Abstract
The UK Lung Screen (UKLS) is a randomised controlled trial of the use of low-dose multidetector CT for lung cancer screening. It completed the Health Technology Appraisal (HTA)-funded feasibility stage in October 2009 and the pilot UKLS will be initiated in early 2011. The pilot will randomise 4000 subjects to either low-dose CT screening or no screening. The full study, due to start in September 2012, if progression criteria are met, will randomise a further 28 000 subjects from seven centres in the UK. Subjects will be selected if they have sufficient risk of developing lung cancer according to the Liverpool Lung Project risk model. The UKLS employs the ‘Wald Single Screen Design’, which was modelled in the UKLS feasibility study. This paper describes the modelling of nodule management in UKLS by using volumetric analysis with a single initial screen design and follow-up period of 10 years. This modelling has resulted in the development and adoption of the UKLS care pathway, which will be implemented in the planned CT screening trial in the UK.
Keywords: Health Economist, imaging/CT MRI etc, lung cancer
Introduction
In 2008 in the UK, >33 500 patients died from lung cancer and there were >38 500 new diagnoses.1 2 Five-year survival was only 6.5%, improved since 2005 but still among the lowest in Europe. There are many hypotheses about the reasons for this poor outcome. The delivery of treatment with curative intent is known to vary in the UK. The surgical resection rate may be as low as 4% in some centres and, in others, >25%.3 It is argued that by ensuring a universally high standard of care the overall outcomes will be improved. The stage and fitness of the patient are other factors that are linked by the timeliness of presentation. There are initiatives to increase public awareness of lung cancer symptoms which aim to encourage earlier presentation. However, data suggest that in most patients, these symptoms commence only a few months prior to the first contact with secondary care and therefore there is only a narrow time frame in which to make a difference.4 Lung cancer screening is therefore an important area for study since this offers the potential to detect presymptomatic cancer at a much earlier stage when it is more likely to be curable and when it has not had a chance to take its toll on the patient's fitness.
Early screening studies conducted in the 1950s to 1970s employed chest radiographs and sputum cytology as screening tools and, although survival was improved in some studies in the screened group, there was no overall improvement in mortality from lung cancer.5 6 A number of important lessons were learnt about the influence of bias in this type of study and the importance of using mortality as the principal outcome measure. The lung component of the National Cancer Institute (NCI) PLCO (Prostate, Lung Colorectal and Ovarian) screening trial recruited patients in the USA between 1993 and 2001. In this trial, a total of 77 464 subjects aged 55–74 years were randomly assigned to the active arm of the trial that included a baseline followed by three repeat chest radiographs. A total of 564 lung cancers (0.72%) were detected. The initial results show that for non-small cell lung cancer (NSCLC), 59.6% of screen-detected and 33.3% of interval cancers were stage I–II.7 The study is due to report on mortality in 2015.
CT is a much more sensitive method of detecting lung cancer at an early stage,8 but for this reason is potentially more susceptible to lead time and overdiagnosis bias than chest radiography.9 Thus apparent differences in survival are not enough to ensure efficacy as a screening test, and studies must measure mortality. Benefit in terms of mortality must outweigh the harms that may result from screening. Screening studies in other cancers have shown the importance of taking into account the harms that may result from a screen. This includes not just the physical harm of multiple exposures to radiation, but the less easily measured psychological harm that may result from screening, especially in the vulnerable.10 11 Screening must also be cost-effective, and this consideration strongly influences the design of a screening programme.12
There are now a number of CT-based screening studies that have been published and several randomised trials underway. The first major lung cancer randomised controlled trial (RCT) screening trial utilising low-dose CT (LDCT) was the National Lung Cancer Screening Trial (NLST), which is a combination of two trials, one set up by the US NCI and the other by the American College of Radiology Imaging Network (ACRIN).13 14 Between 2002 and 2004, 53 456 former and current smokers were randomised to either LDCT or chest radiograph annually for 3 years. The major objective was to determine whether LDCT reduces lung cancer mortality compared with chest radiography. This trial has recently been stopped as it has reached the primary end point of a 20% reduction in mortality in the LDCT arm.15 16 The full report is to be published in the next few months. A number of smaller randomised trials have recently reported their preliminary findings. These include the ItaLung and Dante Trials in Italy,17 18 the French randomised pilot study, Depiscan, comparing LDCT and chest radiography,19 and the Danish lung cancer screening trial.20
These studies employ conventional methods to analyse the images, but the Dutch–Belgian NELSON (NEderlands-Leuvens longkanker Screenings ONderzoek) uses volumetric analysis to classify both the initial characteristics and growth behaviour of nodules.21 The UKLS trial design team used the data available from NELSON to develop the nodule management protocol described below.
