Abstract
The role of nucleotide-binding oligomerization domain-1 (NOD1) and nucleotide-binding oligomerization domain-2 (NOD2), cytoplasmic receptors which detect bacterial cell wall molecules, in pulmonary innate immune responses is poorly understood. We determined that both NOD1 and NOD2 detect heat-killed Legionella and stimulate NF-κb and IFN-β promoter activity using an in vitro luciferase reporter system. We next infected NOD1- and NOD2-deficient animals with aerosolized Legionella pneumophila. At 3 days post infection, Nod1–/– mice had impaired bacterial clearance compared to WT controls. In addition, at 4 h and 24 h, Nod1–/– mice had impaired neutrophil recruitment to the alveolar space. In contrast, increased lung neutrophils were seen in the Nod2–/– animals at 24 h. Analysis of cytokine production at 4 h post infection revealed a significant decrease in proinflammatory cytokines in the Nod1–/– animals when compared to WT animals. In contrast, increased 4-h proinflammatory cytokines were seen in the Nod2–/– animals. Furthermore, the lungs of both Nod1–/– and Nod2–/– mice had significantly increased pro-inflammatory cytokine levels at 24 h, suggesting possible suppressive roles for later stages of infection. Together, our data suggest that although both NOD1 and NOD2 can detect Legionella, these receptors modulate the in vivo pulmonary immune response differently.
Keywords: Legionella pneumophila, NOD1, NOD2, Pneumonia
Introduction
The immune response to intracellular pathogens in the lung initially involves detection of the organisms through a set of receptors located on the cell surface or endosomal compartment (Toll-like receptors (TLR)) or in the cytoplasm (Nod-like receptors (NLR)) and retinoic acid inducible gene I-like receptors (RLR). Based on the type of foreign material (dsRNA, peptidoglycan, lipopolysaccharide) and location (extracellular, endosomal, cytoplasm), pathogens stimulate distinct sets of receptors to activate the immune response. Legionella pneumophila (Lp), an organism known to persist within water-borne amoeba, usually infects humans as a terminal host after exposure to contaminated water systems [1]. Lp replicates within the phagolysosome of the macrophage and secretes bacterial products into the cytosol of the cell through a type IV secretion system (T4SS) which is known to translocate both DNA and proteins that impair the destruction of the organism [2].
Previous work has identified several innate immune receptors that are responsible for the detection of Lp in the murine model of infection. NAIP5 (Baculoviral inhibitor of apoptosis repeat-containing 1e protein (Birc1e)), NLRC4 (IL-1b converting enzyme-protease activating factor (Ipaf)), and caspase-1 have been shown to be important in restriction of Lp replication both in vivo and in vitro [3–6]. TLR5, TLR2, and TLR9 detect Lp and regulate the in vivo immune response to Lp [7–10]. Mice deficient in myeloid differentiation factor 88 (Myd88), an adaptor protein for many TLR, are highly susceptible to in vivo Lp infection with lack of an early immune response, inability to control bacterial replication, and enhanced mortality [8, 10]. More recently, receptor-interacting serine-threonine kinase (RIP2), an adaptor for nucleotide-binding oligomerization domain-1 (NOD1) and nucleotide-binding oligomerization domain-2 (NOD2), was found to regulate Lp replication in the lung, but only on a Myd88–/– genetic background [11]. Since Lp is known to replicate intracellularly and can translocate substances to the cytosol via its type IV secretion system, we hypothesized that the cytosolic NLR may be important in control of the innate immune response to Lp.
NOD1 and NOD2 are cytosolic receptors that detect the microbial cell wall byproducts diaminopimelate (DAP) and muramyl dipeptide (MDP), respectively [12, 13]. Both NOD1 and NOD2, which contain caspase-1 recruitment domains, activate NF-κb signaling through RIP2 kinase [14]. NOD2 is expressed mainly in myeloid cells and is important in the immune response to pathogenic organisms including Mycobacterium tuberculosis and Toxoplasmosis gondii [15, 16]. NOD1 is expressed in both epithelial and myeloid derived cells, and contributes to recognition of a variety of pathogens [17–20]. However, little is known of the in vivo role of NOD1 and NOD2 in host defense.
Although there is evidence that NOD1 and NOD2 detect Lp [21], the functional consequences remain poorly understood. Lp-induced cytokine production in NOD1 and/or NOD2-deficient macrophages is selectively impaired [22], yet NOD1 deficiency does not allow for permissive replication in murine macrophages [23]. Although these results suggest that NOD1 and NOD2 detect Lp microbial components, further studies are needed to determine the contribution of NOD1 and NOD2 to the host response in vivo. Nod2–/– mice are more susceptible to both Listeria monocytogenes and Yersinia pseudotuberculosis with in vivo models of GI infection [24, 25]. NOD2 is also important for survival and IFN-γ production in a murine model of T. gondii [16]. NOD1 is also required for IFN-γ-mediated elimination of Trypanosoma cruzi [26]. Both NOD1 and NOD2 promote clearance of the intracellular pathogen Chlamydophila pneumonia from the lung [27] and NOD2 mediates survival and adaptive immunity to M. tuberculosis [28, 29]. The role of NOD1 and NOD2 activation during in vivo Lp infection has not been examined.
