Abstract
Regardless of age, a marked elevation in circulating IL-6 levels correlates with increased mortality after injury or an inflammatory challenge. We previously reported that aged IL-6 knockout mice given LPS have improved survival and reduced inflammatory response than LPS-treated aged wild type (WT) mice. Herein, we analyzed the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury: dorsal scald (burn) injury versus intraperitoneal LPS administration. At 24 h after burn injury, circulating alanine aminotransferase and hepatic neutrophil accumulation were comparable regardless of age or IL-6 deficiency. However, at this same time point, these indicators of liver damage, in addition to hepatic levels of KC, a neutrophil chemoattractant, were increased in aged WT mice given LPS relative to young WT mice given LPS. The hepatic injury was drastically reduced in aged IL-6 knockout mice given LPS as compared with LPS-exposed aged WT mice. Our results suggest that the nature of the insult will determine the degree of remote injury in aged animals. In addition, the role of IL-6 as a contributing factor of tissue injury may be insult specific.
Keywords: Aging, ALT, burn, IL-6, liver, LPS
INTRODUCTION
Regardless of the type of insult, the elderly are more susceptible to death than younger individuals. For example, a moderate-sized burn covering 20% of the total body surface area (TBSA) causes a mortality rate of only 5.5% in healthy young adult patients, whereas elderly patients with the same burn size have a mortality rate of 75% (1). Besides the increased mortality after injury, the elderly are more susceptible than young adults to viral and bacterial infections, opportunistic infections, and reactivation of latent viruses (2, 3).
Using murine models, we have found that a 15% TBSA caused a higher mortality rate in aged mice than that of young adult mice (4). Similarly, aged mice given a low dose of LPS were approximately six times more sensitive to the lethal toxicity than young mice (5). In these two experimental models, advanced age was associated with the presence of a hyper-reactive systemic inflammatory response characterized, in part, by elevated circulating levels of the cytokine IL-6 (6, 7). In an attempt to better define the role of IL-6 in the systemic inflammatory response in the aged, we challenged young and aged wild type (WT) and IL-6 knockout (KO) BALB/C female mice with LPS. After 24 h, aged IL-6 KO mice had improved survival when compared with aged WT mice (7). Moreover, aged IL-6 KO mice showed a decreased acute-phase response when compared with that of aged WT mice (7). Taken together, these observations have suggested a crucial role for IL-6 as a component of the aberrant systemic innate immune responses of the aged after an inflammatory challenge or injury.
After severe injury, the persistence of a systemic inflammatory response syndrome may lead to multiple organ failure (MOF) (8), which is a leading cause of death in response to both injury and sepsis. After LPS administration (9) or burn injury (10), aged mice had a hyperreactive inflammatory response in their lungs relative to younger mice. Clinically, the lungs are often the first organ to fail after systemic inflammation. Hepatic dysfunction is another component of MOF during sepsis (11) or burn injury (12). With greater hepatic injury, an uncontrolled inflammatory response can trigger the release of high levels of multiple mediators that lead to sequential organ failure, and eventually, death (13).
In the liver, IL-6 has been associated with the development of acute and chronic hepetic injury. For example, elevated IL-6 levels in serum and liver were found to correlate directly with disease progression in patients with fulminant hepatic failure (14). In addition, increased hepatic expression of IL-6 messenger RNA was found in end-stage human cirrhosis (15). Overall, these observations suggest that low to moderate levels of IL-6 are beneficial, whereas elevated or continuous IL-6 exposure can override its protective effects and lead to pathogenesis and progression of disease. Here, we analyzed the inflammatory response in the livers of young and aged WT and IL-6 KO mice after burn injury or administration of LPS. Our results indicate that after 24 h, aged IL-6 KO mice given LPS have reduced hepatic inflammation when compared with aged WT animals given the same treatment. However, in the burn injury model, the magnitude of hepatic injury was comparable regardless of age or IL-6 presence. These findings suggest that the evolution of organ injury in aged mice and the role of IL-6 as a contributing factor of tissue injury may be insult specific.
MATERIALS AND METHODS
Animals
Young (2- to 3-month-old) and aged (18- to 20-month-old) WT female BALB/C mice were purchased from the National Institute of Aging colony at Harlan Laboratories (Indianapolis, Ind). IL-6 KO mice were kindly provided by Dr Manfred Kopf, Molecular Biomedicine, ETH Zurich, Switzerland, backcrossed onto the BALB/C background, bred, maintained, and aged at the Taconic Laboratories (Germantown, NY). Young (2- to 3-month-old) and aged (15- to 23-month-old) female BALB/C IL-6 KO mice were used in the experiments described here. Animals were housed under similar conditions at their respective facilities. They were free of potential endemic viral pathogens that could influence their inflammatory response. All animals where maintained in an environmentally controlled facility at Loyola University Medical Center for at least 1 week before experimentation. At the time of killing, all mice were dissected, and the organs were screened for visible tumors and/or gross abnormalities. If found, these animals were removed from the study. The experimental protocols described here followed the guidelines established by the publication Principles of Laboratory Animal Care (NIH publication No. 86-23, revised 1985) and were approved by the Loyola University Chicago Institutional Animal Care and Use Committee.