The UKLS study objectives
The objective of the UKLS trial is to assess whether LDCT screening and treatment of early lesions will decrease lung cancer mortality compared with a control group without screening while ensuring any benefit exceeds harms in a cost-effective manner. For the screening activity to be cost-effective, the population screened needs to be at sufficiently high risk to ensure detection of enough cancers at a curable stage. This will also ensure that the benefits of the screening will outweigh the likely harms. The Liverpool Lung Project (LLP)22 has recently developed a method to calculate absolute risk of lung cancer over a defined period, based on age, sex, smoking duration, family history of lung cancer, history of non-pulmonary malignant tumour, history of pneumonia and occupational exposure to asbestos. The LLP risk model has been successfully validated both internally and in three independent data sets: EUELC, HARVARD and LLP COHORT samples, and gave a reciever operating characteristic area under the curve (ROC-AUC) of 0.66, 0.78 and 0.82 in EUELC.23 The risk model has the advantage of simplicity of use and interpretation, and identifies some subjects who are at high risk due to risk factors other than smoking. Subjects will be selected if their risk of lung cancer is ≥5% over a 5 year period. Using this selection criterion, it is likely that cancer will be detectable in ∼1.5% of subjects at the first screen as more cancers are detected at the first screen than at a 1 year incidence screen.
Study design and methods
UKLS employs the ‘Wald Single Screen Design’ which was modelled during the UKLS feasibility study. Subjects are randomised either to LDCT, with predefined management protocols to manage the findings, or to the control arm where subjects are given usual care. Both arms are followed up for lung cancer incidence and mortality. This design was chosen for the following reasons:
It is the most economical approach in terms of the number of CT screening examinations needed for a fully powered trial.
It will provide early data on rates of cancers in the years following a screen, to inform ‘interval’ for subsequent screens in a national screening programme.
It will produce mortality results in a similar time frame to the other major international multicentre screening trials, and allow us to synchronise our data with the multicentre groups for analysis.
The single screen design does not have the problem of long-term compliance.
Recruitment
Individuals 50–75 years of age are selected at random from National Health Service (NHS)/Strategic Health Authority (SHA) records and approached with an invitation letter and questionnaire.
The responses to the questionnaire are analysed, based on the LLP,21 to select those with a 5% risk of developing lung cancer in 5 years.
These high-risk individuals are contacted with a further questionnaire to establish details of their medical history and to provide detailed information about the UKLS trial.
Individuals responding to the second questionnaire are invited to one of the recruitment centres where they are given further details and meet a research nurse. Subjects are consented and undergo lung function testing as well as providing blood, buccal swab and sputum specimens. All smokers are provided with smoking cessation advice sheets and a list of local NHS Stop Smoking services.
The recruits are randomised into the CT screen group and no screen group.
Exclusion criteria
Subjects who are unable to give consent including any condition that precludes written informed consent.
Co-morbidity which would unequivocally contraindicate either screening or treatment if lung cancer were detected.
Technical reasons (eg, weight >200 kg (too heavy for the CT table), unable to lie flat, etc.)
CT performed within 1 year of the invitation to be screened.
The study is powered to detect a significant and clinically worthwhile effect of the intervention. The full trial has 90% power to detect a 31% reduction in mortality in subjects with a 5 year incidence of 5%.
