Abstract
Kisspeptin is distributed in brain areas regulating reproduction but also in nuclei involved in feeding control. Whether kisspeptin alters food intake is unknown in mice. We examined how kisspeptin-10 influences feeding after intracerebroventricular injection in mice using automated monitoring. Kisspeptin-10 (0.3, 1 and 3µg/mouse) dose-dependently inhibited the feeding response to an overnight fast by 50%, 95% and 90% respectively during the 2–3 h period post injection. The 1µg/mouse dose reduced the 4-h cumulative food intake by 28% while intraperitoneal injection (10µg/mouse) did not. The decreased 4-h food intake was due to reduced meal frequency (−45%/4h), whereas meal size and gastric emptying were not altered. These data suggest that kisspeptin may be a negative central regulator of feeding by increasing satiety.
Keywords: automated food monitoring, feeding pattern, gastric emptying, intracerebroventricular, intraperitoneal, microstructure
Introduction
The 54 amino acid kisspeptin was discovered in 1996 as the gene product of the metastasis-suppressor gene [1]. The name derives from its initial function as a suppressor sequence and the letters KI were added to form KISS, in keeping with the place of its discovery, Hershey (production site of the “Hershey’s chocolate kisses”). Kisspeptin binds to the G-protein-coupled receptor, GPR54 [2], now referred to as kisspeptin receptor [3]. Since the discovery that mutations of the human KISS1R gene were associated with idiopathic hypothalamic hypogonadism and impaired pubertal maturation [4,5], a finding confirmed in transgenic mice [6], kisspeptin has been identified as a major regulator governing the mammalian hypothalamic-pituitary-gonadal axis and puberty onset (for review see [6]).
Besides the full length C-terminally amidated 55 amino-acid peptide, kisspeptin-54 (also referred to KP-54 [3]), the C-terminal cleavage fragments such as kisspeptin-10, kisspeptin-13 and kisspeptin-14 also activate the receptor with the same affinity and possess biological activity [3,7]. Kisspeptin is widely distributed in the rat and mouse brain including the amygdala, hypothalamic arcuate nucleus (Arc), paraventricular nucleus (PVN), dorsomedial hypothalamus (DMH) and locus coeruleus [8,9]. Likewise, Kiss1r gene was reported to be widely expressed in the mouse brain encompassing sites such as the hippocampus, posterior hypothalamus, medial preoptic area, medial septum and cerebellum [10,11]. However, in the mouse Arc divergent reports showed either no expression in transgenic GPR54 LacZ knock-in mice [10] or expression in all Arc rostrocaudal regions assessed by quantitative real-time PCR [11]. Kisspeptin’s distribution, especially its expression in key food intake regulatory nuclei such as Arc and PVN [12], suggests a role for kisspeptin in the regulation of energy homeostasis [13] and food intake which has been little explored and so far only in rats [14,15].
Therefore, in the present study we investigated the central action of kisspeptin-10 to influence food intake in mice fed ad libitum or fasted overnight using acute injection of the peptide into the brain ventricle (intracerebroventricularly, ICV) compared to a 10-times higher dose injected intraperitoneally (IP). As knowledge of the food intake microstructure is essential to characterize the feeding modulatory properties of a compound, we examined the influence of ICV kisspeptin-10 on the feeding micropattern assessed by an automated episodic food intake monitoring device adapted for mice that was established in our previous studies [16]. In view that slowing of gastric emptying and prolonged gastric distention impact on subsequent food intake [17], we also examined whether kisspeptin injected ICV alters gastric emptying of a solid meal in overnight fasted mice.
Materials and methods
Animals
Adult male C57Bl/6 mice (6–8 weeks, Harlan Laboratories) were group housed 4/cage under controlled illumination (6 am – 6 pm) and temperature (21–23 °C). Animals had ad libitum access to purified standard rodent diet (AIN-93M, Research Diets, Inc., Jules Lane, New Brunswick, NJ) and tap water. Protocols were approved by the Veterans Administration Institutional Animal Care and Use Committee (# 99127-07).
