Abstract
Cytotoxic T cells play a critical role in the control of HIV and the progression of infected individuals to AIDS. 2B4 (CD244) is a member of the SLAM family of receptors that regulate lymphocyte development and function. The expression of 2B4 on CD8+ T cells was shown to increase during AIDS disease progression. However, the functional role of 2B4+CD8+ T cells against HIV infection is not known. Here, we have examined the functional role of 2B4+CD8+ T cells during and after stimulation with HLA B14 or B27 restricted HIV epitopes. Interestingly, IFN-γ secretion and cytotoxic activity of 2B4+ CD8+ T cells stimulated with HIV peptides were significantly decreased when compared to influenza peptide stimulated 2B4+ CD8+ T cells. The expression of the signaling adaptor molecule SAP was downregulated in 2B4+ CD8+ T cells upon HIV peptide stimulation. These results suggest that 2B4+CD8+ T cells play an inhibitory role against constrained HIV epitopes underlying the inability to control the virus during disease progression.
Keywords: Cytotoxic T lymphocyte (CTL), cell surface receptor, 2B4, HIV epitopes, B lymphoblastoid cell line (BLCL
Introduction
An effective cytotoxic T lymphocyte (CTL) response is essential for the immune system to control HIV [1,2]. A unique set of seropositive individuals that are capable of containing HIV infection and delaying the progression to AIDS, termed Long Term Non Progressors (LTNPs) [3], have been shown to have potent anti-HIV CD8+ T cell responses [1,2]. Many LTNPs possess human leukocyte antigen (HLA) B14 or B27 and several studies have shown that individuals that express B14 or B27 have an overall low severity of disease during viral infection. This is likely due to its ability to present epitopes of the virus that are shared among different strains and present it with complex stability [4]. Furthermore, this presentation causes heightened activation of killer T cells that can kill more viral particles. Thus, allowing for immune control of HIV in infected individuals to control viral replication for long periods of time, although not completely eliminating the virus.
HIV possesses many genes that allow it to infect, evade and replicate in a host. The group-specific antigen (Gag) gene in HIV codes for many structural proteins needed for the virion itself. Although, Gag is a late structural protein in the replication cycle of HIV, it has been shown that Gag-specific CD8+ T cells recognize infected cells around 2 hours post infection with SIV-mac239 and are able to eliminate infected cells as early as 6 hours after infection [5]. Conversely, Tat and Env proteins were not able to generate this heightened early response. Specifically we are interested in the p17 and p24 Gag region which makes up the protective matrix and viral capsid, respectively. It has been suggested that the resistance caused by HLA B14 and B27 are due to strong CTL responses against the p17 and p24 Gag proteins [1,6]. However, in non-progressors that are not linked with HLA B27, a p24 specific proliferative response was associated with control of HIV when combined with CTL, additionally highlighting the importance of p24 targeting [7].
2B4 (CD244) is a member of the signaling lymphocyte activation molecule (SLAM/CD150) family of receptors that regulate immune responses [8,9]. 2B4 was first identified in mice as an NK cell activating receptor [10]. However, it is now well established that 2B4 can function as an activating or inhibitory receptor in both mice and human [11,12]. In addition to NK cells, 2B4 is expressed on basophils, eosinophils, monocytes and a subpopulation of CD8+ T cells [13,14]. In human NK cells, this receptor usually acts as an activator of cytotoxic function and IFN-γ secretion, although it has been found to act as an inhibitory receptor when expressed at high levels [8,11]. CD48 is the physiological ligand for 2B4 and its interaction causes cell proliferation when induced between NK cells or with nearby T cells [11,15,16]. 2B4+ CD8+ T cells represent a population of activated or memory T cells, usually about 50% or less of the total amount of the whole CD8+ T cell population [17,18]. Little is known about this receptor's functional role in HIV infection and the progression to AIDS. Interestingly, the levels of 2B4+CD8+ T cells have been shown to increase during the course of HIV disease [19]. The expression of the 2B4 ligand CD48 is downregulated in HIV infected cells [20]. During HIV infection, NK cells have been shown to upregulate 2B4 but have lowered killing activity of HIV infected cells, despite the infected cell downregulation of MHC molecules [21]. SLAM –associated protein (SAP), an intracellular adaptor molecule that mediates signal transduction through 2B4, is expressed in NK and T cells. Previous studies highlight the importance of this signaling molecule in the activating function of 2B4 [17,22]. Examination of 2B4+ CD8+ T cells in relation to Human T-Lymphocyte Virus -1 (HTLV-1) demonstrate that 2B4 and the SAP pathway may be involved in cytotoxic activity of CD8+ T cells [23]. Blocking 2B4 or knocking down SAP expression resulted in inhibition of killing activity, implying that 2B4 is involved in the recognition and killing of HTLV-1 infected cells [23].