Imaging protocol
All participating sites will use a 16 or higher channel multidetector CT (MDCT). The rationale for using a 16 or higher channel MDCT platform is that the majority of screen-detected nodules are small (3–10 mm) and require optimal spatial resolution for accurate and reproducible evaluation including nodule volume measurement. Imaging will be performed during suspended maximal inspiration. No intravenous contrast material will be administered. Thin detector collimation (0.5–0.625 mm) will be used with a pitch of 0.9–1.1. Scan time will be in the region of 5 s but no longer than 10 s to avoid respiratory motion artefact. Radiation exposures will be as low as possible while maintaining good image quality. The effective radiation dose is well below 2 mSv (table 1).
Table 1.
Imaging protocol employed in UKLS
| Slim subjects | Standard | Obese | |
| (<50 kg body weight) | (50–80 kg body weight) | (>80 kg body weight) | |
| kVp setting | 90 kVp | 120 kVp | 140 kVp |
| mAs settings* | *Depending on the scanner type adjusted to achieve the volume CT dose index (CTDIvol) given. | ||
| CTDIvol | 0.8 mGy | 1.6 mGy | 3.2 mGy |
| Effective dose | <0.4 mSv | <0.8 mSv | <1.6 mSv |
All images will be read independently by two readers according to a detailed predefined protocol and, where there is a discrepancy in the findings, a third reader will arbitrate.
Nodule management protocol
Nodule definition
A nodule is defined as a small approximately spherical, non-linear circumscribed focus of abnormal soft tissue. A non-calcified nodule is classified as non-calcified in the absence of a benign pattern of calcification. Where there are multiple nodules, each is classified separately up to a maximum of 20 per subject.
Nodule categorisation
Nodules are classified into four categories that reflect their probability of being malignant (table 2).
Table 2.
UK Lung Sceeen (UKLS) nodule categories
| Solid | Non-solid or part solid | |
| Category 1 | Nodules containing fat or with a benign pattern of calcification are considered benign. Nodules <15 mm3 or if pleural or juxta pleural ≤3 mm | |
| Category 2 | Intraparenchymal nodules with a volume of 15–49 mm3. Pleural or juxtapleural nodules with a maximal diameter of 3.1–4.9 mm. | Nodules with a maximal non-solid component diameter <5 mm. Where there is a solid component, the component volume is <15 mm3 |
| Category 3 | Intraparenchymal nodules with a volume of 50–500 mm3. Pleural or juxtapleural nodules with a maximal diameter of 5–9.9 mm. | Nodules with a maximal non-solid component diameter of >5 mm. Where there is a solid component, the component volume is 15–500 mm3 |
| Category 4 | Intraparenchymal nodules with a volume >500 mm3. Pleural or juxtapleural nodules with a maximal diameter of ≥10 mm. | Nodules with a solid component with a volume >500 mm3 |
Solid nodules are areas of homogeneous soft tissue attenuation. Solid nodules may have different outlines, and these are classified as smooth, polylobulated, spiculated or irregular. Smooth is defined as a continuous regular outline. Lobulation is defined as areas of bulging of the lesion contour. Spiculation is defined as the presence of strands extending from the lung margin into the lung parenchyma. Irregular is defined as not smooth, polylobulated or spiculated. Part-solid nodules are those of both ground-glass and soft tissue attenuation. Non-solid/ground-glass opacity refers to a nodule composed of a focal area of hazy increased lung opacity.
