Abstract
Purpose
We conducted a phase II study to assess the efficacy of continuous dosing of sunitinib in patients with FDG-PET avid, iodine refractory, well-differentiated thyroid carcinoma (WDTC) and medullary thyroid cancer (MTC), and to assess for early response per FDG-PET.
Experimental Design
Patients had metastatic, iodine-refractory WDTC or MTC with FDG-PET avid disease. Sunitinib was administered at 37.5 mg daily on a continuous basis. The primary end-point was response rate per RECIST criteria. Secondary end-points included toxicity, overall survival (OS), and time to progression (TTP). We conducted an exploratory analysis of FDG-PET response after 7 days of treatment.
Results
Thirty-five patients were enrolled (7 MTC;28 WDTC), and 33 patients were evaluable for disease response. The primary endpoint, objective response rate per RECIST criteria, was 11 patients, 31% (95% CI: 16% to 47%). There was one complete response (3%),10 partial responses (28%), and 16 patients (46%) with stable disease. Progressive disease was seen in 17% (6 patients). The median TTP is 12.8 months (95%CI, 8.9 months – not reached). Repeat FDG-PET was performed on 22 patients, the median percent change in average SUVs was −11.7%, −13.9%, and 8.6% for patients with RECIST response, stable, and progressive disease respectively. Differences between response categories were statistically significant (p=0.03). The most common toxicities seen included: fatigue (11%), neutropenia (34%), hand/foot syndrome (17%), diarrhea (17%), and leukopenia (31%). One patient on anticoagulation died of gastrointestinal bleeding.
Conclusion
Continuous administration of sunitinib was effective in patients with iodine refractory WDTC and MTC. Further study is warranted.
Keywords: thyroid cancer, FDG-PET, sunitinib, tyrosine-kinase inhibitors
Introduction
Thyroid carcinoma is a common malignancy with more than 30,000 cases predicted in the United States in 2008.1 The survival of patients with metastatic medullary carcinoma of thyroid (MTC) or radioiodine-refractory, well differentiated thyroid carcinoma (WDTC) is variable.2,3 While some patients may have indolent disease, even when widely metastatic, others may experience rapid disease progression. Recent work has demonstrated that patients with iodine-refractory WDTC that is hypermetabolic on flurodeoxyglucose positron emission topography (FDG-PET), are more likely to have progressive disease and have a median survival of less than 5 years.4 Thus, in iodine-refractory cancer, FDG-PET is increasingly used to select patients in whom systemic therapy should be considered.5 In this regard, the requirement for uptake on FDG-PET may select for more aggressive disease and provides a basis for more uniform patient selection compared to more traditional selection criteria such as evidence of disease progression by changes in tumor size. In addition, by detecting metabolic changes within the tumor, FDG-PET may be able to provide an early assessment of treatment effect.
Sunitinib is a multi-targeted tyrosine kinase inhibitor. Targets of the drug include vascular endothelial growth factors (VEGFRs) types 1 and 2, platelet-derived growth factor receptors, c-KIT, FLT3 and RET.6 The drug’s inhibitory effect on VEGF and RET makes it a rational candidate for the therapy of WDTC and MTC. Somatic mutations of the proto-oncogene RET are important in the development of MTC. Point mutations and rearrangements of RET are also present in 2-60% of patients with papillary carcinoma of the thyroid (PTC).7,8 In addition, elevated serum levels of vascular endothelial growth factor are also associated with poor prognosis in PTC.9 Sunitinib is currently approved for the therapy of renal cell carcinoma and gastrointestinal stromal tumor on an intermittent treatment schedule.10 Recent data suggest that the continuous administration of 37.5 mg/day may be a reasonable alternative to the intermittent dosing of the drug.11
We hypothesized that sunitinib would be effective in treating MTC and iodine-refractory WDTC. We conducted a single institution, phase II trial of continuous administration sunitinib in patients with metastatic, iodine refractory, WDTC or metastatic MTC. The primary goal of our study was objective response to sunitinib by sized-based (RECIST) criteria. Evidence of metastatic, iodine-refractory thyroid cancer, or metastatic medullary thyroid cancer was required for participation. A positive FDG-PET scan was also an entry criterion for the study. In addition we performed a FDG-PET scan after one week of sunitinib therapy as an exploratory study to assess early response based upon data from FDG PET in gastrointestinal stromal tumors treated with sunitinib.
Methods
Patients
Patients were eligible for the study if they were older than 18 years-old; had metastatic WDTC or MTC, evidence of refractoriness to iodine therapy for WDTC (documented by a combination of nuclear imaging and thyroglobulin levels), an ECOG performance status of 0 to 3, evidence of FDG-PET uptake by tumor lesions, measurable disease defined by RECIST criteria, resolution of all acute toxic effects from prior therapy to grade ≤ 1 (NCI-CTCAE, version 3.0), total serum bilirubin ≤ 1.5 x upper limit of normal (ULN), serum transaminases < 2.5 x ULN or < 5.0 X ULN if secondary to liver metastases, serum creatinine ≤ 1.5 x ULN; absolute neutrophil count ≥ 1.5 × 109/L; platelet count ≥ 100,000/μL, and hemoglobin ≥ 9.0 g/dL. FDG-PET or PET/CT used in the entry criteria was reviewed by an experienced Nuclear Medicine physician to confirm positivity prior to patient entry, (see PET/CT interpretive criteria below). All patients signed a written informed consent form prior to enrollment. The institution’s Internal Review Board approved the study protocol.