In this study, we generated an in vitro method using HIV negative HLA B14 and B27 donors in order to compare the responses of 2B4+ and 2B4− T cell populations after priming with conserved HIV antigens with known affinity for the corresponding HLA types. We observed upregulation of the 2B4 receptor in both populations of cells. However, IFN-γ secretion and cytotoxic activity by the 2B4+ CD8+ T cell population that was stimulated with HIV peptide was significantly decreased when compared to influenza peptide stimulated controls. Also, SAP was downregulated in 2B4+CD8+ T cells after HIV peptide pulsed APC stimulation. This suggests that 2B4 may have a key role in the suppressed immune function of 2B4+ CD8+ T cells during HIV infection.
Materials and Methods
Identification of HLA B27 Supertype Donors and Cell Lines
Initial screening of subjects was done by obtaining buccal swabs from healthy volunteers. These samples were HLA typed at Tepnel Lifecodes, Stamford, CT. Four volunteers were identified as having HLA B14 or B27. Blood samples were obtained from these donors and the respective B Lymphocyte Cell Lines (BLCL) were generated at the tissue culture facility at UNC Lineberger Comprehensive Cancer Center, Chapel Hill, NC. Arbitrarily, BLCL-2 expresses class I HLA B14 and B8, BLCL-3 expresses class I HLA B27 and B44, and BLCL-4 expresses class I HLA B27 and B8. All cell lines were cultured in RPMI 1640 supplemented with 10% FBS and antibiotic (Invitrogen, Carlsbad, CA).
Conserved HIV Peptides
Synthetic HIV peptides were selected using the Los Alamos database. These peptides were chosen based on known affinity for HLA B14 and B27. All peptides are from conserved regions of the HIV protective matrix and capsid structural protein, p17 and p24 Gag respectively. Synthetic peptides were synthesized by Celtek Peptides, Nashville, TN. Six conserved HIV peptides including escape variants and one influenza virus peptide used in this study are listed in table 1.
Table 1.
Influenza and HIV peptides and escape variants
| Name | Epitope sequence | Binding | Region of virus |
|---|---|---|---|
| FL-8 | F L K E KG G L | Influenza peptide | |
| DE-10-1 | D R F Y K T L R A E | B14 | p24 gag 166–174 |
| DE-10-2 | D R F Y K I L R A E | Escape variant | |
| IK-9 | I R L R P G G K K | B27 | p17 gag 19–27 |
| IY-11 | I L G L N K I V R M Y | B7, presumed to bind B27 | p24 gag 135 – 145 |
| KK10-1 | K R W I I L G L N K | B27 | p24 gag 131–140 |
| KK10-2 | K R W I I M G L N K | Escape variant |
abold italicized amino acids denote mutations
Monocyte induced Differentiation to Dendritic Cells
Whole blood was obtained from the same donors previously used to prepare the BLCL. PBMC's were purified by ficol separation using a histopaque gradient and monocytes were isolated by magnetic activated cell sorting (MACS) using a monocyte isolation kit (Miltenyi Biotec, Auburn, CA) or by allowing adherence to a polyethylene plate as previously described [24]. Monocytes were differentiated into dendritic cells by culturing for 10 days in the presence of 800 U/ml recombinant human GM-CSF and 500 U/ml recombinant human IL-4 (R&D systems, Minneapolis, MN). Monocyte conditioned media (MCM) was added for dendritic cell maturation on day 7 at a 50% concentration. MCM was made as previously described [24].
