Abstract
The trematode Schistosoma mansoni is the primary cause of schistosomiasis, a devastating neglected tropical disease that affects 200 million individuals. Identifying novel therapeutic targets for the treatment of schistosomiasis is therefore of great public interest. The catecholamines norepinephrine (NE) and dopamine (DA) are essential for the survival of the parasite as they cause muscular relaxation and a lengthening in the parasite and thereby control movement.
Here we characterize a novel dopamine/norepinephrine transporter (SmDAT) gene transcript, from Schistosoma mansoni. The SmDAT is expressed in the adult form and in the sporocyst form (infected snails) of the parasite, and also in the egg and miracidium stage. It is absent in the cercaria stage but curiously a transcript missing the exon encoding transmembrane domain 8 was identified in this stage.
Heterologous expression of the cDNA in mammalian cells resulted in saturable, dopamine transport activity with an apparent affinity for dopamine comparable to that of the human dopamine transporter. Efflux experiments reveal notably higher substrate selectivity compared with its mammalian counterparts as amphetamine is a much less potent efflux elicitor against SmDAT compared to the human DAT. Pharmacological characterization of the SmDAT revealed that most human DAT inhibitors including psychostimulants such as cocaine were significantly less potent in inhibiting SmDAT. Like DATs from other simpler organisms the pharmacology for SmDAT was more similar to the human norepinephrine transporter. We were not able to identify other dopamine transporting carriers within the completed parasite genome and we hypothesize that the SmDAT is the only catecholamine transporter in the parasite and could be responsible for not only clearing DA but also NE.
Keywords: Dopamine transporter, Parasite, DAT, NET, Gene expression, splice variant
1. INTRODUCTION
Schistosoma mansoni is one of four major Schistosoma species that infect over 200 million people in tropical and subtropical regions worldwide [1]. Infection of humans is initiated when free-swimming cercariae penetrate human skin to gain access to its definitive human host. Following penetration the cercariae transform into schistosomulae, entering the vascular system and migrate to their final locus in mesenteric veins where males and females mate continuously. Eggs exit the human host in feces and hatch on contact with water, releasing miracidia, which infect the intermediate snail hosts. The infected snails, bearing schistosomal sporocysts release cercariae into the water which, in turn, infect humans.
Schistosomiasis has serious health costs and the parasitic infections are associated with malnutrition and impaired growth and development [1–2]. One drug, praziquantel, cures 60 to 90 percent of infections but is not active on immature worms and eggs [2]. Like is the case for many other drugs directed against microbes there are reports of an increase in resistance to praziquantel and the development of novel therapeutic strategies are of major interest [3].
The catecholamines norepinephrine (NE) and dopamine (DA) are present in Schistosoma mansoni [4] and are inhibitory neurotransmitters causing a lengthening of the worm through muscular relaxation [5–7]. These transmitters are therefore of great importance to the movement of the organism both within and between its two hosts. The enzyme responsible for the first step in the biosynthetic pathway of NE and DA the tyrosine hydroxylase has been cloned [8] and so has a DA receptor [9]. Following release, DA and NE signaling is terminated by the clearance via dopamine (DAT) and norepinephrine transporters (NET) from the extracellular space using the sodium gradient as thermodynamic driving force [10].
Specific inhibitors for these transporters, including the abused psychostimulants cocaine and amphetamine and several clinically used antidepressants, exert their physiological effects by interfering with uptake and thus prolonging the actions of the monoamines. Because DAT and NET are the targets of humanly abused psychostimulants it is of great importance to enhance our understanding of the interaction of the psychostimulants with their target transporters DAT and NET.
In the present study we have cloned and pharmacologically characterized a catecholamine transporter gene from S. mansoni by expressing the cDNA in mammalian cells. We also show by RT-PCR that the transporter is expressed at various levels in some of the distinct stages of the parasite life cycle. Like the recently cloned serotonin transporter from this species (Fontana et al., 2009) the SmDAT is less promiscuous toward exogenous substrates compared to its mammalian counterparts.
2. MATERIALS AND METHODS
2.1. Parasites
An LE strain of Schistosoma mansoni is routinely maintained by passage through Biomphalaria glabrata snails and BALB/c mice. B. glabrata-infected snails were induced to shed cercariae by exposing them under artificial illumination in a 30°C water bath for 1h. Schistosomula were obtained by mechanical transformation of cercariae according to the procedure described by [11]). Adult worms were recovered from mice previously infected with cercariae by perfusion of the livers and mesenteric veins. All animal experiments were treated according to the guidelines for use of animals in research approved by the Ethics and Animal Experimentation committee of Ribeirão Preto School of Medicine, University of São Paulo, Brazil.
2.2. Materials
[3H]-dopamine, [3H]-serotonin and [3H]-noradrenalin were obtained from Perkin Elmer (Boston, MA, USA). Reagents for uptake and binding buffers, unlabelled dopamine, uptake inhibitors and substrates were from Sigma-RBI (St. Louis, MO, USA). Racemic citalopram was a gift from Lundbeck (Copenhagen, Denmark). Cell culture media, MEM, FCS, horse serum, penicillin/streptomycin, glutamine and D-PBS were from Invitrogen Life Technologies (Carlsbad, CA, USA). All other reagents were of analytical grade or better. The Generacer kit used for cloning was from Invitrogen Life Technologies (Carlsbad, CA, USA). The Phusion polymerase was from Finnzymes (Espoo, Finland).
2.3. Cloning procedures
Total RNA was isolated from adult Schistosoma mansoni. 5 µg of total RNA was used in the RNA ligase-mediated RACE protocol according to the manufacturer (Invitrogen, Carlsbad, CA, USA). PCR was performed using the following primers to isolate Smp_156320/ XP_002577603.1:
SmDAT5: 5′-atgctcgagggATGGCAGAAGAATCAAACAAG-3′
SmDAT3: 5′-atgctgcagtctagaTTATATTGAAGATTCAAAATGATTAC-3′
To isolate Smp_193800/ XP_002571308.1 the following primers were used:
smp193800-5: 5′-atgctcgagggATGAAATCTGAGATATCCGATC-3
smp193800-3: 5′-atgctgcagtctagaTTATACATTTCTAACGACTTTATAAC-3′
The generated fragment was cloned into the pEGFP-C2 (Clontech, Mountain View, CA, USA) for sequencing and for N-terminal tagging with GFP and expression in mammalian cells.