Patients were ineligible for the study if they had symptomatic, untreated brain metastasis; a second primary malignancy; type I diabetes mellitus; type II diabetes mellitus with fasting glucose levels out of the 80 to 150 mg/dL range; uncontrolled hypertension; major surgery or radiation therapy within 4 weeks of starting the study treatment. Pregnant or breast feeding women and patients who had any of the following events within the 6 months prior to study enrollment were excluded: myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, symptomatic congestive heart failure, cerebrovascular accident or transient ischemic attack, pulmonary embolism, or ongoing cardiac arrhythmias of grade ≥ 2. The protocol was amended to exclude patients receiving full dose anticoagulation or with history of hemoptysis (of at least 2.5 cc per episode) within 3 months unless definitively treated with surgery or radiation.
Study Treatment
Patients received sunitinib orally at an initial dose of 37.5 mg to be taken daily until disease progression, unacceptable toxicity, or consent withdrawal. Patients with grade ≥ 3 toxicities discontinued sunitinib until recovery to grade ≤ 2. Sunitinib was held for absolute neutrophil count (ANC) < 0.75 × 109 cells/L. After holding sunitinib, patients restarted sunitinib at the initial dose or reduced to 25 mg daily. Patients who had a dose reduction could not re-escalate the dose to 37.5 mg. Patients who had sunitinib held for longer than 14 days were withdrawn from the study.
Evaluation and Follow-up
Screening evaluation consisted of history and physical examination; CT scans of involved sites, serum chemistries, pregnancy test, complete blood counts (CBC), TSH, free T4, and tumor markers (thyroglobulin for WDTC, calcitonin for MTC). Follow-up evaluations consisted of monthly history and physical examination; assessment of adverse events; CBC, and chemistries. Imaging and tumor marker follow-up studies consisted of CT scans every 3 months or sooner if clinically indicated. CT scans used for response evaluation were fully diagnostic CT scans that included all sites of metastases. These studies were done separately from low-dose non-contrast CT used for attenuation correction and anatomic localization for PET/CT. Contrast-enhancement was used for most of the diagnostic CT scans, especially for patients with regional nodal or liver metastases, where contrast is important for tumor delineation. Response was categorized by the change in lesion diameter based upon CT, using standard RECIST criteria.12 PET scans were repeated at one week after the initial dose, at the option of the patient and referring physician, as an exploratory early indicator of response. Patients were followed until tumor progression, death, or study discontinuation for unacceptable toxicity or consent withdrawal, up to 2 years from the date of the last dose of sunitinib.
PET Imaging and Analysis
FDG-PET and PET/CT patient preparation and image acquisition was performed according to the NCI consensus guidelines.13 Patients fasted at least 6 hours prior to imaging and had plasma glucose assayed prior to FDG injection to rule out significant hyperglycemia. All imaging was performed on either an ADVANCE PET tomography or Discovery STE PET/CT Tomograph (GE Medical Systems, Waukesha, WI). The majority of baseline studies were performed using PET/CT. Follow-up studies were performed on the same device as baseline where possible; however, even in the cases where a different device was used, tomographs were regularly cross-calibrated to assure reproducible standardized uptake values (SUVs). Imaging was performed over 5-6 adjacent imaging fields covering the skull to mid-thighs starting 60 +/− 10 minutes after injection. Images included 7-minute emission scans axial field-of-view. For PET/CT, low-dose non-contrast CT was used for attenuation correction and anatomic localization. For PET-only scans, 3-minute transmission scans per axial field-of-view with image segmentation were used for attenuation correction. All baseline images underwent initial review by Nuclear Medicine physicians experienced in PET as part of standard clinical care. For entry into the study, all baseline images were reviewed by a single reviewer with significant experience in PET interpretation. Clear-cut evidence of uptake greater than blood-pool in a location consistent with thyroid cancer metastasis and correlating to findings on diagnostic CT or PET/CT were considered evidence of FDG-positive thyroid cancer for entry into the study.
For the exploratory study of FDG-PET as a predictor of response to sunitnib, the maximum lesion SUV and the average SUV of up to 9 lesions served as a quantitative measure of FDG uptake. We recorded baseline and one-week maximum SUVs for up to the 9 most avid individual lesions per patient and categorized the lesions as nodal, lung, bone, liver, or neck mass metastasis. Since there are no accepted criteria for response, the percent change in FDG uptake from the baseline to 1-week scan was recorded using both the single lesion with the highest SUV on the baseline scans, as well as the average of maximum SUV of up to 9 recorded lesions. Images were also reviewed qualitatively to assure that subjective impressions matched quantitative results.
