Abstract
Background
Host genetic factors are important determinants for risk of HIV-1 infection and disease progression. This study examined associations of host genetic variants and neurocognitive impairment in Chinese subjects infected through contaminated blood products.
Methods
201 HIV-infected subjects from Anhui, China had neuropsychological (NP) tests at baseline and 12 months. DNA was genotyped for APOE ε2, ε3 and ε4 alleles, MBL2-A/O,CCR5-wt/Δ32, CCR5-59029-G/A, CCR2-180-G/A, SDF-1-G/A, IL4-589-C/T, MCP-1-2518-A/G, CX3CR1-745-G/A, -849-C/T polymorphisms and CCL3L1 copy number variants (CNVs) using real-time PCR. Univariate and multivariate analyses were performed.
Results
The cohort was 61% males, mean education: 5.5 years, AIDS diagnosis: 113(55%), on antiretrovirals: 114(56%), mean baseline CD4+ count: 349/mm3 and mean log10 RNA 4.09. At baseline, 37% had global NP impairment increasing to 44% after 12 months. Of 43 subjects with the APOE ε4 allele, 58% were cognitively impaired versus 31% without the ε4 allele (P=0.001, OR: 3.09; 95% CI: 1.54, 6.18). The mean GDS for ε4 positive participants on antiretrovirals for 12 months was 0.88 (0.55) versus 0.63 (.54) for ε4 negatives (P = 0.053, 95%CI: -0.004, 0.51). For MBL2, 52% of subjects with the O/O genotype declined in cognitive function over 12 months versus 23% with A/A (OR = 3.62. 95% CI: 1.46, 9.03; P=0.004). No associations were observed for the other genetic variants.
Conclusions
The APOE ε4 allele was associated with increased risk for cognitive deficits, while the MBL2 O/O genotype was associated with increased risk for progressive cognitive decline in Chinese subjects infected with HIV through contaminated blood products.
Keywords: APOE, MBL-2, HIV, cognitive impairment, host genetics, China
INTRODUCTION
Recent estimates are that 33 million people are living with HIV-1 infection worldwide [1]. Although estimates of HIV-1 infection in China have been difficult to establish, approximately 700,000 people were living with HIV-1 in China in 2007 with an estimated prevalence of 0.05%. An estimated 85,000 Chinese have AIDS with 35,000 of those infected through blood donation and transfusion. In 2007, there were an estimated 50,000 new infections and 20,000 deaths.
The initial recognition of HIV-1 infection in China was among injection drug users (IDUs) in the city of Ruili located in Yunnan’s Dehong prefecture [2, 3]. Unregulated commercial blood/plasma collection with re-used needles among farmers between 1992 and 1995 in several provinces in central China, including Henan, Anhui and Shanzi, resulted in rapid spread of infection among blood donors and recipients of blood products. In many cases, blood products were also contaminated with hepatitis C virus (HCV) resulting in transmission of both viruses. These early transmissions led to an increased risk for HIV-1 transmission among gay men and heterosexual contacts. Estimates are that of the 1.3 billion people living in mainland China, 30-50 million are at high-risk for infection including IDUs and their sexual partners, sex workers their clients and their partners, and men who have sex with men.
The central nervous system (CNS) is an important target of HIV-1 and a common cause of neurological impairment. With the increased use of combination antiretroviral therapy (ART), severe dementia is less common; however, milder forms of HIV-associated neurocognitive disorders (HAND) remain an important complication of HIV-1 infection, affecting 30% to 50% of person living with HIV-1[4-8]. Until recently, no study has systematically evaluated the neurobehavioral effects of HIV-1 among people in China. Recently, our group reported the results of the initial comprehensive evaluation of 201 HIV-infected former plasma donors in Anhui Province [9]. Using a global summary score, neuropsychological impairment was found in 37% of persons infected with HIV-1 and 13% of an uninfected control group. As expected, persons with AIDS were more likely to be impaired than those HIV-infected who had less advanced disease. Of interest, persons with neuropsychological impairment were more likely to report cognitive complaints and increased dependence in daily functioning. In a 12 month follow-up assessment, 27% of individuals in the original HIV-infected cohort developed significant cognitive decline that was predicted at baseline by AIDS status, lower nadir CD4+ count and lower performance in processing speed[10]. These findings indicate that HAND is an important clinical syndrome in China and emphasize the importance of developing measures to treat and prevent HIV-related central nervous system (CNS) disease.
An abundance of data from persons living in the U.S. and other Western countries indicates that virus-host interactions play a central role in HIV-1 pathogenesis, and host genetic factors are important determinants for risk of infection and disease progression. Host genetic variants are also associated with the presence and development of HAND in adults and children [11-15]. From the emerging data, it is clear that no single genetic variant is the causal risk factor for HAND, and multiple genes affecting innate and adaptive immunity, and neuronal susceptibility can alter the risk for impairment. No data exist for the evaluation of host genetic factors that affect HIV-associated neuropsychological impairment in Chinese. For this reason, our original study was designed to assess the association of host genetic variants that have been linked to HIV-related disease progression and/or cognitive impairment in U.S. and European cohorts. Our findings suggest that variants in genes encoding apolipoprotein E protein (apoE) and mannose binding lectin-2 (MBL2) are associated with neuropsychological disorders in Chinese infected predominantly through contact with contaminated blood products.