For the 5’RACE reaction the following primers were used:
5'SmDATrace: 5′-CAAACATTTGCCAAATCCAC-3′
5'SmDAT-nested: 5′-GCAAACCCAATAATAGATAATAAGAAATC-3′
Together with GeneRacer™ 5′ Primer: 5′-CGACTGGAGCACGAGGACACTGA-3′ GeneRacer™ 5′ Nested Primer: 5′-GGACACTGACATGGACTGAAGGAGTA-3′
2.4. cDNA synthesis and PCR amplifications
Total RNA from different stages of S. mansoni was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. 3 µg of RNA from infected snails (sporocysts), uninfected snails (control), cercariae, schistosomulae, adult worms and eggs were reverse-transcribed using oligo (dt)18 anchor primer and SuperScript II (Invitrogen, Carlsbad, CA, USA).
The specific primers to amplify dopamine transporter transcript were: SmDAT618F: 5’-CCTGGTAAAATTCAATGGCAAA-3’ SmDAT1298R: 5’-GCTCCGATCCCAATGTAGAA-3’ The Smα-tubulin specific primers were: α-Tubulin F: 5′ GAAATGCTTGTTGGGAGTTG 3′ α-Tubulin R: 5′ TTATCACTTGGCATCTGTCC 3′
2.5. Transport assays in COS-7 cells
COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum supplemented with penicillin and streptomycin in a humidified atmosphere with 5% CO2 at 37°C. Uptake experiments were performed two days after transfecting DNAs (hDAT, hNET and GFP-SmDAT) with TransIT-LT1 (Mirus Bio LLC, Madison, WI, USA) transfection reagent and plating the cells in 96 wells-plates. The media was removed and the cells were washed with phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4) containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBSCM). Following washing, cells were incubated at room temperature for 10 min in PBSCM, including 50 µM ascorbic acid, 10 mM D-glucose and 5 µM of the COMT inhibitor RO 41-0960 (PBSCM-AGR) with a constant concentration of radiolabeled substrate (50 nM [3H]DA) and increasing concentrations of unlabeled substrate (0.05 to 100 µM DA). The assay was performed using 12 different substrate concentrations run in duplicate. Washing with PBSCM terminated the uptake. Specific uptake was determined as the difference between uptake counts from cells transfected with SmDAT, hDAT or hNET-containing constructs and from mock-transfected cells. Counts from inhibition of a fixed radiolabeled substrate concentration were converted to total uptake by multiplying with the ratio of ([radiolabeled substrate] + [unlabeled substrate]) / [radiolabeled substrate].
For IC50 determinations, following the initial washing step, cells were incubated for 10 min with 12 increasing concentrations of drug (in duplicate) to reach equilibrium. Substrates were not pre-incubated with the cells. Uptake was then initiated by the addition of a solution of [3H]DA to give a final [3H]DA concentration of 50 nM. The uptake was allowed to continue for 10 min at room temperature and terminated by washing with PBSCM. All washing steps were carried out using an automated plate washer. Cells were solubilized in Optiphase scintillation cocktail (Perkin Elmer, Waltham, MA, USA) and counted on a Wallac 1450 microbeta liquid scintillation counter (Perkin Elmer, Waltham, MA, USA). Assuming Michaelis-Menten kinetics the data were analyzed using non-linear regression using GraphPad Prism version 5.03 for Windows (Graphpad software, La Jolla, CA, USA). Vmax and KM are given as mean ± SD of three independent experiments.
2.6. Calculation of IC50 and EC50 for the effects of the inhibitors and substrates
The concentration-curves generated from the inhibition experiments were fitted to a Hill equation by non-linear regression analysis using GraphPad Prism version 5.03 for Windows (Graphpad software, La Jolla, CA, USA). IC50’s are given as Mean ± SEM of at least five independent experiments. Statistical significance was assessed using Student t-test or two-way ANOVA. A value of p<0.05 was considered to be significant.
2.7. Efflux experiment in COS-7 cells
COS-7 cells were maintained under the same conditions as above. Efflux experiments were performed two days after cell transfection and plating of the cells in 24 wells-plates. Cells were washed with room temperature PBSCM and then loaded with 50 nM [3H]-dopamine for half an hour. After pre-loading cells were washed twice with buffer and then incubated for 10 min with substrates at room temperature. After the incubation the supernatant was collected, liquid scintillation fluid added and the samples counted (Beckman LS-6500 Multi-purpose Scintillation Counter). The cells were solubilized in a solution of 0.1% SDS and 0.1M NaOH, liquid scintillation fluid was added and tubes were counted as above. All assays were done in quadruplicate.
The statistical analyses used consider the percentage of [3H]-dopamine efflux among different treatments as the dependent variable. To analyze this the total amount of radioactivity in the samples was calculate by adding counts of supernatant plus cell lysis, the supernatant counts were then divided by the total and multiplied by 100 to yield the percentage of efflux.
Bar graphics and statistics (t-student test comparing vehicle and treated cells) was performed using GraphPad Prism version 5.03 for Windows (Graph Pad software, La Jolla, CA, USA).