Dendritic Cell Presentation and CD8+ T Cell Activation
CD8+ T cells were isolated from whole blood by MACS using a CD8+ T cell kit (Miltenyi Biotec, Auburn, CA). After incubation with anti-2B4 (C1.7, Beckman Coulter, Brea, CA), 2B4− and 2B4+ T cell populations were separated by Cytopeia influx cell sorting. After 10 day incubation, monocyte-derived dendritic cells (mDC), discussed above, were cultured without (deemed “no peptide” cultured) or with 50 μg/ml of each peptide (Table 1) and 3μg/ml β□-Microglobulin (β□M) (Calbiochem, Gibbstown, NJ) in AIM V medium (Invitrogen, Carlsbad, CA) for 2–4 hours at 37°C. After peptide pulsing, mDC's were treated with 100 μg/ml mitomycin C (Sigma, St. Louis, MO), immediately washed and resuspended into 4+RPMI with 10% human AB serum. CD8+ T cells were resuspended and layered onto the mDC's with a final volume of 1.5ml. 10ng/ml IL-7 was added to the cultures on day 1 and 10 units/ml IL-2 was added on day 2 (R&D systems, Minneapolis, MN) and cultured for 8 days at 37°C. After the first 8 day stimulation, new mDC's were added in a similar fashion for the second stimulation and cultured for another 8 days. After each stimulation, supernatant was harvested from each well and an Interferon-gamma Enzyme Linked ImmunoSorbent Assay (IFN-γ ELISA) was performed (BD Biosciences, San Jose, CA).
Cell Surface Expression of 2B4
2B4− and 2B4+ CD8+ cells were harvested from each stimulated population, as discussed previously, after two rounds of stimulation. After incubation with anti-2B4 (C1.7), flow cytometry was performed and 2B4 expression was determined by mean fluorescence intensity (MFI).
Cytotoxicity Assay Comparison of 2B4− and 2B4+ CD8+ T Cells
After two stimulations with APC's, a 4 hour cytotoxicity assay was performed. CD8+ T cells were harvested and used to make serial dilutions such that there would be an effector to target ratio of 20:1. For making target cells, BLCLs discussed previously were used and were pulsed at a concentration of 50μg/ml with each of the individual peptides (same as used for CD8+ T cell priming) overnight in AIM-V medium at 37°C prior to performing this assay. After pulsing overnight, BLCLs were resuspended into 4+ RPMI with 10% human AB serum and incubated for 90 minutes at 37°C with radioactive sodium chromate (51Cr) (Perkin Elmer, Waltham, MA) at a concentration of 1/5 chromate to 4/5 medium and were washed and incubated with the effector cells for 4 hrs. The supernatants were then collected and analyzed for radioactive chromium (51Cr) released.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
2B4+ and 2B4− CD8 T cells were retained prior to first stimulation, 8 days after first stimulation and 8 days after second stimulation in order to generate cDNA to analyze SAP expression. The cells were dissolved with RNA STAT-60 (Tel-test Inc. Friendswood, TX) in 5 million cells per 1 ml and kept at −80°C until RNA extraction. Total RNA was isolated as per standard procedures. 2μg of RNA was used for cDNA synthesis in the presence of primer mixture of random hexamer (New England Biolabs, Ipswich, MA) and oligo dT (Integrated DNA Technologies, Coralville, IA). cDNA was analyzed by PCR for SAP expression. Beta actin primers were utilized as a control. SAP primers used were hSAP FP -5'-GAC GCA GTG GCT GTG TAT- 3' and hSAP RP -5'-TCA TGG GGC TTT CAG GCA GAC-3'. PCR products were analyzed by electrophoresis on a 1.2% agarose gel with ethidium bromide and visualized by UV fluorescence. SAP PCR product is approxiametly 384 bp. Beta Actin PCR product is approximately 540 bp. The bands were subjected to densitometry analysis and the HIV peptide and influenza peptide stimulated samples were normalized with respect to no peptide sample and the data was represented as fold change.
Statistical Analysis
The statistical significance of differences between groups was determined by ANOVA. A probability of p< 0.05 was considered to be significant.