MATERIAL AND METHODS
Study participants
Details of the subjects who participated in this study have been described previously [9]. The study was approved by the Institutional Review Boards from the China Center for Disease Control/National Center for AIDS, the Peking University and the University of California, San Diego. Written informed consent was obtained from the participants after the research procedure had been fully explained to them. HIV-1 infection was confirmed by the HIV Quick Test (OraSure Technologies, Bethlehem, PA). Participants shared the infection risk of being a former plasma donor and only one participant in the HIV-1 infected group reported homosexual contact as an additional risk for HIV-1 transmission. Individuals with co-morbidities that might cause neurocognitive impairment (other than co-infection with HCV) were excluded from the study. Briefly, of 203 HIV-1 infected individuals enrolled in the study, 201 had blood spots obtained for extraction of DNA for genetic studies, the basis for the analyses in this manuscript. Hepatitis C virus (HCV) serostatus was determined using routine clinical HCV antibody testing. Of note, 93% of the HIV-infected cohort and 62% of the HIV-uninfected controls were HCV positive. Although there was not an increased rate of neurobehavioral impairment in the HIV/HCV co-infected majority versus the small HIV mono-infected group 36.9% versus 35.7%, respectively), a modest HCV effect was seen among the HCV positive/HIV-negative compared to the HCV negative/HIV negative subjects (23.5% versus 12.5%, respectively).
Demographic and medical history information was obtained from each participant. Each subject underwent comprehensive neurocognitive and neuromedical evaluations, a structured psychiatric examination, and an assessment of daily functioning [9]. Details of the NP battery selection and adaptation for use in China have been described previously by our group [9, 16]. Briefly, the tests used of neurobehavioral function were decided jointly by U.S. and Chinese investigators who evaluated the entire HNRC neurobehavioral protocol to gauge each test’s appropriateness for use in China. The battery was carefully reviewed by Chinese mental health professionals, who considered the U.S. tests to be culturally appropriate with only minor modifications for the study populations in China. The tests were translated by the Chinese investigators from English to Mandarin. Back-translation was performed independently by a professional translator with no prior knowledge of these instruments. The test battery used evaluates multiple cognitive–motor ability domains that repeatedly have been found to be affected by HIV-associated brain disease in the U.S. [17-19]. All test scores in the battery were adjusted for age, education and gender using data from demographically comparable HIV seronegative former plasma donors from the same rural Chinese region [9].
Genomic DNA Extraction and Genotyping
Genomic DNA extracted from dried blood spots using QIAamp DNA Mini Kit (Qiagen, Valencia, CA) was genotyped for CCR5-wt/Δ32, CCR5-59029-G/A, CCR2-180-G/A, SDF-1-G/A, IL4-589-C/T, MCP-1-2518-A/G, MBL2, APOE 2060-T/C and 2198-C/T (distinguishing ε2, ε3 and ε4 alleles), CCL3L2 copy number variants (CNVs), and CX3CR1-745-G/A, -849-C/T polymorphisms using real-time PCR (LightCycler, Roche) as described previously [13, 20, 21]. These genetic variants were selected because each has previously been found to be associated with HIV-related disease progression and/or neurocognitive impairment.
Statistical Methods
For bivariate analyses, categorical variables were compared using either the Chi square test or the Fisher’s exact test. Continuous variables were compared using the Student’s t test for parametric data or the Mann-Whitney U test for non-paramteric data. All genetic variant comparisons analyzed were based on differences between genotypes or haplotypes previously identified in other cohorts to be associated with HIV-1 related disease. P-values were considered significant if <0.05 two-tailed.
RESULTS
Demographic Data and Genotype Frequencies
Table 1 shows the demographic, laboratory and cognitive function data of the HIV-infected subjects at baseline (N = 201) and at 12 months of follow-up (N = 192). The cohort was comprised of 60.7% males with a mean education of 5.5 years. AIDS was diagnosed in 113 subjects at baseline and 114 were receiving antiretroviral therapy. The mean CD4+ lymphocyte count at baseline was 349/mm3 which increased to 400/mm3 at 12 months. The plasma HIV-1 RNA was detectable in 127 subjects at baseline with the mean viral load of 4.09 log10 copies/mL. At baseline, 74 (37%) subjects were globally impaired which increased to 85 (44.2%) after 12 months of follow-up. Table 2 gives the frequencies for each of the genes assessed. Of note, no subjects were found to have the CCR5Δ32 allele.
Table 1.