Study End-Points
The primary end-point was the overall response rate (RR) based on RECIST criteria at the time of maximal response.12 Secondary endpoints included evaluation of time to tumor progression (TTP), overall survival (OS), and the safety and toxicity profile of continuous daily treatment with sunitinib in this population. Tumor marker change from baseline was also recorded. A tumor marker response was defined empirically as a 50% decrease from the baseline, given the absence of an established cut-off for response.14 Patients with elevated thyroglobulin antibodies were not considered evaluable for thyroglobulin tumor marker response.15 We conducted exploratory analyses of early FDG-PET and tumor marker responses and their association with radiographic response and TTP.
Statistical Analysis
We calculated a sample size of 35 patients with the assumption that 30 patients would undergo evaluation for tumor response. This confers a power of 92% if the null hypothesis is a RR ≤ 10% and the alternative hypothesis is a RR ≥ 33% at a two-sided alpha level of 0.05. We postulated that a true response rate < 10% was not likely to be clinically important. We reported adverse events as proportions for each adverse event of NCI-CTCAE grade 3 or higher. We reported response rate as the proportion and 95% confidence interval (CI) of patients who achieved a CR or PR. We reported duration of response, TTP, and OS as median values with their respective 95% CI.
FDG-PET scan exploratory analysis
For the exploratory analysis of serial FDG-PET SUV changes, we used one-way analysis of variance (ANOVA) to test the association of RECIST response (categorized as response, stable, or tumor progression) with percent SUV changes for both the most PET avid lesion and the average maximum of up to 9 lesions at one week. We estimated the pairwise Pearson correlation coefficient between average percent SUV changes and changes in tumor size measured at the time of RECIST assessment. For the analysis of FDG-PET changes by type of metastatic lesion, we a used a chi square test for the association of dichotomized FDG-PET response with the type lesion (nodal, lung, bone, liver, or neck mass). Based upon published estimates of SUV precision, a decline of 20% or more in the average maximum SUV was considered a PET response to the purpose of this analysis.16 We used a one-way ANOVA to test the association of average SUV percent changes with type of metastatic lesion. We applied Cox proportional hazards regression model to test the association of PET changes, (average percent SUV changes from baseline as a continuous variable) and time-to-tumor progression.
Tumor marker exploratory analysis
we calculated the PPV and NPV for thyroglobulin response and response by RECIST in patients with WDTC. We used a Fisher’s exact test for the association of response by thyroglobulin and by RECIST criteria. We did not test the validity of calcitonin response in MTC because there were only 6 patients with MTC.
We considered results as statistically significant for p–values < 0.05. All statistical tests are two-sided. We performed all statistical analyzes with STATA SE11 software.
Results
Baseline Characteristics
Between August 2007 and February 2009, 35 patients with recurrent or metastatic, WDTC or MTC were enrolled in the study. All patients had disease with increased metabolic activity per FDG-PET and all patients with WDTC were radioiodine refractory. A total of 33 patients were available for evaluation of response; one patient did not have measurable disease per RECIST at baseline, and one patient was removed from study due to an adverse event prior to evaluation. 24 patients underwent repeat evaluation with FDG-PET following seven days of therapy. The lesions with the highest SUV per FDG-PET were most often located in the lung and lymph nodes. Patient demographics are listed in Table 1. The study population was primarily composed of WDTC subtypes (80%, predominately papillary thyroid carcinoma). The patients had good performance status and were heavily pretreated with radioiodine therapy. Four patients had been previously treated with a tyrosine kinase inhibitor.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients N = 35 (%) |
|---|---|
| Sex | |
| Female | 18 (51) |
| Male | 17 (49) |
|
| |
| Age (years) | |
| Median | 61 |
| Range | 34-73 |
|
| |
| Race/Ethnic Group | |
| White | 29 (76) |
| Asian | 2 (6) |
| Hispanic/Latino | 4 (11) |
|
| |
| Histologic Subtype | |
| Papillary | 18 (51) |
| Follicular | 4 (11) |
| Hurthle Cell | 5 (14) |
| Insular | 1 (3) |
| Medullary | 7 (20) |
|
| |
| ECOG Performance Status | |
| 0/1 | 33 (94) |
| 2/3 | 2 (6) |
|
| |
| Prior Therapy | |
| Surgery | 34 (97) |
| Iodine 131 treatment | 27 (77) |
| External Beam Radiation | 21 (60) |
| Chemotherapy | 6 (17) |
| Tyrosine Kinase Inhibitor (2 sorafenib, 2 motesanib) |
4 (11) |
|
| |
| Location of Primary FDG- PET Avid Lesion |
N=24 |
| Lymph Node | 9 (38) |
| Liver | 1 (4) |
| Bone | 5 (21) |
| Lung | 7 (29) |
| Neck | 2 (8) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FDG-PET, fluorodeoxyglucose-positron emission topography
Efficacy
A total of 35 patients were treated with sunitinib and all are included in the intent-to-treat analysis. Two patients are not evaluable for response for reasons described above. The median duration of treatment for all patients is 8.5 months with 11 patients remaining on trial. The median follow-up is 15.5 months (range 1 to 25.5 months). The primary endpoint, objective response rate per RECIST criteria, was documented in 11 patients (RR=31%, 95% CI: 16% to 47%) Table 2. Eight of 29 patients with WDTC and 3 of 6 patients with MTC achieved a RECIST response (RR=28% and 50% for WDTC and MTC, respectively). There was one complete response (3%) and 10 partial responses (28%). In addition, 16 patients (46%) had stable disease. Thus 77%, (95% CI: 63% to 91%) did not have disease progression, at first evaluation at three months, while progressive disease was seen in 17%, (95% CI: 7%-35%). The maximum percentage change in tumor size from baseline for each patient is illustrated in Figure 1A. One patient was not considered evaluable for response due to lack of measurable disease at baseline. At treatment initiation, this patient had multiple, subcentimeter pulmonary nodules associated with symptomatic dyspnea requiring 2 liters of supplemental oxygen per nasal canula. Although early review of his CT scans suggested evaluable disease; subsequent re-review found no lesions of sufficient size to meet RECIST criteria. After 3 months on study drug he was off supplemental oxygen with significant decrease in size and number of his pulmonary nodules.