Induction of burn injury and exposure to LPS
Mice were subjected to a 15% TBSA dorsal scald or sham injury according to a modified protocol of Walker and Mason (16). The animals were anesthetized with Nembutal (50 mg/kg intraperitoneally [i.p.]), and then clippers were used to remove the hair from the dorsum of each animal. Anesthetized mice receiving scald injuries were placed on their dorsum in an appropriate plastic template designed to expose a 15% TBSA and immersed in a boiling water bath for 8 s. After burn injury, mice were immediately dried off to prevent any further scalding. As a control, sham-injured animals were anesthetized, shaved, held in a plastic template, but a room temperature water bath was used instead for the same period. Both burn and sham-injured animals were resuscitated with 1.0 mL of 0.9% isotonic sodium chloride solution i.p. per 20 g body weight. The animal cages were then placed on heating pads to prevent circulatory collapse and cardiovascular shock. After recovery from anesthesia, mice were able to eat, drink, groom, and ambulate to their preinjury capacity.
In other studies, animals were given 1.5 μg LPS/g of body weight i.p. (7, 9), using LPS that was derived from Pseudomonas aeruginosa (serotype 10, lot number: 123K4144, Sigma Chemical Co, St Louis, Mo) and dissolved in sterile saline. Control mice received saline alone. We have previously demonstrated that i.p. LPS produces a mortality rate close to 25% in aged mice and no mortality in young animals (7).
For most of the experiments described here, animals were killed at 24 h after each treatment. An additional group of mice was euthanized at 3 h of LPS administration. The mice were killed by carbon dioxide inhalation and subsequent cervical dislocation. No additional therapeutic intervention was provided, as administration of anti-inflammatory or analgesic drugs may introduce complications into the assessment of inflammatory responses. To avoid confounding factors related to circadian rhythms, all injury procedures were administered between 8:00 AM and 10:00 AM. At the time of killing, cardiac blood was collected and serum was obtained and aliquoted. Liver was flash frozen in liquid N2. All samples were kept at −80°C until use.
Analysis of serum alanine aminotransferase
Serum alanine aminotransferase (ALT) activity was assessed using a commercial kit (Biotron Diagnostics, Inc, Hemet, Calif). Ten microliters of serum samples from each mouse were assayed for ALT, as recommended by the manufacturer. Data are reported as ALT International Units (IUs) per milliliter of serum.
Histological evaluation of neutrophil content
Briefly, liver was fixed in 10% (wt/vol) phosphate buffer saline–buffered formalin solution and embedded in paraffin. Five-micron sections of liver were stained with hematoxylin and eosin (H&E) and evaluated by light microscopy. Ten high-power fields per section were analyzed for neutrophils on the basis of their distinct morphologic features (granular cytoplasm with a multilobed nucleus) and scored in a blinded manner. Data are reported as number of cells in ten 630× fields.
Analysis of myeloperoxidase activity
Myeloperoxidase (MPO), a neutrophil-specific enzyme, was used as an index of neutrophil infiltration using the method of Suzuki et al. with modifications (17). The weight of liver was determined before homogenization, and the levels of MPO activity were measured and compared with a commercial standard (Sigma Chemical Co, St Louis, Mo). Data are reported as mean MPO activity in units per milligram tissue.
Analysis of chemokine and IL-6 levels
Liver homogenates were prepared as described by Colantoni and colleagues (18) with minor modifications. Briefly, 50 mg of tissue was homogenized in 1.0 mL of cold phosphate buffer saline containing protease inhibitor cocktail (Hoffmann-La Roche Ltd, Basel, Switzerland). Single-use aliquots of the homogenates were stored at −80°C before measurements. Liver KC, macrophage inflammatory protein 2 ([MIP-2] R&D Systems, Minneapolis, Minn), and IL-6 (BD Biosciences, San Diego, Calif) were determined with commercially available Enzyme-Linked ImmunoSorbent Assay (ELISA) kits according to the manufacturers’ instructions. Cytokine standard curves were prepared in ELISA buffer, and samples from the tissue homogenates were calculated from these standard curves. Results were normalized to total protein content in the liver samples, determined by the BioRad Protein Assay (BioRad Laboratories, Hercules, Calif). Data are reported as cytokine or chemokine per milligram protein.
Statistical analysis
Data are expressed as mean ± SEM. ANOVA and Tukey-Kramer multiple comparisons were used to determine statistical significance using GraphPad Prism Version 2.0 statistical package (San Diego, Calif). A value of P < 0.05 was considered significant.
