Abstract
Nuclear receptors (NRs) are ligand-activated transcriptional factors that are involved in various physiological, developmental, and toxicological processes. Farnesoid X receptor (FXR) is a NR that belongs to the NR superfamily. The endogenous ligands of FXR are bile acids. FXR is essential in regulating a network of genes involved in maintaining bile acid and lipid homeostasis. It is clear that FXR is critical for liver and intestinal function. In mice FXR deficiency leads to the development of cholestasis, gallstone disease, nonalcoholic steatohepatitis, liver tumor, and colon tumor. Using mouse models where FXR is deleted either in the whole-body, or selectively in hepatocytes or enterocytes, we start to reveal the importance of tissue-specific FXR function in regulating bile acid and lipid homeostasis. However, a great challenge exists for developing tissue-specific FXR modulators to prevent and treat diseases associated with bile acid or lipid disorders. With further understanding of FXR function in both rodents and humans, this nuclear receptor may emerge as a novel target to prevent and treat liver, gastrointestinal and systemic diseases.
1. Introduction to nuclear receptor superfamily
Nuclear receptors (NRs) are a superfamily of ligand-activated transcription factors that regulate diverse biological functions. The NR superfamily is classified into three groups: the classical endocrine NRs, the orphan nuclear receptors (ONRs), and the adopted ONRs. Farnesoid X receptor (FXR) is an adopted ONR. The adopted ONR family, including the peroxisome proliferators-activated receptors (PPARs), liver X receptor, FXR, pregnane X receptor (PXR), and constitutive androstane receptor (CAR), is essential for the body to respond to endogenous or exogenous nutritional and environmental factors. Because the function of FXR in humans is not clear, the current review will mainly focus on research findings obtained from mice.
2. FXR cloning and structure
The FXR gene (gene symbol: NR1H4/Nr1h4) was first cloned from mouse and rat liver by using a degenerative probe corresponding to the highly conserved DNA binding domain of the NR superfamily [1, 2]. The FXR gene is evolutionally conserved and similarity among different species is high. In drosophila the counterpart of FXR is the ecdysone receptor, EcR [3].
The human NR1H4 and the murine Nr1h4 gene are located on chromosome 12 and 10, respectively. Using alternative promoters and splicing, four FXR isoforms in humans and mice have been identified, FXRα1, FXRα2, FXRβ1, and FXRβ2 [4, 5]. In C57BL/6J mice, FXRα is highly expressed in liver, kidney, intestine, and adrenal gland (www.nursa.org/10.1621/datasets.02001), and FXRβ is highly expressed in liver and testis (www.nursa.org/10.1621/datasets.02001). In humans, FXRα is highly expressed in adrenal and liver while FXRβ is highly expressed in colon, duodenum and kidney with a much lower expression level in liver [5].
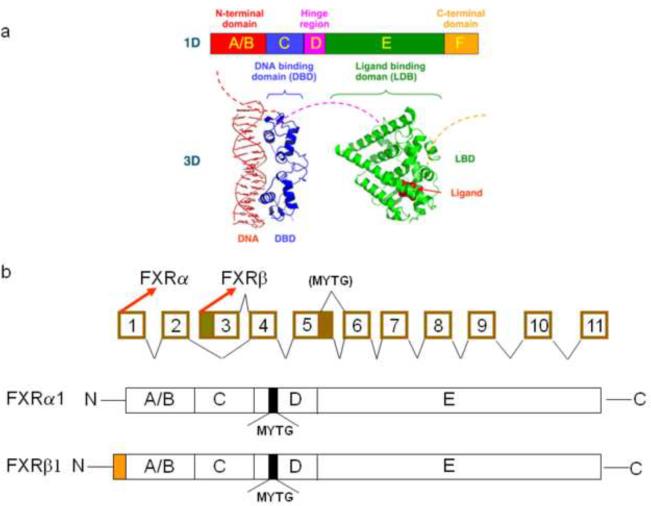
FXR protein shares the common structure with other NR superfamily members (Fig. 1), including the DNA-binding domain in the N-terminal region and the ligand-binding domain in the C-terminal region. The ligand-independent activation function-1 (AF1) domain is located in the N-terminal region and the ligand-dependent activation function-2 domain (AF2) is located in the C-terminal region [6, 7].
Fig 1. Gene and protein structure of FXR.
