Abstract
Interpretation of adrenal cortex phenotypes is greatly facilitated by simultaneous examination of multiple markers at single cell resolution. However the availability of multiple appropriate antibodies can be rate limiting, while their cognate antigens are often subject to variable accessibility. Specific markers not subject to these constraints thus have obvious utility. Here we report that endogenous biotin, when detected in fixed, frozen tissue sections using fluorescent streptavidin, is a specific marker of apparently all cells with steroidogenic potential in the murine adrenal cortex. While streptavidin stains presteroidogenic and mature cortical cells, it does not label the adrenal capsule, medulla or vascular endothelium. Developmental profiles reveal adrenal endogenous biotin labeling from E13.5 through adulthood. Comparisons with zonal markers, hypothalamic-pituitary-adrenal (HPA) axis-remodeled tissue, transgenic Shhn-LacZ or Gli1-nLacZ animals, and Shh mutant embryos further demonstrate the utility of this approach. Fluorescent streptavidin applied using a simple one-step staining protocol thus provides a potent counterstain for use in adrenal analyses.
Keywords: adrenal cortex, steroidogenic, endogenous biotin, streptavidin, fluorescence microscopy
1. Introduction
The adrenal cortex is divided into multiple functional steroid-producing zones arranged concentrically within a non-steroidogenic mesenchymal capsule, and surrounding a neuroendocrine medulla. In mice these zones include an outer zona glomerulosa (ZG) that produces mineralocorticoids, a more central zona fasciculata (ZF) that produces glucocorticoids, and a transient fetal/X-zone of indeterminate function that is present embryonically and in immature animals but regresses at sexual maturity in males, and at the first pregnancy in females (Kim et al., 2009; Val and Swain, 2010).
Cortical cells with steroidogenic potential express distinguishing combinations of proteins, some of which are pan-steroidogenic, while others are more regionally restricted. Pan-steroidogenic proteins are required for common early steps in steroidogenesis, and are expressed in all cortical zones. They include the cholesterol side chain cleavage enzyme (scc or Cyp11A1), required for the conversion of cholesterol to pregnenolone, the initial step in steroid synthesis, and the transcription factor SF1/Ad4BP, which regulates the expression of steroidogenic enzymes. In the murine ZG at least two cell populations can be identified: cells we refer to as presteroidogenic, and mature ZG cells. The presteroidogenic cells possess characteristics of mature steroid producing cells, but have not completely differentiated. They express SF1 and scc, but not Cyp11B2, the terminal enzyme in the aldosterone biosynthetic pathway. Significantly, most, possibly all, of these cells also express sonic hedgehog (Shh), and the Shh-expressing population contains progenitors of all more differentiated cortical cell populations (King et al., 2009). Murine presteroidogenic cells have similarities to rat undifferentiated zone cells located between the ZG and ZF, many of which express Shh but express neither Cyp11B2 nor Cyp11B1 (Mitani et al., 2003; Guasti et al., 2010). Mature ZG cells express zonally-restricted Cyp11B2 as well as SF1 and scc, but most do not express Shh. ZF cells express pan-steroidogenic markers and Cyp11B1 but not Shh or Cyp11B2 (Domalik et al., 1991; Hatano et al., 1994; King et al., 2009; Luo et al., 1994; Val and Swain, 2010). Fetal/X-zone cells express SF1 and scc, as well as the zonal marker 20-alpha-hydroxysteroid dehydrogenase (20aHSD; Hershkovitz et al., 2007).
Molecular genetic analyses have begun to characterize the lineage relationships among these different cell populations, as well as the functional consequences of mutating various signaling molecules, transcription factors and ion channels (Ching and Vilain, 2009; Davies et al., 2008; Heitzmann et al., 2008; Huang et al., 2010; Kim et al., 2009; Kim et al., 2008; King et al., 2009; Val and Swain, 2010; Zubair et al., 2008). Interpreting the phenotypes in these experiments at the cellular level is greatly facilitated by the ability to assess the expression of multiple markers simultaneously, most effectively using multichannel fluorescence microscopy, where three or four markers, often detected immunologically, can simultaneously be assessed. Fixed cryosectioned tissue is a useful substrate for these analyses, as many epitopes are preserved. However such multilabel experiments often depend on the use of antibodies raised in different species, and the availability of appropriate antibody combinations is limited. Furthermore the adrenal gland is heavily vascularized, and IgG binding proteins severely limit the usefulness of monoclonal antibodies on murine tissue, unless they are conjugated directly to fluors or of the relatively rare IgG2a or IgG2b subtypes. Thus additional non-immunological markers that can be used in conjunction with specific antibodies would be helpful tools.
While analyzing the murine adrenal cortex, we attempted to immunostain cryosectioned tissue using a biotinylated primary antibody, in combination with the biotin binding protein streptavidin conjugated to a fluorescent molecule. However we could not detect staining that correlated with the known expression pattern of the antigen. We instead observed labeling throughout the cortex, but not the medulla (not shown). Excluding the primary antibody resulted in the same staining pattern. This indicated the streptavidin alone was apparently detecting endogenous biotin in the adrenal tissue. Biotin is a coenzyme for four mammalian carboxylases, three of which are mitochondrial (Hollinshead et al., 1997). These enzymes function in intermediate metabolic pathways associated with gluconeogenesis, lipogenesis and amino acid catabolism. The phenomenon of streptavidin detecting endogenous biotin in cells that are rich in mitochondria, such as those of the adrenal cortex, was previously described for stains of paraffin embedded tissue sections (Bussolati et al., 1997; Shelton and Jones, 1971; Wood and Warnke, 1981; Zelander, 1957). Most reports have focused on methodologies to reduce this background signal, although Bussolati et al. (1997) suggested that endogenous biotin might be a useful marker in certain experimental contexts. However it is not widely used in analyses of adrenal tissue. This perhaps reflects the variability of staining in paraffin-embedded sections, or alternatively, it might be because the specificity of the adrenal staining pattern is not well characterized.