Here, we show that Lp induces pro-inflammatory cytokines in a NOD1- and NOD2-dependent manner. In addition, we demonstrate that NOD1 regulates the pulmonary cytokine response and phagocytic recruitment during Lp infection. Furthermore, at 3 and 10 days, delayed clearance of Lp is seen in mice lacking NOD1 receptor. These data suggest that detection of Lp by NOD1 receptor is important in the host response to this intracellular pathogen and delayed clearance at 10 days may suggest NOD1 alters the adaptive immune response.
Results
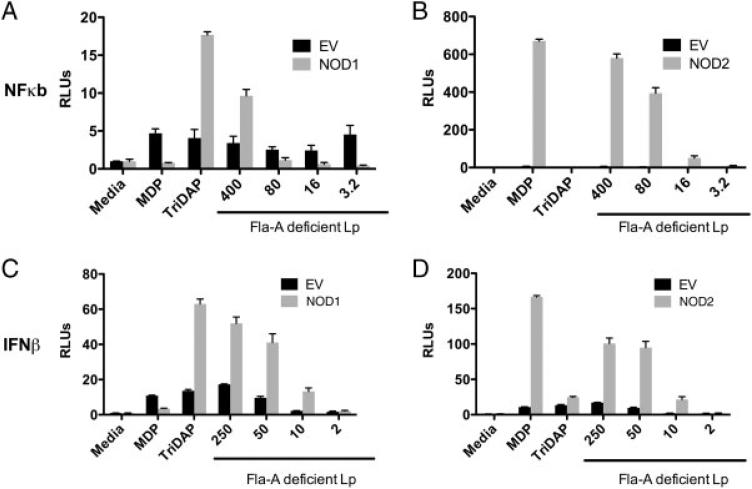
To determine whether Lp signals through NOD1 and NOD2, we co-transfected human embryonic kidney (HEK) cells with either human NOD1 or NOD2 expression vectors simultaneously with either endothelial-leukocyte adhesion molecule (ELAM) or an IFN-β promoter firefly luciferase reporter construct (Fig. 1A–D). Transfected cells were stimulated with heat killed Lp FlaA (deficient in flagellin protein), to avoid stimulation through endogenously expressed TLR5 (the receptor for bacterial flagellin) in the presence of transfection reagent to optimize cytoplasmic delivery [30]. We found that overexpression of human NOD1 and NOD2 stimulated Lp dependant ELAM promoter activity after 24 h when compared to empty vector control (Fig. 1A and B). Only NOD1 was able to stimulate promoter activity at the highest MOI. In addition overexpression of both NOD1 and NOD2 stimulated Lp dependant IFN-β promoter activity, as compared to empty vector control. As controls, MDP and l-alanyl-γ-d-glutamyl-meso-DAP (Tri-DAP) activated ELAM and IFN-β promoter activity through NOD2 and NOD1, respectively. Relative levels of induction using NOD1 purified stimuli Tri-DAP were considerably less due to higher baseline stimulation in both empty vector controls and untreated cells due to known expression of NOD1 in HEK cells. These data suggest that both human NOD1 and NOD2 proteins can detect Legionella in vitro.
Figure 1.
Heat-killed Legionella is detected by NOD1 and NOD2. HEK293 cells were transfected with expression vectors containing human NOD1 (A, C) and NOD2 (B, D) (grey) or empty vector (EV) controls (black). Firefly luciferase reporter constructs for the NF-κB (ELAM-pGL2) (A, B) and IFN-β promoters (IFNβ-pGL2) (C, D) were simultaneously transfected. Cells were then exposed to NOD1 ligand (Tri-DAP, 1 μg/mL), NOD2 ligand (MDP, 1 μg/mL), or heat-killed flagellin-deficient Lp (Lp FlaA, Corby strain) at decreasing heat-killed MOI as determined by OD 600 nm. HEK cells were incubated overnight (16 h) and then lysed. Transfection efficiency was compared between wells by co-transfecting the expression vector for Renilla luciferase. Data are mean ± SD, n = 3, representative of three experiments. Lp: Legionella pneumophila, RLU: relative light units.
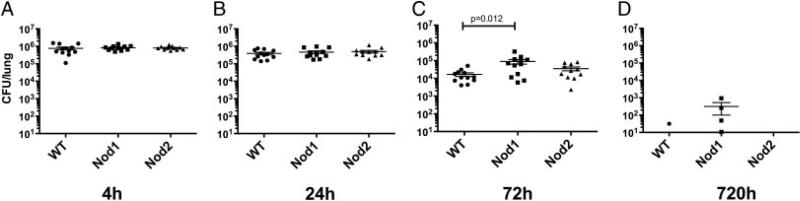
To examine the in vivo role of NOD1 and NOD2 in pulmonary host defense to intracellular pathogens, we used a murine model of airborne infection with Lp. At 4 and 24 h after infection, no significant differences in Lp CFU were seen between WT and Nod1–/– and Nod2–/– animals (Fig. 2A and B). At 72 h, however, a significant increase in Lp CFU was seen in Nod1–/– animals (mean±SEM: 8.9 × 104 CFU/lung±2.6 × 104) compared to WT animals (1.7 × 104 CFU/lung±3.9 × 103) (Fig. 2C). There were no significant CFU differences observed in Nod2–/– animals compared to WT. Lastly, at 10 days, there was late defect in clearance in the Nod1–/– mice that trended toward significant (p = 0.054) (Fig. 2D). To determine whether the CFU difference was due to differences in apoptotic cell death, we examined lungs at 4 and 24 h for terminal deoxynucleotidyl transferase dUTP nick end labeling and saw no difference between Nod1–/– animals and WT controls (our unpublished observations). These results suggest that NOD1 regulates clearance of Lp from the lung after aerosolized exposure.