(a) General structure of nuclear receptors (NRs). A typical NR contains several domains. The N-terminal region (A/B) contains the ligand-independent AF1 transactivation domain. The C region contains the conserved DNA binding domain. A hinge region (D) connects the DNA- and ligand-binding domain. The E region contains the ligand-binding domain. (b) Schematic diagram of the murine Nr1h4 genomic structure. The mouse Nr1h4 gene is composed of 11 exons and 10 introns. FXRα and FXRβ are transcribed from exon 1 and 3, respectively. A 12 base-pair (bp) insert is located at the 3′ end of exon 5. Alternative splicing from exons 5 and 6 produces two FXR isoforms that either contain the 12-bp insert or not. FXRα and FXRβ have the same amino acid sequence, except for an additional 37 amino acid at the N-terminal of FXRβ. FXRα1 and FXRβ1 have a 4 amino acid insert (MYTG) that is located at the hinge region.
The most recognized DNA binding sequence by FXR is an inverted repeat separated by one nucleotide (IR1). This FXR response element (FXRE) has been detected in many FXR target genes [1, 8–13]. In addition, FXR has been reported to bind to a few other response elements, such as an everted repeat separated by 8 nucleotides (ER8) in the Abcc2 gene [14], but the binding affinity may be lower compared to binding to IR1. Recently, our group has identified a novel FXR motif, ER2, which is present in many FXR binding sites, in addition to IR1 [15]. FXR normally binds to FXRE in gene regulatory regions as a heterodimer with 9-cis-retinoid acid receptor α (RXRα). It is commonly considered that without ligand binding, a co-repressor complex may be associated with the FXR/RXR dimer, which prevents the recruitment of the transcriptional activation machinery to get access to FXR target genes. Upon ligand binding, FXR undergoes conformational changes, which may result in the release of co-repressor(s) and subsequent recruitment of co-activator complex. The best characterized co-activators for the FXR/RXR dimer include steroid receptor coactivator-1, protein arginine(R)methyltransferase-1, PPARγ coactivator-1α and histone acetyl transferase [16, 17]. How FXR interacts with co-activators (and co-repressors) and the mechanism by which FXR interacts with co-factors to regulate gene-specific transcription is largely unknown.
3. FXR modulators
3.1. Endogenous ligands of FXR
Bile acids are endogenous ligands of FXR [18–20], although FXR may be activated by high concentrations of farnesol and juvenile hormone III [1]. It is clear that several bile acids activate FXR with high potency, including chenodeoxycholic acid (CDCA) and its conjugates, lithocholic acid (LCA) and its conjugates, deoxycholic acid (DCA) and its conjugates and cholic acid (CA) and its conjugates. Synthesis and excretion of bile acids represent the most important mechanism to remove cholesterol from our body. The field of bile acid research has been greatly advanced by identifying bile acids as signaling molecules that activate a cohort of NRs, including FXR, PXR, CAR and vitamin D receptor [21–24]. Activating these receptors by bile acids collectively maintains bile acid homeostasis by regulating bile acid synthesis, transport and metabolism. In addition to activating NRs, bile acids activate a G-protein coupled membrane receptor, TGR5 [25]. Activation of TGR5 is critical in modulating thyroid hormone function and thermogenesis in the brown adipose tissue as well as regulating glucagon-like peptide production in the L cells in small intestine to regulate glucose homeostasis [26, 27].
3.2. Synthetic FXR ligands
Because of the innate detergent property, bile acids are toxic to cells at higher concentrations. In addition, bile acids activate multiple signaling pathways, thus acting as non-specific FXR ligands. A significant effort has been devoted to develop non-bile acid FXR modulators that may be used to treat bile acid and/or lipid related disorders. Increasing numbers of non-bile acid modulators that activate FXR with high potency have been developed, including GW4064 [28], 6α-ethyl-CDCA [29], fexaramine [30], methyl cholate, methyl deoxycholate, 5β-cholanic acid, 5β-cholanic acid-7α,12α-diol, NIHS700, marchantin A and marchantin E, and MFA-1 [31, 32]. In animal studies some of these synthetic FXR modulators have been shown to reduce serum triglycerides [28], prevent cholestasis [33], and suppress liver fibrosis [34–36]. A comprehensive review of synthetic FXR ligands has been recently published for an update status of FXR modulator development [37]. However, without a comprehensive understanding of the biological functions of FXR, the clinical use of FXR full agonists needs to be cautioned because FXR full agonists may result in undesired toxicities. Based on prior experience and success in developing selective estrogen and androgen receptor modulators, it is reasonable to predict that selective FXR modulators will be more useful in the clinic. Indeed, effort has been spent in identifying gene-selective FXR modulators [38], however their pharmaceutical development and clinical use are not clear.