Results and Discussion
Production of IFN-gamma by 2B4+ CD8+ T cells is inhibited by dendritic cells presenting HIV peptides
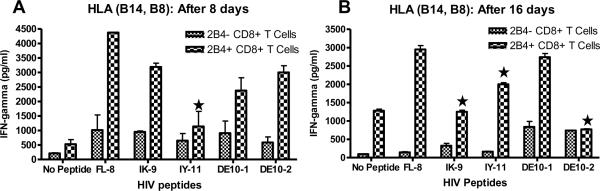
We examined the effects of stimulation with HIV peptide pulsed APCs on 2B4− and 2B4+ CD8+ T cell on cytokine secretion. Because IFN-γ is the predominant cytokine secreted by this population of immune cells in response to viral infections, we determined IFN-γ production. PBMCs from two HIV negative individuals that possess HLA-B14 and B27 (BLCL-2 and 3 respectively) were sorted into 2B4+CD8+ T cell and 2B4−CD8+ T cell populations. These cells were then cultured for 16 days with HIV peptide-pulsed dendritic cells that presented epitopes known to bind to the respective HLA types. The first and second round of stimulations in which autologous peptide pulsed APC's were added to the culture of 2B4− and 2B4+ CD8+ T cells were approximately 8 days each, and ELISAs were performed on day 8 and day 16. In order to confirm that the levels of secretion were due to the HIV peptide stimulation itself and not just due to viral antigen, we compared the secretion of IFN-γ released by HIV peptide activated cells with that of Influenza peptide activated cells. Both after the first 8 days and 16 days of in vitro stimulation, IFN-γ secretion was decreased in all HIV peptide stimulated 2B4+ CD8+ T cells from HLA- B14 donor when compared to the 2B4+ T cells that were stimulated with influenza peptide (Fig. 1). Similar data was also obtained from HLA-B27 donors (data not shown). This data suggests that 2B4+ CD8+ T cells lose their ability to produce sufficient amounts of IFN-γ during immune responses to HIV.
Fig. 1.
HIV antigen pulsed APC stimulation inhibits IFN-g production in 2B4+ CD8+ T cells. 2B4− and 2B4+ CD8+ T cells were primed either without peptide or with Influenza and HIV-peptide pulsed APCs. IFN-γ released was measured by ELISA after first and second stimulation with APCs, approximately 8 and 16 days respectively. PBMCs from HIV-negative HLA B14 donor (BLCL-2) after 8 days (A) and 16 days of stimulation (B). (*- p<0.05; 2 way ANOVAs detected significant differences between 2B4+ CD8+ T cells stimulated with HIV peptides and FL-8).
Cytolytic activity of 2B4+ CD8+ T cells is inhibited by dendritic cells presenting HIV peptides
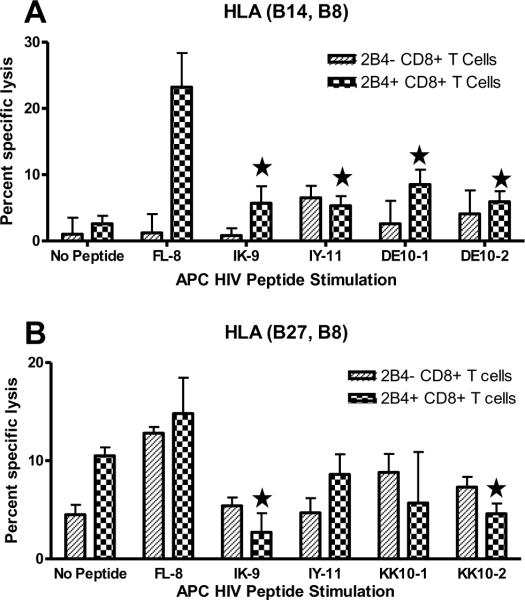
In order to determine the cytolytic activity of 2B4+ CD8+ T cells towards HIV infected cells, we performed a cytotoxicity assay to compare the killing activity between 2B4− and 2B4+ CD8+ T cells that were primed using the influenza peptide verses those that were primed using the HIV peptides. The two populations were stimulated and restimulated as described before. At the end of this time period they were used as effector cells and tested for their cytotoxic activity on non-peptide pulsed and influenza or HIV peptide-pulsed autologous BLCL targets. As shown in Fig. 2, both the individuals showed marked decrease in HIV primed 2B4+ CD8+ T cell cytotoxicity when compared to the same type of cells that were primed with influenza peptide. Therefore, the decrease in cytotoxicity was influenced by the priming with HIV epitopes themselves, and not due to priming with viral antigen as a whole, implying that priming with HIV peptides specifically suppresses killing activity of 2B4+ CD8+ T cells.
Fig. 2.