Injections
Acute injection into the lateral brain ventricle (5 µl) was performed under short isoflurane anesthesia (2–3 min, 4.5% vapor concentration in oxygen; VSS, Rockmart, GA) as in our previous studies [16,18]. The injection site was localized at the apex of the equal triangle between the eyes and the back of the head, cleaned with Povidone-Iodine 10% (Aplicare Inc., Meriden, CT) and the skull punctured manually at the point of least resistance with a 30-gauge needle equipped with a polyethylene tube (leaving 4 mm of the needle tip exposed) and attached to a Hamilton syringe. Mice completely recovered from anesthesia within 5 min. The accuracy of the injections was confirmed in our previous studies by injecting cresyl violet dye ICV under similar conditions in 50 mice [18]. IP injections (100 µl) were performed in conscious hand restrained mice handled for this position on three days during the week before the experiment.
Food intake measurement
The microstructure analysis of food intake was assessed by the BioDAQ episodic Food Intake Monitor for mice (BioDAQ, Research Diets, Inc., New Brunswick, NJ) as described in detail in our previous study [16]. This system allows the continuous monitoring of meal patterns in undisturbed mice with minimal human interference. Mice habituated to these cages and feeding from the hopper within 3–4 days, indicated by normal food intake and regular body weight gain.
The system weighs the food hopper (± 0.01 g) second by second and detects not eating as weight stable and eating as weight unstable. Meals consist of one or more bouts (changes in stable weight before and after a bout) separated by an inter-meal interval (IMI, ≥5 min). The minimum meal amount was defined as 0.02 g. Therefore, food intake was considered as one meal when the feeding bouts occurred within 5 min of the previous response and their sum was ≥ 0.02 g; if feeding bouts were ≥5 min apart, they were considered as a new meal. Meal parameters include meal/bout frequency, meal size and duration, total time spent eating (time in min or %), latency to first meal, inter-meal interval, rate of ingestion and satiety ratio (average inter-meal interval divided by the average meal size).
Experiments were started after one week of habituation. Overnight fasted mice were ICV injected with kisspeptin-10 (0.3, 1 or 3 µg/mouse in saline containing 0.1% bovine serum albumin, BSA, Phoenix Pharmaceuticals, Inc., Burlingame, CA) or vehicle (saline containing 0.1% BSA) at 9 am and microstructure of food ingestion was recorded for 24 h. The maximal effective ICV dose of 1 µg/mouse was used for all other studies. Ad libitum fed mice received kisspeptin-10 or vehicle at the onset of the dark phase and food intake microstructure was assessed for 24 h. In another study, ad libitum fed mice were ICV injected with kisspeptin-10 or vehicle during the light phase at 9 am and food intake microstructure monitored for 24 h. In one study, overnight fasted mice were injected IP with kisspeptin-10 (10 µg/mouse) or vehicle at 9 am and food intake microstructure was assessed for 24 h. Experiments were repeated in a crossover design in the same batch of mice for each treatment.
Gastric emptying
Gastric emptying of rodent diet was determined as in our previous studies [18]. Briefly, mice were fasted overnight and re-fed with pre-weighed standard chow starting at 8 am for 1 h. Then, food and water were removed, mice injected ICV with kisspeptin-10 (1 µg/mouse) or vehicle and gastric emptying assessed 3 h later. Mice were euthanized by cervical dislocation, the stomach quickly removed, the stomach content weighed and gastric emptying calculated as (1-gastric content/food intake) × 100.
Statistical analysis
Data are expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test or two-way ANOVA followed by Holm-Sidak method. Differences between groups were considered significant when P<0.05.