Research and development on FXR antagonists is limited. A main ingredient extracted from resins of the guggle tree, guggulsterone, has been identified as a potent FXR antagonist [39]. Further studies showed that the action of guggulsterone is not specific. In addition to antagonizing FXR, this guggle tree extract also acts on several steroid hormone receptors, including the mineralocorticoid receptor, the androgen receptor, as well as the glucocorticoid receptor [40].
4. Tissue-specific function of FXR in regulating bile acid, cholesterol, and triglyceride homeostasis
4.1. Synthesis and enterohepatic circulation of bile acids
The maintenance of cholesterol homeostasis is vital to survival and development. Disruption of cholesterol homeostasis results in serious cardiovascular and neurological disorders. The most important route in the body for cholesterol elimination is the conversion of cholesterol into bile acids in the liver [41]. Two pathways are responsible for bile acid synthesis in the liver: the neutral or classical pathway and the acidic or alternative pathway. Cholesterol-7α-hydroxylase (CYP7A1) is the rate-limiting enzyme in the classic pathway and sterol 27-hydroxylase (CYP27A1) is the rate-limiting enzyme in the alternative pathway. The classical pathway generates both CA and CDCA, collectively called primary bile acids. The acidic pathway mainly synthesizes CDCA. Primary bile acids are conjugated to glycine or taurine before their excretion into the bile mediated by two canalicular transporters, bile salt efflux pump (Bsep) and multi-drug resistance-associated protein 2 (Mrp2) [42, 43]. Bile is released into the small intestine upon food consumption. In the ileum, after facilitating lipid and lipid-soluble vitamin absorption, most of the primary bile acids are absorbed from intestinal lumen into enterocytes by an intestinal bile acid uptake transporter (Asbt) [44]. In ileal enterocytes bile acids are presumably bound to intestinal bile acid binding proteins (Ibabp) before being excreted into the portal vein from the basolateral side of enterocytes by the organic solute transporter (Ost) α and β heterodimer [45]. Through the portal circulation, bile acids reach the liver and are taken up mainly by the Na+/taurocholate cotransporting polypeptide (Ntcp) [46]. Most of the primary bile acids undergo continuous enterohepatic circulation and only 5% enter the colon where conversion from primary to secondary bile acids occurs by deamination and 7α-dehydroxylation by the intestinal bacteria with CA converted to DCA and CDCA to LCA. Under physiological conditions, DCA enters the enterohepatic circulation and LCA is mainly excreted from the feces.
4.2. Intestinal FXR is mainly responsible for FXR-mediated suppression of bile acid synthesis under physiological condition
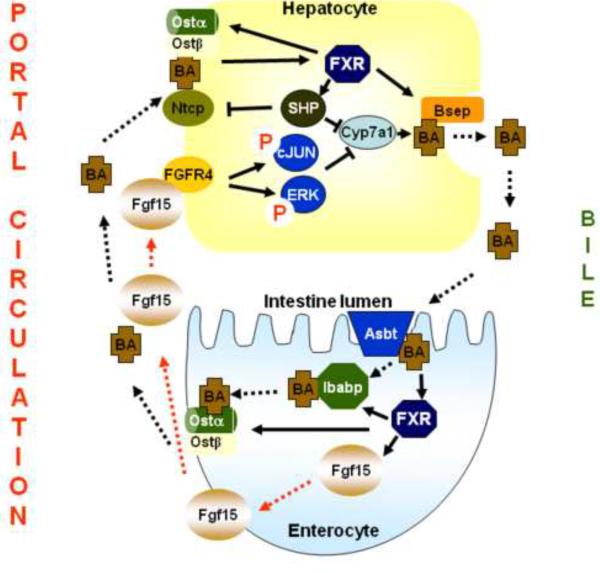
Bile acids have detergent-like properties thus their synthesis is highly regulated. Feedback suppression of bile acid synthesis by bile acids is one of the major mechanisms in maintaining bile acid homeostasis. In ileum, where FXR is highly expressed, bile acids activate FXR to induce a growth factor-like peptide in the intestine, fibroblast growth factor 15 (Fgf15) in mice and FGF19 in humans [10, 11]. Fgf15 is secreted from the enterocytes and travels to the liver, presumably via the portal circulation. In liver Fgf15 binds and activates its membrane receptor, FGFR4, which possesses endogenous tyrosin kinase activity. Activation of FGFR4 leads to activation of a cascade of signaling molecules, including cJun and ERK, which quickly turn off Cyp7a1 gene transcription [10, 47, 48]. The detailed signaling pathways in hepatocytes after FGFR4 activation still remain not clear. While FXR negatively regulates bile acid synthesis, this nuclear receptor induces bile acid efflux transporters, including Bsep and Ostα/β heterodimer, in both liver and intestine. This upregulation is believed to promote the enterohepatic bile acid circulation [49, 50]. Under physiological condition, activation of intestinal FXR may be the major mechanism to suppress Cyp7a1 gene transcription by bile acids (Fig. 2).