Demographic, laboratory and cognitive function data of HIV-1 infected Chinese subjects at baseline for entire cohort (N = 201) and separated by those who experienced a decline in global deficit score (GDS) score at 12 months and those who did not decline (N = 192 for cohort at 12 months).
| At 12 Months |
||||
|---|---|---|---|---|
| Neurocognitive | ||||
| All | Decline | No Decline | P-Value | |
| Number | 201 | 53 | 139 | |
| Age (yrs-mean ± SD) | 40.3 (6.4) | 41.6 (6.6) | 39.6 (6.2) | 0.08 |
| Education (yrs-mean ± SD) | 5.5 (2.3) | 5.2 (2.4) | 5.6 (2.2) | 0.34 |
| Male (%) | 60.7 | 60.4 | 61.9 | 0.85 |
| Baseline NP-impaired (GDS ≥0.5) | 74 (36.8%) | 20 (37.7%) | 51 (36.7%) | 0.89 |
| GDS baseline (mean ± SD) | .49 (0.49) | .52 (0.4) | .49 (0.5) | 0.65 |
| NP-impaired at 12 months (GDS ≥0.5-corrected) | 85 (44.2%) | 43 (81.1%) | 42 (30.2%) | <.0001 |
| GDS follow-up (corrected) (mean ± SD) | .56 (.49) | .93 (0.48) | 0.41 (0.41) | <.0001 |
| AIDS baseline (%) | 113 (56.0) | 36 (68.0) | 73 (52.5) | 0.09 |
| On ART at baseline (%) | 114 (57.0) | 35 (66.0) | 73 (52.5) | 0.09 |
| On ART at 12 months (%) | 122 (63.5) | 39 (73.6) | 83 (59.7) | 0.07 |
| CD4+/mm3 baseline (mean ±SD) | 349 (193) | 324 (186) | 365 (203) | 0.19 |
| CD4+/mm3 12 months (mean ±SD) (N = 190) | 400 (218) | 367 (182) | 422 (222) | 0.09 |
| Log 10 HIV RNA baseline (±SD) (n=127 with detectable RNA) | 4.09 (0.8) | 4.08 (0.8) | 4.09 (0.85) | 0.97 |
Table 2.
Genotype frequencies among HIV-infected subjects in Anhui, China.
| Genotype | Frequency N (%) |
|---|---|
| APOE* | |
| ε2/ε3 | 20 (10) |
| ε2/ε4 | 4 (2) |
| ε3/ε3 | 138 (69) |
| ε3/ε4 | 37 (18) |
| ε4/ε4 | 2 (1) |
| CCR2-180-G/A (V/I) | |
| A/A | 12 (6) |
| G/A | 71 (35) |
| G/G | 118 (59) |
| CCR5-59029-G/A | |
| A/A | 50 (25) |
| G/A | 89 (45) |
| G/G | 61 (31) |
| CCR5-wt/Δ32 | 0 (0) |
| wt/wt | 201 (100) |
| CCL3L1-copy number (mean = 3) | |
| <2 | 92 (45) |
| 2 – 4 | 64 (31) |
| 4 – 6 | 32 (16) |
| >6 | 16 (8) |
| CX3CR1-745-G/A (V/I) | |
| G/A | 14 (7) |
| G/G | 187 (93) |
| CX3CR1-849-C/T (T/M) | |
| C/C | 9 (4) |
| C/T | 77 (39) |
| T/T | 114 (57) |
| MCP1-2518-A/G | |
| A/A | 31 (15) |
| G/A | 101 (50) |
| G/G | 69 (34) |
| MBL2-ABCD | |
| AA | 104 (52) |
| AB | 30 (15) |
| AC | 42 (21) |
| BB | 4 (2) |
| BC | 8 (4) |
| CC | 13 (6) |
| MBL2-HL | |
| HH | 94 (47) |
| HL | 71 (35) |
| LL | 35 (17) |
| MBL2-PQ | |
| PP | 159 (79) |
| PQ | 38 (19) |
| 4 (2) | |
| MBL2-XY | |
| XX | 4 (2) |
| XY | 56 (28) |
| YY | 141 (70) |
For APOE alleles:
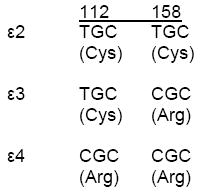
Genetic associations
Association of APOE ε4 genotype with HIV-related cognitive impairment
Subjects with the ε4 allele had less education than those without the ε4 allele (mean 4.6 versus 5.7 years, respectively); however, all GDS assessment where adjusted for age, gender and education. Because the ε4/ε4 genotype was present in only 2 (1%) subjects, all analyses that examined the association of ε4 were performed using the presence or absence of the ε4 allele. Presence of the ε4 allele was more likely to be associated with cognitive impairment (adjusted for age, gender and education). Of the 43 subjects with the ε4 allele, 25 (58%) were cognitively impaired compared to 49 (31%) of 158 without the ε4 allele (P = 0.001, OR: 3.09; 95% CI 1.54, 6.18) (Table 3A). The increased likelihood of impairment held whether subjects were on antiretroviral therapy at baseline (P=0.042, OR=2.64; 95% CI: 1.04, 6.68) or not (P=0.018, OR=3.75; 95%CI: 1.28, 11.0). The mean GDS (+/-SD) at baseline was 0.61 (0.51) for subjects with the ε4 allele and 0.46 (0.47) for those without ε4 (P = 0.015)
Table 3.