Growth characteristics
Nodules in categories 2 and 3 will have a repeat CT at 1 year, and at 3 months and 1 year, respectively. A volume doubling time of <400 days is considered consistent with malignancy and so these nodules will be referred for further diagnostic incestigation.24 25
Nodule management and categories
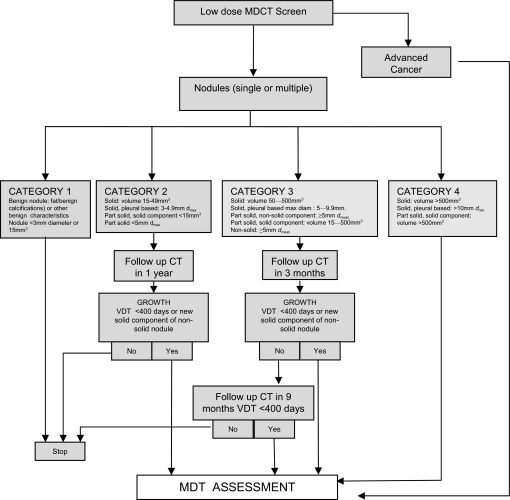
Figure 1 shows the algorithm for the management of findings on CT relating to possible lung cancer. In NELSON, the probability of malignancy in all ‘negative’ nodules was 0.24%. In NELSON, ‘negative’ nodules were not subject to any further screening, except for the fact that those detected on earlier screens would have a repeat CT as part of the study design. How this relates to the UKLS categories can only be estimated from published data. Table 3 summarises the proportions of nodules in different categories that were negative on first screen in NELSON.26 The UKLS categories are given for comparison. UKLS category 1 nodules are considered a negative finding—that is, no further follow-up is indicated. The rationale for this is that these nodules have a low risk of being malignant owing to their benign features or tiny size. The important difference between UKLS and NELSON categories is that 15–49 mm3 nodules are a separate category in UKLS (category 2). This was to ensure that the single screen design did not miss cancers in nodules <50 mm3. Thus a further CT scan at 12 months for UKLS category 2 is part of the UKLS protocol. Category 3 nodules have a repeat scan at 3 months and, if the volume doubling time is >400 days, they are scanned again after a further 9 months. The logic behind this is to intercept the faster growing cancers early enough for curative treatment to be offered while still not missing the more indolent cancers in this group that may not quite reach the criteria for significant growth at 3 months. The cut-off of <400 days to trigger referral is thought to exclude those indolent cancers most likely to be overdiagnosed.24 25 Category 4 nodules have a sufficiently high pretest probability of malignancy to be referred directly to the lung cancer multidisciplinary team.
Figure 1.
UK Lung Screen nodule care pathway management protocol. MDCT, multidetector CT; MDT, multidisciplinary team; VDT, volume doubling time.
Table 3.
Comparison of the nodule categories in UKLS and NELSON including the findings in 8309 nodules that tested negative in NELSON
| Nodule characteristic n (%) | ||||
| NELSON | Benign | <50 mm3 | 50–500 mm3 | Lung cancers on subsequent scan, n (%) |
| 1395 (16.8) | 4861 (58.5) | 2053 (24.7) | 20 (0.24) | |
| Equivalent UKLS categories | Category 1 plus <15 mm3 | Category 2 if >15–49 mm3 | UKLS category 3 | |
The UKLS single screen design required some modification to the nodule classification in NELSON to include more nodules in category 2 (those 15–49 mm3) that were subject to further scanning. Please see table 2 for a full description of UKLS categories.
NELSON, NEderlands-Leuvens longkanker Screenings ONderzoek; UKLS, UK Lung Screen.
Modelling of timing of interval CT for UKLS category 2 nodules
Category 2 nodules have a low probability of malignancy (<0.24% in NELSON, although this included some category 1 and 3 nodules) but, despite this, some of the cancers detected at baseline will be category 2 nodules. The purpose of the repeat CT is to detect growth, but also to detect cancer at a curable stage. Thus if the interval is too long there may have been so much growth that the window for cure has closed. If the interval is too short, some of the slower growing nodules may not be recognised.
For this reason we have modelled growth using average volume doubling times expected in NSCLC and small cell lung cancer (SCLC) (table 4). We have modelled this for the minimum and maximum size in category 2.
Table 4.