Figure 2.
NOD1-deficient animals show impaired clearance of Legionella pneumophila in lungs at 3 days. Mice were infected with aerosolized L. pneumophila (Philadelphia strain) and then euthanized at 4 h (A), 24 h (B), 72 h (C), and 240 h (D). Bacterial CFU were determined by serial dilution of tissue homogenates plated on buffered yeast charcoal extract agar. Nod1–/– knockout mice (■), WT controls (•), Nod2–/– knockout mice (▲). Initial deposition was approximately 1 × 106 Lp CFU per lung. A Student's t-test was used to determine significance. Bars represent mean ± SEM range; results combined from three separate experiments with four mice per time point (n = 12 total) except for the 720-h time point, which was one experiment (n = 4).
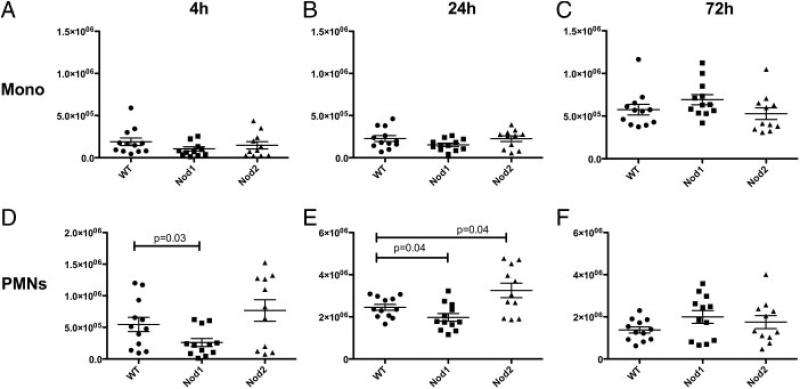
Next, we examined recruitment of inflammatory cells to the pulmonary airspaces by performing bronchoalveolar lavage on WT and Nod1–/– , and Nod2–/– animals. At 4 h, we saw significantly impaired recruitment of PMN in the Nod1–/– (Mean±SEM: 2.6 × 105 PMN/lung±6.3 × 104) animals compared to WT (5.5 × 105 PMN/lung±1.1 × 105) (Fig. 3D). At 24 h, these differences persisted, although the magnitude was smaller (Fig. 3E) (Nod1–/–, 2.0 × 106 ± 1.4 × 105; WT, 2.5 × 106 ± 1.4 × 105). Interestingly, at the same 72-h time point where increased CFU of Lp was present, Nod1–/– animals showed a borderline increased level of PMN recruited to the alveolar space compared to WT controls (Fig. 3F, p = 0.07). For Nod2–/– animals, increased PMN were recruited to the bronchoalveolar space at 24 h compared to WT animals. In addition, no significant differences were seen in total monocytic recruitment to the lung following infection at 4, 24, and 72 h in the NOD1- or NOD2-deficient animals (Fig. 3A–C).
Figure 3.
NOD1 and NOD2 regulate neutrophil recruitment to the lung in Lp-infected mice. Bronchoalveolar lavages were performed on mice infected with Legionella pneumophila and euthanized at the indicated time points: 4 h (A, D), 24 h (B, E), and 72 h (C, F). Total neutrophil (PMN) (D, E, F) and monocyte (Mono) (A, B, C) counts were compared in WT (•), Nod1–/– (■) and Nod2–/– animals (▲). A Student's t-test was used to determine significance as represented on the graph. Bars represent mean ± SEM. Combined results from three separate experiments with four mice per time point (n = 12).
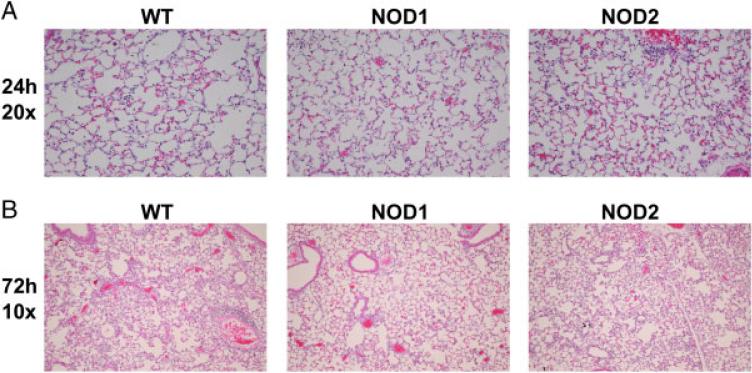
We examined histologic lung sections from 24- and 72-h time points to determine if visual differences were seen in lung samples. Six lungs from 24 h (Fig. 4A) and 72 h (Fig. 4B) were scored in ten separate high-powered fields for percentage of airspace involved. Significant decreases in inflammation were seen in NOD1-deficient animals at 24 h (p = 0.01, n = 6) compared to WT controls (Table 1). Only a trend toward a decrease in cellular infiltration was seen at 72 h (p = 0.11) in the NOD1-deficient animals when compared to WT controls. These data suggest that NOD1 deficiency impairs recruitment of inflammatory cells to the lung during Lp infection.