3. RESULTS
3.1. Molecular cloning of catecholamine transporter from Schistosoma mansoni
A search in the recently completed genome of the Schistosoma mansoni v4.0 for sodium dependent neurotransmitter transporters (solute carrier family 6 – slc6) identified several putative transporter proteins. Based on homology to other well characterized transporters from other species within this family two proteins had sequences similar to dopamine and norepinephrine transporters. We decided to isolate and investigate these two proteins and characterize their biochemistry and pharmacology. The protein (SmDAT) with the highest homology to DAT and NET was Smp_156320 (Schistosoma mansoni GeneDB ID)/ XP_002577603.1 (Genbank ID). The other putative catecholamine transporter although with significantly lower homology was the Smp_193800/ XP_002571308.1. Other transporter proteins were identified in this search and homology analyses suggested that they were the recently characterized serotonin transporter [12](smSERT) (Smp_157420/XP_002577786.1; Smp_157430/XP_002577787.1), glycine transporters (Smp_131890/XP_002569602.1; Smp_028690/XP_002574310.1), and creatine/taurine transporters (Smp_160360/XP_002578233.1; Smp_078930/XP_002579022.1; Smp_143800/XP_002575336.1)
Similar to what we found in the smSERT [12], we identified two different SmDAT isoforms that differed in two amino acids located in the protein’s predicted intracellular N-terminal. In isoform A, residue 54 is a lysine and residue 59 is an asparagine. The corresponding residues in isoform B are glutamine and aspartate (Fig. 1). Several independent clones were sequenced to eliminate the possibility of PCR artifacts. The resulting sequence has been deposited in Genbank (Accession no: HM775215). A scan of the genomic sequences deposited in the genome databases of TIGR and the Sanger Institute covering this region identifies independent shotgun reads and reveals the existence of both the A (shisto9833f11) and B (shisto3760b02) isoforms in the parasite genome.
Fig. 1. N-terminus of SmDAT.
The intracellular N-terminal part of SmDAT is shown above. The repeated poly-asparagines string is underlined. The two polymorphic residues at position 54 and 59 are marked with asterisks (*). The amino acids for isoform A are shown. The corresponding residues in isoform B are glutamine and aspartate. Arginine 123 is marked with a caret (^). This arginine is conserved among monoamine transporters (it corresponds to arginine 60 in human DAT) and forms the beginning of trans-membrane domain 1.
The SmDAT version we initially isolated was based on the longest in silico predicted open reading frames within the parasite genome that could be isolated with RT-PCR based methods. To determine if longer versions of SmDAT were produced by the parasite and could be isolated we performed 5’RACE experiments. The 5’RACE procedure generated a main product that contained sequence that would translate into a shorter version of SmDAT than the in silico predicted version because it would employ as start codon the methionine at position 102 that is highlighted in Fig. 1. The resulting shorter N-terminal version is visualized with a box in Fig. 1. This result suggests that several N-terminal versions of SmDAT are expressed by the parasite.
The sequence reveals a protein with the typical structure of sodium/chloride dependent neurotransmitter transporters (slc6). It has a long 120 residue intracellular N-terminal compared to the human DAT (60 amino acids). The sequence of the N-terminal has an unusual repeat-like sequence that could be important for protein interactions and trafficking of the transporter. The repeat contains 4 to 5 asparagines in a row separated by a serine and an isoleucine and places more than 10 asparagines within a short stretch of less than 20 amino acids in the intracellular N-terminus (Fig. 1). The transporter contains potential sites for glycosylation in the second extracellular loop.
3.2. Functional characterization of SmDAT in mammalian cells
To identify the substrates of the cloned transporters, we expressed SmDAT and also the Smp_193800/ XP_002571308.1 gene product heterogeneously in COS-7 cells. Similar to what we previously found with the parasite serotonin transporter attaching GFP to the N-termini greatly enhanced the functional expression of the transporters (Data not shown).
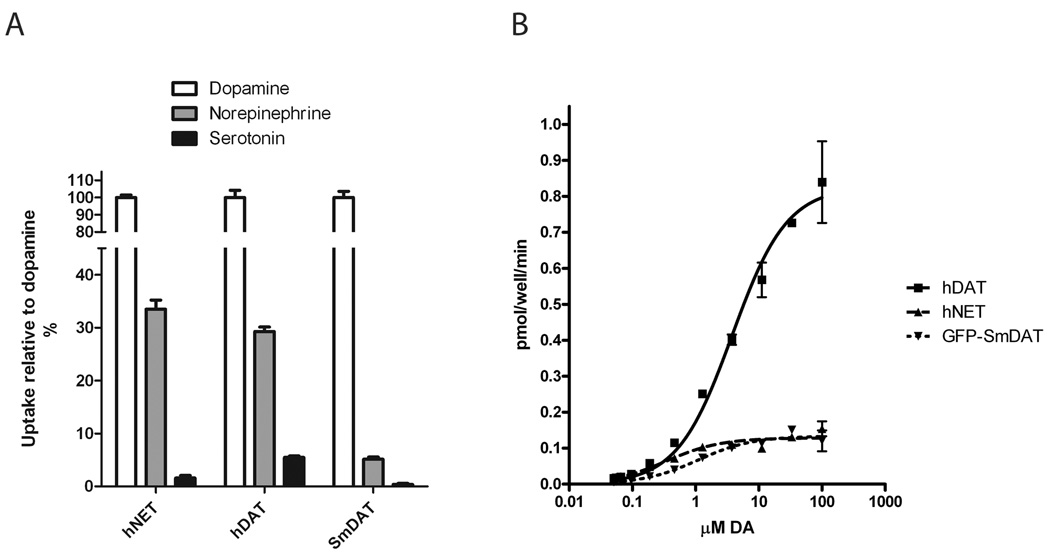
GFP-transporter fusion proteins of several carriers in the slc6 family have been shown to function normally when compared to wild type transporters [13]. We were not able to detect any significant dopamine uptake activity by the Smp_193800/ XP_002571308.1 gene product (data not shown). On the other hand uptake experiments verified that the Smp_156320/ XP_002577603.1 gene product (SmDAT) is a catecholamine transporter as SmDAT, like human DAT and NET, transport tritiated dopamine and low but significant levels of norepinephrine (Fig. 2A). We could not detect any detectable levels of serotonin uptake. Kinetic analyses of dopamine uptake by hDAT, hNET and GFP-SmDAT (Fig. 2B) revealed similar apparent affinity (KT) for dopamine between GFP-SmDAT (2.0 ± 0.3 µM) and hDAT human DAT (4.2 ± 0.5 µM) and higher affinity for hNET (0.47 ± 0.08 µM).
Fig. 2. Expression of SmDAT in mammalian COS-7 cells.