Fig 2. Regulation of bile acid homeostasis by FXR.
Under physiological conditions, bile acids in the intestine activate FXR that induces Fgf15, which reaches hepatocytes and activates FGFR4. Activation of FGFR4 leads to activation of signaling cascades, including cJUN and ERK, which collectively turn off the transcription of the Cyp7a1 gene. FXR not only suppresses bile acid-mediated feedback regulation of Cyp7a1, but also induces bile acid transport in both the liver and intestine. In the liver, FXR induces the expression of Bsep and in the intestine FXR induces the expression of Ibabp and Ostα/β dimer, to promote enterohepatic bile acid circulation. Under cholestatic conditions, increased bile acids in the liver activate FXR as well as other signaling pathways. Activated FXR induces SHP, which inhibits the transcription of Cyp7a1 and Ntcp. Meanwhile, FXR induces Bsep and Ostα/β dimer in the liver to promote hepatic efflux of bile acids into the bile and blood, respectively.
4.3. FXR-mediated regulation of bile acid synthesis and transport under cholestatic condition
During cholestasis, bile flow is impaired and bile acids accumulate in the liver. In this situation, hepatic FXR plays a partial role in suppressing bile acid synthesis, likely via inducing an ONR in the liver, small heterodimer partner (SHP). SHP binds to liver receptor homologue 1 (LRH-1) to inhibit Cyp7a1 gene transcription [12, 13]. In fact, suppression of bile acid synthesis during cholestasis may be mainly achieved by NR-independent signaling pathways, such as inflammatory cytokines, activated by higher concentrations of bile acids [41]. Although partially responsible for suppressing bile acid synthesis, activation of FXR during cholestasis may protect the liver by reducing hepatic bile acid levels via a direct induction of the gene expression of Bsep, the bile acid efflux transporter to promote biliary efflux, as well as via inhibition of the gene expression of Ntcp through the FXR-SHP-LRH-1 cascade to prevent bile acid uptake into the liver [51]. Ntcp is the major bile acid uptake transporter expressed in the hepatic sinusoids and is responsible for taking up the majority of conjugated bile acids into the liver [46]. Therefore the overall effect of FXR activation during cholestasis is to protect the liver from overt bile acid accumulation (Fig. 2). Toxic concentrations of bile acids in the liver also activate PXR and CAR to promote bile acid detoxification and sinusoidal efflux [21, 24]. The use of non-bile acid FXR activators may be useful in the future to treat intrahepatic cholestasis to suppress bile acid synthesis and to promote bile acid efflux from hepatocytes. However, for extrahepatic cholestasis where the bile ducts are blocked, the use of FXR activators may be detrimental because increased bile acid release into a blocked bile duct will result in more damage. In agreement with this concept, FXR deficiency results in less severe hepatic injury following common bile-duct ligation [52]. Overall, activation of FXR serves two functions in regulating bile acids: to maintain bile acid homeostasis under physiological condition and to protect the liver from overt bile acid toxicity during cholestasis.
4.4. FXR in lipid metabolism
Deficiency of FXR in mice highly indicates that an important function of FXR is to maintain cholesterol and triglyceride homeostasis [53]. Although deficiency of FXR increases bile acid synthesis, which in turn should have removed more cholesterol from the body, FXR deficiency leads to increased serum levels of VLDL, LDL, and HDL, as well as triglycerides. The increased serum cholesterol levels in FXR knockout mice indicate FXR regulates cholesterol homeostasis in addition to regulating the conversion of cholesterol to bile acids [53, 54]. It has been reported that FXR promotes reverse transport of cholesterol by increasing hepatic uptake of HDL-cholesterol via two independent mechanisms. The first is FXR-mediated suppression of hepatic lipase expression [55]. Hepatic lipase reduces HDL particle size by hydrolyzing its triglycerides and phospholipids in hepatic sinusoids, which facilitates hepatic uptake of HDL-cholesterol. The second mechanism is that FXR has been shown to induce the gene expression of scavenger receptor B1, the HDL uptake transporter in the liver [54]. Therefore one of the mechanisms by which FXR deficiency leads to increased serum cholesterol levels may be to decrease reverse cholesterol transport to the liver.
