagnosis in developing countries: A cross-cultural validation study. Lancet. 2003;361(9361):909–17. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 88.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimers Disease (CERAD), Part1: Clinical and neuropsychological assessment of Alzheimer disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 89.Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, et al. CAMDEX: A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 90.Baiyewu O, Unverzagt FW, Lane KA, Gureje O, Ogunniyi A, Musick B, et al. The stick design test: A new measure of visuoconstructional ability. J Int Neuropsychol Soc. 2005;11:598–605. doi: 10.1017/S135561770505071X. [DOI] [PubMed] [Google Scholar]
- 91.Unverzagt FW, Hall KS, Torke AM, Rediger JD, Mercado N, Gureje O, et al. Effects of age, education, and gender on CERAD neuropsychological test performance in an African American sample. Clinical Neuropsychologist. 1996;10:180–90. [Google Scholar]
- 92.Hendrie HC, Lane KA, Ogunniyi A, Baiyewu O, Gureje O, Evans R, et al. The development of a semi-structured home interview (CHIF) to directly assess function in cognitively impaired elderly in two cultures. Int Psychogeriatr. 2006;18:653–66. doi: 10.1017/S104161020500308X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, et al. Incidence of dementia and Alzheimer’s disease in 2 communities: Yoruba residing in Ibadan Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–47. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 94.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 95.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 96.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–3. doi: 10.1001/archneur.62.7.1160. discussion 1167. [DOI] [PubMed] [Google Scholar]
- 97.Dubois B, Feldman HH, Jacova C, Dekosky ST, et al. Research Criteria for the diagnosis of Alzheimer’s disease. Revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 98.Breitner JC, Welsh KA, Robinette CD, Gau BA, Folstein MF, Brandt J. Alzheimer’s disease in the NAS-NRC registry of aging twin veterans. II. Longitudinal findings in a pilot series. National Academy of Sciences. National Research Council Registry. Dementia. 1994;5:99–105. doi: 10.1159/000106703. [DOI] [PubMed] [Google Scholar]
- 99.Breitner JC, Welsh KA, Gau BA, McDonald WM, Steffens DC, Saunders AM, et al. Alzheimer’s disease in the National Academy of Sciences-National Research Council Registry of Aging Twin Veterans. III. Detection of cases, longitudinal results, and observations on twin concordance. Arch Neurol. 1995;52:763–71. doi: 10.1001/archneur.1995.00540320035011. [DOI] [PubMed] [Google Scholar]
- 100.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC, Hayden K, et al. Conversion to dementia from mild cognitive disorder: The Cache County Study. Neurology. 2006;67:229–34. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 101.Crook T, Bartus R, Ferris S, Whitehouse P, Cohen G, Gershon S. Age-associated Memory Impairment: proposed diagnostic criteria and measures of clinical change: Report of the National Institute of Mental Health Work Group. Developmental Neuropsychology. 1986;2:261–76. [Google Scholar]
- 102.Levy R. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in colIaboration with the World Health Organization. Int Psychogeriatr. 1994;6:63–8. [PubMed] [Google Scholar]
- 103.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly: Results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52:612–9. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 104.Sacuiu S, Gustafson D, Johansson B, Thorvaldsson V, Berg S, Sjögren M, et al. The pattern of cognitive symptoms predicts time to dementia onset. Alzheimers Dement. 2009;5:199–206. doi: 10.1016/j.jalz.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 105.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–6. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 106.Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64:443–50. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 108.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 109.Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) Project. Am J Geriatr Psychiatry. 2010;18:674–83. doi: 10.1097/JGP.0b013e3181cdee4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saxton J, Snitz BE, Lopez OL, Ives DG, Dunn LO, Fitzpatrick A, et al. J Neurol Neurosurg Psychiatry. 2009;80:737–47. doi: 10.1136/jnnp.2008.160705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–56. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jin Y-P, Gatz M, Johansson B, Pedersen NL. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology. 2004;63:739–41. doi: 10.1212/01.wnl.0000134604.48018.97. [DOI] [PubMed] [Google Scholar]