A: Dopamine, norepinephrine, and serotonin uptake by human DAT, NET and SmDAT. Uptake was assayed at a concentration of 100 nM for each substrate and normalized to the dopamine uptake level. B: Kinetic analysis of dopamine uptake in COS-7 cells expressing hDAT (■), hNET (▲), or GFP-SmDAT (▼). Shown here is a representative experiment using twelve substrate concentrations with two replicates. Data from four independent experiments generated values of 4.2 ± 0.5 µM for the KM and 0.9 ± 0.2 pmol/well/min for the Vmax for hDAT; 0.47 ± 0.08 µM for the KM and 0.17 ± 0.03 pmol/min for the Vmax for hNET and 2.0 ± 0.3 µM for the KM and 0.16 ± 0.04 pmol/min for the Vmax for GFP-SmDAT. Values are given as Mean ± SEM. Non-specific uptake was determined by parallel uptake in mock-transfected cells and subtracted.
Similar to what was found for the parasite SERT the Vmax was found to be significantly diminished for the GFP-SmDAT with a Vmax for GFP-SmDAT of 0.16 ± 0.04 pmol/well/min compared to the Vmax for human DAT (0.9 ± 0.2 pmol/well/min) but comparable to human NET (0.17 ± 0.03 pmol/min).
3.3. SmDAT expression during the life cycle of the S. mansoni
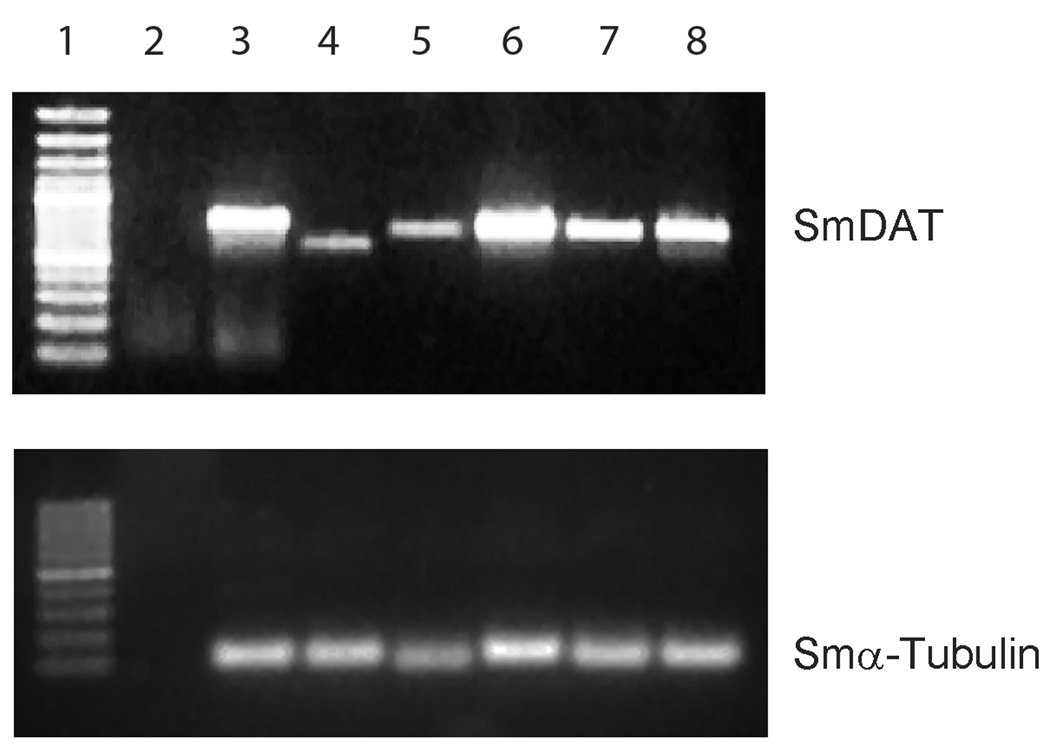
In order to evaluate the presence of SmDAT transcripts in cercariae, schistosomulae, adult worms, eggs, and the sporocyst life stage found in infected snails, RT-PCR was performed using specific primers for SmDAT and S. mansoni α-tubulin as described in material and methods. As shown in Fig. 3 significant levels of the SmDAT were detected in the adult worms and in sporocysts—the two stages of the parasite life cycle when it resides in host organisms (human and snail, Fig. 3). It was also found to be expressed in the egg and miracidium stage. It is absent in the cercaria stage but interestingly a fragment with a smaller than expected size was found in this stage. Sequencing of this fragment revealed that it was the SmDAT but a full exon was missing explaining the smaller size. This exon encodes the transmembrane domain 8 and when this transcript is expressed will result in a protein with this transmembrane domain missing and could result in the normally intracellular C-terminus to be located extracellular. 24 hours after the mechanical transformation of cercaria to schistosomula we observe a complete disappearance of the alternatively spliced transcript and the appearance of the full length transcript.
Fig. 3. SmDAT expression in the parasitic lifestages of the parasite developmental cycle.
- MW – 100bp DNA ladder
- Non-infected snail (control without infection)
- Infected snail 45 days (sporocysts)
- Cercariae
- Schistosomula (24 hours)
- Adult worms
- Eggs
- Miracidium
3.4. Pharmacological characterization of inhibitors and substrates on SmDAT compared to human DAT and NET
We investigated the pharmacology of the SmDAT and compared its sensitivity to inhibitors to that of hDAT. Table 1 shows the IC50 values for inhibiting dopamine uptake by hDAT, hNET and GFP-SmDAT expressed in COS-7 cells for the following compounds: Norepinephrine, d-Amphetamine, p-Tyramine, β-Phenethylamine, Cocaine, Benztropine, Bupropion, Nisoxetine, Desipramine, GBR-12909, and Citalopram. The majority of the drugs were weaker inhibitors of [3H]-dopamine uptake by SmDAT than by hDAT (Table 1), with nisoxetine, desipramine and citalopram being the exceptions. The mammalian DAT and NET display very distinguishable pharmacologies with drugs such as nisoxetine and desipramine displaying higher affinities for the NET and GBR-12909, benztropine and bupropion displaying higher affinity for DAT. In the case of SmDAT the NET specific drugs nisoxetine and desipramine display lower affinity than for hNET, but more significantly they display higher affinity for these two compounds compared to hDAT. For the DAT specific drugs, GBR- 12909, benztropine, and bupropion, SmDAT display lower affinity for these three drugs compared to both hDAT and hNET. It therefore appears that SmDAT has a more NET-like pharmacology. This has also been observed in other catecholamine transporters from species such as Drosophila melanogaster [14]. The SERT specific inhibitor citalopram has low affinity to all three transporters, hNET, hDAT and SmDAT. The psychostimulants cocaine and d-amphetamine display a dramatic and close to 100 fold lower affinity for the SmDAT compared to both hNET and hDAT. Interestingly, the affinity for putative substrates including p-Tyramine and β-Phenethylamine is significantly lower for the SmDAT compared to hNET and hDAT. This is different from what was found with the apparent affinity for the endogenous substrates DA (See section 3.2) and NE as we find that the affinity of SmDAT for NE is comparable to the affinity of NE for hDAT and slightly lower than the affinity of NE to hNET.