Bile acids are essential in facilitating intestinal absorption of lipid and lipid-soluble vitamins but the role of FXR in intestinal absorption of lipid remains unknown. However, it is known that activation of FXR reduces liver de novo fatty acid synthesis and VLDL transport. Studies have shown that FXR suppresses sterol regulatory element-binding protein-1c (SREBP-1c) and microsomal triglyceride transfer protein (MTP) via a SHP-mediated inhibition of co-activator recruitment to the SREBP1c and MTP promoters [56, 57]. SREBP-1c is a central transcription factor in activating hepatic de novo fatty acid synthesis. MTP is a microsomal transport protein facilitating VLDL efflux from the liver. Furthermore, bile acids induce PPARα in humans via a FXR-dependent mechanism [58]. PPARα activation reduces lipid levels by increasing fatty acid oxidation. In addition, FXR activation induces the expression of apoCII [59], but inhibits the expression of apoCIII [60]. ApoCII is a co-activator for lipoprotein lipase and promotes lipoprotein lipase-mediated hydrolysis of triglycerides. On contrary, apoCIII is a co-inhibitor for lipoprotein lipase. Therefore FXR activation results in lowering circulating triglycerides by reducing hepatic fatty acid synthesis and transport as well as enhancing oxidation, meanwhile FXR activation may result in promoting VLDL and chylomicron triglyceride hydrolysis in the circulation.
5. Implication of FXR in hepatic, gastrointestinal, and systemic diseases
As mentioned above, FXR is clearly important in the liver and intestine to maintain bile acid, cholesterol and triglyceride homeostasis. Studies in experimental animals, especially using genetically modified mice, demonstrate that dysfunction of FXR is implicated in several hepatic, gastrointestinal and systemic abnormalities, including gallstone disease, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), liver fibrosis, liver regeneration, liver tumors, intestinal tumors, and atherosclerosis.
5.1. Functional FXR prevents gallstone formation
By quantitative trait locus analysis the Nr1h4 gene has been identified as one of the litho-related genes contributing to gallstone formation [61]. In mice, activation of FXR has been shown to prevent gallstone formation by increasing the bile acid to cholesterol ratio in bile [62]. However, in humans polymorphism of the NR1H4 gene has not been linked to increased gallstone disease incidence [63]. One reason would be that humans have different ethnic background, which may lead to more complicated interaction with other risk factors for gallstone formation.
5.2. FXR is implicated in NAFLD and NASH development
NAFLD is a well recognized component of the metabolic syndrome, characterized by increased serum levels of lipids and glucose, increased incidence of type II diabetes, atherosclerosis, hypertension, and breast and colon cancer. Although many NAFLD cases have benign prognosis, some develop NASH, liver fibrosis, cirrhosis, and tumor. The disruption of the Nr1h4 gene in mice showed that FXR deficiency results in fatty liver formation following feeding with a high-cholesterol diet [53]. In addition, FXR deficiency renders the mice more susceptible to NASH formation in a diet-induced obese mouse model [64]. The exact mechanism by which FXR deficiency enhances NAFLD to NASH transition is not clear, but likely involves a FXR-dependent disruption of lipid and bile acid homeostasis, which leads to lipid accumulation and bile acid-induced chronic injury in the liver. FXR deficiency also results in increased collagen expression [64], and increased collagen expression is an early event in liver fibrosis development. In agreement, activation of FXR has been shown to suppress liver fibrosis development [36]. Advanced liver fibrosis leads to cirrhosis, portal hypertension and liver failure. The treatment of choice is liver transplantation because no effective pharmaceutical agents are available to halt or reverse liver fibrosis. Liver fibrosis is associated with activation of hepatic stellate cells. FXR is expressed in stellate cells and activation of FXR interacts with the PPARγ to suppress stellate cell activation, which collectively reduces the expression of pro-fibrotic genes, including transforming growth factor β1 and αI collagen [34].
5.3. FXR has been implicated in hepatic and gastrointestinal cancer development
FXR deficiency in mice leads to spontaneous cancer development in the liver, including hepatocellular adenoma, carcinoma and hepatocholangiocellular carcinoma [65, 66]. Moreover, in contrast to other liver tumor models where the male gender is mainly affected, 100% of both male and female FXR knockout mice develop liver tumors, indicating FXR deficiency disrupts estrogen-protected pathway(s) in the liver to promote tumor development. Studies have shown that a gender specific expression of IL-6 in the liver determines female-specific resistance to hepatocarcinoma development [67]. Both FXR deficient male and female mice express similar amount of IL-6 mRNA in the liver (unpublished data), whereas male wild-type mice have more IL-6 than female wild-type mice. These data indicate that FXR may regulate IL-6 expression and thus subsequently affecting gender-dependent development of liver tumor.