The simplicity of staining frozen tissue sections for endogenous biotin, combined with the apparent ubiquity of staining in the steroidogenic cortex, led us to further investigate the utility of conjugated streptavidin for coimmunofluorescence studies. We compared the staining of streptavidin with a range of adrenal markers, and found that it labels apparently all presteroidogenic and steroidogenic cells of the cortex, but not medulla, capsule or endothelial cells, thus establishing streptavidin as a useful counterstain for more region-specific gene expression. We performed a developmental time course of streptavidin staining on embryonic adrenal tissue, and used it as a marker in comparison to non-steroidogenic Gli1, presteroidogenic Shh and steroidogenic Cyp11B2, Cyp11B1 and 20aHSD expression. We also evaluated it in the context of adrenocortical remodeling by HPA axis modulation, and in Shh null mutant embryos. Our results validate and demonstrate the utility of endogenous biotin as a marker of cells with steroidogenic potential in the adrenal cortex.
2. Materials and methods
2.1 Animals
2.1.1 Husbandry and mouse strains
All live animal procedures were approved and performed according to Columbia University Institutional Animal Care and Use Committee guidelines. Mice were maintained on mixed 129.B6 or 129.B6.Sw backgrounds and fed standard laboratory chow. For timed pregnancies noon on the day of vaginal plug detection was designated embryonic day (E) 0.5. The following alleles were used: Gli1LacZ (Gli1-nLacZ; gift of A. Joyner) (Bai et al., 2002), ShhnLacZ (Shh-nLacZ; gift of A. Kottmann and T. Jessell) (Lewis et al., 2004), Shh− (St-Jacques et al., 1998).
2.1.2 Genotyping
Mice were genotyped by PCR using tail snip DNA prepared by incubation at 55°C overnight in 10mM Tris pH 7.5, 10mM EDTA, 100mM NaCl, 0.5% (w/v) N-lauryl-sarkosyl, 100µg/ml Proteinase K (Roche Diagnostics, Indianapolis, IN). Crude lysates were spun for 5 min, RT at maximum speed in a microcentrifuge, and supernatant diluted 1:50 in 10 mM Tris pH 7.5, 1mM EDTA prior to using 1 µl in the PCR reaction.
The Gli1LacZ and ShhnLacZ alleles were detected using LacZ primers (For: CATCTGGTCGCTGGGGAATGAATC; Rev: GGAACTGGAAAAACTGCTGCTGGTG; 95°C, 2 min; [94°C, 30 sec; 60°C, 1 min; 72°C, 1 min] × 35; 72°C, 2 min; ~500bp band); and the Shh− allele was detected using Shh primers (ShhL1: CCTGTTGTTACTGCATCCCTTCCATC; ShhL3: CGCTGCTAGGTGCACTTTTA; 95°C, 2 min; [95°C, 30 sec; 59°C, 30 sec; 72°C, 1 min] × 35; 72°C, 2 min; ~550bp band). Shh−/− embryos were identified by morphology.
2.1.3 Hypothalamic-pituitary-adrenal axis modulation
For dexamethasone treatments, three adult mice were injected IP with dexamethasone USP (Sigma-Aldrich, St Louis, MO) in saline at 5mg/kg, once/day (Huber et al., 2005) for four days. For ACTH treatments, three adult mice were injected IM with 1.2 U Synacthen Depot (Alliance Pharmaceuticals, Wiltshire, England), once/day (Martinez et al., 2003) for four days. Control animals were injected IP with saline, daily for four days.
2.2. Immunostaining
Tissue was fixed in fresh 4% paraformaldehyde (PFA) in PBS at 4°C for 45 min to 2 h, except in long term fixation experiments, which were fixed overnight. Following extensive washes in PBS on ice, tissue was dehydrated in 30% sucrose for 4 h to overnight at 4°C and embedded in OCT (TissueTek, VWR). Serial 5 µm cryosections were collected on Superfrost plus slides (Fisher Scientific) and air-dried prior to storage at −80°C. Upon thawing sections were postfixed in 4% PFA/PBS for 5 min at room temperature (RT), rinsed in PBS, treated with NaBH4 (2mg/ml in PBS) for 15 min at RT to reduce autofluorescence and rinsed in PBS. They were then incubated with primary antibodies diluted in blocking solution (2% horse serum, 0.1% Triton X-100 in PBS) in Antibody Amplifier chambers (Prohisto, Columbia, SC) overnight at 4°C. Slides were then washed in PBS at RT (3 × 3 min) prior to incubation with secondary antibodies diluted in blocking buffer for 1 h to 2 h at RT in Antibody Amplifier chambers. Streptavidin-conjugated fluors were included in either the primary or secondary antibody mixtures as required. The slides were then washed in PBS (3 × 3 min) prior to mounting with either Fluoromount G (Fisher) or Vectashield (Vector Labs). Hoechst 33342 (2µg/ml) or Toto 3 (0.5µM) was included in the secondary antibody mixture as a DNA counterstain when required.
The following primary antibodies were used at the indicated dilution: mouse anti-Cyp11B1, Oregon Green conjugate, 1:1000 (gift of C. Gomez-Sanchez (Wotus et al., 1998)); rabbit anti-Cyp11B2, 1:1000 (gift of C. Gomez-Sanchez (Wotus et al., 1998)); rabbit anti-Cyp11A1 (scc), 1:4000 (Millipore, Billerica, MA); rabbit anti-SF1/Ad4BP, 1:4000 (gift of K. Morohashi); rabbit anti-20alphaHSD, 1:4000 (gift of Y. Weinstein (Hershkovitz et al., 2007)); rabbit anti-tyrosine hydroxylase, 1:1000 (Millipore); rat anti-PECAM, 1:16,000 (BD Pharmingen, San Diego, CA); rabbit anti-beta galactosidase, 1:10,000 (MP Biomedicals, Irvine, CA).
Streptavidin was used at the following dilutions: Alexa488 conjugate, 1:2000 (Invitrogen), Cy3 conjugate, 1:2000 (Jackson ImmunoResearch Labs, West Grove, PA), Cy5 conjugate, 1:2000 (Jackson ImmunoResearch Labs).