Figure 4.
NOD1-deficient mice have impaired inflammatory cell infiltrates at 24 h. Lungs from WT, NOD1- and NOD2-deficient mice were fixed in paraformaldehyde and stained in H and E. Representative slides for 24 h (A) and 72 h (B) are shown. Ten high-power fields for each lung were examined and the percentage of airspace involvement was scored by a pathologist blinded to condition. Student's t-test was used to determine significance. Scores are shown in Table 1 (mean ± SEM, n = 6).
Table 1.
Decreased inflammation in lungs of Nod1–/– mice
p = 0.01 by Student's t-test compared to WT controls 9 (n = 6).
p = 0.11 by Student's t-test compared to WT controls (n = 6).
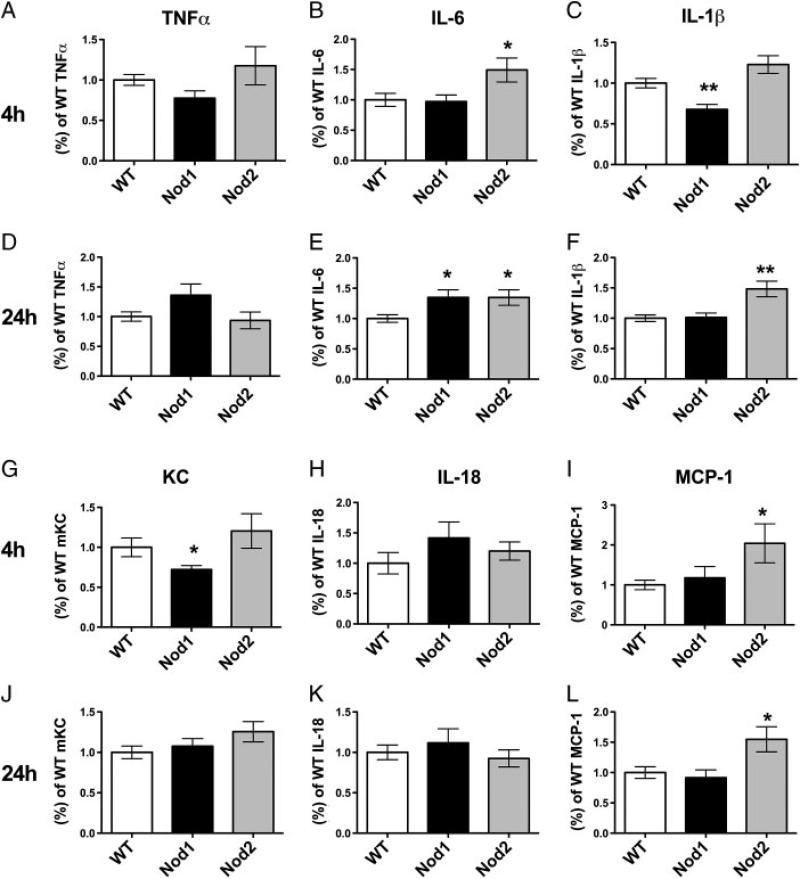
We next measured levels of cytokines and chemokines to examine the mechanism of NOD1-mediated protection. Cytokine levels from lung homogenates from WT, Nod1–/– , and Nod2–/– animals were measured for TNFα, IL-1β, IL-6, KC, IL-18, and MCP-1 to determine if there were significant differences in WT compared to Nod1–/– and Nod2–/– animals (Fig. 5). At 4 h, there was significantly decreased production of IL-1β (WT 1.00 ± 0.06 versus NOD1 0.68 ± 0.06 (mean ± SEM)), KC (WT 1.00 ± 0.12 versus NOD1 0.72 ± 0.05), and trend toward decreased TNFα (WT 1.00 ± 0.07 versus NOD1 0.78 ± 0.09, p = 0.06) in the Nod1–/– animals, when compared to WT controls (Fig. 5A, C, and G). In contrast, at 4 h, there was no change in IL-6, IL-18, or MCP-1 levels in the Nod1–/– animals (Fig. 5B, H, and I). At 24 h, Nod1–/– animals exhibited significantly increased levels of IL-6 production (WT 1.00 ± 0.06 versus NOD1 1.35 ± 0.13) compared to WT controls and a trend toward increased TNFα production (WT 1.00 ± 0.08 versus NOD1 1.36 ± 0.19, p = 0.06). The only significant change seen in the Nod2–/– animals compared to WT controls at 4 h was a significantly increased production of IL-6 (WT 1.00 ± 0.36 versus NOD2 1.49 ± 0.66) and MCP-1 (WT 1.00 ± 0.12 versus NOD2 2.04 ± 0.49). In addition, significant increases were seen in Nod2–/– animals compared to WT in IL-1β (WT 1.00 ± 0.19 versus NOD2 1.49 ± 0.43), IL-6 production (WT 1.00 ± 0.11 versus NOD2 1.49 ± 0.20), and MCP-1 production (WT 1.00 ± 0.10 versus NOD2 1.55 ± 0.21) at 24 h (Fig. 5E, F, and L). In addition, IFN-γ was analyzed at the 24-h, 72-h and 10-day time points and only minimal production was seen in lung homogenates (our unpublished observations). The levels of IFN-γ were not different when comparing WT, Nod1–/– , and Nod2–/– mice. These data demonstrate an early impaired production of proinflammatory cytokines KC and IL-1β seen in the absence of NOD1 protein and a later increase in proinflammatory markers (IL-1β, IL-6, and MCP-1) in Nod2–/– and (IL-6) Nod1–/– animals.