FXR deficiency also enhances intestinal tumor development in mice. Using both adenomatosis polyposis coli multiple intestinal neoplasia mice (APCmin mice), and mice treated with azoxymethane, a well-known colon carcinogen, FXR deficiency increased intestinal tumor incidence and tumor size [68, 69]. The mechanism by which FXR reduces tumorigenesis has been investigated and it is likely that activated FXR mediates both a pro-apoptotic as well as anti-inflammatory effects to suppress tumor development [69, 70]. In humans, FXR expression is associated with colon cancer development. The expression of intestinal FXR and its target genes are decreased with the progression of colon cancer as well as with increased malignancy of colon cancer cell lines [71]. This association indicates FXR may play a role in colon cancer development or at least the loss of FXR expression may be used as a marker for colon cancer formation and the degree of malignancy.
5.4. FXR in atherosclerosis
FXR regulates lipid homeostasis and deficiency of FXR in mice increases systemic and liver lipid levels. However, FXR deficiency has been shown to increase atherosclerotic plaque formation in male ApoE knockout mice but protect female ApoE mice from atherosclerotic plaque formation [72–74]. The reduction of atherosclerotic plaque in the aorta area of female mice may be due to a decreased CD36 expression and foam cell formation. CD36 is a long-chain fatty acid transporter and is mainly responsible for taking up oxidized LDL into macrophages. Lipid-laden macrophages become foam cells, the hall mark for atherosclerosis plaque development. This gender difference in the role of FXR in atherosclerosis development indicates again that FXR may interact with estrogen-related pathway(s) to modulate biological responses.
6. Novel genome-wide research tools for cutting-edge FXR research
FXR is a transcription factor that binds to DNA, likely[75], in the entire genome. In the past, we have made substantial advances in indentifying FXR target genes and regulated pathways. However the complicated mechanisms by which FXR and its target gene products both regulate transcription make it difficult to identify direct target genes, especially at the genomic level. The emerging cutting-edge technology, chromatin immunoprecipitation coupled with deep sequencing (ChIP-seq), is a non-biased method to identify genomic regions bound by transcription factors [76]. We and others have recently published FXR ChIP-seq analysis [15, 77]. These ChIP-seq studies show that FXR binds to broader genomic regions, promoters, introns, exons and intergenic regions. In addition, there is only 11% FXR binding sites overlap between liver and intestine, indicating epigenetic mechanism that may be responsible for tissue-specific FXR binding. The novel ChIP-seq data allow us to discover that binding of FXR to both upstream and downstream gene regulatory regions forms head-to-tail chromatin looping to increase the efficiency in gene transcriptional activation [78]. Motif analysis of FXR binding site indicates pioneer transcription factors may facilitate FXR-mediated gene transcriptional activation. Binding of a pioneer transcription factor to DNA helps to remodel chromatin structures and convert heterochromatin to euchromatin, to which other transcription factors will bind. The important roles of pioneer factors in facilitating estrogen receptor binding to DNA have been recently documented [79, 80]. In agreement, Chong et al. showed that LRH-1 binds adjacently to FXRE and enhances FXR-mediated transcriptional activation [77]. Using the cutting edge technology we have provided an unbiased and comprehensive analysis of potential FXR target genes, which may be used as the basis to discover novel biology pathways regulated by FXR.
7. Future directions for FXR research
The last decade has just turned the page for an in-depth research on nutrition-related NRs, including FXR. The intensive research on FXR regulated biological pathways made breakthroughs in understanding bile acid, liver, and intestinal biology. It is likely that FXR is involved in regulating diverse biological functions to affect hepatic, gastrointestinal and systemic homeostasis. It is clear that there are multiple layers of regulation for FXR function. Besides regulating FXR ligand availability and concentration, the regulation of FXR expression and function also likely leads to more insight into bile acid homeostasis and pathophysiology of diseases associated with bile acid and lipid abnormalities [81]. In addition, the interaction of FXR with other components of the transcriptional machinery at the chromatin level will likely add profound understanding of FXR target gene regulation. With an in-depth understanding of FXR function and regulation at the cell-, gene- and tissue-specific levels, we will have more tools and be more confident in designing FXR modulators in the future to prevent and/or treat bile acid and lipid related abnormalities in humans.