Table 1. Inhibitory constants (IC50) for transport inhibitors in hDAT, hNET, and GFP-SmDAT.
Transiently transfected COS-7 cells were incubated for 10 min with increasing concentrations of the indicated inhibitor, followed by the addition of [3H]DA to give a final concentration of 50 nM [3H]DA. Putative substrates were not pre-incubated but instead incubated directly with 50 nM [3H]DA. The uptake was allowed to continue for 10 min at 20°C before washing. IC50 values were calculated from at least five experiments performed in duplicate. Values are given in µM and represent Mean ± SEM.
| Human DAT | Human NET | GFP-SmDAT | |
|---|---|---|---|
| Norepinephrine | 9.80±1.50 | 1.87±0.30 | 17.8±1.8 |
| d-Amphetamine | 0.251±0.049 | 0.0670±0.0100 | 10.0±0.6 |
| p-Tyramine | 4.12±0.81 | 1.03±0.40 | 46.3±4.6 |
| b-Phenethylamine | 2.74±0.44 | 0.385±0.053 | 42.5±1.4 |
| Cocaine | 0.292±0.036 | 0.714±0.095 | 29.4±3.9 |
| Benztropine | 0.509±0.070 | 2.05±0.18 | 21.6±1.5 |
| Bupropion | 1.06±0.22 | 9.21±1.19 | 242±36 |
| Nisoxetine | 0.862±0.084 | 0.00146±0.00065 | 0.114±0.010 |
| Desipramine | 42.9±6.17 | 0.0281±0.0034 | 1.41±0.16 |
| GBR-12909 | 0.00244±0.00137 | 0.743±0.168 | 3.08±0.50 |
| Citalopram | 157±25 | 17.1±4.1 | 50.5±4.7 |
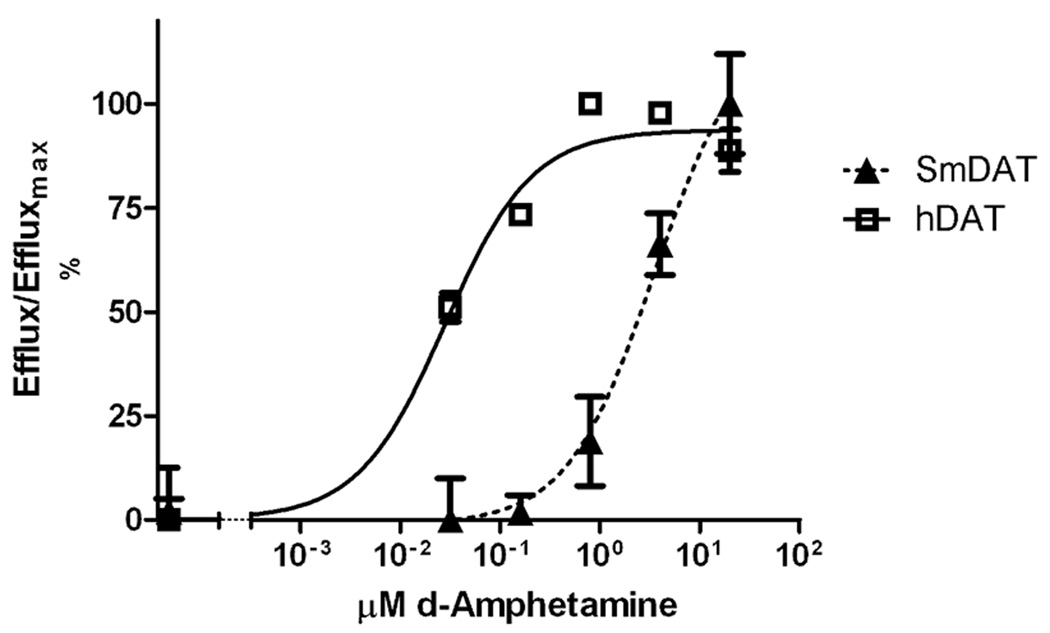
An inhibition assay does not distinguish if a compound is an inhibitor or a competitive substrate of the transporter and we performed efflux (substrate exchange) experiments to further investigate if d-amphetamine is an actual substrate of the transporter. In these experiments the efflux of preloaded dopamine is induced by compounds that are substrates of the carrier. Fig. 4 shows efflux experiments on hDAT and SmDAT expressed in COS-7 cells. d-amphetamine evoked efflux of previously loaded dopamine through both carriers but the potency was dramatically altered. We found EC50 values of 0.071 ± 0.021 µM for hDAT and 3.5 ± 0.15 µM for GFP-SmDAT signifying a 50-fold lower efficacy of d-amphetamine at eliciting efflux against SmDAT compared to hDAT. This is comparable to what we found in the uptake based inhibition assay described above. Because the N-terminal has been implicated in amphetamine evoked efflux [15] we also tested the shorter version that uses Methionine-102 as the start codon. We could not detect any difference in amphetamine-mediated efflux between this and the longer version of SmDAT (Data not shown)
Fig. 4. Substrate efflux from human and parasite DAT.
Efflux experiments in hDAT (■) and GFP-SmDAT (▲) transfected COS-7 cells after pre-loading with 50 nM [3H]- dopamine. Shown here is a representative experiment using five d-amphetamine concentrations with three replicates. Data from four independent experiments generated EC50 values of 0.071 ± 0.021 µM for hDAT and 3.5 ± 0.15 µM for GFP-SmDAT. Values are given as Mean ± SEM.