Figure 5.
Cytokine production in lung homogenates of Lp-infected mice. WT (white), Nod1–/– (black), and Nod2–/– (grey) mice were infected with Lp and at 4 and 24 h after infection lungs were homogenized. Total levels of TNFα (A,D), IL-6 (B,E), IL-1β (C,F), mKC (G,J), IL-18 (H,K), and MCP-1 (I,L) were measured. To combine experiments, Nod1–/– and Nod2–/– cytokine levels were normalized to average of WT controls for each cytokine. *p<0.05 compared with WT, **p≤0.01 compared with WT. Statistical significance was determined by performing Student's t-test. Data combined from three separate experiments with n = 12 total for each group except Nod2–/– animals, which had n = 11. Bars represent mean ± SEM.
Our data herein suggest that both NOD1 and NOD2 can detect Lp, but only NOD1 regulates in vivo bacterial clearance at 72 h. In addition, NOD1-deficient animals display early decreases in PMN recruitment to the alveolar space of the lung at 4 and 24 h and NOD2-deficient animals display a significant increase in PMN recruitment at 24 h. NOD1- and NOD2-deficient mice also show altered pulmonary inflammatory cell infiltration and cytokine responses to Legionella.
Discussion
In our aerosolized animal model, we identified higher Lp CFU in Nod1–/– mice compared to WT controls. Delayed bacterial clearance of Lp has been a characteristic of other knockout systems. Myd88–/– and to a lesser extent Tlr2–/– animals have impaired bacterial clearance that is associated with delayed inflammatory cytokine production and decreased early neutrophil recruitment to the lungs [7, 10]. Tlr9–/– and Tlr5–/– deficient animals, however, show little difference in Lp clearance compared to WT controls (unpublished observations) [7, 9]. In addition, NAIP5 and NLRC4 limit growth of Legionella both in vitro and in vivo through the detection of intracellular flagellin [3, 31]. The mechanism of delayed Legionella clearance in Nod1–/– infected lung may be due to multiple factors. One possible explanation could be that Nod1–/– animals have impaired early recruitment of PMN to the alveolar space leading to later impaired Lp phagocytic clearance. Alternatively, NOD1 may either directly or indirectly regulate replication of Lp in macrophages. Studies in bone marrow derived macrophages suggest, however, that NOD1 does not regulate Lp replication through direct detection [23]. Interestingly RIP2-deficient animals show little difference in organism clearance, suggesting the mechanism of increased CFU seen in Nod1–/– animals may be due to a RIP2-independent mechanism [11]. Whether the mechanism of Lp clearance by NOD1 is due to increased phagocytic killing versus impaired replication in cells containing NOD1 is currently unknown.
Recruitment of neutrophils to the lung may be important in clearance of Legionella and help to develop a protective Th1 response to the pathogen [32]. In addition, inhibition of chemotactic receptors important for neutrophil recruitment has been associated with enhance mortality of mice infected with Lp [33]. Impaired early neutrophil recruitment was previously observed in the lungs of Myd88–/– , and to a lesser extent in Tlr2–/– and Tlr5–/– deficient animals [9, 10]. In our model, we demonstrated that decreased PMN recruitment and impaired Lp clearance in the Nod1–/– animals was associated with decreased early IL-1β, and KC levels in the lungs of Nod1–/– mice as compared to WT controls. Impaired production of KC (CXCL1) may account for the impaired PMN recruitment seen in Nod1–/– mice [34]. Also, NOD may be important in regulation of IL-1β not only by inducing pro-IL-1β transcription but also by activating caspase-1 directly to cleave pro-IL-1β to the active form [35, 36]. At 24 h, we also observed increased IL-6 levels and a trend toward increased TNFα in the Nod1–/– lung in comparison to WT mice. These data suggest that NOD1 regulates suppression of later proinflammatory cytokine signaling. Together, our data suggest that NOD1 detection of Lp contributes to early cytokine and chemokine responses, early recruitment of PMN, and effective clearance of Lp from the lungs.
While NOD2 deficiency was not associated with impaired bacterial clearance in our study, alterations in inflammatory cell recruitment and cytokine responses were seen in Nod2–/– compared to WT. At 4 h after infection, increases in MCP-1 and IL-6 were observed in Nod2–/– animals compared to WT and at 24 h there was an increase in PMN recruitment and an increase in IL-1β, IL-6 and MCP-1 levels that was observed in the Nod2–/– mice. These data suggest that NOD2 may counteract the proinflammatory response to Lp by decreasing cytokine production at 4 and 24 h and PMN recruitment at 24 h. The mechanism of this early decrease in proinflammatory response by NOD2 to Lp is unknown. One possibility is that through heterotypic association of the caspase-1 recruitment domains, NOD2 protein may inhibit other inflammasomes, such as those containing NAIP5/NLRC4 [37]. Alternatively, NOD2 may modulate IL-1β production through interaction with RIP2 or TLR-pathway intermediates.