Table 2.
Response to Treatment
| Response | No. of Patients (N=35) |
% | Median % Change in Average SUV (range) |
|---|---|---|---|
| Complete Response | 1 | 3 | −78.8% (n=1) |
| Partial Response | 10 | 28 | −10.0% (−0.5 to −22.9) (n=5) |
| Stable Disease Total Stable Disease > 6 months |
16 13 |
46 37 |
−13.9% (−34.4 to 11.5) (n=12) |
| Progressive Disease | 6 | 17 | 8.6% (−0.8 to 36.1) (n=4) |
| Not Assessable | 2 | 6 | n =13 |
| Objective Response Rate 95% CI |
11 | 31 (16 to 47) |
|
| Disease Control Rate 95% CI |
27 | 77 (63 to 91) |
|
| Disease Control by Histology WDTC MTC |
22/28 5/7 |
78 71 |
Abbreviations: CI, Confidence Interval; WDTC, Well-differentiated thyroid cancer; MTC, Medullary thyroid cancer
Disease control rate includes patients with complete response, partial response, and stable disease at 3 months.
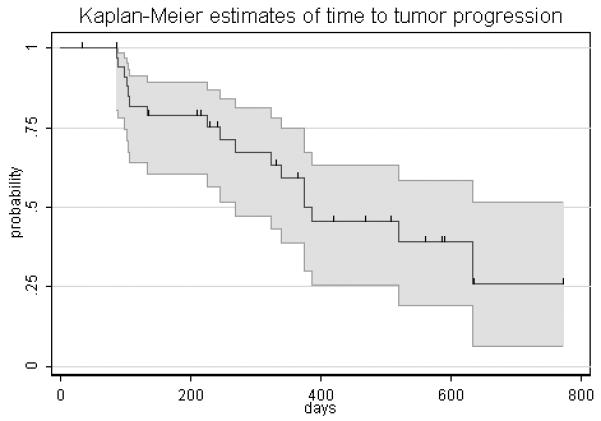
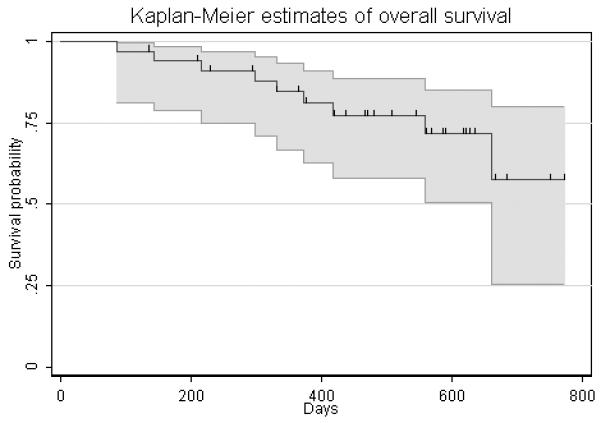
Figure 1.
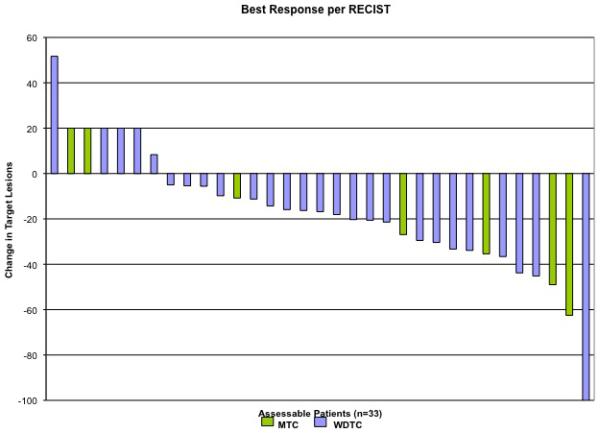
(A) Maximum percentage change in target lesions from baseline in all patients with evaluable disease (n=33). (1 patient removed from study due to adverse event prior to evaluation, 1 patient did not have measurable disease per RECIST criteria at baseline). Blue bars: well-differentiated thyroid cancer; Green bars: medullary thyroid cancer.