Demographic, laboratory and cognitive function data of HIV-1 infected Chinese subjects at baseline and at 12 months of follow-up separated by: A. APOE ε4(+) versus APOE ε4(-) and B. MBL2 –A/A versus –O/O.
| A. | |||
|---|---|---|---|
| Baseline | APOE ε4(+) | APOE ε4(-) | P-value |
| Total N | 43 (21%) | 158 (79%) | |
| Gender M/F | 23/20 | 99/59 | 0.28 |
| Educational level (mean +/- SD) | 4.58 (1.9) | 5.70 (2.3) | 0.004 |
| Age (years) (mean +/- SD) | 40.8 (7.4) | 40.1 (6.1) | 0.50 |
| GDS (mean +/- SD) | .65 (.51) | .46 (.47) | 0.015 |
| Impairment status (GDS > 0.50) | 25 (58%) | 49 (31%) | 0.001 |
| Baseline CD4 (cells/mm3 +/- SD) | 414.9 (414.9) | 331.6 (179.5) | .016 |
| Nadir CD4 (cells/mm3 +/-SD) | 274.1 (172.6) | 246.5 (153.1) | 0.31 |
| AIDS diagnosis | 23 (54%) | 90 (57%) | 0.68 |
| Plasma HIV RNA (mean log10 +/-SD) | 4.99 (1.43) | 5.08 (1.54) | 0.76 |
| At 12 months | |||
| Total N | 42 (22%) | 150 (78%) | |
| Gender M/F | 23/19 | 95/55 | 0.31 |
| Educational level (mean +/- SD) | 4.61 (1.9) | 5.71 (2.3) | 0.005 |
| Age (years) (mean +/- SD) | 40.8 (7.4) | 40.0 (6.0) | 0.49 |
| GDS (mean +/- SD) | .63 (.55) | .54 (.48) | 0.28 |
| Impairment status (GDS ≥ 0.50) | 20 (48%) | 65 (43%) | 0.62 |
| GDS (mean +/- SD) ART 0-12 months | .88 (.55) | .63 (.54) | 0.05 |
| CD4 at 12 months (cells/mm3 +/- SD) | 421.1 (228.8) | 336.8 (179.4) | .016 |
| AIDS diagnosis | 22 (52%) | 84 (56%) | 0.67 |
| Plasma HIV RNA (mean log10 +/-SD) | 3.55 (1.57) | 3.14 (1.37) | 0.10 |
| B. | |||
| Baseline | MBL2-A/A | MBL2-O/O | P-value |
| Total N | 104 | 25 | |
| Gender M/F | 60/44 | 20/5 | .039 |
| Educational level (mean +/- SD) | 5.44 (2.3) | 5.88 (2.2) | 0.39 |
| Age (years) (mean +/- SD) | 40.3 (6.5) | 41.2 (7.1) | 0.52 |
| GDS (mean +/- SD) | .48 (.46) | .42 (.47) | 0.51 |
| Impairment status (GDS ≥ 0.50) | 40 (38.5%) | 8 (32%) | 0.55 |
| Baseline CD4 (cells/mm3 +/-SD) | 365 (198.4) | 367.1 (198.7) | 0.96 |
| Nadir CD4 (cells/mm3 +/-SD) | 263.4 (159.6) | 264.3 (158.7) | 0.98 |
| AIDS diagnosis | 58 (55.8%) | 13 (52%) | 0.73 |
| Plasma HIV RNA (mean log10 +/-SD) | 3.30 (1.3) | 3.42 (1.3) | 0.66 |
| At 12 months | |||
| Total N | 100 (80%) | 25 (20%) | |
| Gender M/F | 58/42 | 80/20 | 0.04 |
| Educational level (mean +/- SD) | 5.47 (2.3) | 5.68 (2.2) | 0.68 |
| Age (years) (mean +/- SD) | 40.05 (6.4) | 41.2 (7.1) | 0.43 |
| GDS (mean +/- SD) | .53 (.49) | .70 (.57) | 0.15 |
| Impairment status (GDS > 0.50) | 41 (41%) | 15 (60%) | 0.09 |
| Decline in cognitive score | 23 (23%) | 13 (52%) | 0.004 |
| CD4 at 12 months (cells/mm3 +/-SD) | 367.2 (199.8) | 367.1 (193.7) | 0.99 |
| AIDS diagnosis | 56 (56%) | 13 (52%) | 0.72 |
| Plasma HIV RNA (mean log10 +/-SD) | 3.26 (1.4) | 3.37 (1.4) | 0.75 |
Because high viral load and low CD4+ lymphocyte counts have been associated with HAND, we examined the CD4+ counts and viral loads of subjects with and without the ε4 allele. Of note, subjects with the ε4 allele had higher baseline CD4+ lymphocyte counts (414.9 ± 229.0) versus 331.6 ± 179.3, respectively; P = 0.016) and their mean nadir CD4+ count did not differ from those without the ε4 allele (274.1 versus 246.5, respectively; P=0.31). The proportion of subjects with an AIDS diagnosis and HIV viral load did not significantly differ between the two groups. The associations between APOE ε4 genotypes at baseline held in multivariate analyses controlling for CD4+ lymphocyte count, HIV viral load or both (P <0.01 for all comparisons).