All secondary antibodies were raised in donkeys, cross-absorbed for multilabeling, and used at the following dilutions: Alexa488 conjugates, 1:2000 (Invitrogen); Cy3 conjugates, 1:2000 (Jackson ImmunoResearch Labs); Cy5 conjugates, 1:1000 (Jackson ImmunoResearch Labs).
2.3. Imaging
Sections were imaged following staining either on an Eclipse E800 compound microscope (Nikon Americas, Melville, NY) equipped with narrow bandpass filters and a Retiga 1300i or Retiga Exi camera (Q Imaging, Vancouver, BC, Canada), or in multichannel mode on a Zeiss 510 confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY). Composite images were compiled using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA) and OmniGraffle (Omni Group, Seattle, WA) software.
3. Results and Discussion
3.1. Endogenous biotin labels specifically steroidogenic cells in postnatal adrenal tissue
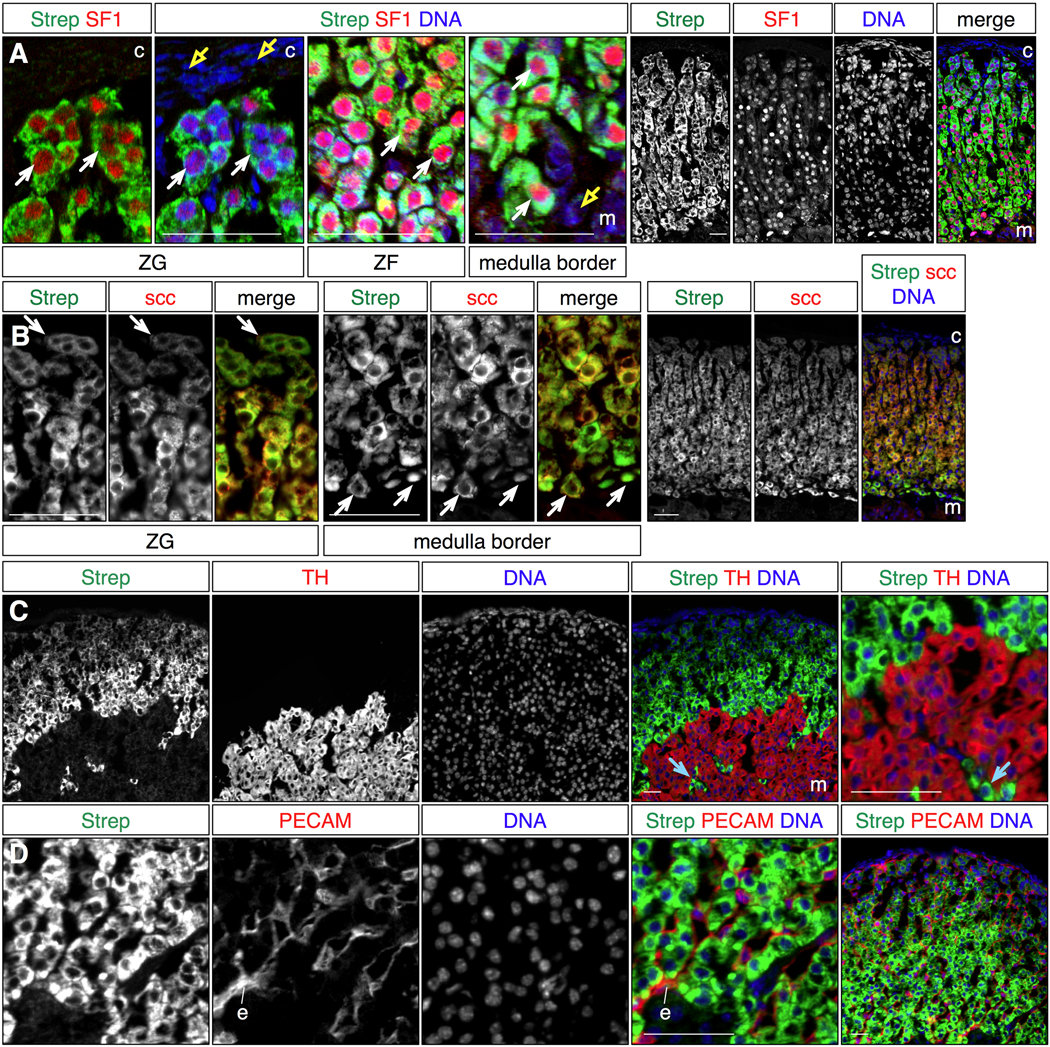
To determine how the streptavidin staining pattern compares to that of other adrenal steroidogenic cell markers, we costained adult adrenal sections with fluorescent streptavidin and antibodies that detect adrenal antigens, and visualized the expression patterns by multichannel fluorescent imaging (Fig 1). Comparison with the steroidogenic transcription factor SF1/Ad4BP (Fig 1A) revealed nuclear SF1 expression surrounded by cytoplasmic streptavidin signal in cells extending from just beneath the capsule to the border of the medulla. Interestingly the streptavidin signal is graded, with strongest signal near the medulla, and weakest peripherally. The correlated expression between SF1+ cells and streptavidin was visible at all intermediate locations in the ZG and ZF. Comparison of streptavidin staining with scc showed non-nuclear labeling of the same cells throughout the cortex (Fig 1B). These results indicate that streptavidin appears to mark all presteroidogenic and mature steroidogenic cells in the cortex.
Figure 1. Endogenous biotin marks steroidogenic cells in the adrenal cortex.
Biotin accumulation was detected by streptavidin staining of cryosectioned adult adrenal gland tissue, and compared to adrenal markers by multichannel immunofluorescent labeling. All images are representative micrographs.
A. Streptavidin stains the same cells as the nuclear transcription factor SF1, a steroidogenic cell determinant, in the zona glomerulosa, zona fasciculata and at the boundary with the medulla (white arrows). Neither medulla nor capsule cells are labeled with streptavidin (yellow arrows).