Modelling of timing of interval CT for UKLS category 2 nodules
| Volume doubling time (days) | Initial volume (mm3) (diameter) | 1 year interval CT volume (diameter) | 2 year interval CT volume (diameter) |
| 100 (NSCLC) | 15 (3) | 180 (7 mm) | 1920 (15.4 mm) |
| 100 (NSCLC) | 49.9 (4.4) | 600 (10.4 mm) | 6400 (23 mm) |
| 30 (SCLC) | 15 (3) | 61 440 (49 mm) | 126 000 000 (622 mm) |
| 30 (SCLC) | 49.9 (4.4) | 102 400 (58 mm) | 419 000 000 (928 mm) |
| 400 | 15 (3) | 28 (3.8) | 53.5 (4.7) |
| 400 | 49.9 (4.4) | 94 (5.6) | 178 (7) |
The values in bold indicate patients that are likely to be incurable.
NSLC, non-small cell lung cancer; SCLC, small cell lung cancer.
For SCLC, an interval of 2 years will be too long and other clinical events will supervene long before the magnitude of growth at 2 years. At a 1 year interval it is likely that some patients will have presented before the screen. SCLC will probably represent a small proportion of nodules (<10%).
For NSCLC, by extending the interval CT to 2 years there will be significant upstaging at the extreme of the size range (T1a to T1b). Screening studies have also suggested better survival for sub 1 cm nodules, so the subjects with nodules at the lower limit of category 2 may also be at a survival disadvantage by using an interval of 2 years rather than 1 year.
If the model is applied to the maximum permitted volume doubling time of 400 days, the changes in volume of nodules are still within the limits of resolution after an interval of 1 year.
Multidisciplinary team management of pulmonary nodules
The UKLS team recognised the importance of patient choice in the management of CT findings. Obvious lung cancer that appears at a relatively advanced stage would normally be managed according to the potential treatment that might be offered and in accordance with NICE (National Instiute for Health and Clinical Excellence) guidelines. The management of the smaller pulmonary nodule is less well worked out but, from the preliminary results of NELSON, the risk of malignancy is 36% in all subjects that have a positive test (UKLS category 4 nodules and category 3 that show significant growth). In UKLS, category 2 nodules that show significant growth will also be referred for further management. A strong argument can be made for either biopsy or removal of these lesions. Further observation (that might be offered in smaller nodules to detect growth) would not usually be indicated as the UKLS protocol will have already detected growth as defined by a volume doubling time of <400 days. The UKLS team will offer advice on the management of nodules, including an estimate of the proportion of nodules in a given category that are expected to be malignant. This will enable patients to make an informed choice about their management. It is hoped that most subjects will be fit enough to undergo treatment with curative intent, and thus further imaging will mainly be indicated for staging purposes (eg, standard dose CT with contrast or positron emission tomography (PET)-CT).
Discussion
The objective of the UKLS trial is to assess whether LDCT screening and treatment of early lesions will decrease lung cancer mortality compared with a control group without screening and whether this is achievable without unacceptable harms and in a cost-effective manner. Thus the group selected is one where it is thought that the risk of lung cancer is sufficiently high to ensure the screening activity detects enough tumours for the benefits of the screening to outweigh the likely harms. There has been increasing interest in developing methods for individual risk prediction for lung cancer. Models have been developed for use within high-risk groups,27 and for the general population,28 based mainly on age and smoking. The predictive accuracy of lung cancer risk models may be further improved by the addition of other epidemiological risk factors.29 The LLP22 23 30 calculates absolute risk of lung cancer over a defined period. It has been chosen for UKLS because the predictor variables are all explicitly defined and can be readily assessed at the time of patient presentation and, secondly, patients can be assigned to their appropriate risk class on the basis of information from the initial history alone. The screening process confers potential harms as well as potential benefits. In a randomised trial and in any future national screening service, the screening would be provided only to those whose risk was sufficiently high that the likely benefits outweigh the likely harms. The risk of cancer in UKLS is 5% over 5 years as set by the LLP model.