Results
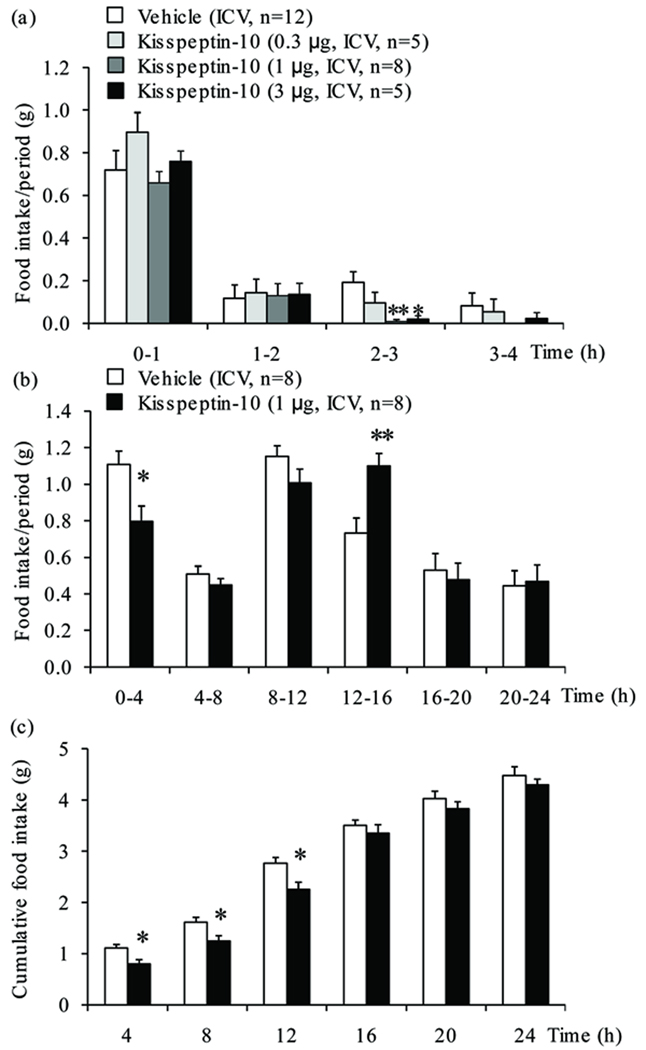
Kisspeptin-10 (0.3, 1 and 3 µg/mouse) injected ICV in overnight fasted mice induced a dose-related decrease in light phase food intake (−50%, −95% and −90% respectively) during the 2–3 h period post injection reaching statistical significance at 1 and 3 µg/mouse (P<0.05; Fig. 1a), whereas there was no inhibition at the 0–1, 1–2 and 3–4 h periods post injection. Two-way ANOVA showed a significant influence of treatment (F(3,104)=4.1, P<0.01), time (F(3,104)=222.4, P<0.001) and treatment × time (F(9,104)=2.1, P<0.05). Based on these results, the dose of 1 µg/mouse was selected for further analyses. The reduction during the 2–3 h period resulted in a significant decrease in the 4-h cumulative food intake (−28%, P<0.05; Fig. 1b) which was still observed at 12 h compared to vehicle (−19%, P<0.05; Fig. 1c). During the 12–16 h period, there was a compensatory increase in food intake (Fig. 1b) resulting in similar 24-h food intake values in ICV kisspeptin-10 and vehicle injected groups (Fig. 1c).
Figure 1.
Intracerebroventricular injection of kisspeptin-10 reduces the food intake response to an overnight fast in mice. Animals deprived of food for 17 h were ICV injected with kisspeptin-10 (0.3, 1 or 3 µg/mouse) or vehicle at 9 am and food ingestion was automatically recorded for 24 h and expressed as (a) food intake/1 h periods during the first 4 h, (b) food intake/4 h periods over 24 h and (c) cumulative food intake over 24 h. *P<0.05 and **P<0.01 vs. vehicle.