B. Streptavidin staining labels the same cells as cholesterol side chain cleavage enzyme (scc, Cyp11A1; white arrows), across the depth of the cortex.
C. Tyrosine hydroxylase expression, which marks neuroendocrine cells of the medulla, and streptavidin staining are mutually exclusive. Note that streptavidin also labels scattered TH- cells intermingled in the medulla (blue arrows).
D. Streptavidin staining and PECAM expression, which labels endothelial cells (e), are mutually exclusive, indicating that streptavidin does not label adrenocortical blood vessels.
Note that streptavidin staining is graded, with stronger signal near the medulla, and weaker towards the cortical periphery (see also Fig 2).
Strep: streptavidin, c: capsule, m: medulla, ZG: zona glomerulosa, ZF: zona fasciculata. Scale bars: 50 microns.
To confirm that streptavidin labels only cells with steroidogenic potential, we compared the streptavidin staining pattern with the expression of tyrosine hydroxylase (TH, Fig 1C), which is expressed by neuroendocrine cells of the medulla, and PECAM (Fig 1D), which marks endothelial cells. Streptavidin staining and TH expression were mutually exclusive, demarcating a sharp boundary between the cortex and medulla. Furthermore streptavidin-labeled cells intermingled in the medulla were readily identifiable as discrete islands surrounded by cells that expressed TH. Cells marked with streptavidin were similarly distinct from the endothelium, except that in this case cells that express PECAM are visible in the interstitial spaces immediately adjacent to columns of streptavidin-labeled cortical cells. Together these data provide multiple lines of evidence that streptavidin staining is specific for presteroidogenic and steroidogenic cells, detects apparently all of these cells in the adrenal cortex, and is an effective counterstain for cells in all mature adrenocortical zones.
3.2. Streptavidin staining of fixed, cryosectioned adrenocortical tissue is robust
Having determined the specificity of streptavidin staining, we next determined the reproducibility of staining under different fixation and staining protocols used in conjunction with fixed frozen sections. Some antigenic epitopes and analytical techniques are sensitive to the time of exposure to paraformaldehyde fixative, with particular epitopes requiring relatively short fixation of less than 2 h, while non-radioactive in situ hybridization analysis requires overnight fixation. Furthermore depending on experimental particulars, it would be useful if streptavidin staining could be performed simultaneously with the application of either primary or secondary antibodies. We therefore fixed adult adrenal tissue in paraformaldehyde for either short (45 min) or long (overnight) incubation periods, and stained cryosections with streptavidin at either the primary (4°C, overnight) or secondary (room temperature; 1 h) antibody stage of the staining procedure (Fig 2). Examination of the streptavidin signal revealed that in all cases the signal was similar, with robust staining exclusively throughout the cortex, and stronger intensity signal more centrally. Thus streptavidin detects endogenous biotin in adrenal tissue equally well under all of these experimental conditions. However we have not compared these results to those generated using other fixatives, or in conjunction with harsher post-sectioning procedures, such as following an in situ hybridization protocol.
Figure 2. Streptavidin staining is insensitive to duration of fixation, and can be combined with primary or secondary antibody staining.
Adult adrenal glands were fixed for 45 minutes (A, B) or overnight (C, D), and streptavidin conjugates were applied at the same time as primary (A, C) or secondary (B, D) antibodies. See text for details. Left panels: streptavidin signal, short exposure to emphasize signal near the medulla, Center panels: streptavidin signal in yellow, DNA counterstain in blue. Right panels: streptavidin signal, long exposure to emphasize signal near the periphery. Note that a similar streptavidin signal is detected under each staining or fixation condition. All images are representative compound micrographs.
O/N: overnight, scale bars: 50 microns.
3.3. Developmental time course of adrenocortical endogenous biotin accumulation
We next asked how early during embryonic development we could use streptavidin staining to distinguish adrenocortical cells. We performed a developmental time course of streptavidin staining from E11.5 through E16.5, and compared to expression of SF1, the adrenocortical steroidogenic cell lineage determinant (Fig 3). While we could detect some adrenocortical cells expressing SF1 at E11.5, at this age we could not detect streptavidin staining above background (not shown). We first detected streptavidin staining above background at E12.5, and this signal did label cells that expressed SF1 (Fig 3A). However there was also significant staining of SF1-negative cells in the region of the adrenal gland, sometimes to the same degree as the bona-fide adrenocortical cells. We attribute the labeling of SF1-negative cells primarily to the relatively weak streptavidin signal and low apparent signal/noise ratio, rather than labeling of presteroidogenic cells that have undetectable levels of SF1. At E13.5 the specific streptavidin signal associated with SF1+ cells was substantial, although there was still some degree of background staining (Fig 3B). Streptavidin signal was even more robust at E14.5 (Fig 3C) and did not increase substantially by E16.5 (Fig 3D). At these ages the adrenal signal was very strong, and within the gland we could readily identify segregating medullary cells that did not stain with streptavidin or SF1. We also observed that the central to peripheral gradient of streptavidin staining was apparent by E14.5. In wild type embryos we could confidently identify the adrenal primordium by E13.5 using the pattern of streptavidin staining alone, although in mutant contexts this might not be possible until E14.5. This developmental profile is delayed relative to SF1 expression, and roughly parallels the expression profile of basal steroidogenic enzymes such as scc and Cyp21B (Kim et al., 2009; Val and Swain, 2010). This is consistent with endogenous biotin accumulation reflecting the differentiation state of cells with steroidogenic potential, rather than their specification to an adrenocortical identity.
Figure 3. Developmental progression of biotin accumulation in the adrenal gland.
Cryosections from embryonic ages E12.5 through E16.5 were labeled with fluorescent streptavidin and immunostained for the pan-steroidogenic cell marker SF1. Panel A: representative confocal micrograph, Panels B–D: representative compound micrographs. The leftmost column of panels shows SF1 expression and nuclei (DNA) in low magnification images of each section.