4. DISCUSSION
The two Schistosoma mansoni DAT isoforms reported in this study have amino acid sequences highly similar to other cloned DATs. They have a much longer N-terminus compared to most homologs in other species, but this region is generally the least conserved between the members of the dopamine transporter family. The two isoforms differ by two amino acid substitutions in the N-terminus and genomic sequence analysis suggests that the two isoforms are products of different alleles (Fig. 1) and that only a single DAT gene exists in the parasite. The function of the N-terminus of DAT and other neurotransmitter transporters is not completely understood, but it has been found to interact with a number of other proteins and to regulate transporter function [15–18]. These data suggest that proteins that interact with the SmDATs in an isoform specific manner could be important for regulating the localization and trafficking of the transporters in their native environment. We identified a very unusual sequence repeat in the N-terminus of both isoforms. This consisted of a string of asparagines followed by a serine and an isoleucine. It has been suggested that asparagine and glutamine rich regions may behave as modular mediators of protein-protein interactions termed "polar zippers" [19]. This is based on their capacity to provide flexibility and of their side chains to form hydrogen bond networks. Similar strings of asparagines are found in zinc finger nucleases and transcription factors such as Pdr1p in yeast [20]. A search for proteins containing similar poly-asparagines tracts identified several proteins known to anchor membrane proteins to the cytoskeleton including ankyrins, neurabin, spinophilin, synphilin and Xin repeat containing proteins. Though the role of the asparagine strings within these proteins has not been determined, the finding that such asparagine strings are more common in proteins that interact with the cytoskeleton suggest that the function of the poly-asparagine repeats in smDAT could be to form interactions with the cytoskeleton through "polar zipper" mediated interactions.
SmDAT was expressed at the highest levels in the host stages of the parasite implying the highest need for control of catecholaminergic signaling when the parasite is in its host. Both dopamine and norepinephrine has also been found in the parasite [4] and the catecholamines are believed to be actual neurotransmitters in Schistosoma mansoni. Treating adult worms with dopamine cause a relaxation of muscles and a lengthening of the worms [5–7]. Further support of a role for the catecholamines is the recent isolation of the rate limiting enzyme in catecholamine synthesis the tyrosine hydroxylase [8] and the report of the presence of a D2 receptor [9]. A dopamine β-hydroxylase (DBH) that converts dopamine to norepinephrine also appears to be present in the genome (Smp_163900/ XP_002578917.1). The source of catecholamines for the parasite is not completely understood but the parasite has the capability to synthesize endogenous catecholamines as its genome contains tyrosine hydroxylase [8] and DBH. It has been speculated that in some circumstances the parasite will obtain another biogenic monoamine, serotonin, from its host and with the higher expression of SmDAT in the host stages of the parasites life cycle we speculate if scavenging circulating catecholamines from their hosts could be a way for schistosomes to reduce a metabolic cost. However, it also poses the more interesting question of whether catecholamines serve as a signal from the host that regulates parasite biology and their interactions with the host.
A surprising alternative splice variant was observed in the cercaria stage. This is the stage where the parasite has abandoned its snail host and has to find a human host within hours as the half life of the cercaria is believed to be only a few hours. At this stage there is very little over all gene expression and the parasite is using glycogen as energy source. The alternative splice variant will result in a truncated version of the transporter with the trans-membrane domain 8 absent and the normally intracellular C-terminus would then become extracellular. The protein, if expressed, will most likely not be functional. Interestingly, comparable splice variants of the human DAT and NET have recently been described [21]. These variants resulted in the absence of trans-membrane domain 6 and it was shown that they are expressed at the cell surface with the C-terminus located extracellularly but are not functional and displays a dominant negative effect on co-expressed wild type transporters. The expression of the truncated splice variant in the cercaria could therefore be a way for the parasite to eliminate the function of residual SmDAT proteins left in the cell membrane from the sporocyst stage.
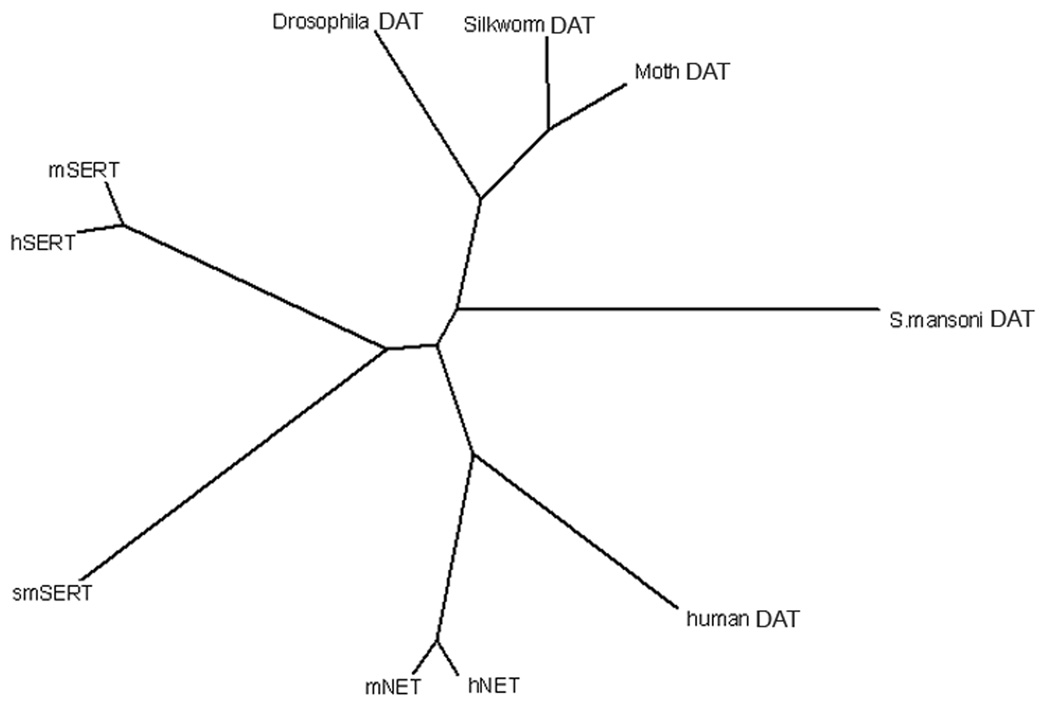
Most drugs showed lower potency against the SmDATs compared to human DAT and NET. Interestingly the rank potencies of inhibitors are different from the mammalian DAT and NETs. Though the affinity for the NET specific drugs nisoxetine and desipramine are lower in SmDAT compared to hNET they are higher compared to hDAT. It therefore appears that SmDAT has a similar pharmacology to NET. This has also been observed in other catecholamine transporters from species such as Drosophila melanogaster [14]. We searched the completed parasite genome for other putative catecholamine carriers, and only one other gene had sequence characteristics suggesting it to be a catecholamine transporter. We were unable to detect any monoamine uptake mediated by this gene. The NE uptake activity of SmDAT is rather low, but there is strong evidence for the presence of NE [4] in the parasite and it would be expected that the parasite requires some mechanism for clearing extracellular NE. SmDAT is the only transporter in the genome of the parasite that would have any significant NE uptake