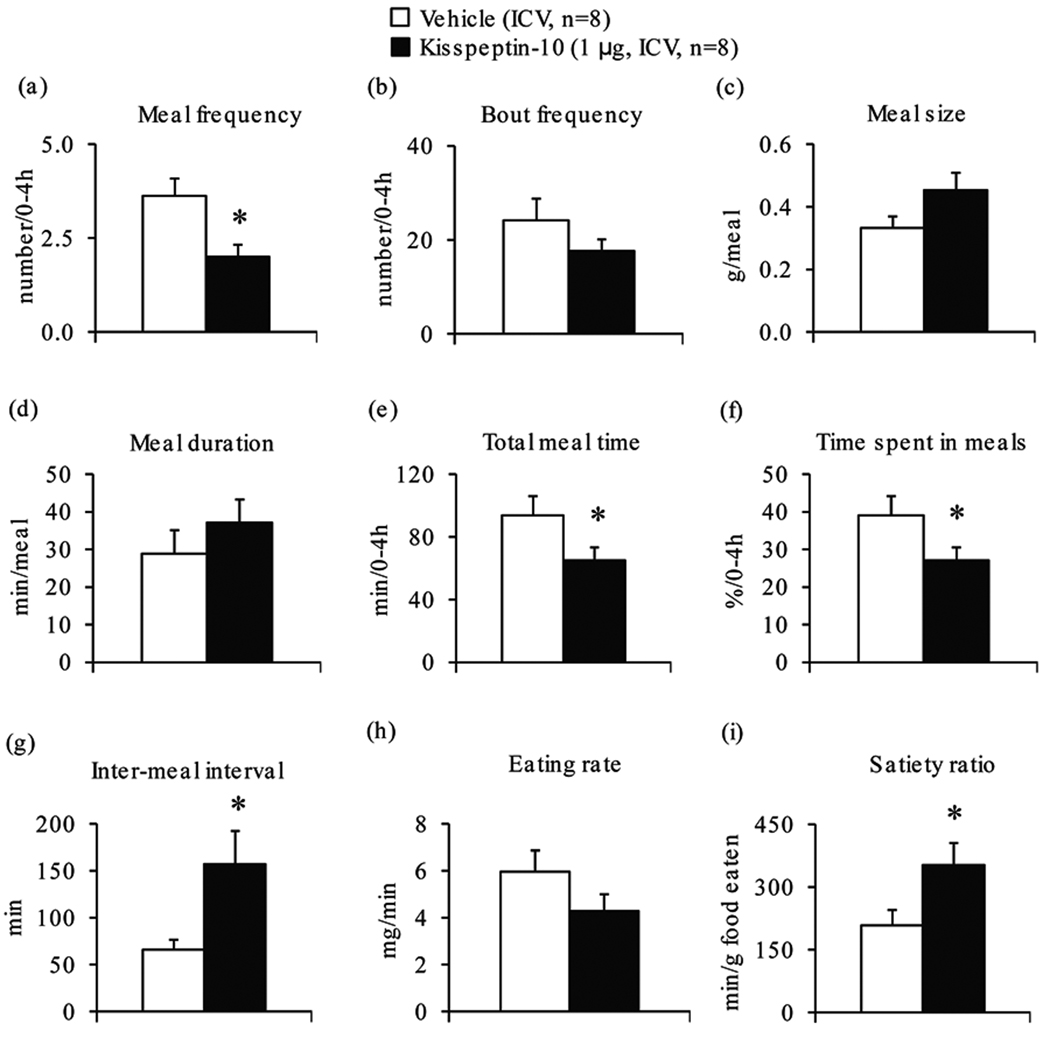
Since the main effect was observed during the first 4 h post injection, the food intake microstructure was assessed in this period. Kisspeptin-10 injected ICV significantly decreased meal frequency (−45%, P<0.05; Fig. 2a), total meal time (−31%, P<0.05; Fig. 2e) and time spent in meals (−31%, P<0.05; Fig. 2f), increased inter-meal intervals (+137%, P<0.05; Fig. 2g) and satiety ratio (+69%, P<0.05; Fig. 2i) compared to vehicle, whereas bout frequency (Fig. 2b), meal size (Fig. 2c), duration of meals (Fig. 2d) and eating rate (Fig. 2h) were not significantly changed (P>0.05).