A. Streptavidin labels SF1+ cells (yellow arrows) at E12.5, although the adrenal-specific signal is not much above background, and numerous SF1-negative cells are associated with streptavidin signal (blue arrows).
B. By E13.5 streptavidin labeling of SF1+ adrenocortical cells is significant (yellow arrows) although there is still some background labeling (blue arrows).
C,D. At E14.5 (C) and E16.5 (D) streptavidin labeling of SF1+ adrenocortical cells is robust (yellow arrows), and the streptavidin pattern readily identifies the adrenal tissue. Unlabeled medulla cells are detected (magenta arrows) segregating from the SF1+ and streptavidin+ cortical cells. Note that at E14.5 there is already a center to periphery gradient of decreasing streptavidin signal.
Labels: a: adrenal gland, g: gonad, c: coelom, d: dorsal aorta, v: vertebral body, M: axial midline, Strep: streptavidin. In all images dorsal is up and medial to right, although the medium and high magnification images in B–D are rotated slightly clockwise to better fit the panels. Dotted white lines in A, B outline the nascent adrenal gland, based on SF1 staining and cell morphology. Scale bars: 50 microns.
3.4. Examples of endogenous biotin as a steroidogenic cell marker
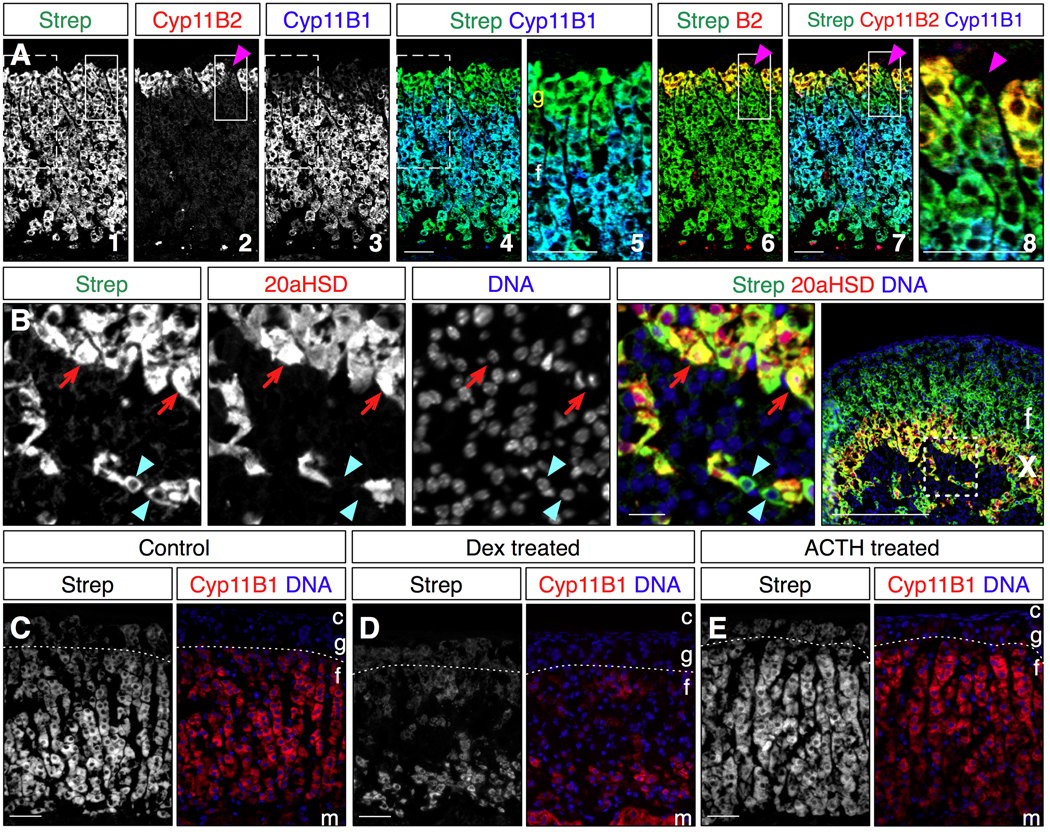
We next compared the pattern of streptavidin staining with more regionally restricted cortical markers to assess the utility of streptavidin as a pan-steroidogenic cell counterstain. Cyp11B1 marks ZF cells, and Cyp11B2 marks mature ZG cells (Kim et al., 2009; King et al., 2009). Triple labeling of adult mouse adrenal tissue with streptavidin and antibodies to Cyp11B1 and Cyp11B2 allowed a direct comparison of the three expression patterns (Fig 4A). Streptavidin staining extended the width of the cortex, while Cyp11B1 expression did not extend into the ZG near the cortical periphery, nor did it extend completely to the medullary boundary. Thus streptavidin in combination with Cyp11B1 allowed us to distinguish steroidogenic cells within the ZF from ZG cells. Interestingly, examination of the ZG staining pattern for all three markers clearly revealed cell clusters expressing neither Cyp11B2 nor Cyp11B1, but that did label with streptavidin. As all ZG cells colabel with scc and streptavidin (Fig 1), and all Shh-expressing cells also colabel with streptavidin (see below and Fig 5), we conclude these cells are the presteroidogenic cells that include the cortical progenitors (King et al., 2009).
Figure 4. Streptavidin staining compared to regional adrenocortical markers and following HPA axis-mediated adrenal remodeling.
All images are representative compound micrographs.
A. Comparison of Cyp11B2 expression in the zona glomerulosa, and Cyp11B1 expression in the zona fasciculata with cortical streptavidin staining. Streptavidin labels cells in the zona glomerulosa that do not express Cyp11B1, and thus can identify pan-steroidogenic cells in the ZG (panels 4,5). Streptavidin also labels ZG cells that express neither Cyp11B2 nor Cyp11B1 (panels 6,7,8), and are thus presteroidogenic (magenta arrowheads). Speckles at the bottom of panels 2,3,4,6 and 7 are non-specific signal, likely due to antibody binding to cell debris at the cortex/medulla boundary. The dotted boxes mark the region enlarged in panel 5, while the solid boxes mark the region enlarged in panel 8.