Our study is unique in that it is one of the few to do a side-by-side comparison of both NOD1 and NOD2 in a murine infection model. In comparison, other studies demonstrated unilaterally that NOD1 is important in the gastrointestinal and intravenous immune response to organisms such as L. monocytogenes and Salmonella Pathogenicity Island 1-deficient Salmonella enterica serovar Typhimurium [38, 39]. Furthermore, in isolated lung epithelium, Moraxella catarrhalis-induced IL-8 production is in part due to detection by NOD1 [40]. Although replication of C. pneumoniae in vitro and clearance of organisms has been shown to be dependent upon NOD1 and NOD2, increased in vivo mortality was only demonstrated in RIP2 kinase-deficient animals [27]. Lastly, although no significant phenotype was seen in Lp infection with the NOD2-deficient mouse, its importance has been demonstrated in other mouse pulmonary models of intracellular infections such as M. tuberculosis [28, 29]. Together, these studies suggest that NOD1 and NOD2 regulate distinct aspects of the in vivo immune response to pathogens.
Materials and methods
Materials
DMEM media, l-glutamine, penicillin, and streptomycin were obtained from Invitrogen. FCS was from Hyclone Thermo-Fisher (Waltham, MA, USA). FUGENE HD was from Roche Applied Bioscience (Mannheim, Germany). Buffered charcoal yeast extract components were from Sigma (St. Louis, MO USA) and Difco/BD Biosciences (San Jose, CA, USA).
Bacteria
Lp Corby (serogroup 1) and Lp Corby Flagellin deficient were gifts from K. Heuner [41]. Lp, Philadelphia 1 (American Type Culture Collection – ATCC 33152) was stored as previously described [42, 43]. NOD1 and NOD2 expression plasmids were a kind gift from Gabriel Nuñez. Legionella were grown in buffered charcoal yeast extract plates and harvested by scraping into PBS. Bacterial concentrations were estimated by correlating OD600 values with CFU counts on agar plates.
Model of murine legionellosis
All experiments were approved by Institutional Animal Care and Use Committee at the University of Washington. Nod2–/– mice (strain designation B6.129S1-Nod2tm1Flv/J) were derived and backcrossed for eight generations as previously described [44]. Nod1–/– mice were derived and backcrossed against eight generations of mice as previously described [12]. WT control mice were from a C57BL/6 background (Jackson Laboratory, Bar Harbor, ME, USA). Lp (Philadelphia strain) used for mouse aerosol infections was grown and prepared as described [9]. The mice were exposed to bacterial aerosols generated by twin jet nebulizers (Salter Laboratories, Arvin, CA, USA) for 30 min in a whole animal exposure chamber as described [45]. At each time point, mice were euthanized with intraperitoneal pentobarbital and exsanguinated by cardiac puncture. The left lung was homogenized for quantitative culture and measurement of cytokines as described [9]. The right lung was lavaged for cell counts [9]. Cytospins were performed on cells from bronchoalveolar lavages and cell types were determined following a modified Wright-Giemsa stain (Diff-Quick, Dade Behring, Dudingen, Switzerland). For histologic preparation, the lung was inflated to 15 cm pressure with 4% paraformaldehyde, fixed in the same solution, embedded in paraffin, and 4-mm sections were generated. Sections stained with hemotoxylin and eosin were examined by a pathologist blinded to mouse genotype and time following infection. Inflammation was scored as a percentage of the airspaces involved derived from the examination of ten high-power fields.
NOD1 and NOD2 transfection experiments
Human NOD1 and NOD2 constructs (gift from Gabriel Nuñez) were subcloned into the pEF6 expression vector (Promega, Madison, WI, USA). The region of the murine IFN-β promoter (–43 bp to –218 bp upstream the transcription site) containing interferon response factor-1 and NF-κb binding sites was placed upstream a luciferase reporter construct (pGL2-IFNβ) (gift from Pierre-Yves Bochud) (Invitrogen, Carlsbad, CA, USA). pGL2–ELAM was used as previously described [46], and pRL-TK was purchased from Promega. HEK293 cells were transfected with FUGENE HD (Roche Diagnostics, Basel, Switzerland) using the manufacturer recommended protocol. ELAM promoter-firefly luciferase and IFN-β promoter-firefly luciferase reporter constructs were co-transfected with plasmid expression constructs containing human NOD1 and NOD2 along with Renilla luciferase expression constructs driven by the HSV thymidine kinase promoter to control for transfection efficiency. Cells were simultaneously exposed to heat-killed FlaA and WT (Corby strain) Lp and incubated overnight at 37°C in order to potentiate cytoplasmic delivery of the pathogen by the transfection reagent. Firefly luciferase activity was measured after lysis of cells using Dual Luciferase Reporter Assay System as per the manufacturer's recommended instructions (Promega). Total luminescence over one second was measured using luciferin (to measure firefly luciferase) followed by a second reading with coelenterazine (to measure Renilla luciferase activity) with simultaneous administration of an inhibitor to firefly luciferase. Transfection efficiency of the reporter promoter was adjusted for each well by dividing the relative light units of firefly luciferase by the relative light units of Renilla luciferase.