Figure 2.
Intracerebroventricular injection of kisspeptin-10 alters the microstructure of feeding response to an overnight fast in mice. Animals deprived of food for 17 h were injected with kisspeptin-10 (1 µg/mouse, ICV) or vehicle and food intake microstructure was recorded for 24 h. The feeding microstructure parameters encompassing (a) meal frequency, (b) bout frequency, (c) meal size, (d) meal duration, (e) total meal time, (f) time spent in meals, (g) inter-meal interval, (h) eating rate and (i) satiety ratio are displayed for the first 4 h post injection. *P<0.05 vs. vehicle.
The decreased food intake was not associated with alterations of gastric emptying. Indeed, under the same conditions, kisspeptin-10 (1 µg/mouse, ICV) did not modify gastric transit of a solid meal in overnight fasted mice assessed at 3 h after injection during the light phase compared to vehicle (34.9 ± 8.1% vs. 37.0 ± 5.6 %, n=6/group, P>0.05). In contrast to ICV injection, when injected IP at a 10-times higher dose (10 µg/mouse) than ICV kisspeptin-10 did not influence the light phase food intake in overnight fasted mice (Table 1).
Table 1.
Effect of ICV or IP injection of kisspeptin-10 on 4-h meal pattern.
| Parameter | Fed ad libitum in dark phase | Fed ad libitum in light phase | Overnight fasted/re-fed in light phase |
|||
|---|---|---|---|---|---|---|
| Vehicle (ICV) |
Kisspeptin-10 (1 µg, ICV) |
Vehicle (ICV) |
Kisspeptin-10 (1 µg, ICV) |
Vehicle (IP) |
Kisspeptin-10 (10 µg, IP) |
|
| Food intake/4h (g) | 0.47 ± 0.08 | 0.48 ± 0.10 | 0.04 ± 0.02 | 0.11 ± 0.03 | 1.25 ± 0.06 | 1.16 ± 0.05 |
| Meal frequency (number/4h) |
4.57 ± 1.00 | 5.29 ± 1.04 | 1.25 ± 0.48 | 1.75 ± 0.48 | 1.43 ± 0.20 | 1.63 ± 0.26 |
| Bout frequency (number/4h) |
17.00 ± 2.31 | 18.71 ± 1.69 | 4.00 ± 0.82 | 7.25 ± 2.59 | 24.00 ± 2.37 | 21.00 ± 1.56 |
| Meal size (g/meal) | 0.11 ± 0.02 | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.00 | 0.98 ± 0.13 | 0.82 ± 0.11 |
| Meal duration (min) |
12.13 ±2.51 | 9.64 ± 1.13 | 4.02 ± 2.88 | 7.06 ± 2.22 | 117.49 ±19.72 | 109.02 ± 18.06 |
| Total meal time (min/4h) |
48.78 ± 8.66 | 47.81 ± 9.19 | 4.87 ± 3.80 | 13.82 ± 5.75 | 147.66 ± 11.67 | 147.83 ± 10.24 |
| Time spent in meals (%/4h) |
20.30 ± 3.59 | 19.92 ± 3.83 | 1.68 ± 1.20 | 5.76 ± 2.40 | 61.52 ± 4.86 | 61.59 ± 4.27 |
| Latency to 1st meal (min) |
40.89 ± 23.62 | 43.71 ± 27.12 | 22.66 ± 13.22 | 43.23 ± 31.66 | 5.66 ± 1.21 | 4.65 ± 1.73 |
| Duration 1st meal (min) |
23.95 ± 4.49 | 15.10 ± 2.48 | 4.86 ± 2.70 | 6.75 ± 1.77 | 135.27 ± 14.50 | 110.54 ± 17.27 |
| Inter-meal interval (min) |
73.01 ± 21.00 | 48.73 ± 10.32 | 183.10 ± 94.97 | 176.46 ± 84.29 | 111.40 ± 13.83 | 94.57 ± 8.94 |
| Eating rate/4h (mg/min) |
2.33 ± 0.44 | 2.60 ± 0.45 | 0.71 ± 0.59 | 0.64 ± 0.29 | 4.32 ± 0.27 | 4.39 ± 0.34 |
| Satiety ratio (min/g) |
875.61 ± 424.19 | 572.51 ± 139.90 | 2714.58 ± 2396.25 | 2270.11 ± 1230.59 | 117.95 ± 11.59 | 124.52 ± 15.57 |
Each group had vehicle controls tested at the same time of kisspeptin-10 also injected ICV or IP. Data are mean ± SEM; n=6–8 mice/group.