We next assessed the association of the ε4 allele on cognitive impairment at 12 months of follow-up. For the 192 HIV-infected subjects with neurocognitive evaluations at 12 months, those with the ε4 allele were no more likely to be impaired than those without ε4 (48% versus 43%, respectively; P = 0.73). Notably, subjects with the ε4 allele were no more likely to be on antiretroviral therapy at baseline or during the 12 months of follow-up compared to those without the ε4 allele. However, whereas the 23 ε4 positive participants on treatment during the 12 months had a mean (+/-SD) GDS score of 0.88 (0.55), the mean GDS for the ε4 negatives was 0.63 (.54) (P = 0.053, 95%CI: -0.004, 0.51). This association held in multivariate analyses controlling for baseline CD4+ lymphocyte count (P = 0.036), but failed to reach statistical significance controlling for viral load (P = 0.08) or CD4+ count and viral load (P = 0.067). Similar findings were observed when controlling for 12 month CD4 count, viral load or both (P = 0.031, 0.073 and 0.054, respectively).
Because genetic variants in APOE have been found to have a profound effect on the development of Alzheimer’s disease and other neurodegenerative disease, we examined the impact of age on the association of ε4 variants with cognitive impairment at baseline. For these analyses, we compared subjects between 20-39 years of age (n=22) to those ≥50 years old (n = 8). For the 20-39 year old group of those positive for the ε4 variant 13 (59%) were impaired at baseline compared to 5 (63%) in the ≥50 year old group (OR = 0.87. 95% CI: 0.16, 4.58; P=1.00). Whereas being ε4 positive was associated with cognitive impairment in the 20-39 year old group (n=78; OR = 0.31, 95% CI: 0.12, 0.82; P=0.02), the association was not present in the ≥50 year olds (n = 24; OR = 0.6, 95% CI: 0.11, 3.4; P = 0.68).
Association of MBL2 genotypes with HIV-related cognitive impairment
At baseline, no significant differences in rates of cognitive impairment were observed for study subjects with different MBL2 genotypes (Table 3B). For the A/A group 40 of 104 (38.5%) were impaired compared to 26 of 72 (36.1%) of the A/O group and 8 of 25 (32%) for the O/O group (P=0.64 for A/A versus O/O). By the end of 12 months of follow-up, 41%, 43% and 60% of subjects were impaired in the A/A, A/O and O/O groups, respectively. There was a trend toward increased impairment in the O/O group compared to the A/A group (OR: 2.13; 95%CI: 0.8829, 5.28; P = 0.09). Of note, whereas 13 (52%) of subjects with the O/O genotype experienced a decline in cognitive function over the 12 month period, only 23 (23%) of subjects in the A/A group demonstrated declining cognition (OR = 3.6. 95%CI: 1.46, 9.03; P = 0.004). This association held in multivariate analyses controlling for CD4+ lymphocyte count, viral load or both at baseline (P < 0.006 for each).
Genetic variants CCR2-180G/A (V/I), CCR5-59029-G/A, IL4-589C/T, MCP1-2518-A/G and CCL3L1 copy number variation (CNV) fail to show any association with global neuropsychological impairment
Genetic variants in genes encoding for MCP-1 (CCL2) and its receptor CCR2, CCL3L1 CNV and receptor CCR5, and IL-4 have each been associated with HIV disease progression and/or cognitive impairment in some HIV-infected cohorts generally from the U.S. or Western Europe. Table 2 summarizes the allelic and haplotype frequencies of each of the additional genetic variants evaluated. In our HIV-infected Chinese cohort, no association was observed with any of these genetic variants with the global deficit score at baseline or after 12 months of follow-up (Table 4).
Table 4.
Association of cognitive impairment at baseline with CCR2-180G/A (V/I), CCR5-59029-G/A, IL4-589C/T, MCP1-2518-A/G, MBL2-H/L, P/Q and X/Y, and CCL3L1 copy number at baseline.