Protein analysis
For cytokine analysis, in vivo lung homogenates were used and levels of specific cytokines were measured by sandwich ELISA (Duoset) (R&D systems, Minneapolis, MN, USA) according to the manufacturer's recommendations.
Statistical analysis
Student's t-test was used to assess statistical significance. A value of p<0.05 was considered significant. Statistics were calculated with Prism version 5.0c (GraphPad).
Acknowledgements
Funding support was from the National Institutes of Health (NIH) for WRB (K08 AI080952), SJS and TRH (R01 AI061464). The authors would like to acknowledge Malinka Jansson-Hutson and Destry Taylor for technical assistance.
Abbreviations
- DAP
diaminopimelate
- ELAM
endothelial-leukocyte adhesion molecule
- FlaA
flagellin deficient
- HEK293
human embryonic kidney (cell line)-293
- Lp
Legionella pneumophila
- MDP
muramyl dipeptide
- Myd88
myeloid differentiation factor 88
- NAIP5
neuronal apoptosis inhibitory protein 5
- NOD1
nucleotide-binding oligomerization domain-1
- NOD2
nucleotide-binding oligomerization domain-2
- NLR
NOD-like receptors
- NLRC4
NLR family, CARD domain containing 4
- RIP2
receptor -interacting serinethreonine kinase g 4
- Tri-DAP
l-alanyl-γ-d-glutamyl-meso-DAP
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Swanson MS, Hammer BK. Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 2000;54:567–613. doi: 10.1146/annurev.micro.54.1.567. [DOI] [PubMed] [Google Scholar]
- 2.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell. Microbiol. 2008;10:1209–1220. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 3.Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect. Immun. 2009;77:1981–1991. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molofsky A, Byrne B, Whitfield N, Madigan C, Fuse E, Tateda K, Swanson M. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamboni D, Kobayashi K, Kohlsdorf T, Ogura Y, Long E, Vance R, Kuida K, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 6.Diez E, Lee S, Gauthier S, Yaraghi Z, Tremblay M, Vidal S, Gros P. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 2003;33:55–60. doi: 10.1038/ng1065. [DOI] [PubMed] [Google Scholar]
- 7.Archer KA, Alexopoulou L, Flavell RA, Roy CR. Multiple MyD88-dependent responses contribute to pulmonary clearance of Legionella pneumophila. Cell. Microbiol. 2009;11:21–36. doi: 10.1111/j.1462-5822.2008.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archer K, Roy C. MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires’ disease. Infect. Immun. 2006;74:3325–3333. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawn TR, Berrington WR, Smith IA, Uematsu S, Akira S, Aderem A, Smith KD, Skerrett SJ. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J. Immunol. 2007;179:6981–6987. doi: 10.4049/jimmunol.179.10.6981. [DOI] [PubMed] [Google Scholar]
- 10.Hawn T, Smith K, Aderem A, Skerrett S. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J. Infect. Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- 11.Archer KA, Ader F, Kobayashi KS, Flavell RA, Roy CR. Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila. Infect. Immun. 2010;78:2477–2487. doi: 10.1128/IAI.00243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 13.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi KS, Inohara N, Hernandez LD, Galán JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 15.Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, van Crevel R, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw MH, Reimer T, Sanchez-Valdepenas C, Warner N, Kim YG, Fresno M, Nunez G. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat. Immunol. 2009;10:1267–1274. doi: 10.1038/ni.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosa A, Trumstedt C, Eriksson E, Soehnlein O, Heuts F, Janik K, Klos A, et al. Non-hematopoietic cells control the outcome of infection with Listeria monocytogenes in a NOD1-dependent manner. Infect. Immun. 2009;77:2908–2918. doi: 10.1128/IAI.01068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derbigny WA, Kerr MS, Johnson RM. Pattern recognition molecules activated by Chlamydia muridarum infection of cloned murine oviduct epithelial cell lines. J. Immunol. 2005;175:6065–6075. doi: 10.4049/jimmunol.175.9.6065. [DOI] [PubMed] [Google Scholar]
- 19.Viala J, Chaput C, Boneca I, Cardona A, Girardin S, Moran A, Athman R, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 20.Girardin S, Tournebize R, Mavris M, Page A, Li X, Stark G, Bertin J, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa M, Yang K, Hashimoto M, Park J, Kim Y, Fujimoto Y, Nuñez G, et al. Differential release and distribution of Nod1 and Nod2 immunostimulatory molecules among bacterial species and environments. J. Biol. Chem. 2006;281:29054–29063. doi: 10.1074/jbc.M602638200. [DOI] [PubMed] [Google Scholar]