RESULTS
Serum ALT levels in aged IL-6–deficient mice after LPS or injury
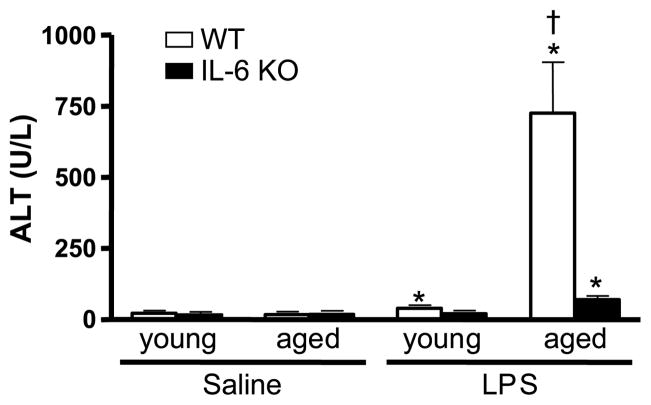
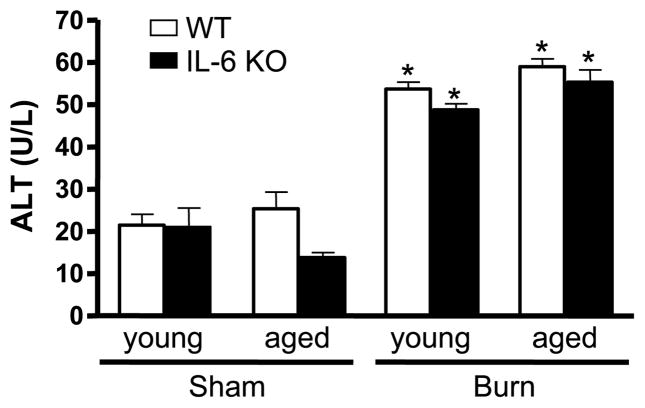
The major source of ALT is the liver and, because of this, elevation in serum level of this enzyme has been used as an index of hepatic injury (19). Serum ALT levels were examined at 24 h after LPS administration or burn injury (Figs. 1 and 2). Detectable serum levels of ALT were found in all experimental groups. In the LPS model, animals given saline did not show differences in serum ALT (17.6 – 22.4 IU/L) despite age or IL-6 deficiency (Fig. 1). LPS induced an increase in serum ALT levels in young WT mice versus young saline-treated mice (39.7 ± 4.9 U/L, P < 0.05), but the concentration was within the reference range for mice (20). In contrast, LPS-treated aged WT mice had a 40-fold elevation over the level observed in aged mice given saline (P < 0.05). After LPS exposure, young IL-6 KO mice did not show an elevation in serum ALT when compared with young IL-6 KO mice receiving saline. Aged IL-6 KO mice administered LPS had a moderate increase in serum ALT levels (70.0 ± 12.8 U/L, P < 0.05), which was 10% of the level observed in aged WT animals after the same treatment (P < 0.05). In the burn model, animals receiving a sham injury did not show significant differences in serum ALT (13.8 – 21.8 IU/L) regardless of age or the presence of IL-6 (Fig. 2). At 24 h after burn, there was a 2- to 4-fold increase of serum ALT levels in all experimental groups (48.9 – 58.9 IU/L), P < 0.05. However, ALT levels were comparable regardless of age or IL-6 deficiency.
Fig. 1. ALT was measured in serum obtained from young and aged WT and IL-6 KO mice given LPS (1.5 μg LPS/g body weight) or saline at 24 h after treatment.
Data are expressed as mean ALT units (units per milliliter serum ± SEM. n = 5 – 11 per group). *P < 0.05 from age-matched saline groups; †P < 0.05 from all other groups.
Fig. 2. ALT was measured in serum obtained from young and aged WT and IL-6 KO mice 24 h after sham or burn injury.
Data are expressed as mean ALT units (units per milliliter serum ± SEM. n = 5 to 11 per group). *P < 0.05 from age-matched saline groups.
Effect of aging and IL-6 deficiency on hepatic neutrophil infiltration after LPS or injury
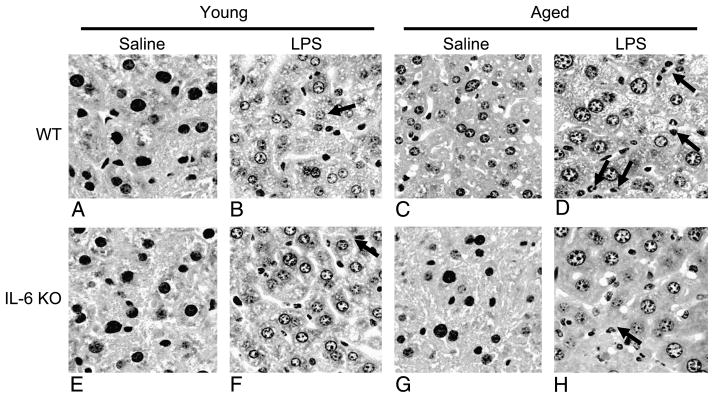
Hepatic neutrophil transmigration is another index of hepatic injury (19). Histological analysis of H&E-stained sections of formalin-fixed livers (Fig. 3) revealed an absence of neutrophils in the livers of young WT, young IL-6 KO, and aged IL-6 KO animals receiving saline (Fig. 3A, E, and G, respectively). Similarly, neutrophils were not found in the livers of aged WT control mice (Fig. 3C). Young WT mice given LPS had elevated hepatic neutrophils compared with saline controls (Fig. 3B). In contrast, aged WT mice given LPS had markedly elevated neutrophil infiltration in their livers (Fig. 3D). In young IL-6 KO animals receiving LPS, elevated hepatic neutrophils were observed when compared with saline controls (Fig. 3F). The number of neutrophils was similar to that of young saline-treated WT mice. IL-6 KO aged mice given LPS exhibited elevated hepatic neutrophils (Fig. 3H), but their presence was lower when compared with aged WT mice administered with LPS. In animals given burn injury, no changes in hepatic neutrophils were observed regardless of age, treatment, or IL-6 deficiency (data not shown).