| ODDS RATIO | 95% CI lower | 95% CI upper | P-value | |
|---|---|---|---|---|
| CCR2-180-G/A(V/I) | ||||
| GA vs. GG | 0.94 | 0.51 | 1.72 | 0.84 |
| AA vs. GG | 0.74 | 0.37 | 1.45 | 0.38 |
| GA+AA (either GA or AA) vs. GG | 0.87 | 0.49 | 1.56 | 0.64 |
| CCR5-59029-G/A | ||||
| GA vs. GG | 0.73 | 0.37 | 1.44 | 0.36 |
| AA vs. GG | 0.94 | 0.64 | 1.38 | 0.75 |
| GA+AA (either GA or AA) vs. GG | 0.78 | 0.42 | 1.45 | 0.44 |
| IL4-589-C/T | ||||
| CT vs. TT | 1.18 | 0.65 | 2.15 | 0.59 |
| CC vs. TT | 1.22 | 0.61 | 2.41 | 0.57 |
| CT+CC (either CT or CC) vs. TT | 1.21 | 0.68 | 2.16 | 0.52 |
| MCP1-2518-A/G | ||||
| GA vs. GG | 0.90 | 0.48 | 1.69 | 0.74 |
| AA vs. GG | 0.86 | 0.55 | 1.35 | 0.51 |
| GA+AA (either GA or AA) vs. GG | 0.86 | 0.47 | 1.57 | 0.62 |
| MBL2_HL | ||||
| HL vs. HH | 1.33 | 0.70 | 2.54 | 0.39 |
| LL vs. HH | 1.43 | 0.96 | 2.13 | 0.08 |
| HL+LL (either HL or LL) vs. HH | 1.54 | 0.86 | 2.75 | 0.15 |
| MBL2_PQ | ||||
| PQ vs. PP | 0.67 | 0.31 | 1.45 | 0.31 |
| QQ vs. PP | 2.23 | 0.71 | 6.98 | 0.17 |
| PQ+QQ (PQ or QQ) vs. PP | 0.83 | 0.40 | 1.69 | 0.60 |
| MBL2-XY | ||||
| XY vs. YY | 1.23 | 0.65 | 2.32 | 0.52 |
| XX vs. YY | no estimate possible | |||
| XY+XX (XY or XX) vs. YY | 1.10 | 0.59 | 2.05 | 0.98 |
| CCL3L1 | ||||
| <3 copies vs. ≥3 copies | 1.35 | 0.76 | 2.42 | 0.31 |
| 1 copy vs. >4 copies | 0.67 | 0.29 | 1.51 | 0.33 |
| 1 copy vs. >5 copies | 0.60 | 0.22 | 1.63 | 0.31 |
DISCUSSION
An increasing body of data supports an important role for host genetics in the pathogenesis of HIV-1 disease. Most studies, to date, have been performed in cohorts from countries where there is little representation of persons of Chinese origin and none have examined genetic associations on HAND in persons living in China. Thus, our findings indicating that genetic variants in APOE and MBL2 are associated with cognitive impairment in HIV-infected Chinese who acquired their infection through exposure to contaminated plasma are novel, and are consistent with the important roles that apoE and MBL2 are thought to play in the pathogenesis of HIV infection.
ApoE has a central role in lipid metabolism and genetic variants have been implicated in the development and progression of cardiovascular, neurological and infectious diseases [22]. ApoE has a particularly important role in the plasma clearance of trigyceride- and cholesterol-rich lipoproteins and is the major ligand for the LDL receptor. It is also the ligand for other members of the LDL receptor family including the LDL-receptor-related protein, which contributes to lipoprotein remnant clearance. ApoE binds to heparin sulfate proteoglycans which also clear apoE-containing remnant lipoproteins (for review see [23, 24]). The finding of an association of APOE genetic variants with an impaired GDS is of particular interest. ApoE is an important carrier of cholesterol in the CNS and has been identified as a risk factor in Alzheimer’s disease (for review see [23]). Detrimental effects of the apoE4 isoforms correlate with the structure of apoE (for review see [24]). ApoE has two structural domains separated by a hinge region; the N-terminal domain contains the receptor binding region while the C-terminal domain contains the major lipid binding region. Amino acid variations in these regions have been shown to have dramatic effects on apoE structure. Persons heterozygous or homozygous for APOE ε4 are at increased risk for Alzheimer’s disease compared to those with other apoE isoforms. The presence of the ε4 allele also decreases the age at which persons are at risk for development signs of Alzheimer’s disease. Additionally, it appears that there is a synergistic negative effect between the ε4 allele and Amyloid-β on the risk for Alzheimer’s disease [24].
Corder et al [25] were the first to examine the association of APOE genetic variants and HIV-related dementia. In their small predominantly Caucasian cohort, the presence of the ε4 allele was associated with twice the risk for HIV-related dementia as well as an excess risk for peripheral neuropathy. Also, in a study by Valcour and colleagues [26] the presence of APOE ε4 was associated with increased risk for HIV-associated dementia in older subjects but not younger patients. More recently, Burt and colleagues [27] found that ε4 homozygosity in a U.S. cohort was associated with more rapid HIV-related disease progression and death when compared to those homozygous for ε3. However, no association was observed with the presence of the ε4 allele and HIV-associated dementia. In the current study, the ε4 allele was found to be present in a greater proportion of cognitively impaired HIV-infected Chinese than those with the other apoE isoforms. Moreover, for those receiving antiretroviral treatment over the 12 month period, the ε4 allele remained associated with cognitive impairment. The association of ε4 with impairment was unaffected by age in our study; however because only 8 subjects were ≥50 years old, we cannot definitively exclude that the association with the APOE ε4 with HIV-related cognitive impairment does not increase with age.