- 22.Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, Flavell RA, Roy CR, Zamboni DS. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 2008;4:e1000220. doi: 10.1371/journal.ppat.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amer A, Franchi L, Kanneganti T, Body-Malapel M, Ozören N, Brady G, Meshinchi S, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 24.Kim YG, Park JH, Daignault S, Fukase K, Nunez G. Cross-tolerization between Nod1 and Nod2 signaling results in reduced refractoriness to bacterial infection in Nod2-deficient macrophages. J. Immunol. 2008;181:4340–4346. doi: 10.4049/jimmunol.181.6.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meinzer U, Esmiol-Welterlin S, Barreau F, Berrebi D, Dussaillant M, Bonacorsi S, Chareyre F, et al. Nod2 mediates susceptibility to Yersinia pseudotuberculosis in mice. PLoS ONE. 2008;3:e2769. doi: 10.1371/journal.pone.0002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva GK, Gutierrez FR, Guedes PM, Horta CV, Cunha LD, Mineo TW, Santiago-Silva J, et al. Cutting edge: Nucleotide-binding oligomerization domain 1-dependent responses account for murine resistance against Trypanosoma cruzi infection. J. Immunol. 2010;184:1148–1152. doi: 10.4049/jimmunol.0902254. [DOI] [PubMed] [Google Scholar]
- 27.Shimada K, Chen S, Dempsey PW, Sorrentino R, Alsabeh R, Slepenkin AV, Peterson E, et al. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog. 2009;5:e1000379. doi: 10.1371/journal.ppat.1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandotra S, Jang S, Murray PJ, Salgame P, Ehrt S. Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis. Infect. Immun. 2007;75:5127–5134. doi: 10.1128/IAI.00458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi KS, et al. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J. Immunol. 2008;181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- 30.Hawn T, Verbon A, Lettinga K, Zhao L, Li S, Laws R, Skerrett S, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires’ disease. J. Exp. Med. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamkanfi M, Amer A, Kanneganti T, Muñoz-Planillo R, Chen G, Vandenabeele P, Fortier A, et al. The Nod-Like Receptor Family Member Naip5/Birc1e Restricts Legionella pneumophila Growth Independently of Caspase-1 Activation. J. Immunol. 2007;178:8022–8027. doi: 10.4049/jimmunol.178.12.8022. [DOI] [PubMed] [Google Scholar]
- 32.Tateda K, Moore TA, Deng JC, Newstead MW, Zeng X, Matsukawa A, Swanson MS, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 2001;166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 33.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, et al. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect. Immun. 2001;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frevert CW, Goodman RB, Kinsella MG, Kajikawa O, Ballman K, Clark-Lewis I, Proudfoot AE, et al. Tissue-specific mechanisms control the retention of IL-8 in lungs and skin. J. Immunol. 2002;168:3550–3556. doi: 10.4049/jimmunol.168.7.3550. [DOI] [PubMed] [Google Scholar]
- 35.Ferwerda G, Kramer M, de Jong D, Piccini A, Joosten LA, Devesaginer I, Girardin SE, et al. Engagement of NOD2 has a dual effect on proIL-1beta mRNA transcription and secretion of bioactive IL-1beta. Eur. J. Immunol. 2008;38:184–191. doi: 10.1002/eji.200737103. [DOI] [PubMed] [Google Scholar]
- 36.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. USA. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Damiano JS, Oliveira V, Welsh K, Reed JC. Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses. Biochem. J. 2004;381:213–219. doi: 10.1042/BJ20031506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bourhis L, Magalhaes JG, Selvanantham T, Travassos LH, Geddes K, Fritz JH, Viala J, et al. Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2009;77:4480–4486. doi: 10.1128/IAI.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosa A, Trumstedt C, Eriksson E, Soehnlein O, Heuts F, Janik K, Klos A, et al. Nonhematopoietic cells control the outcome of infection with Listeria monocytogenes in a nucleotide oligomerization domain 1-dependent manner. Infect. Immun. 2009;77:2908–2918. doi: 10.1128/IAI.01068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slevogt H, Seybold J, Tiwari K, Hocke A, Jonatat C, Dietel S, Hippenstiel S, et al. Moraxella catarrhalis is internalized in respiratory epithelial cells by a trigger-like mechanism and initiates a TLR2- and partly NOD1-dependent inflammatory immune response. Cell. Microbiol. 2007;9:694–707. doi: 10.1111/j.1462-5822.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich C, Heuner K, Brand BC, Hacker J, Steinert M. Flagellum of Legionella pneumophila positively affects the early phase of infection of eukaryotic host cells. Infect. Immun. 2001;69:2116–2122. doi: 10.1128/IAI.69.4.2116-2122.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skerrett SJ, Martin TR. Roles for tumor necrosis factor alpha and nitric oxide in resistance of rat alveolar macrophages to Legionella pneumophila. Infect. Immun. 1996;64:3236–3243. doi: 10.1128/iai.64.8.3236-3243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skerrett SJ, Bagby GJ, Schmidt RA, Nelson S. Antibody-mediated depletion of tumor necrosis factor-alpha impairs pulmonary host defenses to Legionella pneumophila. J. Infect. Dis. 1997;176:1019–1028. doi: 10.1086/516530. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 45.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 2004;172:3377–3381. doi: 10.4049/jimmunol.172.6.3377. [DOI] [PubMed] [Google Scholar]
- 46.Schindler U, Baichwal VR. Three NF-kappa B binding sites in the human E-selectin gene required for maximal tumor necrosis factor alpha-induced expression. Mol. Cell. Biol. 1994;14:5820–5831. doi: 10.1128/mcb.14.9.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]