Cytotoxic activity against peptide pulsed targets is decreased after HIV-peptide pulsed APC stimulation. 2B4+ and 2B4− CD8+ T cells primed with two rounds of HIV-peptide pulsed APCs were analysed for their cytotoxic activity against peptide pulsed BLCLs in a standard 4 hr51Cr release assay. CD8+ T cells used as effectors and the target cells BLCL-2 were from HLA B14 donor (A), CD8+ T cells used as effectors and the target cells BLCL-3 were from HLA B27 donor (B). Data shown is at an E:T ratio of 20:1. Each data point is the mean ± SEM of two independent experiments done in triplicates. (*- p<0.05; 2 way ANOVAs detected significant differences between 2B4+ CD8+ T cells stimulated with HIV peptides and FL-8).
SAP is downregulated in 2B4+ CD8+ T cells upon HIV peptide stimulation
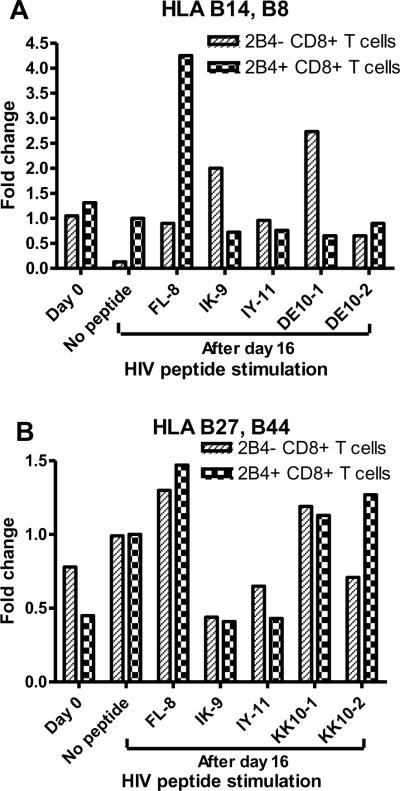
The signaling adaptor protein SAP/SH2D1A plays a crucial role in regulating signals through SLAM-related receptors [9]. SAP is essential for 2B4-mediated activation signaling in NK cells [22]. It has been shown that in the absence of SAP other SAP-related adaptor molecules that can transduce inhibitory signals bind to the immunoreceptor tyrosine-based switch motif (ITSM) of 2B4 [25]. In order to get an insight into the reduced functional activity of 2B4+ CD8+ T cells, we determined the expression of SAP upon HIV peptide stimulation. As seen in Fig. 3, the expression of SAP was significantly reduced in 2B4+ CD8+ T cells upon HIV antigen presentation by dendritic cells.
Fig. 3.
SAP is downregulated in 2B4+ CD8+ T cells upon HIV peptide stimulation. 2B4+ and 2B4− CD8+ T cells before stimulation (day 0) and after HIV peptides and influenza peptide (FL-8) stimulation (after day 16) were harvested and total RNA was extracted and SAP expression was evaluated by RT-PCR for HLA B14, B8 donor (A) and HLA B27, B44 donor (B). The bands were subjected to densitometry analysis and the relative density of HIV peptide and influenza peptide stimulated samples were normalized with respect to no peptide sample and the data was represented as fold change.