(B) Kaplan-Meier estimate of time to progression. The median time to progression per Kaplan-Meier analysis is 12.8 months (95%CI, 8.9 months – not reached).
(C) Kaplan-Meier estimate of overall survival. The median overall survival has not been reached, 9 patients have died during the follow up period.
The current study included 4 patients previously treated with oral tyrosine kinase inhibitors. Two were enrolled in a clinical trial with motesanib and two were treated with sorafenib. The two patients treated with motesanib had stable disease on the drug and were on therapy for more than one year. One of these patients had a partial response to sunitinib and the other had progressive disease at initial evaluation. The two patients previously treated with sorafenib had the drug discontinued secondary to toxicity. One of these patients had a 29% reduction of tumor by RECIST (SD) in the current study and was on therapy for 12 months.
Of the 11 patients with an objective disease response, 6 have subsequently had progressive disease per RECIST. The median duration of response was 8.0 months, (95% CI, 3.2 months to 15.6 months). The median overall survival has not been reached, nine patients have died during the follow up period. The median time to progression per Kaplan-Meier analysis is 12.8 months (95% CI, 8.9 months – not reached). (Figure 1B)
FDG PET Response and exploratory analysis
FDG PET and RECIST response
Twenty-four patients underwent evaluation by FDG-PET both at baseline and after seven days of sunitinib therapy. Of these patients, 22 were evaluable by RECIST criteria. The remaining patients either chose not to undergo repeat FDG PET (exploratory and optional for this study) or had undergone outside FDG PET studies at baseline, which were useful for determining evidence of tumor FDG uptake for study entry criteria but not for quantitative comparison to follow-up PET performed at our center. The median number of PET lesions per patient was 4 (SD=± 1.87; range 1-9). At baseline, the median average SUV and SUV for the most avid lesion were 7.9 (SD=± 16.2; range 3.3 to 59.6) and 13.0 (SD=± 20.5; range 3.8 to 67.0), respectively, indicating highly metabolically active lesions. The median percent change from baseline in average SUVs was − 11.7% (SD± 29.5%; n=6) in patients who had a RECIST response, − 13.9% (SD± 13.2%; n=12) in patients with stable disease, versus 8.6% (SD± 15.9%; n=4) in patients with progressive disease by RECIST criteria (figure 2). The association of average SUV percent changes by type of RECIST response was statistically significant (P=0.03). Patients with RECIST response and stable disease had a statistically significant decline in average SUVs compared to patients with progressive disease (P=0.02 and 0.01, respectively). The average SUV percent changes did not differ statistically between RECIST responders (PR/CR) and non-responders (SD/PD) (P=0.2). The median percent changes in SUVs for the most PET avid lesion was −3.9% (SD±15.2%; n=5), −17.2% (SD±17.2%; n=12), and 10.8% (SD±22.3%; n=4) for patients with RECIST response, stable, and progressive disease, respectively. These results were statistically similar to the analysis for the average SUV changes. There was no statistically significant association of PET SUVs at baseline and RECIST response (P=0.58; n=22). In 19 patients with WDTC, the association of percent changes in SUV and RECIST response remained statistically significant (median % SUV change of −13.5, −17.5, and 9.0% for responders, stable disease, and non-responders, respectively; P=0.03). There was a statistically significant correlation between percent changes in SUV and tumor size (pairwise Pearson coefficient r=0.58; P=0.005).
Figure 2.
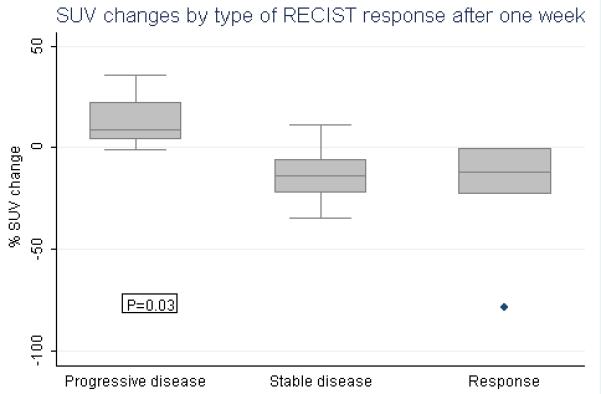
Average SUV percent change by type of RECIST response Percent changes in average SUVs from baseline to one week of sunitinib by RECIST response criteria, categorized as responsive (n=6), stable (n=12), or progressive disease (n=4). The P-value was obtained from a global F-test.
FDG PET and Time-to-tumor Progression (TTP)
We performed analyzes of TTP by average percent SUV change from baseline as a continuous variable. There was a statistically significant association of TTP with increases in average percent SUV (HR=1.04; 95% CI= 1.01-1.09; P=0.02). The risk of tumor progression increased by 4% for each 1% increase in average SUV from baseline.