How apoE variants are associated with an increased risk for infectious diseases is an area of active investigation. However, apoE isoforms have been associated with susceptibility to a broad spectrum of microorganisms including malaria, bacterial infections and viral infections including herpes simplex and HIV-1 [28]. Additionally, in vitro studies demonstrate that in the presence of apoE4 cells are more susceptible to HIV-1 infection than cells treated with apoE3 [29]. The susceptibility of cells to HIV-1 infection appears to be mediated by the amphiphatic helices of apoE interacting with the HIV gp41 protein promoting viral fusion. The structure of apo4 leading to a decreased capacity to block the binding of gp41 to the cell or the differential effects on the cholesterol content or other lipids on the target cell may be responsible for the enhanced replication. Thus, it is possible that the apoE4 isoform both enhances HIV-1 infection and provides an increased susceptibility to cognitive impairment. Of interest, the apoE4 isoform is more frequent in African populations where its role in the HIV/AIDS epidemic has not been examined.
Mannose binding lectin-2 (MBL2) is an evolutionarily conserved, circulating host protein encoded by the MBL2 gene. It is an important component of the innate immune system and binds to the mannose residues present on some bacteria, parasites, yeast and viruses, mediating complement activation, opsonization and phagocytosis [30-32]. MBL2 exon 1 variants result in single amino acid changes affecting oligomerization of MBL. Homozygous wild-type (A/A) sera contain predominantly fully functional MBL, whereas homozygous mutant sera (O/O = any combination of B, C, or D alleles) contain mostly low-molecular-weight MBL. Sera containing heterozygous variant alleles (A/O, O = B, C, or D) contain both high-molecular-weight and low-molecular-weight MBL, with the ratio determined by the promoter type on the normal haplotype (A allele). Low MW MBL2 can result in opsonic defects, and has been linked to an increased risk for several inherited immunodeficiencies, autoimmune diseases and risk for serious infectious diseases including acute respiratory infections and meningococcal disease [31, 33, 34]. Additionally, MBL2 genetic variants have been associated with HIV-related disease progression in both adults and children [35, 36]. Of note, Singh et al have identified an increased risk for progression to CNS impairment in HIV-1 infected children in the U.S. with the O/O genotype compared to those with either the A/O or A/A genotypes [36]. Little is known of the effect of MBL2 variants in the Chinese population or the association of MBL with HIV-related cognitive impairment. Thus, the findings presented here of an increased risk for development of cognitive impairment in a cohort of HIV-infected Chinese adults over the course of 12 months are novel, but are consistent with previous association studies performed in the U.S.
The current study has a number of limitations. First, the timing of HIV-1 infection was not established so that participants could have been infected at any time when participants were exposed to contaminated blood drawing equipment. Second, there was not documentation of when antiretroviral therapy was initiated for those on treatment. However, there was consistency of the drugs used for treatment with first line therapy in China with most study participants receiving two nucleoside reverse transcriptase inhibitors and nevirapine. Third, because such a large proportion of the subjects were HCV seropositive, it is possible that the effects seen may reflect an additive contribution to impaired cognitive performance and is not representative of HIV-1 infection alone. Finally, because the participants in this study were infected through blood products, these findings may not be applicable to persons infected with HIV-1 through sexual transmission. In total, however, our findings do suggest that the APOE ε4 allele and MBL2 O/O genotypes are associated with neurobehavioral outcomes in HIV-infected Chinese. These findings will need to be confirmed in additional cohort studies in China to define further the associations between these genetic variants and HIV-related CNS impairment.
Acknowledgments
This study was supported by the NIMH grant 5 RO1 MH073433, the HIV Neurobehavioral Research Center (HNRC) MH 62512 and NIMH 5R01MH085608. We thank Rodney Trout and Patricia Riggs for their technical assistance and help with data collection, respectively.
References
- 1.UNAIDS W. 2007 AIDS epidemic update. 2009 http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 2.Yu ES, Xie Q, Zhang K, Lu P, Chan LL. HIV infection and AIDS in China, 1985 through 1994. Am J Public Health. 1996;86:1116–1122. doi: 10.2105/ajph.86.8_pt_1.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X, Tian C, Choi KH, Zhang J, Cheng H, Yang X, et al. Injecting drug use and HIV infection in southwest China. AIDS. 1994;8:1141–1147. doi: 10.1097/00002030-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- 5.Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. Int Rev Psychiatry. 2008;20:3–13. doi: 10.1080/09540260701862086. [DOI] [PubMed] [Google Scholar]
- 6.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Nath A, Sacktor N. Influence of highly active antiretroviral therapy on persistence of HIV in the central nervous system. Current Opinion in Neurology. 2006;19:358–361. doi: 10.1097/01.wco.0000236614.51592.ca. [DOI] [PubMed] [Google Scholar]