People that have progressively worse AIDS disease have lower CD4+ T cells and a higher population of 2B4+ CD8+ T cells [19]. A recent study of HTLV-1 patients showed 2B4 as an important receptor in the recognition and killing of infected cells by CTL [23]. 2B4 upregulation during the later stages of HIV disease may cause further disease progression by inhibiting the killing of the virus, or it may serve as the final effort by the immune system to upregulate the molecules necessary to fight the virus. The present study suggests that 2B4+ CD8+ T cell activation with APCs is inhibited during in vitro HIV peptide stimulation, leading to decreased killing activity. This finding is surprising because 2B4 expression on CD8+ T cells may highlight a population with high cytotoxic function [26]. As shown in Figure 1, after 8 and 16 days of priming with APCs, the HIV stimulated 2B4+ CD8+ T cells secreted less IFN-γ when compared to influenza peptide stimulated 2B4+ CD8+ T cells. This inhibition suggests that 2B4 expression on CD8+ T cells plays a role in antigen recognition and activation via MHC class I-HIV epitope complex. This data provides a rationale for the finding that upregulation of 2B4 in CD8+ T cells correlates with worsening HIV disease progression [19]. As this molecule is being overexpressed, likely due to repeated activation of CD8+ T cells with HIV epitopes (shown to be a factor over the course of two stimulations, data not shown), cytokine secretion is downregulated. Cytokines are needed in order to dictate the function of immune cells, and without sufficient amounts of IFN-γ, CD8+ T cells may not function as efficiently in terms of their cytotoxic activity. We have observed an upregulation of the 2B4 receptor after two stimulations with influenza or HIV peptide pulsed dendritic cells (data not shown), nevertheless inhibition of IFN-γ secretion and killing activity only occurred in HIV primed cells. The decreased expression of SAP in 2B4+ CD8+ T cells upon HIV peptide stimulation implicate that SAP is involved in functional activity of these cells. Lowered expression of SAP in these cells could allow other adaptor molecules such as SHP-1, SHP-2, EAT-2 to bind to the ITSM motif of 2B4 and facilitate inhibitory signaling. In contrast to HIV infection, in HTLV-1 disease SAP expression was upregulated in 2B4+ CD8+ T cells [23].
In conclusion, we have shown that priming with HIV epitopes leads to decreased IFN-γ secretion and cytotoxic activity of 2B4+ CD8+ T cells. This could explain the inability of cytotoxic T lymphocytes to control the virus replication in HIV infection and disease progression.
Acknowledgements
This study was supported by the EXPORT center pilot grant # P20MD001633 from the National Center for Minority Health and Health Disparities (NCMHD) and a grant from Miller Brewing Company, Fort Worth, TX. We thank Dr. Xiangle Sun for assistance with flow cytometry. Flow cytometry was performed in the Flow Cytometry and Laser Capture Microdissection Core Facility at the University of North Texas Health Science Center. Flow Cytometry facility is supported by National Institutes of Health award ISIORR018999-01A1.
Abbreviations used in this paper
- (BLCL)
B Lymphoblastoid Cell Lines
- (Gag)
Group-Specific Antigen
- (mDC)
monocytes-derived Dendritic Cells
- (MCM)
Monocyte Conditioned Media
- (SAP)
SLAM -associated protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Schneidewind A, Brockman MA, Sidney J, Wang YE, Chen H, Suscovich TJ, Li B, Adam RI, Allgaier RL, Mothe BR, Kuntzen T, Oniangue-Ndza C, Trocha A, Yu XG, Brander C, Sette A, Walker BD, Allen TM. Structural and functional constraints limit options for cytotoxic T-lymphocyte escape in the immunodominant HLA-B27-restricted epitope in human immunodeficiency virus type 1 capsid. J. Virol. 2008;82:5594–5605. doi: 10.1128/JVI.02356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].den Uyl D, van der Horst-Bruinsma IE, van Agtmael M. Progression of HIV to AIDS: a protective role for HLA-B27? AIDS Rev. 2004;6:89–96. [PubMed] [Google Scholar]
- [3].Imami N, Gotch F. Mechanisms of loss of HIV-1-specific T-cell responses. J. HIV Ther. 2002;7:30–34. [PubMed] [Google Scholar]
- [4].Brooks JM, Colbert RA, Mear JP, Leese AM, Rickinson AB. HLA-B27 subtype polymorphism and CTL epitope choice: studies with EBV peptides link immunogenicity with stability of the B27:peptide complex. J. Immunol. 1998;161:5252–5259. [PubMed] [Google Scholar]