Fig. 3. Livers from young and aged WT and IL-6 KO mice obtained at 24 h after LPS (1.5 μg LPS/g body weight) or saline treatment were formalin-fixed.
Five-micron sections of liver were stained with H&E and then observed by light microscopy. Representative sections of livers are shown from young WT given saline (A), young WT given LPS (B), aged WT given saline (C), aged WT given LPS (D), young IL-6 KO given saline (E), young IL-6 KO given LPS (F), aged IL-6 KO given saline (G), and aged IL-6 KO given LPS (H). Black arrows indicate neutrophils (original magnification ×400).
Quantitation of the number of neutrophils showed that in the LPS model (Table 1), few neutrophils were found in the saline controls, irrespective of age or IL-6 deficiency. Basal levels of neutrophils in the liver of aged WT mice were elevated when compared with the other control groups, however, the difference did not reach statistical significance. Administration of LPS elevated hepatic neutrophil numbers in all experimental groups. Aged WT mice given LPS showed an increase in neutrophil infiltration that was 4-fold higher than that of young LPS-treated WT animals (P < 0.05). In contrast, aged IL-6 KO mice given LPS had only a 1.5-fold increase in hepatic neutrophils as compared with young IL-6 KO mice given the same stimulus (P < 0.05). In addition, aged IL-6 KO mice had 44% less neutrophils when compared with aged WT LPS-treated mice (P < 0.05).
Table 1.
Liver neutrophils in young and aged WT and IL-6 KO mice given LPS or burn injury*
| WT | IL-6 KO | |||
|---|---|---|---|---|
| LPS model | ||||
| Saline | LPS | Saline | LPS | |
| Young | 1.2 ± 0.6 (n = 6) | 32.1 ± 7.2† (n = 7) | 2.5 ± 1.0 (n = 4) | 48.6 ± 6.7† (n = 5) |
| Aged | 7.2 ± 3.1 (n = 6) | 132.5 ± 27.7†‡ (n = 11) | 1.4 ± 0.7 (n = 6) | 72.0 ± 9.8 (n = 6)† |
| Burn model | ||||
| Sham | Burn | Sham | Burn | |
| Young | 1.0 ± 0.0 (n = 4) | 4.6 ± 0.5 (n = 5) | 2.0 ± 1.1 (n = 4) | 2.3 ± 1.3 (n = 8) |
| Aged | 1.3 ± 0.8 (n = 4) | 3.3 ± 0.6 (n = 7) | 1.3 ± 0.6 (n = 4) | 3.5 ± 1.3 (n = 6) |
Values are expressed as mean number of neutrophils per 10 fields ± SEM for young or aged WT or IL-6 KO female BALB/C mice at 24 h after either an i.p. injection of LPS (1.5 μg/g body weight), saline, a 15% TBSA scald or sham injury. Number of animals are listed inside parentheses.
P < 0.05 from corresponding age control groups.
P < 0.05 from all other experimental groups.
In the burn model, similar levels (1 – 2 neutrophils in 10 fields) of neutrophils were found in the sham-injured controls, irrespective of age or IL-6 deficiency. Burn triggered a modest increase in hepatic neutrophil counts in young and aged WT and IL-6 KO mice (2.3 – 4.6 neutrophils in 10 fields), but this was not significant. Overall, the results shown in Figures 1–3 and Table 1 indicate that under our experimental conditions, there is an insult-specific development of hepatic injury in aged mice. In the burn injury model, the magnitude of hepatic injury was comparable regardless of age or IL-6 deficiency. However, in the LPS model, the hepatic injury seen in aged mice was IL-6 dependent.
LPS-induced elevation in hepatic MPO activity in aged WT mice is reduced in aged IL-6 KO mice
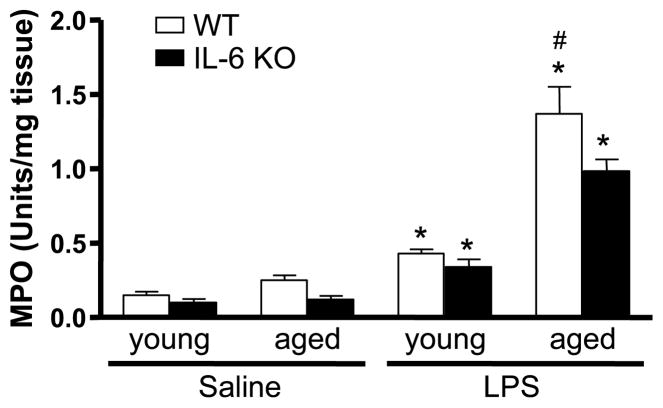
As an additional index of neutrophil infiltration, MPO activity was measured in liver homogenates. Because burn-injured mice did not show effects of aging or IL-6 on hepatic inflammation, we elected to continue our studies only in the LPS model. Neutrophil infiltration was similar in young and aged WT and IL-6 KO mice given saline. The MPO level in livers of aged saline-treated WT animals was slightly higher than the other control groups, but this was not significant (Fig. 4). Administration of LPS was associated with a higher level of MPO activity in the livers of young WT mice compared with their saline controls, but this level was one-fourth that of LPS-treated aged WT mice (P < 0.05). The livers of young IL-6 KO and young WT mice given LPS had similar levels of MPO when compared with young IL-6 KO mice given the same stimulus. In contrast, the elevated MPO seen in the liver of aged IL-6 KO mice receiving LPS was reduced by 30% relative to that of aged WT animals given LPS (P < 0.05). These results indicate that in the absence of IL-6, the LPS-induced increase in MPO activity observed in aged mice is attenuated, thus corroborating our findings of increased hepatic neutrophils (Fig. 3 and Table 1).