capacity and we hypothesize that SmDAT is employed by the parasite to clear DA and NE. From an evolutionary standpoint we also believe this means that having specific carriers for dopamine and norepinephrine respectively is something unique to organisms within the vertebrate phylum. This idea is supported by a recent extensive study of transporters from the major branches in the metazoan evolution [22]. In that study it was indeed found that mammalian NET and DAT are paralogous and have arisen through gene duplication. This becomes evident from an evolutionary tree of dopamine and norepinephrine transporters (Fig. 5) as it is apparent the split producing the two different transporters has occurred at a point in evolution where the line leading to mammalian organisms have split from lines that eventually would lead to invertebrates including insects and platyhelminthes including Schistosoma mansoni.
Fig. 5. Phylogenetic analysis of SmDAT.
Phylogenetic tree based on ClustalW alignment. Human (hSERT), mouse (mSERT) and Schistosoma mansoni (smSERT) serotonin transporters form one separate group and human (hNET) and mouse (mNET) norepinephrine transporters form another separate group together with the human dopamine transporter (human DAT). Insect (drosophila DAT, moth DAT and silkworm DAT) and parasite (S. mansoni DAT) monoamine transporters are all located on another evolutionary branch.
The evolutionary divergence of drug interactions among similar transporter from the same species and the same transporter from different species can be taken advantage of to identify structural features within the transporters that are important for the interaction with specific inhibitors and substrates. For example, the difference in inhibitor sensitivity between DAT and NET has proven useful for the identification of amino acid residues within the transporters involved in the interaction with inhibitors. Studies on the interaction with benztropine, a classical antagonist of the muscarinic acetylcholine receptor and used as a drug to treat Parkinson's disease, and bupropion, an antidepressant with higher affinity for DAT than NET has identified several residues within the transporters that are important for their interaction with these two drugs. Some of these differ in SmDAT and could be responsible for the decreased affinity for these two drugs with SmDAT. One study identified two residues (A279 and S359) to be important for the interaction with both bupropion and benztropine [23]. The amino acids at these two positions differ from both hDAT and hNET in the SmDAT and could therefore be determinants of the lowered affinity for these two drugs in SmDAT. I352 and L432 in SmDAT correspond to A279 and S359 in hDAT, respectively. Another study found that the residue corresponding to T216 in SmDAT, V152 in hDAT, is important for interaction with benztropine analogs [24]. Interestingly the same position in hSERT, I172, has been found to be important for interactions with antidepressants [25–26]. Finally, studies have found the highly conserved residues W147, D386 [27–28], D142 [29], and Y408 [30] to also be important for interactions with benztropines but these residues are also conserved in SmDAT and therefore most likely not responsible for the differences in affinity we observe.
We also observe a dramatic almost 100 fold decrease in the cocaine affinity of SmDAT compared to human DAT. A recent study used mutagenesis to produce a DAT variant with comparably lowered affinity to cocaine [31]. This was achieved by mutating three amino acids in the transmembrane domain 2. One of these, L104/L167 is conserved between the mammalian and parasite DAT, but the two others differ between the two: F105/M168 and A109/G172.
Results from inhibition and efflux experiments suggest substrate specificity between mammalian DATs and SmDAT differs and that SmDAT is more selective for substrates than human DAT. Amphetamine, a well established exogenous substrate of human DAT, display dramatically lowered potency in inhibiting dopamine uptake by SmDAT compared to hDAT (Table 1) and also is much less effective at eliciting efflux of previously loaded dopamine from SmDATs (Fig. 4). We made a similar observation with the Schistosoma mansoni SERT, which also appears less promiscuous for exogenous substrates than human SERT [12]. A comparison of Schistosoma and human DATs and SERT could be used to determine structural features of the transporters involved in substrate recognition and selectivity and could also further our knowledge on the mechanism of reversed transport.
Because of the role of catecholamines in the mobility of the parasite the difference in substrate selectivity could also be profited from therapeutically to identify molecules that interact with and modulate the function of SmDAT. We tested relatively few drugs on SmDAT to identify substrates of SmDAT but as we found some molecules such as amphetamine to be a less potent substrate on SmDAT compared with hDAT we hypothesize the opposite also can occur. SmDAT-selective substrates might be identified and could serve as a novel avenue of anti-schistosomal pharmacotherapy. We propose SmDAT could be used as a delivery vehicle for targeting toxic SmDAT specific molecules to catecholaminergic parasite cells.
In conclusion we here report the isolation of the gene for a catecholamine transporter from the human parasite Schistosoma mansoni. The catecholamines are important for the mobility of the animal [5–7], and dopaminergic proteins are potential targets for novel drug treatments of schistosomiasis. Considering that the current drug of choice praziquantel only cures 60 to 90 percent of patients and there are reports of worm resistance to praziquantel, novel therapeutic strategies are necessary. The SmDAT could represent a novel target for therapeutic intervention. Thus the cloning and characterization of SmDAT function could facilitate development of novel drugs to treat schistosomiasis.