Exploratory analysis of FDG PET by type of lesion
The total number of PET metastatic lesions was 86. Twenty-eight (33%) lesions were in lymph-nodes, 26 (30%) in lungs, 15 (17%) in bones, 11 (13%) in the neck or thyroid bed, and 6 (7%) in the liver. Twenty-eight (33%) lesions demonstrated a PET response (>20% SUV decline). The number of PET responses of > 20% by type of lesion was 9 (32%) for lymph-nodes, 12 (46%) for lung, 5 (33%) for bone, 2 (18%) for neck or thyroid bed, and zero (0%) for liver metastasis. There were no statistically significant associations between the type of metastatic lesion and dichotomized PET response (>20% decline in SUV) or changes in average SUV from baseline (P=0.19 and 0.91, respectively). The lack of association between the type of metastatic lesion and the PET response suggests that analysis of a single index lesion may be feasible.
Tumor Marker Analysis
A total of 19 patients had baseline and serial thyroglobulin levels drawn while on sunitinib. Of these, 58% (11 patients) had a decrease in serum thyroglobulin levels of 50% or greater, (mean decrease of 56%). Of the patients with WDTC, a 50% change in thyroglobulin levels had a PPV for RECIST response of 54% and a NPV of 75%. There was no significant association between a 50% change in serum tumor marker and response per RECIST (p=.35). Six patients with MTC had baseline and serial calcitonin levels drawn during treatment. Although there are too few patients to determine statistical significance, all three patients who had a 50% decrease in calcitonin levels while on sunitinib had disease control (lack of progression).
Safety
Patients were treated with continuous dosing of sunitinib at 37.5 mg per day. Dose reduction to 25 mg per day was required in 21 of 35 treated patients (60%). There was no association between previous therapy, including use of TKIs, and the need for dose reduction. Sixteen patients (45%), had their dose reduced after the protocol was modified to allow for drug continuation with an ANC > 0.75 × 109 cell/L. These patients had been on therapy for a median of 3.5 months. There were no incidents of neutropenic fever. All patients who had a dose reduction remained at 25 mg per protocol requirements. Four patients discontinued treatment due to toxicity: two patients developed grade 2 hemoptysis, one patient developed grade 3 diarrhea. One patient died on study due to gastrointestinal bleeding, this patient was on full dose anticoagulation with low molecular weight heparin for a previous pulmonary embolism. Two other patients required admission for bleeding episodes. One was on warfarin for atrial fibrillation. After these events, the exclusion criteria were modified to exclude patients on full dose anticoagulation. The reasons for dose reduction include the following grade 3 toxicities: fatigue (11%), dehydration (3%), mucositis (3%), diarrhea (17%), gastrointestinal bleeding (6%), hand/foot syndrome (17%), cytopenias (46%), hypocalcemia (3%), peripheral neuropathy (3%), supraventricular tachycardia (6%), laryngeal edema (3%), odynophagia (3%), and infection without neutropenia (3%). The most common adverse events were fatigue, diarrhea, hand/foot syndrome, and neutropenia. These events have been reported in previous studies with sunitinib.11
Discussion
The response rates to conventional chemotherapy are low in patients with WDTC requiring palliation of symptoms.2,3 In addition, many patients have asymptomatic progressive disease and it is important that palliative treatments do not worsen a patient’s quality of life. Recently, multi-targeted tyrosine kinase inhibitors have shown significant activity in patients with iodine-refractory WDTC and MTC.15,17,18,19,20 The activity of these agents has been consistently superior to conventional chemotherapy and associated with a favorable side effect profile. Table 4 summarizes efficacy results from these trials. The studies have shown long intervals of progression free survival that suggest a change in the natural history of the disease. Because many patients with these tumors can have an indolent course that does not require therapy, most trials have attempted to select patients with more aggressive disease. Some trials have required documentation of disease progression by RECIST over 6 or 12 months prior to enrollment. or the presence of symptomatic disease.15,17,20 However, the definition of progressive disease in studies requiring disease progression for entry was either unclear or evaluated individually by each investigator in a multi-center trial.15,17 The typically slow progression of even refractory thyroid cancer can make documentation of disease progression challenging. The current trial used a simple objective criterion for entry, namely, the presence of at least one FDG-PET avid lesion with uptake clearly above blood pool background. Although the entry criteria for this study may not be directly comparable to prior studies of other TKIs in thyroid cancer, the presence of FDG-avid tumors is strongly predictive of a more aggressive course of the disease, and associated with 5-year overall survival of less than 50%.4 Tumors were highly metabolically active by FDG PET, with median lesion SUV of 7.9, indicating an aggressive phenotype.
Table 4.
| Study Medication | Response Rate (%) |
Progression Free Survival (months) |
Median Survival (months) |
|---|---|---|---|
| Sunitinib* | 31 | 12.8 | NR |
| Sorafenib17 | 23 | 19.3 | NR |
| Sorafenib18 | 14 | 16/10† | 23/37.5† |
| Axitinib19 | 30 | 18.1 | NR |
| Motesanib15 | 14 | 9.2 | NR |
| Motesanib20‡ | 2 | 11 | NR |
Current study
Study had 2 arms with different inclusion criteria
Study only included patients with medullary carcinoma
Abbreviations: NR, Not Reached
As in some prior published studies, we included both patients with iodine-refractory WDTC and MTC, both diseases for which there are few, if any, accepted systemic therapy choices. Although we reported results from both cancers combined, the RECIST response rates were similar (WDTC = 28%; MTC = 50%), and sunitinib clearly had activity in both tumor types.