Fig. 4. Young and aged WT and IL-6 KO mice were given LPS or saline.
Livers harvested at 24 h after LPS administration were homogenized, and MPO levels were determined. Data are expressed as MPO activity in units per milligram tissue ± SEM. n = 4 to 11. *P < 0.05 from age-matched saline groups; †P < 0.05 from all other groups.
Hepatic production of the chemokine KC is elevated in WT aged mice given LPS, but lower in aged IL-6 KO mice given LPS
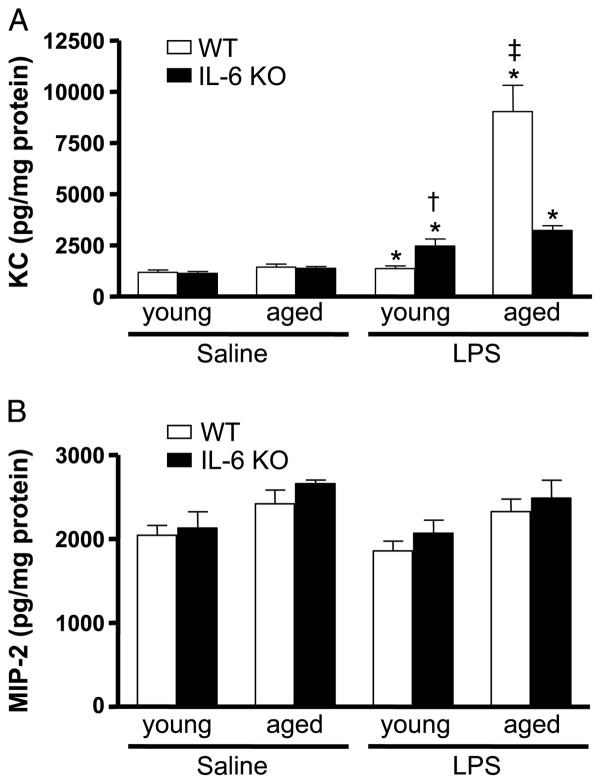
Because the elevated hepatic inflammatory response seen in LPS-treated aged WT mice was reduced in the livers of aged IL-6 KO mice exposed to LPS, we elected to investigate whether these differences were associated with reduced hepatic levels of the neutrophil chemokines KC and MIP-2. Both KC and MIP-2 were detectable in all samples examined (Fig. 5, A and B). The expression of KC in the livers was similar in all saline groups. LPS stimulation of KC was not evident in livers from young WT mice, whereas livers of aged WT mice given LPS had an 8-fold increase relative to age-matched controls. An elevation in hepatic KC was also seen in young and aged WT mice at 3 h after LPS treatment, however, there was no age difference (data not shown). After LPS exposure, young and aged IL-6 KO mice showed similar increases in hepatic KC levels. Livers of young IL-6 KO mice given LPS had twice as much KC as did livers of young WT mice given LPS (P < 0.05). In contrast, hepatic KC levels in LPS-treated aged IL-6 KO mice were reduced by 75% when compared with aged WT animals given LPS (P < 0.05). Unlike KC, we found that liver levels of MIP-2 were similar, regardless of age, IL-6 deficiency, or LPS treatment. According to these results, the neutrophil chemokine KC is markedly elevated in the liver after LPS administration in aged WT mice relative to young mice given the same stimulus, suggesting that it may play a role in the infiltration of neutrophils after LPS exposure. Interestingly, the elevation in hepatic KC seen in aged mice receiving LPS is dependent, in part, on the presence of IL-6.
Fig. 5. Livers were harvested at 24 h after LPS or saline administration.
KC (A) and MIP-2 (B) levels were measured in liver homogenates by ELISA. Data were normalized to total protein content in the homogenates and are shown as mean chemokines in picograms per milligram protein ± SEM. n = 4 to 11. *P < 0.05 from age-matched saline groups; †P < 0.05 from young WT + LPS group; ‡P < 0.05 from all other groups.