When tested in ad libitum fed mice either during either the light or at the onset of the dark phase, kisspeptin-10 (1 µg/mouse, ICV) had no effect on food intake or microstructure parameters compared to vehicle (Table 1).
Discussion
In the present study, we show for the first time that kisspeptin-10 injected into the lateral brain ventricle at a low dose (1 µg = 0.8 nmol) during the light phase induced a peak 95% inhibition of the hourly food intake response to an overnight fast in mice. The inhibitory action was dose-related, occurred mainly during the 2–3 h period post injection reflecting a delayed onset, was long-lasting as shown by the 12-h reduction of cumulative food intake post injection and not associated with delayed gastric emptying of ingested food. The lack of effect of a 10-times higher dose injected IP supports that the anorexigenic effect of kisspeptin-10 represents a centrally mediated action and not a leakage of the peptide into the periphery. Therefore, the delayed onset of feeding inhibition may point towards time needed to modulate mRNA/protein expression of anorexigenic brain signaling. These data, coupled with the well documented suppression of hypothalamic Kiss-1 mRNA expression by fasting or other states of negative energy balance in rats [13,14], may collectively suggest an anorexigenic role for kisspeptin in the central regulation of food intake.
In contrast, the same low ICV dose was ineffective to alter food intake when injected at the onset of the dark phase in non-fasted mice. These data indicate that the food intake-suppressive effect of kisspeptin-10 is specific to brain circuitries involved in the feeding response to a fast and not the nocturnal drive to eat under ad libitum feeding conditions. Fasting is well established to increase the orexigenic hypothalamic neuropeptide Y (NPY), whereas mRNA expression of the food intake suppressing proopiomelanocortin (POMC) is decreased [19]. A recent in vitro electrophysiological study in mice showed that kisspeptin activates POMC hypothalamic green fluorescent protein expressing neurons, whereas NPY neurons are inhibited [11]. Immunohistochemical and RT-PCR expression studies showed that kisspeptin positive fibers are located in close proximity to POMC neurons as well as that Kiss1r is expressed in these neurons [11]. In addition, the posterior hypothalamus is a prominent expression site of the Kiss1r [10]. Moreover, the orexigenic melanin-concentrating hormone (MCH) activates neurons that are inhibited by kisspeptin [20]. Taken together, these data suggest that the inhibitory effect of kisspeptin-10 on the re-feeding response to a fast may be exerted via an inhibition of central NPY and MCH signaling as well as the recruitment of POMC-derived alpha-melanocyte-stimulating hormone (α-MSH). In addition, almost half of the kisspeptin positive neurons in the murine Arc express the leptin receptor [21] suggesting the interaction with another key hormone regulating food intake and energy homeostasis that reduces food intake by decreasing the number of meals but not meal size [22]. However, the POMC-derived α-MSH decrease food intake by selectively reducing meal size but not frequency [23], whereas NPY increases the number of meals [24]. In the present study we showed that kisspeptin-10 decreases food intake by increasing inter-meal intervals leading to the reduction of meal number, whereas the meal size was not altered. This pattern is indicative of inducing satiety (mechanisms causing later onset of the next meal after one completed meal [25]), whereas satiation (mechanisms causing meal termination [25]) is not altered. Therefore, on the basis of previous and present findings, it may be speculated that a negative central interaction between kisspeptin and orexigenic NPY pathways along with a positive interaction with anorexigenic leptin signaling exists. However, it cannot be ruled out that kisspeptin also acts by modulating the processing of other gut-derived satiety signals reaching the brain or potentiate their interactions [12] and thereby induces satiety.