Although it is not possible to directly compare results from the current trial with those previously published, therapy with continuous sunitinib was associated with a high rate of disease control. Seventy seven percent of patients had no evidence of disease progression 3 months after trial initiation. The median time to disease progression was 12.8 months and the median survival has not been reached. These results seem to compare favorably with what would be expected in this population with FGD-PET avid tumors.4 Furthermore, 78% of patients had some degree of tumor reduction. This is very important as patients with stable disease per RECIST criteria represent a heterogeneous group, which includes patients with a 29% reduction and a 19% increase in tumor size. Radiological response and disease control were seen both in WDTC and MTC. In addition, in those patients with measurable serum tumor markers, 58% of patients with WDTC and 50% with MTC had a significant decrease from baseline levels.
We attempted to correlate the results of a FDG-PET scan one week after therapy initiation with subsequent response to therapy, based upon data showing that a decline in FDG uptake could be an early indicator of response in other diseases treated by sunitinib.21,22 This could provide a very useful method to predict treatment benefit, particularly when using an expensive therapy in a clinical situation where stable radiologic disease is of unclear significance. We observed a significant association of average SUV percent change and RECIST response. Patients with partial/complete response and stable disease had a significant decline in average SUVs compared to patients with progressive disease. This is also true of mean percent change in the SUV of the most PET avid lesion. This is somewhat similar to, but of somewhat lower magnitude compared to results for sunitinib treatment in GIST, upon which the exploratory analysis was based and where early changes in FDG uptake have been shown to predict response.21 The early repeat FDG PET was an exploratory analysis; however, results suggest that, as in the use of sunitinib in GIST, FDG PET may provide an early indication of response, and importantly a lack of response. Thus early FDG PET might help identify patients unlikely to respond to sunitinib, sparing them the expense and toxicity of treatment. This would need to be studies more formally in future trials, but early results appear somewhat promising. It is possible, and perhaps likely, that a FDG-PET done later than one week from treatment initiation would have been a better predictor of benefit and may merit further investigation. This trial also showed that tumor markers were unreliable predictors of treatment benefit compared to objective response measures (RECIST). This result has also been shown in other trials using multi-targeted tyrosine kinase inhibitors.19
Dose reductions from 37.5 mg of continuous sunitinib were common, however a majority of patients requiring dose reduction, for non-hematologic toxicity, had documented disease control on 37.5 mg. Only four toxicities grades 3 and 4 were seen in more than 10% of patients: fatigue, diarrhea, hand/foot syndrome and leukopenia/neutropenia. The most serious complication was bleeding, with one treatment related death. Although the use of anticoagulation is not a contraindication for the use of sunitinib, our experience suggests that continuous administration of sunitinib in patients with well differentiated thyroid carcinoma may be associated with significant risk of serious bleeding episodes, particularly in the setting of full-dose anticoagulation. This trial also had two patients with grade 2 hemoptysis and subsequently we excluded patients with prior history of hemoptysis. We also observed more neutropenia than previously reported with sunitinib. This may be a consequence of our schedule of continuous administration. It is also possible that prior marrow radiation during radioactive iodine therapy may have contributed to this toxicity. Despite the frequent neutropenia, no patient in the trial developed fever in the setting of neutropenia. Data from this trial support the cut-off of 0.75 × 109cells/ L neutrophil count used to allow for treatment continuation.
In conclusion, continuous oral administration of sunitinib was effective in patients with iodine-refractory well-differentiated thyroid carcinoma and medullary carcinoma of the thyroid. Gastrointestinal bleeding and hemoptysis were the most serious complications of therapy and may be associated with the use of anticoagulation. Multi-targeted tyrosine kinase inhibitors have changed the therapy of WDTC and MTC. Although phase III clinical trials are necessary to define their precise clinical benefit, phase II trials conducted to date show a toxicity profile favorable to that of cytotoxic chemotherapy, with more consistent response rates and response duration.
Statement of Translational Relevance.
Until recently, the treatment of advanced thyroid cancer has been limited by the poor efficacy of available chemotherapy agents. The introduction of tyrosine kinase inhibitors (TKIs), is leading to a paradigm shift in how this disease is treated. Our work focuses on two important aspects of TKI therapy to improve the future use of these agents in thyroid cancer. The first is the use of continuous dosing of sunitinib to improve response and better understand the toxicity in this unique patient population. The second is the investigation of FDG-PET imaging early in the treatment course to assess response. The use of functional imaging to assess treatment response and aid in clinical decision-making is a powerful tool that has the potential to be broadly applicable in the future practice of oncology.
Table 3.