Hepatic production of the proinflammatory cytokine IL-6 is affected by age
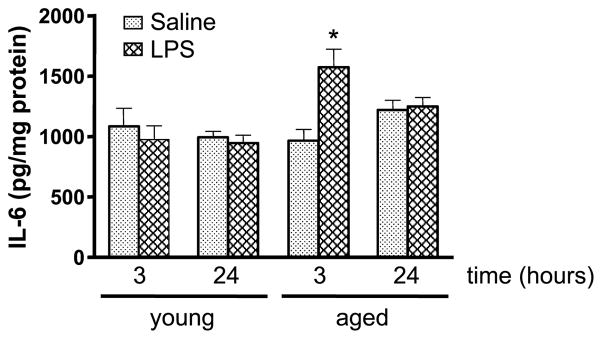
In the liver, proinflammatory cytokines, such as IL-6, have key roles in the development and progression of inflammation (21). To see the effect of aging on the hepatic levels of IL-6 in response to LPS, the levels of the cytokine were measured in liver homogenates (Fig. 6). No differences in hepatic IL-6 levels were found between young and aged WT saline controls. A similar result was found at 24 h after LPS treatment. As expected, no IL-6 was detectable in IL-6 KO mice (data not shown). Because others reported an elevation in IL-6 in the liver at 3 h after LPS exposure (22), we examined hepatic levels of this cytokine at an earlier time point. Young and aged saline control mice showed comparable liver IL-6 levels, and there was no change in the level of this cytokine in livers obtained from young mice at 3 h after LPS. In contrast, LPS-treated aged WT mice showed a 38% increase of hepatic IL-6 over that of saline controls (P < 0.05). Thus, early after LPS administration, hepatic production of IL-6 is elevated in aged but not young WT mice.
Fig. 6. IL-6 levels were measured in liver homogenates.
At 3 and 24 h after LPS or saline treatment, livers were collected from young and aged WT mice. Levels of IL-6 in homogenates of livers were determined by ELISA. These values were normalized to the total protein content. Data are expressed as mean IL-6 in picograms per milligram protein ± SEM. n = 5 to 11. *P < 0.05 from all other groups.
DISCUSSION
In this study, we analyzed the effects of aging and IL-6 on the hepatic inflammatory response in two models of inflammation and injury: dorsal scald injury versus i.p. LPS administration. We found a similar degree of hepatic injury in burn-injured mice, independent of age or IL-6 deficiency. However, aged WT mice given LPS had greater hepatic injury relative to young WT mice. When compared with aged WT mice, aged IL-6 KO mice receiving LPS had reduced hepatic injury. Overall, our results indicate that the occurrence of organ injury in aged mice depends on the nature of the insult. In addition, the role of IL-6 as a contributing factor to hepatic injury during the inflammatory response of aged mice is insult specific.
The “two-hit” inflammatory model establishes that an initial serious injury primes the immune system for an excessive inflammatory response after a subsequent stimulus (23). The exacerbated response to the second hit would be a decisive factor in triggering the induction of MOF, as has been demonstrated by epidemiological studies (24) and in laboratory investigations (25, 26). In the context of aging, the presence of a proinflammatory state (inflamm-aging) characterized, in part, by elevated circulating levels of IL-6 in healthy aged subjects previous to insult may well represent the first hit. Just as in humans, our published results show that, before burn injury (6) or LPS exposure (7), aged WT mice exhibit detectable IL-6 in their serum, thus indicating the presence of inflamm-aging in the two animal models used herein.
After a second hit, such as burn injury or LPS administration, aged individuals exhibit an augmented response beyond that of young mice receiving a comparable insult and concomitantly are more prone to the development of MOF, relative to their younger counterparts. Interestingly, the remote organ damage in the two animal models presented here has distinct characteristics. Analysis of the lungs revealed an exacerbated pulmonary inflammatory response in aged mice given LPS compared with that of young mice receiving LPS (9). This response was characterized by a 6-fold higher neutrophil infiltration, higher pulmonary levels of the neutrophil chemotactic cytokines MIP-2 and KC (100-fold for MIP-2, and a 25-fold for KC, respectively), and 1.5-fold increase in levels of the proinflammatory cytokine IL-1β compared with those of the lungs of young mice given LPS (9). Meanwhile, 24 h after burn injury, the lungs of aged mice showed 5-fold higher neutrophil content and 3 times more KC, but no changes in lung MIP-2 and IL-1β compared with young burn-injured mice (10). In total, these results indicate that remote tissue injury in aged mice will depend on the nature of the second hit. Under our experimental conditions, LPS administration may represent a different second hit compared with burn injury, as suggested by the parameters of pulmonary inflammation.
Here we have found that hepatic injury in aged mice showed some similarities, but also profound differences between our two experimental models. A similar increase in serum ALT was found in young WT mice given either burn injury or LPS. However, a lack of effect of age in the induction of serum ALT in aged WT mice given burn injury was found. In addition, aged WT animals given LPS exhibited elevated hepatic neutrophil infiltration compared with their age matches receiving burn injury. Independent of age, the liver has been identified as one of the most frequently affected organs in burn-injured patients (12), as well as in animal models (25, 27) of systemic inflammation. We have previously characterized the hepatic acute-phase response in the livers of young mice receiving a 15% TBSA burn (28). This response involved elevated early production of hepatic TNF-α and IL-1β (with a peak at 2 h after burn injury), whereas IL-6 was not involved until later (increasing until 12 h after injury and beginning to decline at 24 h). These studies suggest differential levels and kinetics of the production of proinflammatory cytokines in young mice receiving burn injury. In the current studies, we have begun to unravel the contribution of IL-6 to burn-induced hepatic injury in the context of advanced age. Our results indicating a lack of effect of IL-6 in hepatic inflammation seem to rule out an involvement of IL-6 as a contributor to hepatic injury after burn injury, even in the presence of a first hit (aging). Alternatively, the hepatic damage caused by the burn injury could be of lesser magnitude, and hence, an effect of IL-6 could not be determined. This second possibility also needs to be considered.