- 113.Ganguli M, Rodriguez E, Mulsant B, Richards S, Pandav R, Vander Bilt J, et al. Detection and management of cognitive impairment in primary care: the Steel Valley Seniors Survey. J Am Geriatr Soc. 2004;52:1668–75. doi: 10.1111/j.1532-5415.2004.52459.x. [DOI] [PubMed] [Google Scholar]
- 114.U.S. Preventive Services Task Force. Screening for dementia: recommendation and rationale. Ann Intern Med. 2003;138:925–926. doi: 10.7326/0003-4819-138-11-200306030-00014. [DOI] [PubMed] [Google Scholar]
- 115.Carr DB, Gray S, Baty J, Morris JC. The value of informant vs. individual’s complaints of memory impairment in early dementia. Neurology. 2000;55:1724–6. doi: 10.1212/wnl.55.11.1724. [DOI] [PubMed] [Google Scholar]
- 116.Dodge HH, Meguro K, Ishii H, Yamaguchi S, Saxton JA, Ganguli M. Cross-cultural comparisons of the Mini-Mental State Examination between Japanese and U.S. cohorts. Int Psychogeriatr. 2009;21:113–22. doi: 10.1017/S1041610208007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beeri MS, Schmeidler J, Sano M, Wang J, Lally R, Grossman H, et al. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology. 2006;67:1006–10. doi: 10.1212/01.wnl.0000237548.15734.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manly JJ. Deconstructing race and ethnicity: Implications for measurement of health outcomes. Medical Care. 2006;44:S10–S16. doi: 10.1097/01.mlr.0000245427.22788.be. [DOI] [PubMed] [Google Scholar]
- 119.Ramirez M, Teresi JA, Holmes D, Gurland B, Lantigua R. Differential item functioning (DIF) and the Mini-Mental State Examination (MMSE): Overview, sample, and issues of translation. Medical Care. 2006;44:S95–S106. doi: 10.1097/01.mlr.0000245181.96133.db. [DOI] [PubMed] [Google Scholar]
- 120.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–22. doi: 10.1038/nature08538. PMC2810658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Miller JP, Storandt M, Morris JC. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65:559–64. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 123.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 124.Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 125.Galvin JE, Fagan AM, Holtzman DM, Mintun MA, Morris JC. Relationship of dementia screening tests with biomarkers of Alzheimer’s disease. Brain. 2010;133:3290–3300. doi: 10.1093/brain/awq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-β peptide deposition identified with Pittsburgh compound B: Cognitive decline associated with Aβ deposition. Arch Neurol. 2009;66:1476–81. doi: 10.1001/archneurol.2009.272. PMC2796577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johnson DK, Storandt M, Morris J, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66:1254–59. doi: 10.1001/archneurol.2009.158. PMC2795328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Howieson DB, Holm LA, Kaye JA, Oken BS, Howieson J. Neurologic function in the optimally healthy oldest old: Neuropsychological evaluation. Neurology. 1993;43:1882–86. doi: 10.1212/wnl.43.10.1882. [DOI] [PubMed] [Google Scholar]
- 129.Silwinski M, Lipton RB, Buschke H, Stewart W. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J Gerontol B Psychol Sci Soc Sci. 1996;51:P217–P225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- 130.Tuokko H, Garrett DD, McDowell I, Silverberg N, Kristjansson B. Cognitive decline in high-functioning older adults: reserve or ascertainment bias? Aging and Mental Health. 2003;7:259–270. doi: 10.1080/1360786031000120750. [DOI] [PubMed] [Google Scholar]
- 131.Storandt M, Morris JC. Ascertainment bias in the clinical diagnosis of Alzheimer’s disease. Arch Neurol. 2010;67:1364–1369. doi: 10.1001/archneurol.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]