The NELSON trial21 26 is a Dutch–Belgian randomised trial of LDCT screening that compares LDCT at baseline, 1 year and 3 years with no screening. It is designed to determine whether, after 10 years of follow-up, CT screening has reduced mortality by ≥25%. The design of UKLS differs from that of NELSON in that there is a single screen rather than multiple screens. This is primarily to maximise the yield of the screening test and make the screening more cost-effective, but will also allow the option of combining the results of the first screen with that of NELSON. The UKLS design team were concerned to ensure that as many cancers as possible were detected that could benefit from early diagnosis. This meant that nodules that could potentially develop into cancer and shorten life expectancy had to be followed up with a repeat CT after an appropriate period. In NELSON, the later round scan effectively follows up all nodules that are detected by earlier scans, and it was found that after a negative test lung cancer was subsequently detected in 20 (0.24%) subjects. The investigators found that the initial screen detected 70 lung cancers in patients with positive tests. The 20 subsequently detected in those subjects with negative tests comprised 3 interval cancers and 17 in the round 2 screening. This represents a significant proportion of the lung cancers detected and so it is important that the UKLS protocol minimises the chance of missing these cancers. The UKLS protocol therefore includes a repeat scan for nodules that are 15–49 mm3 (classified as ‘negative’ by NELSON). We modelled average growth rates for NSCLC and SCLC to estimate whether nodules in the 15–49 mm3 category (UKLS category 2) would still be curable after 1 year and 2 years, and found that a 1 year repeat scan was appropriate for the majority of this category of nodules. If nodules were SCLC, they would be likely to be incurable after a year but in the UK only 11% of all lung cancers are small cell and because they grow quickly they are more likely to present as interval cancers. Furthermore, only 2% of screen-detected cancers in NELSON were small cell cancers26 (R van Klaveren, personal communication). The NELSON investigators found that the 17 lung cancers detected in round 2 were found after an interval of 384±59 days, which supports the UKLS modelling. The cost-effectiveness of detection of lung cancers in subjects that initially test negative is an important question and will be addressed in both NELSON and UKLS. These subjects come from a group whose pretest probability of malignancy is only ≤0.24%, so the value of screening has to be clearly established and compared with the value of using the resource in a higher risk population. The application of further markers of lung cancer to stratify patients into risk categories may help the cost-effectiveness of screening in lower risk populations. This might involve the use of a variety of biomarkers, currently under evaluation, within risk models.
Thus UKLS and NELSON both employ postprocessing volumetric assessment of nodules detected by CT screening, but with differences in design will provide information about relative cost-effectiveness of a multiple screen and a single screen. The design similarities mean that there will be the facility for joint reporting of the single screen element.
The nodule management protocols used in these studies may be adapted for clinical use now: the preliminary results of NELSON indicate that the protocol works and the protocol could therefore be adapted to incidentally detected nodules. The protocol results in less radiation by reducing the number of follow-up scans and employing low-dose techniques.
Acknowledgments
Respiratory Physician, Imaging and Thoracic Surgery Review Groups were set up by DRB, MHH and RP, respectively, when developing the relevant aspects of the UKLS protocol. Members of these Groups are thanked for their help and support and are listed below. R van Klaveren and M Prokop are members of the NELSON investigators group. Respiratory Review Group: D R Baldwin, Nottingham (Chair); R van Klaveren, The Netherlands; M Walshaw, Liverpool; R Milroy, Glasgow; M Peake, Leicester. Radiology Review Group: D M Hansell, London (Chair); J J Entwisle, Leicester; F V Gleeson, Oxford; M Prokop, The Netherlands; N J Screaton, Cambridge. Surgical Review Group: R Page, Liverpool (Chair); D Waller, Leicester; S Barnard, Newcastle.