In the liver, neutrophil infiltration into the hepatic parenchyma is one of the markers of LPS-mediated hepatic damage (19). In our experiments, elevated hepatic neutrophil numbers and MPO activity were observed in aged WT mice given LPS relative to young WT animals receiving the same stimulus. Although significant, these indices of neutrophil accumulation differed in magnitude relative to ALT activity. These results suggest that although relevant for hepatic injury in aged animals receiving LPS, IL-6 is not an exclusive contributor to hepatic neutrophil infiltration. Like in other tissues, the accumulation of neutrophils is a process triggered by chemokines, such as the CXC chemokine, IL-8, or the murine functional homologs, KC and MIP-2, produced by multiple cell types including Kupffer cells and hepatocytes (29, 30). In our experiments, the infiltration of neutrophils into the liver observed at 24 h after LPS administration in aged WT mice was accompanied by enhanced hepatic production of KC. An elevation in hepatic KC was also seen in young and aged WT mice at 3 h after LPS treatment (data not shown). Using a high dose of LPS, it was described that hepatic messenger RNA for KC and MIP-2 was rapidly induced in the liver of young mice, at approximately 50- to 100-fold within 3 h of LPS administration (30), then drastically decreased at 6 h. Interestingly, here we show that at 24 h after LPS administration, only hepatic KC remained elevated in aged mice, suggesting a prolonged time course of inflammation in the liver of aged WT mice. In addition, our results in liver differ from our observations in lungs of aged WT mice at 24 h after LPS treatment, where the infiltration of neutrophils was accompanied by elevated production of both MIP-2 and KC (9). It has been proposed that tissue-specific expression of chemokines, such as KC and MIP-2, may account for this variation, which may be a function of differential control of gene expression (31). This evidence, added to the observed aberrant expression and activity of hepatic inflammation-associated transcription factors associated with advanced aging (32), may help explain why LPS fails to elevate MIP-2 levels in the liver in our model.
Because the elevation in hepatic IL-6 was relatively small (1.6-fold) compared with the magnitude of serum IL-6—160 times that of young WT mice after 24 h of LPS administration (7)— and liver injury, assessed by serum ALT, there are likely to be other sources for this cytokine, such as adipose tissue (33) and arteries (34).
Many of the changes in the function of immune cells that occur with advanced aged have been attributed to chronic oxidative stress [for a recent review see (35)]. Oxidative stress leads to the activation of transcription factors (such as nuclear factor–κB) and ultimately the expression of oxidant and inflammatory genes (35). As a result of oxidative injury associated with aging, immune cells may lose their ability to maintain their redox balance. This imbalance may generate a vicious cycle, resulting in a worse outcome after exposure to an inflammatory insult. In the absence of exogenous stimuli, analysis of hepatic levels of glutathione and malondialdehyde revealed increased oxidative stress in the liver of aged WT mice as compared with that of young WT animals (C.R. Gomez, PhD and E.J. Kovacs, PhD, unpublished data). This increase was not observed in aged IL-6 KO mice. Although preliminary, these data suggest that oxidative stress may be a critical component of the pathway leading to aberrant IL-6 and KC production in the livers of aged WT mice given LPS.
The results described here in conjunction with our previous published (7, 10) observations have suggested that the nature of the insult will determine the degree of remote tissue injury in aged animals. Under our experimental setting, LPS administration was a stronger second hit relative to a 15% TBSA burn injury, resulting in a more pronounced inflammatory response systemically (6) and locally in the lungs and in the liver [(9), (10), and the results described here]. Our results demonstrate that the relative difference in the levels of IL-6, 28-fold higher in the serum of aged mice given LPS as compared with that of aged mice receiving a burn injury (2-fold higher) (6), is associated with a greater inflammatory response in the LPS model. This response could in turn catalyze the events leading to hepatic dysfunction and, eventually, to MOF. Although the molecular mechanisms involved in IL-6–mediated tissue injury with age need a better understanding, our results suggest that blocking this critical cytokine’s actions on target cells may diminish organ damage associated with hyperinflammatory responses, especially in the aged population.
Acknowledgments
The authors thank Mr Luis Ramirez and Ms Michelle Morgan for excellent technical assistance; Drs Pamela Witte, Heather Minges-Wols, and Ingrid Espinoza for thoughtful discussions and critical review of this article; and Ms Mary Kay Olson for her expertise in preparing and processing histological specimens.
This study was supported by the National Institutes of Health (grant no. R01 AG018859 to E.J.K., grant no. AG018859-S1 to E.J.K., and grant no. F30 AG029724 to V.N.).