Summary of Treatment Related Adverse Events
| Total | Grade ≥3 | |||
|---|---|---|---|---|
| No. | % | No. | % | |
| Constitutional | ||||
| Fatigue | 9 | 26 | 4 | 11 |
| Dehydration | 2 | 6 | 1 | 3 |
| Anorexia | 1 | 3 | 0 | |
|
| ||||
| Gastrointestinal | ||||
| Mucositis | 2 | 6 | 1 | 3 |
| Xerostomia | 1 | 3 | 0 | |
| Diarrhea | 9 | 26 | 6 | 17 |
| Nausea/Vomiting | 3 | 9 | 0 | |
| Dysphagia | 1 | 3 | 0 | |
| Odynophagia | 2 | 6 | 1 | 3 |
| GI Bleed | 5 | 14 | 2 (1 grade 5) | 6 |
| Ulcer | 1 | 3 | 0 | |
|
| ||||
| Dermatologic | ||||
| Hand/Foot Syndrome | 9 | 26 | 6 | 17 |
|
| ||||
| Pulmonary | ||||
| Hemoptysis | 2 | 6 | 0 | |
|
| ||||
| Cardiovascular | ||||
| Hypertension | 1 | 3 | 0 | 0 |
| Supraventricular Tachycardia | 2 | 6 | 2 | 6 |
|
| ||||
| Hemeatologic | ||||
| Leukopenia | 11 | 31 | 11 | 31 |
| Neutropenia | 12 | 34 | 12 | 34 |
| Anemia | 2 | 6 | 2 | 6 |
| Thrombocytopenia | 1 | 3 | 1 | 3 |
|
| ||||
| ENT | ||||
| Epistaxis | 2 | 6 | 0 | |
| Edema of Larynx | 1 | 3 | 1 | 3 |
|
| ||||
| Endocrine | ||||
| Hypocalcemia | 1 | 3 | 1 | 3 |
|
| ||||
| Neurologic | ||||
| Peripheral Neuropathy | 1 | 3 | 1 | 3 |
| Infection | 2 | 6 | 1 | 3 |
Grade 3 and higher are reported in separate column
Abbreviations: ENT, ear, nose, and throat
Acknowledgements
The authors are indebted to the patients who participated in the study and to Kelly Nguyen for manuscript preparation.
This study was supported by a research grant from Pfizer Inc
Footnotes
This study has been presented in part at the 44th and 45th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008 Chicago, IL and May 29-June 2, 2009, Orlando FL.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8:148–156. doi: 10.1016/S1470-2045(07)70034-7. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez C, Hu MI, Gagel RF. Management of medullary thyroid carcinoma. Endocrinol Metab Clin N Am. 2008;37:481–496. doi: 10.1016/j.ecl.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]Fluoro-2-Deoxy-D-Glucose-Positron Emission Tomography. J Clin Endocrinol Metab. 2006;91:498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 5.Iagaru A, Kalinyak JE, McDougall IR. F-18 FDG PET/CT in the management of thyroid cancer. Clin Nucl Med. 2007;32:690–5. doi: 10.1097/RLU.0b013e318125037a. [DOI] [PubMed] [Google Scholar]
- 6.Chow LQM, Eckhardt S. Sunitinib: From rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 7.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(s2):s37–43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komminoth P. The RET proto-oncogene in medullary and papillary thyroid carcinoma: Molecular features, pathophysiology and clinical implications. Virchows Arch. 1997;431:1–9. doi: 10.1007/s004280050062. [DOI] [PubMed] [Google Scholar]
- 9.Yu XM, Lo CY, Lam AKY. Serum vascular endothelial growth factor C correlates with lymph node metastasis and high-risk tumor profiles in papillary thyroid carcinoma. Ann Surg. 2008;247:483–489. doi: 10.1097/SLA.0b013e31815fa447. [DOI] [PubMed] [Google Scholar]
- 10.Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther. 2007;29:1338–1358. doi: 10.1016/j.clinthera.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 11.George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumor after imatinib failure. Eur J Cancer. 2009;24:1959–1968. doi: 10.1016/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 14.Sherman S, Wirth L, Droz JP, et al. Motesanib Diphosphate in Progressive Differentiated Thyroid Cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 15.Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence of serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–1127. doi: 10.1210/jcem.83.4.4683. [DOI] [PubMed] [Google Scholar]
- 16.Weber WA, Ziegler SI, Thodtmann R, et al. Reproducibility of metabolic measurements in malignan tumore using FDG-PET. J Nucl Med. 1999;40:1771–7. [PubMed] [Google Scholar]
- 17.Gupta-Abramson V, Troxel A, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloos R, Ringel M, Knopp M, et al. Phase II Trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen E, Rosen L, Vokes E. Axitinib is an active treatment for all histologic subytpes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. el al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlumberger M, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794–3801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 21.Prior JO, Montemurro M, Orcurto MV, et al. Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol. 2009;27:439–45. doi: 10.1200/JCO.2008.17.2742. [DOI] [PubMed] [Google Scholar]
- 22.Vercellino L, Bousquet G, Baillet B, et al. 18F-FDG PET/CT imaging for an early assessment of response to sunitinib in metastatic renal carcinoma: preliminary study. Cancer Biother Radiopharm. 2009;24:137–44. doi: 10.1089/cbr.2008.0527. [DOI] [PubMed] [Google Scholar]