Acknowledgements
Research in the authors' laboratory which contributed to this review article was supported in part by NIH grant, DK081343.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- [2].Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid x receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- [3].Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS. The drosophila ECR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- [4].Zhang Y, Kast-Woelbern HR, Edwards PA. Natural structural variants of the nuclear receptor farnesoid x receptor affect transcriptional activation. J Biol Chem. 2003;278:104–110. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
- [5].Huber RM, Murphy K, Miao B, Link JR, Cunningham MR, Rupar MJ, et al. Generation of multiple farnesoid x receptor isoforms through the use of alternative promoters. Gene. 2002;290:35–43. doi: 10.1016/s0378-1119(02)00557-7. [DOI] [PubMed] [Google Scholar]
- [6].Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the x-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- [7].Chawla A, Saez E, Evans RM. Don't know much bile-ology. Cell. 2000;103:1–4. doi: 10.1016/s0092-8674(00)00097-0. [DOI] [PubMed] [Google Scholar]
- [8].Hwang ST, Urizar NL, Moore DD, Henning SJ. Bile acids regulate the ontogenic expression of ileal bile acid binding protein in the rat via the farnesoid x receptor. Gastroenterology. 2002;122:1483. doi: 10.1053/gast.2002.32982. [DOI] [PubMed] [Google Scholar]
- [9].Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [11].Li J, Pircher PC, Schulman IG, Westin SK. Regulation of complement C3 expression by the bile acid receptor FXR. J Biol Chem. 2005;280:7427–7434. doi: 10.1074/jbc.M411473200. [DOI] [PubMed] [Google Scholar]
- [12].Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- [13].Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- [14].Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane x receptor, farnesoid x-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- [15].Thomas A, Hart SN, Kong B, Fang J, Zhong X, Guo GL. Genome-wide tissue specific farnesoid x receptor binding in mouse liver and intestine. Hepatology. 2010;51:1410–1419. doi: 10.1002/hep.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rizzo G, Renga B, Antonelli E, Passeri D, Pellicciari R, Fiorucci S. The methyl transferase prmt1 functions as co-activator of farnesoid x receptor (FXR)/9-cis retinoid x receptor and regulates transcription of FXR responsive genes. Mol Pharmacol. 2005;68:551–558. doi: 10.1124/mol.105.012104. [DOI] [PubMed] [Google Scholar]
- [17].Fang S, Tsang S, Jones R, Ponugoti B, Yoon H, Wu SY, et al. The p300 acetylase is critical for ligand-activated farnesoid x receptor (FXR) induction of shp. J Biol Chem. 2008;283:35086–35095. doi: 10.1074/jbc.M803531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- [19].Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- [20].Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- [21].Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- [24].Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr., Kliewer SA, et al. Complementary roles of farnesoid x receptor, pregnane x receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- [25].Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- [26].Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through tgr5 in a murine enteroendocrine cell line stc-1. Biochem and Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- [27].Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- [28].Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- [29].Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, et al. 6alpha-ethyl-chenodeoxycholic acid (6-eCDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- [30].Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–1092. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Suzuki T, Tamehiro N, Sato Y, Kobayashi T, Ishii-Watabe A, Shinozaki Y, et al. The novel compounds that activate farnesoid x receptor: the diversity of their effects on gene expression. J Pharmacol Sci. 2008;107:285–294. doi: 10.1254/jphs.08006fp. [DOI] [PubMed] [Google Scholar]
- [32].Soisson SM, Parthasarathy G, Adams AD, Sahoo S, Sitlani A, Sparrow C, et al. Identification of a potent synthetic FXR agonist with an unexpected mode of binding and activation. Proc Natl Acad Sci USA. 2008;105:5337–5342. doi: 10.1073/pnas.0710981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, et al. Hepatoprotection by the farnesoid x receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, et al. Crosstalk between farnesoid x receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther. 2005;315:58–68. doi: 10.1124/jpet.105.085597. [DOI] [PubMed] [Google Scholar]
- [35].Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, et al. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314:584–595. doi: 10.1124/jpet.105.084905. [DOI] [PubMed] [Google Scholar]
- [36].Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- [37].Crawley ML. Farnesoid x receptor modulators: a patent review. Expert Opin Ther Pat. 2010;20:1047–1057. doi: 10.1517/13543776.2010.496777. [DOI] [PubMed] [Google Scholar]
- [38].Dussault I, Beard R, Lin M, Hollister K, Chen J, Xiao JH, et al. Identification of gene-selective modulators of the bile acid receptor FXR. J Biol Chem. 2003;278:7027–7033. doi: 10.1074/jbc.M209863200. [DOI] [PubMed] [Google Scholar]
- [39].Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–1706. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- [40].Burris TP, Montrose C, Houck KA, Osborne HE, Bocchinfuso WP, Yaden BC, et al. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol Pharmacol. 2005;67:948–954. doi: 10.1124/mol.104.007054. [DOI] [PubMed] [Google Scholar]
- [41].Chiang JY. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J of Hepatol. 2004;40:539. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- [42].Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007;453:611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- [43].Keppler D, Konig J, Buchler M. The canalicular multidrug resistance protein, cMRP/MRP2, a novel conjugate export pump expressed in the apical membrane of hepatocytes. Adv Enzyme Regul. 1997;37:321–333. doi: 10.1016/s0065-2571(96)00013-1. [DOI] [PubMed] [Google Scholar]