B. P12 mouse adrenal stained with streptavidin and for 20aHSD expression, which marks the fetal/X-zone. Cells positive for 20aHSD staining are also labeled with streptavidin (red arrows). However some cells labeled with streptavidin that appear to be in the X-zone do not also express 20aHSD (blue arrowheads). Note the presence of nuclei for these cells in the image, which indicates that these are not cytoplasmic extrusions. Boxed region in rightmost panel is enlarged in the other panels.
C–E. Adult mice were exposed to four days of chronic dexamethasone (D) or ACTH (E) treatment to induce ZF remodeling. D: Dexamethasone treatment causes coordinate reduction of Cyp11B1 expression and streptavidin staining of the ZF, but does not affect streptavidin staining of the ZG (dotted lines mark the ZG/ZF borders). E: ACTH treatment causes hypertrophy of ZF cells and increased Cyp11B1 expression and streptavidin staining. (N.B. This image was chosen to fit the figure at the same magnification, but the degree of ZF expansion from medulla to ZG compared to controls was typically much larger. Nonetheless the changes in staining are representative.)
Strep: streptavidin, c: capsule, m: medulla, g: zona glomerulosa, f: zona fasciculata, X: fetal/X-zone. Scale bars: 50 microns.
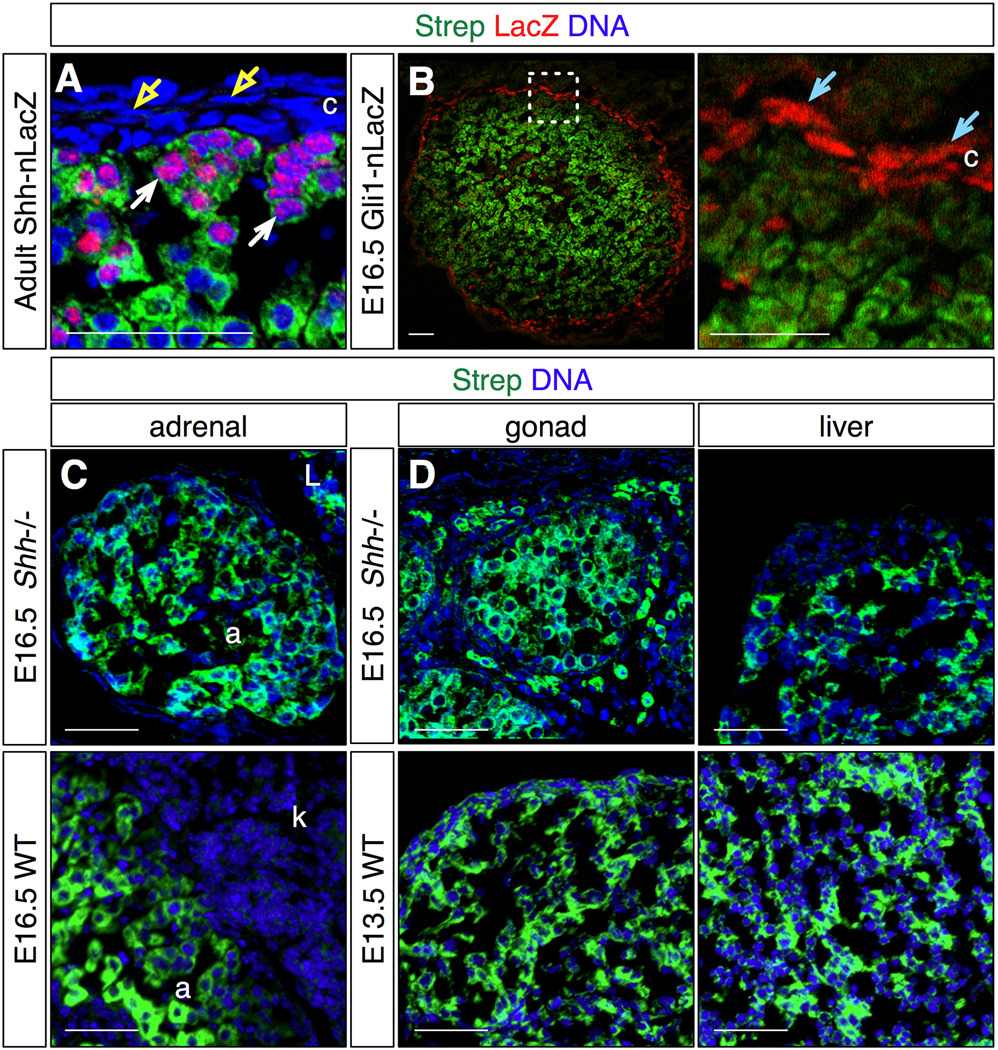
Figure 5. Comparison of streptavidin staining to Shh pathway marker expression, and in Shh null adrenal glands.
Streptavidin staining was compared to the adrenal distribution of Shh signaling (A) or responding Gli1-expressing (B) cells, or in Shh null mutant (C,D) embryos. Representative confocal (A,B) and compound (C,D) micrographs.
A. Comparison of streptavidin stain and LacZ expression in adult Shh-nLacZ adrenal glands reveals that all cells expressing LacZ also stain with streptavidin (white arrows), and thus have steroidogenic potential. Overlying cells in the capsule do not express LacZ or stain with streptavidin (yellow arrows).
B. Comparison of streptavidin stain and LacZ expression in E16.5 Gli1-nLacZ adrenal glands reveals that LacZ is expressed in cells that do not stain with streptavidin (blue arrows). Thus the Shh-responding cells do not display steroidogenic potential.
C. Streptavidin staining is readily detectable in E16.5 Shh−/− adrenal and adjacent liver tissue (upper panel). In wild type embryos the adrenal and adjacent kidney are readily distinguished by their relative streptavidin signals (lower panel).
D. Streptavidin stains cells in tissues other than the adrenal cortex, including gonad and liver of both Shh−/− and wild type embryos.