Acknowledgments
The study was supported by CNPq and FAPESP (V.R.). We thank Dr. Susan G. Amara and her lab for support of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, et al. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 2.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 3.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 4.Gianutsos G, Bennett JL. The regional distribution of dopamine and norepinephrine in Schistosoma mansoni and Fasciola hepatica. Comp Biochem Physiol C. 1977;58:157–159. doi: 10.1016/0306-4492(77)90097-1. [DOI] [PubMed] [Google Scholar]
- 5.Tomosky TK, Bennett JL, Bueding E. Tryptaminergic and dopaminergic responses of Schistosoma mansoni. J Pharmacol Exp Ther. 1974;190:260–271. [PubMed] [Google Scholar]
- 6.Mellin TN, Busch RD, Wang CC, Kath G. Neuropharmacology of the parasitic trematode, Schistosoma mansoni. Am J Trop Med Hyg. 1983;32:83–93. doi: 10.4269/ajtmh.1983.32.83. [DOI] [PubMed] [Google Scholar]
- 7.Pax RA, Siefker C, Bennett JL. Schistosoma mansoni: differences in acetylcholine, dopamine, and serotonin control of circular and longitudinal parasite muscles. Exp Parasitol. 1984;58:314–324. doi: 10.1016/0014-4894(84)90048-1. [DOI] [PubMed] [Google Scholar]
- 8.Hamdan FF, Ribeiro P. Cloning and characterization of a novel form of tyrosine hydroxylase from the human parasite, Schistosoma mansoni. J Neurochem. 1998;71:1369–1380. doi: 10.1046/j.1471-4159.1998.71041369.x. [DOI] [PubMed] [Google Scholar]
- 9.Taman A, Ribeiro P. Investigation of a dopamine receptor in Schistosoma mansoni: functional studies and immunolocalization. Mol Biochem Parasitol. 2009;168:24–33. doi: 10.1016/j.molbiopara.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 11.Harrop R, Wilson RA. Protein synthesis and release by cultured schistosomula of Schistosoma mansoni. Parasitology. 1993;107(Pt 3):265–274. doi: 10.1017/s0031182000079245. [DOI] [PubMed] [Google Scholar]
- 12.Fontana AC, Sonders MS, Pereira-Junior OS, Knight M, Javitch JA, Rodrigues V, et al. Two allelic isoforms of the serotonin transporter from Schistosoma mansoni display electrogenic transport and high selectivity for serotonin. Eur J Pharmacol. 2009;616:48–57. doi: 10.1016/j.ejphar.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid JA, Scholze P, Kudlacek O, Freissmuth M, Singer EA, Sitte HH. Oligomerization of the human serotonin transporter and of the rat GABA transporter 1 visualized by fluorescence resonance energy transfer microscopy in living cells. J Biol Chem. 2001;276:3805–3810. doi: 10.1074/jbc.M007357200. [DOI] [PubMed] [Google Scholar]
- 14.Porzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol Pharmacol. 2001;59:83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- 15.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 17.Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortensen OV, Larsen MB, Prasad BM, Amara SG. Genetic complementation screen identifies a mitogen-activated protein kinase phosphatase, MKP3, as a regulator of dopamine transporter trafficking. Mol Biol Cell. 2008;19:2818–2829. doi: 10.1091/mbc.E07-09-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perutz MF, Johnson T, Suzuki M, Finch JT. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc Natl Acad Sci U S A. 1994;91:5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolaczkowska A, Kolaczkowski M, Delahodde A, Goffeau A. Functional dissection of Pdr1p, a regulator of multidrug resistance in Saccharomyces cerevisiae. Mol Genet Genomics. 2002;267:96–106. doi: 10.1007/s00438-002-0642-0. [DOI] [PubMed] [Google Scholar]
- 21.Sogawa C, Mitsuhata C, Kumagai-Morioka K, Sogawa N, Ohyama K, Morita K, et al. Expression and function of variants of human catecholamine transporters lacking the fifth transmembrane region encoded by exon 6. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caveney S, Cladman W, Verellen L, Donly C. Ancestry of neuronal monoamine transporters in the Metazoa. J Exp Biol. 2006;209:4858–4868. doi: 10.1242/jeb.02607. [DOI] [PubMed] [Google Scholar]
- 23.Mortensen OV, Amara SG. Gain of function mutants reveal sites important for the interaction of the atypical inhibitors benztropine and bupropion with monoamine transporters. J Neurochem. 2006;98:1531–1540. doi: 10.1111/j.1471-4159.2006.04060.x. [DOI] [PubMed] [Google Scholar]
- 24.Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen MB, Elfving B, Wiborg O. The chicken serotonin transporter discriminates between serotonin-selective reuptake inhibitors. A species-scanning mutagenesis study. J Biol Chem. 2004;279:42147–42156. doi: 10.1074/jbc.M405579200. [DOI] [PubMed] [Google Scholar]
- 26.Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou MF, et al. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem. 2006;281:2012–2023. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- 27.Chen N, Zhen J, Reith ME. Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding interaction for GBR12909 and benztropine as opposed to cocaine. J Neurochem. 2004;89:853–864. doi: 10.1111/j.1471-4159.2004.02386.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, et al. Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist-binding properties. J Neurochem. 2008;107:928–940. doi: 10.1111/j.1471-4159.2008.05667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ukairo OT, Bondi CD, Newman AH, Kulkarni SS, Kozikowski AP, Pan S, et al. Recognition of benztropine by the dopamine transporter (DAT) differs from that of the classical dopamine uptake inhibitors cocaine, methylphenidate, and mazindol as a function of a DAT transmembrane 1 aspartic acid residue. J Pharmacol Exp Ther. 2005;314:575–583. doi: 10.1124/jpet.105.085829. [DOI] [PubMed] [Google Scholar]
- 30.Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, et al. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- 31.Chen R, Han DD, Gu HH. A triple mutation in the second transmembrane domain of mouse dopamine transporter markedly decreases sensitivity to cocaine and methylphenidate. J Neurochem. 2005;94:352–359. doi: 10.1111/j.1471-4159.2005.03199.x. [DOI] [PubMed] [Google Scholar]