- [44].Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: Phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- [45].Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, et al. The heteromeric organic solute transporter alpha-beta, OST alpha-OST beta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93:1326–1331. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-jun n-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J Biol Chem. 2005;280:17707–17714. doi: 10.1074/jbc.M411771200. [DOI] [PubMed] [Google Scholar]
- [49].Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid x receptor/bile acid receptor. J Biol Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- [50].Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- [51].Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, et al. The orphan nuclear receptor, SHP, mediates bile acid-induced inhibition of the rat bile acid transporter, NTCP. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- [52].Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, et al. Role of farnesoid x receptor in determining hepatic abc transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- [53].Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- [54].Lambert G, Amar MJ, Guo G, Brewer HB, Jr., Gonzalez FJ, Sinal CJ. The farnesoid x receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- [55].Sirvent A, Claudel T, Martin G, Brozek J, Kosykh V, Darteil R, et al. The farnesoid x receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 2004;566:173–177. doi: 10.1016/j.febslet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- [56].Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hirokane H, Nakahara M, Tachibana S, Shimizu M, Sato R. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J Biol Chem. 2004;279:45685–45692. doi: 10.1074/jbc.M404255200. [DOI] [PubMed] [Google Scholar]
- [58].Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart J-C, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid x receptor. Mol Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- [59].Kast HR, Nguyen CM, Sinal CJ, Jones SA, Laffitte BA, Reue K, et al. Farnesoid x activated receptor induces apolipoprotein c-II transcription: A molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol. 2001;15:1720–1728. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- [60].Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J, et al. Farnesoid x receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–555. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- [61].Wittenburg H, Lyons MA, Li R, Churchill GA, Carey MC, Paigen B. FXR and ABCG5/BCG8 as determinants of cholesterol gallstone formation from quantitative trait locus mapping in mice. Gastroenterology. 2003;125:868. doi: 10.1016/s0016-5085(03)01053-9. [DOI] [PubMed] [Google Scholar]
- [62].Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10:1352–1358. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- [63].Kovacs P, Kress R, Rocha J, Kurtz U, Miquel JF, Nervi F, et al. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J of Hepatol. 2008;48:116. doi: 10.1016/j.jhep.2007.07.027. [DOI] [PubMed] [Google Scholar]
- [64].Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid x receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–122. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid x receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid x receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- [67].Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in myd88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- [68].Maran RRM, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D, et al. Farnesoid x receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328:469–477. doi: 10.1124/jpet.108.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- [70].Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid x receptor antagonizes Nuclear Factor Kappab in hepatic inflammatory response. Hepatology. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G, et al. The bile acid nuclear receptor FXR and the bile acid binding protein Ibabp are differently expressed in colon cancer. Dig Dis Sci. 2004;49:982–989. doi: 10.1023/b:ddas.0000034558.78747.98. [DOI] [PubMed] [Google Scholar]
- [72].Guo GL, Santamarina-Fojo S, Akiyama TE, Amar MJ, Paigen BJ, Brewer B, Jr., et al. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim Biophys Acta. 2006;1761:1401–1409. doi: 10.1016/j.bbalip.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, et al. Fxr deficiency causes reduced atherosclerosis in LDLr−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- [74].Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid x receptor increases atherosclerotic lesions in apolipoprotein e-deficient mice. J Lipid Res. 2005;46:2595–2604. doi: 10.1194/jlr.M500390-JLR200. [DOI] [PubMed] [Google Scholar]
- [75].Hagenbuch B, Stieger B, Foguet M, Lubbert H, Meier PJ. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991;88:10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Park PJ. Chip-seq: Advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chong HK, Infante AM, Seo Y-K, Jeon T-I, Zhang Y, Edwards PA, et al. Genome-wide interrogation of hepatic FXR reveals an asymmetric IR-1 motif and synergy with LRH-1. Nucl Acids Res. 2010;38:6007–6017. doi: 10.1093/nar/gkq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li G, Thomas AM, Hart SN, Zhong X, Wu D, Guo GL. Farnesoid x receptor activation mediates head-to-tail chromatin looping in the Nr0b2 gene encoding small heterodimer partner. Mol Endocrinol. 2010;24:1404–1412. doi: 10.1210/me.2010-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FOXA1. Cell. 2005;122:33. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- [80].Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, et al. Foxa1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, et al. Fxr acetylation is normally dynamically regulated by p300 and Sirt1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]