Box in B left panel indicates area enlarged in right panel. Strep: streptavidin, c: capsule, a: adrenal, L: liver, k: kidney. Scale bars: 50 microns.
We next examined streptavidin staining in adrenal tissue isolated from immature postnatal animals to visualize the X-zone (Fig 4B). We compared streptavidin staining at P12 with the expression 20aHSD, the fetal/X-zone marker (Hershkovitz et al., 2007). Streptavidin again labeled cortical cells extending from the ZG to the medullary boundary. 20aHSD expression marked streptavidin-stained cells near the medulla. However some streptavidin cells at the medulla boundary were not also labeled by 20aHSD, even though adjacent cells were double-labeled. This heterogeneity implies either that a subset of X-zone cells does not express 20aHSD, or that some of these cells are not part of the X-zone. Resolution of this issue will require further investigation of this poorly understood region of the adrenal cortex.
The adrenal cortex can be remodeled in response to changing activity of the HPA axis or renin-angiotensin system. For example chronic HPA axis inhibition leads to ZF atrophy through reduced proliferation and apoptosis (Thomas et al., 2004), and a concomitant decrease in steroidogenic enzyme expression and corticosteroid production, while activation expands the ZF, predominantly through hypertrophy (Ulrich-Lai et al., 2006). To assess endogenous biotin levels during these processes, we chronically treated mice for four days either with dexamethasone, to suppress HPA axis activity, or with ACTH to mimic hyperactivation of the HPA axis. We then compared streptavidin staining to Cyp11B1 expression in sectioned adrenal tissue (Fig 4C–E). Dexamethasone treatment resulted in down-regulation of Cyp11B1 expression, predominantly in the outer two-thirds of the ZF, and coordinate loss of streptavidin staining. Streptavidin staining of the ZG was relatively unaffected. By contrast ACTH treatment resulted in hypertrophy of ZF cells and more robust Cyp11B1 and streptavidin staining. Scc expression was either down- or up-regulated coordinately with the changes in Cyp11B1 expression and streptavidin staining (not shown). Thus endogenous biotin levels under these conditions grossly correlate with the steroidogenic state of the ZF cells.
We previously found that the intercellular signal sonic hedgehog (Shh) is expressed primarily in presteroidogenic cells in the ZG, which also express SF1 and scc, but not the terminal enzyme Cyp11B2. By contrast the cells that respond to the signal, and express the target gene Gli1, do not have obvious steroidogenic potential, as they express neither SF1 nor scc, and are located predominantly in the adrenal capsule (King et al., 2009). We next asked whether we could confirm this relationship using streptavidin staining. We isolated adrenal glands from adult mice carrying a Shh-nLacZ allele, which express nuclear LacZ under the control of the Shh locus (Lewis et al., 2004), or E16.5 embryos carrying a Gli1-nLacZ allele, which express nuclear LacZ under the control of the Gli1 locus (Bai et al., 2002). We compared the streptavidin and LacZ staining patterns for each genotype, and found that all LacZ+ cells in the Shh-nLacZ adrenals also stained with streptavidin (Fig 5A), while the LacZ+ cells in the Gli1-nLacZ adrenals did not (Fig 5B). These results provide additional evidence that the adrenal Shh signal is transmitted from peripheral presteroidogenic cells to adjacent non-steroidogenic mesenchyme cells. Several groups have suggested that adrenocortical stem cells reside in the capsule or just beneath the capsule (Kim et al., 2009; Pignatelli et al., 2002). We have also reported that both Gli1- and Shh-expressing cells have stem cell properties, as each can give rise to more differentiated steroidogenic lineages (King et al., 2009). Our results suggest that only stem cells with steroidogenic potential and residing within the cortex, such as Shh+ cells, should label with streptavidin.
In Shh null embryos the adrenal glands form, but have a small cortex, and the mutant embryos are severely dysmorphic, which can make identifying the mutant adrenal glands difficult (King et al., 2009). We first asked whether there is a relationship between the adrenal growth phenotype and biotin accumulation by staining E16.5 embryonic Shh−/− tissue with streptavidin. We found that as in control adrenal tissue, the mutant adrenal glands stained robustly (Fig 5C), indicating that these cells are differentiating. Thus streptavidin staining can be a useful tool simply for locating the adrenal glands in this mutant context. However biotin accumulates in many other tissues, including the liver and gonads, in addition to the adrenal cortex in both wild type and mutant embryos (Fig 5C,D; (Bussolati et al., 1997)). Thus while endogenous biotin accumulation is a valid marker for adrenocortical cells with steroidogenic potential, streptavidin staining should be used in conjunction with other markers to assign adrenocortical identity to cells that lie outside of a morphologically distinct and unambiguous organ.
4. Conclusions
Our results validate endogenous biotin, detected in a simple, efficient, single step staining procedure on fixed, frozen tissue sections using streptavidin directly conjugated to synthetic fluors, as a specific, pan-steroidogenic cell marker in the adrenal cortex. Streptavidin appears to label all cells in the adrenal cortex with steroidogenic potential, including those that express SF1, scc, Cyp11B1, Cyp11B2, 20aHSD or Shh. Nonetheless we cannot exclude the possibility that a small fraction of presteroidogenic or steroidogenic cells remain unlabeled. The detection of adrenocortical cells in embryonic and mutant tissue, identification of previously unappreciated cellular heterogeneity within the X-zone, the labeling of ZG cells that lack Cyp11B2 expression, changes in staining patterns during zonal remodeling, and documentation that cells expressing Shh have steroidogenic potential all attest to the utility of this reagent. Streptavidin is available conjugated to many different fluors, which adds to its flexibility when used in multiple labeling experiments. Similarly, because direct streptavidin staining can be used in conjunction with antibodies raised in any species, it expands the range of marker combinations that can be examined simultaneously. Conjugated streptavidin is thus a useful addition to the collection of reagents that can be applied to analyses of cells in the adrenal gland.