Footnotes
Funding: The HTA funded the feasibility study.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cancer Research UK Cancer stats. 2010. http://info.cancerresearchuk.org/cancerstats/types/lung/index.htm?script=true#mortality
- 2.Office for National Statistics Mortality statistics: deaths registered in 2008. DR, 2009. http://www.statistics.gov.uk/downloads/theme_health/DR2008/DR_08.pdf (accessed 26 Jan 2011). [Google Scholar]
- 3.National Lung Cancer Audit National Lung Cancer Audit. 2009. http://www.ic.nhs.uk/webfiles/Services/NCASP/audits%20and%20reports/NHS%20IC%20Lung%20Cancer%20AUDIT%202009%20FINAL.pdf (accessed 26 Jan 2011). [Google Scholar]
- 4.Corner J, Hopkinson J, Fitzsimmons D, et al. Is late diagnosis of lung cancer inevitable? Interview study of patients' recollections of symptoms before diagnosis. Thorax 2005;60:314–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brett GZ. Earlier diagnosis and survival in lung cancer. BMJ 1969;4:260–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field JK, Duffy SW. Lung cancer screening: the way forward. Br J Cancer 2008;99:557–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hocking WG, Hu P, Oken MM, et al. Lung cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. J Natl Cancer Inst 2010;102:722–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henschke CI, Yankelevitz DF, Libby DM, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763–71 [DOI] [PubMed] [Google Scholar]
- 9.Mulshine JL, Sullivan DC. Lung cancer screening. N Engl J Med 2005;352:2714–20 [DOI] [PubMed] [Google Scholar]
- 10.Gray JA, Patnick J, Blanks RG. Maximising benefit and minimising harm of screening. BMJ 2008;336:480–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010;102:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whynes DK. Could CT screening for lung cancer ever be cost effective in the United Kingdom. Cost Eff Resour Alloc 2008;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47:9–15 [DOI] [PubMed] [Google Scholar]
- 14.Croswell JM, Kramer BS, Kreimer AR, et al. Cumulative incidence of false-positive results in repeated, multimodal cancer screening. Ann Fam Med 2009;7:212–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.http://www.cancer.gov/newscenter/pressreleases/NLSTresultsRelease
- 16.Aberle DR, Berg CD, Black WC, et al. National Lung Screening Trial Research Team The National Lung Screening Trial: overview and study design. Radiology 2011;258:243–53 http://www.ncbi.nlm.nih.gov/pubmed/21045183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes Pegne A, Picozzi G, Mascalchi M. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34–40 [DOI] [PubMed] [Google Scholar]
- 18.Infante M, Cavuto S, Lutman F, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med 2009;180:445–53 [DOI] [PubMed] [Google Scholar]
- 19.Blanchon T, Brechot JM, Grenier PA, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58:50–8 [DOI] [PubMed] [Google Scholar]
- 20.Pedersen J, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial—overall design and results of the prevalence round. J Thorac Oncol 2009;5:608–14 [DOI] [PubMed] [Google Scholar]
- 21.Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177–84 [DOI] [PubMed] [Google Scholar]
- 22.Cassidy A, Myles J, van-Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98:270–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raji OY, Duffy SW, Agbaje OF, et al. Predictive Accuracy and Clinical Utility of the Liverpool Lung Project Risk Model using Population-based Data from Europe and North America. Liverpool, UK: NCRI Conference, 2010 [Google Scholar]
- 24.Yankelevitz DF, Kostis WJ, Henschke CI, et al. Overdiagnosis in chest radiographic screening for lung carcinoma: frequency. Cancer 2003;97:1271–5 [DOI] [PubMed] [Google Scholar]
- 25.Lindell RM, Hartman TE, Swensen SJ, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology 2007;242:555–62 [DOI] [PubMed] [Google Scholar]
- 26.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221–9 [DOI] [PubMed] [Google Scholar]
- 27.Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003;95:470–8 [DOI] [PubMed] [Google Scholar]
- 28.van Klaveren RJ, de Koning HJ, Mulshine J, et al. Lung cancer screening by spiral CT. What is the optimal target population for screening trials? Lung Cancer 2002;38:243–52 [DOI] [PubMed] [Google Scholar]
- 29.Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst 2007;99:715–26 [DOI] [PubMed] [Google Scholar]
- 30.Field JK, Smith DL, Duffy S, et al. The Liverpool Lung Project research protocol. Int J Oncol 2005;27:1633–45 [PubMed] [Google Scholar]