- [5].Sacha JB, Chung C, Rakasz EG, Spencer SP, Jonas AK, Bean AT, Lee W, Burwitz BJ, Stephany JJ, Loffredo JT, Allison DB, Adnan S, Hoji A, Wilson NA, Friedrich TC, Lifson JD, Yang OO, Watkins DI. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 2007;178:2746–2754. doi: 10.4049/jimmunol.178.5.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johnson RP, Trocha A, Yang L, Mazzara GP, Panicali DL, Buchanan TM, Walker BD. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J. Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- [7].Dyer WB, Zaunders JJ, Yuan FF, Wang B, Learmont JC, Geczy AF, Saksena NK, McPhee DA, Gorry PR, Sullivan JS. Mechanisms of HIV non-progression; robust and sustained CD4+ T-cell proliferative responses to p24 antigen correlate with control of viraemia and lack of disease progression after long-term transfusion-acquired HIV-1 infection. Retrovirology. 2008;5:112. doi: 10.1186/1742-4690-5-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vaidya SV, Mathew PA. Of mice and men: different functions of the murine and human 2B4 (CD244) receptor on NK cells. Immunol. Lett. 2006;105:180–184. doi: 10.1016/j.imlet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- [9].Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat. Rev. Immunol. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- [10].Mathew PA, Garni-Wagner BA, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J. Immunol. 1993;151:5328–5337. [PubMed] [Google Scholar]
- [11].Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular Basis of the Dual Functions of 2B4 (CD244) J. Immunol. 2008;180:8159–8167. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- [12].Mathew PA. Regulation of NK cell function by 2B4 (CD244) receptor. Curr. Trends Immunol. 2008:25–32. [Google Scholar]
- [13].Boles KS, Nakajima H, Colonna M, Chuang SS, Stepp SE, Bennett M, Kumar V, Mathew PA. Molecular characterization of a novel human natural killer cell receptor homologous to mouse 2B4. Tissue Antigens. 1999;54:27–34. doi: 10.1034/j.1399-0039.1999.540103.x. [DOI] [PubMed] [Google Scholar]
- [14].Nakajima H, Cella M, Langen H, Friedlein A, Colonna M. Activating interactions in human NK cell recognition: the role of 2B4- CD48. Eur. J. Immunol. 1999;29:1676–1683. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- [15].Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J. Exp. Med. 1998;188:2083–2090. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Latchman Y, McKay PF, Reiser H. Identification of the 2B4 molecule as a counter-receptor for CD48. J. Immunol. 1998;161:5809–5812. [PubMed] [Google Scholar]
- [17].Assarsson E, Kambayashi T, Persson CM, Chambers BJ, Ljunggren HG. 2B4/CD48-mediated regulation of lymphocyte activation and function. J. Immunol. 2005;175:2045–2049. doi: 10.4049/jimmunol.175.4.2045. [DOI] [PubMed] [Google Scholar]
- [18].Altvater B, Landmeier S, Pscherer S, Temme J, Juergens H, Pule M, Rossig C. 2B4 (CD244) signaling via chimeric receptors costimulates tumor-antigen specific proliferation and in vitro expansion of human T cells. Cancer Immunol. Immunother. 2009;58:1991–2001. doi: 10.1007/s00262-009-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peritt D, Sesok-Pizzini DA, Schretzenmair R, Macgregor RR, Valiante NM, Tu X, Trinchieri G, Kamoun M. C1.7 antigen expression on CD8+ T cells is activation dependent: increased proportion of C1.7+CD8+ T cells in HIV-1-infected patients with progressing disease. J. Immunol. 1999;162:7563–7568. [PubMed] [Google Scholar]
- [20].Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ostrowski SR, Ullum H, Pedersen BK, Gerstoft J, Katzenstein TL. 2B4 expression on natural killer cells increases in HIV-1 infected patients followed prospectively during highly active antiretroviral therapy. Clin. Exp. Immunol. 2005;141:526–533. doi: 10.1111/j.1365-2249.2005.02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J. Immunol. 1999;162:6981–6985. [PubMed] [Google Scholar]
- [23].Enose-Akahata Y, Matsuura E, Oh U, Jacobson S. High expression of CD244 and SAP regulated CD8 T cell responses of patients with HTLV-I associated neurologic disease. PLoS Pathog. 2009;5:e1000682. doi: 10.1371/journal.ppat.1000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zarling AL, Johnson JG, Hoffman RW, Lee DR. Induction of primary human CD8+ T lymphocyte responses in vitro using dendritic cells. J. Immunol. 1999;162:5197–5204. [PubMed] [Google Scholar]
- [25].Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J. Exp. Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rey J, Giustiniani J, Mallet F, Schiavon V, Boumsell L, Bensussan A, Olive D, Costello RT. The co-expression of 2B4 (CD244) and CD160 delineates a subpopulation of human CD8+ T cells with a potent CD160-mediated cytolytic effector function. Eur. J. Immunol. 2006;36:2359–2366. doi: 10.1002/eji.200635935. [DOI] [PubMed] [Google Scholar]