- 8.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 9.Heaton RK, Cysique LA, Jin H, Shi C, Yu X, Letendre S, et al. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol. 2008:1–14. doi: 10.1080/13550280802378880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cysique L, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, Shi C, Yu X, Wu Z, Abramson IS, Grant I, Heaton RK the HIV Neurobehavioral Research Center Group. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. Aids. doi: 10.1097/QAD.0b013e32833336c8. in press:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boven LA, van der Bruggen T, van Asbeck BS, Marx JJ, Nottet HS. Potential role of CCR5 polymorphism in the development of AIDS dementia complex. FEMS Immunol Med Microbiol. 1999;26:243–247. doi: 10.1111/j.1574-695X.1999.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 12.Meyer L, Magierowska M, Hubert JB, Rouzioux C, Deveau C, Sanson F, et al. Early protective effect of CCR-5 delta 32 heterozygosity on HIV-1 disease progression: relationship with viral load. The SEROCO Study Group. AIDS. 1997;11:F73–78. doi: 10.1097/00002030-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Singh KK, Hughes MD, Chen J, Spector SA. Genetic Polymorphisms in CX3CR1 Predict HIV-1 Disease Progression in Children Independently of CD4+ Lymphocyte Count and HIV-1 RNA Load. J Infect Dis. 2005;191:1971–1980. doi: 10.1086/430091. [DOI] [PubMed] [Google Scholar]
- 14.Singh KK, Hughes MD, Chen J, Spector SA. Impact of MCP-1-2518-G allele on the HIV-1 disease of children in the United States. AIDS. 2006;20:475–478. doi: 10.1097/01.aids.0000200540.09856.58. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cysique LA, Jin H, Franklin DR, Jr, Morgan EE, Shi C, Yu X, et al. Neurobehavioral effects of HIV-1 infection in China and the United States: a pilot study. J Int Neuropsychol Soc. 2007;13:781–790. doi: 10.1017/S1355617707071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 18.Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 19.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 20.Singh KK, Barroga CF, Hughes MD, Chen J, Raskino C, McKinney RE, Spector SA. Genetic influence of CCR5, CCR2, and SDF1 variants on human immunodeficiency virus 1 (HIV-1)-related disease progression and neurological impairment, in children with symptomatic HIV-1 infection. J Infect Dis. 2003;188:1461–1472. doi: 10.1086/379038. [DOI] [PubMed] [Google Scholar]
- 21.Singh KK, Hughes MD, Chen J, Phiri K, Rousseau C, Kuhn L, et al. Associations of chemokine receptor polymorphisms With HIV-1 mother-to-child transmission in sub-Saharan Africa: possible modulation of genetic effects by antiretrovirals. Journal of Acquired Immune Deficiency Syndromes. 2008;49:259–265. doi: 10.1097/QAI.0b013e318186eaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobson CB, Sales SD, Hoggard P, Wozniak MA, Crutcher KA. The receptor-binding region of human apolipoprotein E has direct anti-infective activity. J Infect Dis. 2006;193:442–450. doi: 10.1086/499280. [DOI] [PubMed] [Google Scholar]
- 23.Cedazo-Minguez A. Apolipoprotein E and Alzheimer’s disease: molecular mechanisms and therapeutic opportunities. J Cell Mol Med. 2007;11:1227–1238. doi: 10.1111/j.1582-4934.2007.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009;50(Suppl):S183–188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corder EH, Robertson K, Lannfelt L, Bogdanovic N, Eggertsen G, Wilkins J, Hall C. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4:1182–1184. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- 26.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004;157:197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urosevic N, Martins RN. Infection and Alzheimer’s disease: the APOE epsilon4 connection and lipid metabolism. J Alzheimers Dis. 2008;13:421–435. doi: 10.3233/jad-2008-13407. [DOI] [PubMed] [Google Scholar]
- 29.Martin I, Dubois MC, Saermark T, Ruysschaert JM. Apolipoprotein A-1 interacts with the N-terminal fusogenic domains of SIV (simian immunodeficiency virus) GP32 and HIV (human immunodeficiency virus) GP41: implications in viral entry. Biochem Biophys Res Commun. 1992;186:95–101. doi: 10.1016/s0006-291x(05)80780-6. [DOI] [PubMed] [Google Scholar]
- 30.Arora M, Munoz E, Tenner AJ. Identification of a site on mannan-binding lectin critical for enhancement of phagocytosis. J Biol Chem. 2001;276:43087–43094. doi: 10.1074/jbc.M105455200. [DOI] [PubMed] [Google Scholar]
- 31.Jack DL, Klein NJ, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 32.Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38:133–149. doi: 10.1016/s0161-5890(01)00038-4. [DOI] [PubMed] [Google Scholar]
- 33.Garred P, Voss A, Madsen HO, Junker P. Association of mannose-binding lectin gene variation with disease severity and infections in a population-based cohort of systemic lupus erythematosus patients. Genes Immun. 2001;2:442–450. doi: 10.1038/sj.gene.6363804. [DOI] [PubMed] [Google Scholar]
- 34.Turner MW, Hamvas RM. Mannose-binding lectin: structure, function, genetics and disease associations. Rev Immunogenet. 2000;2:305–322. [PubMed] [Google Scholar]
- 35.Catano G, Agan BK, Kulkarni H, Telles V, Marconi VC, Dolan MJ, Ahuja SK. Independent effects of genetic variations in mannose-binding lectin influence the course of HIV disease: the advantage of heterozygosity for coding mutations. J Infect Dis. 2008;198:72–80. doi: 10.1086/588712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh KK, Lieser A, Ruan PK, Fenton T, Spector SA. An age-dependent association of mannose-binding lectin-2 genetic variants on HIV-1-related disease in children. J Allergy Clin Immunol. 2008;122:173–180. 180 e171–172. doi: 10.1016/j.jaci.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