ABBREVIATIONS
- ALT
Alanine aminotransferase
- MIP-2
macrophage inflammatory protein 2
- MOF
multiple organ failure
- MPO
myeloperoxidase
- TBSA
total body surface area
References
- 1.American Burn Association. National Burn Repository 2002 Report. Chicago, IL: American Burn Association; 2002. [Google Scholar]
- 2.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs EJ, Grabowski KA, Duffner LA, Plackett TP, Gregory MS. Survival and cell mediated immunity after burn injury in aged mice. J Am Aging Assoc. 2002;25:3–10. doi: 10.1007/s11357-002-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. J Leukoc Biol. 2004;76:36–41. doi: 10.1189/jlb.1103538. [DOI] [PubMed] [Google Scholar]
- 7.Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25:581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- 8.Baue AE. MOF, MODS, and SIRS: what is in a name or an acronym? Shock. 2006;26:438–449. doi: 10.1097/01.shk.0000228172.32587.7a. [DOI] [PubMed] [Google Scholar]
- 9.Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 10.Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol. 2008;83:1493–1501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 12.Aikawa N, Shinozawa Y, Ishibiki K, Abe O, Yamamoto S, Motegi M, Yoshii H, Sudoh M. Clinical analysis of multiple organ failure in burned patients. Burns Incl Therm Inj. 1987;13:103–109. doi: 10.1016/0305-4179(87)90097-0. [DOI] [PubMed] [Google Scholar]
- 13.Asfar P, De Backer D, Meier-Hellmann A, Radermacher P, Sakka SG. Clinical review: influence of vasoactive and other therapies on intestinal and hepatic circulations in patients with septic shock. Crit Care. 2004;8:170–179. doi: 10.1186/cc2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streetz KL, Tacke F, Leifeld L, Wustefeld T, Graw A, Klein C, Kamino K, Spengler U, Kreipe H, Kubicka S, et al. Interleukin 6/gp130-dependent pathways are protective during chronic liver diseases. Hepatology. 2003;38:218–229. doi: 10.1053/jhep.2003.50268. [DOI] [PubMed] [Google Scholar]
- 15.Napoli J, Bishop GA, McCaughan GW. Increased intrahepatic messenger RNA expression of interleukins 2, 6, and 8 in human cirrhosis. Gastroenterology. 1994;107:789–798. doi: 10.1016/0016-5085(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 16.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62:733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 17.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 18.Colantoni A, Duffner LA, De Maria N, Fontanilla CV, Messingham KA, Van Thiel DH, Kovacs EJ. Dose-dependent effect of ethanol on hepatic oxidative stress and interleukin-6 production after burn injury in the mouse. Alcohol Clin Exp Res. 2000;24:1443–1448. [PubMed] [Google Scholar]
- 19.Rose R, Banerjee A, Ramaiah SK. Characterization of a lipopolysaccharide mediated neutrophilic hepatitis model in Sprague Dawley rats. J Appl Toxicol. 2007;27:602–611. doi: 10.1002/jat.1243. [DOI] [PubMed] [Google Scholar]
- 20.Loeb WF, Das SR, Harbour LS, Turturro A, Bucci TJ, Clifford CB. Clinical biochemistry. In: Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, editors. Pathobiology of the Aging Mouse. Vol. 1. Washington, DC: International Life Sciences Institute; 1996. p. 9. [Google Scholar]
- 21.Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161:III–XIII. 1–151. doi: 10.1007/978-3-642-56553-3. [DOI] [PubMed] [Google Scholar]
- 22.Xie GQ, Jiang JX, Chen YH, Liu DW, Zhu PF, Wang ZG. Induction of acute hepatic injury by endotoxin in mice. Hepatobiliary Pancreat Dis Int. 2002;1:558–564. [PubMed] [Google Scholar]
- 23.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauaia A, Moore FA, Moore EE, Lezotte DC. Early risk factors for postinjury multiple organ failure. World J Surg. 1996;20:392–400. doi: 10.1007/s002689900062. [DOI] [PubMed] [Google Scholar]
- 25.Murphy TJ, Paterson HM, Kriynovich S, Zang Y, Kurt-Jones EA, Mannick JA, Lederer JA. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. J Leukoc Biol. 2005;77:16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 26.Ayala A, Perrin MM, Ertel W, Chaudry IH. Differential effects of hemorrhage on Kupffer cells: decreased antigen presentation despite increased inflammatory cytokine (IL-1, IL-6 and TNF) release. Cytokine. 1992;4:66–75. doi: 10.1016/1043-4666(92)90039-t. [DOI] [PubMed] [Google Scholar]
- 27.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 28.Plackett TP, Colantoni A, Heinrich SA, Messingham KA, Gamelli RL, Kovacs EJ. The early acute phase response after burn injury in mice. J Burn Care Res. 2007;28:167–172. doi: 10.1097/BCR.0b013E31802CB84F. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Klintman D, Liu Q, Sato T, Jeppsson B, Thorlacius H. Critical role of CXC chemokines in endotoxemic liver injury in mice. J Leukoc Biol. 2004;75:443–452. doi: 10.1189/jlb.0603297. [DOI] [PubMed] [Google Scholar]
- 30.Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, Hamilton TA, Vogel SN. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–1544. [PubMed] [Google Scholar]
- 31.Ohmori Y, Fukumoto S, Hamilton TA. Two structurally distinct kappa B sequence motifs cooperatively control LPS-induced KC gene transcription in mouse macrophages. J Immunol. 1995;155:3593–3600. [PubMed] [Google Scholar]
- 32.Walter R, Sierra F. Changes in hepatic DNA binding proteins as a function of age in rats. J Gerontol A Biol Sci Med Sci. 1998;53:B102–B110. doi: 10.1093/gerona/53a.2.b102. [DOI] [PubMed] [Google Scholar]
- 33.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 34.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 35.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]