Acknowledgments
We thank Peter King for helpful advice and discussions. We also thank Celso Gomez-Sanchez, Ken Morohashi, Yakob Weinstein, Alex Joyner, Andreas Kottmann, Thomas Jessell and the late Keith Parker for their generous gifts of reagents. Funding was provided by grants to EL from the NIH (R01 DK 08515), and the American Heart Association (GiA 0855896D). These funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Abbreviations
- HPA
hypothalamic-pituitary-adrenal
- Shh
sonic hedgehog
- ZG
zona glomerulosa
- ZF
zona fasciculata
- scc
cholesterol side chain cleavage enzyme, Cyp11A1
- 20aHSD
20-alpha-hydroxysteroid dehydrogenase
- RT
room temperature
- WT
wild type
- IP
intraperitoneal
- IM
intramuscular
- ACTH
adrenocorticotropic hormone
- PFA
paraformaldehyde
- PBS
phosphate buffered saline, pH 7.4
- SF1/Ad4BP
transcription factor NR5A1
- TH
tyrosine hydroxylase
- E11.5
11.5 days post fertilization
- O/N
overnight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alex Paul, Email: ajp2142@columbia.edu.
Ed Laufer, Email: elaufer@columbia.edu.
References
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Bussolati G, Gugliotta P, Volante M, Pace M, Papotti M. Retrieved endogenous biotin: a novel marker and a potential pitfall in diagnostic immunohistochemistry. Histopathology. 1997;31:400–407. doi: 10.1046/j.1365-2559.1997.3020895.x. [DOI] [PubMed] [Google Scholar]
- Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. genesis. 2009;47:628–637. doi: 10.1002/dvg.20532. [DOI] [PubMed] [Google Scholar]
- Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domalik LJ, Chaplin DD, Kirkman MS, Wu RC, Liu WW, Howard TA, Seldin MF, Parker KL. Different isozymes of mouse 11 beta-hydroxylase produce mineralocorticoids and glucocorticoids. Mol Endocrinol. 1991;5:1853–1861. doi: 10.1210/mend-5-12-1853. [DOI] [PubMed] [Google Scholar]
- Guasti L, Paul A, Laufer E, King P. Localization of Sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.11.010. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano O, Takayama K, Imai T, Waterman MR, Takakusu A, Omura T, Morohashi K. Sex-dependent expression of a transcription factor, Ad4BP, regulating steroidogenic P-450 genes in the gonads during prenatal and postnatal rat development. Development. 1994;120:2787–2797. doi: 10.1242/dev.120.10.2787. [DOI] [PubMed] [Google Scholar]
- Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R, Reichold M, Tegtmeier I, Bendahhou S, Gomez-Sanchez CE, Aller MI, Wisden W, Weber A, Lesage F, Warth R, Barhanin J. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J. 2008;27:179–187. doi: 10.1038/sj.emboj.7601934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148:976–988. doi: 10.1210/en.2006-1100. [DOI] [PubMed] [Google Scholar]
- Hollinshead M, Sanderson J, Vaux DJ. Anti-biotin antibodies offer superior organelle-specific labeling of mitochondria over avidin or streptavidin. J Histochem Cytochem. 1997;45:1053–1057. doi: 10.1177/002215549704500803. [DOI] [PubMed] [Google Scholar]
- Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151:1119–1128. doi: 10.1210/en.2009-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber P, Mallet C, Faure E, Rampon C, Prandini MH, Feraud O, Bouillot S, Vilgrain I. ACTH depletion represses vascular endothelial-cadherin transcription in mouse adrenal endothelium in vivo. Journal of Molecular Endocrinology. 2005;34:127–137. doi: 10.1677/jme.1.01641. [DOI] [PubMed] [Google Scholar]
- Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, Scheys JO, Simon DP, Trovato A, Yang WH, Hammer GD. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30:241–263. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Martinez A, Val P, Sahut-Barnola I, Aigueperse C, Veyssiere G, Lefrancois-Martinez AM. Steroidogenic factor-1 controls the aldose reductase akr1b7 gene promoter in transgenic mice through an atypical binding site. Endocrinology. 2003;144:2111–2120. doi: 10.1210/en.2002-220825. [DOI] [PubMed] [Google Scholar]
- Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim Biophys Acta. 2003;1619:317–324. doi: 10.1016/s0304-4165(02)00490-7. [DOI] [PubMed] [Google Scholar]
- Pignatelli D, Ferreira J, Vendeira P, Magalhaes MC, Vinson GP. Proliferation of capsular stem cells induced by ACTH in the rat adrenal cortex. Endocr Res. 2002;28:683–691. doi: 10.1081/erc-120016987. [DOI] [PubMed] [Google Scholar]
- Shelton JH, Jones AL. The fine structure of the mouse adrenal cortex and the ultrastructural changes in the zona glomerulosa with low and high sodium diets. Anat Rec. 1971;170:147–181. doi: 10.1002/ar.1091700204. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Thomas M, Keramidas M, Monchaux E, Feige JJ. Dual hormonal regulation of endocrine tissue mass and vasculature by adrenocorticotropin in the adrenal cortex. Endocrinology. 2004;145:4320–4329. doi: 10.1210/en.2004-0179. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Val P, Swain A. Gene dosage effects and transcriptional regulation of early mammalian adrenal cortex development. Mol Cell Endocrinol. 2010;323:105–114. doi: 10.1016/j.mce.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Wood GS, Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981;29:1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]
- Wotus C, Levay-Young BK, Rogers LM, Gomez-Sanchez CE, Engeland WC. Development of adrenal zonation in fetal rats defined by expression of aldosterone synthase and 11beta-hydroxylase. Endocrinology. 1998;139:4397–4403. doi: 10.1210/endo.139.10.6230. [DOI] [PubMed] [Google Scholar]
- Zelander T. The ultrastructure of the adrenal cortex of the mouse. Z Zellforsch Mikrosk Anat. 1957;46:710–716. doi: 10.1007/BF00339373. [DOI] [PubMed] [Google Scholar]
- Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28:7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]