In contrast to the present findings in mice, ICV injection of kisspeptin-10 at similar doses (1.3 or 3.8 µg/rat) in rats under fasted or ad libitum feeding conditions did not alter food intake during the light or dark phase while positive peptide controls had the expected effects [14,15]. Whether species differences account for these divergent results or the automated non-interfering food intake monitoring versus manual assessment warrants further investigation.
In conclusion, this study shows in mice that kisspeptin-10 acts centrally to reduce the light phase food intake response to an overnight fast with a delayed onset, whereas the nocturnal food intake is not altered. The reduction of feeding after a fast is exerted via a reduction in meal frequency and associated with prolonged inter-meal intervals. Such changes in microstructure pattern of feeding are indicative of a stimulatory effect on satiety which was not related to alterations in gastric emptying of a meal. These data, along with the expression of kisspeptin in major feeding regulatory brain nuclei and its down-regulation by fasting, unravel a potential negative regulatory role of central kisspeptin in the control of feeding in mice.
Acknowledgments
We are grateful to Mrs. Honghui Liang for the excellent technical support and thank Ms. Eugenia Hu for reviewing the manuscript.
Grant support: This work was supported by Veterans Administration Research Career Scientist Award, R01 NIH DK-33061, Center Grant DK-41301 (Animal Core and Supplement Grant, Y.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 2.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 3.Kirby HR, Maguire JJ, Colledge WH, Davenport AP. International Union of Basic and Clinical Pharmacology. LXXVII. Kisspeptin receptor nomenclature, distribution, and function. Pharmacol Rev. 2010;62:565–578. doi: 10.1124/pr.110.002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 5.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. Physiological roles of the kisspeptin/GPR54 system in the neuroendocrine control of reproduction. Prog Brain Res. 2010;181:55–77. doi: 10.1016/S0079-6123(08)81005-9. [DOI] [PubMed] [Google Scholar]
- 7.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, et al. KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol. 2005;481:314–329. doi: 10.1002/cne.20350. [DOI] [PubMed] [Google Scholar]
- 10.Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- 11.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30:10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–372. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- 13.Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res. 2010;1364:129–138. doi: 10.1016/j.brainres.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 14.Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 15.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 16.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, et al. Activation of brain somatostatin(2) receptors stimulates feeding in mice: Analysis of food intake microstructure. Physiol Behav. 2010;101:614–622. doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766–R769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 18.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poplawski MM, Mastaitis JW, Yang XJ, Mobbs CV. Hypothalamic responses to fasting indicate metabolic reprogramming away from glycolysis toward lipid oxidation. Endocrinology. 2010;151:5206–5217. doi: 10.1210/en.2010-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci U S A. 2009;106:17217–17222. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 22.Zorrilla EP, Inoue K, Valdez GR, Tabarin A, Koob GF. Leptin and post-prandial satiety: acute central leptin more potently reduces meal frequency than meal size in the rat. Psychopharmacology (Berl) 2005;177:324–335. doi: 10.1007/s00213-004-1952-1. [DOI] [PubMed] [Google Scholar]
- 23.Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. doi: 10.1016/s0031-9384(02)00883-1. [DOI] [PubMed] [Google Scholar]
- 24.Baird JP, Gray NE, Fischer SG. Effects of neuropeptide Y on feeding microstructure: dissociation of appetitive and consummatory actions. Behav Neurosci. 2006;120:937–951. doi: 10.1037/0735-7044.120.4.937. [DOI] [PubMed] [Google Scholar]
- 25.Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, et al. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]