Abstract
The peanut plant (Arachis hypogaea L.), when infected by a microbial pathogen, is capable of producing stilbene-derived compounds that are considered antifungal phytoalexins. In addition, the potential health benefits of other stilbenoids from peanuts, including resveratrol and pterostilbene, have been acknowledged by several investigators. Despite considerable progress in peanut research, relatively little is known about the biological activity of the stilbenoid phytoalexins. This study investigated the activities of some of these compounds in a broad spectrum of biological assays. Since peanut stilbenoids appear to play roles in plant defense mechanisms, they were evaluated for their effects on economically important plant pathogenic fungi of the genera Colletotrichum, Botrytis, Fusarium, and Phomopsis. We further investigated these peanut phytoalexins, together with some related natural and synthetic stilbenoids (a total of 24 compounds) in a panel of bioassays to determine their anti-inflammatory, cytotoxic, and antioxidant activities in mammalian cells. Several of these compounds were also evaluated as mammalian opioid receptor competitive antagonists. Assays for adult mosquito and larvae toxicity were also performed. The results of these studies reveal that peanut stilbenoids, as well as related natural and synthetic stilbene derivatives, display a diverse range of biological activities.
Keywords: Arachis hypogaea, peanuts, groundnuts, stilbenoids, resveratrol, phytoalexins, biological activity, antifungal, antitumor, anticancer, cytotoxic, antioxidant, anti-inflamatory, opioid receptor, adult mosquito, mosquito larvae
INTRODUCTION
Stilbene-derived compounds are commonly present in plants and have become of great interest to many research groups worldwide because of the diverse range of biological activities that they tend to display (1, 2). When infected by a microbial pathogen, the peanut plant (Arachis hypogaea) becomes a potent producer of a distinctive set of stilbene-derived phytoalexins, (3–5). Peanut stilbenoids have been considered the major sustaining factor of the plant’s resistance to diseases (3). In addition, the health benefits of resveratrol (24) (Figure 1) from peanuts and other plants have been established by many investigators (1, 2, 6).
Figure 1.
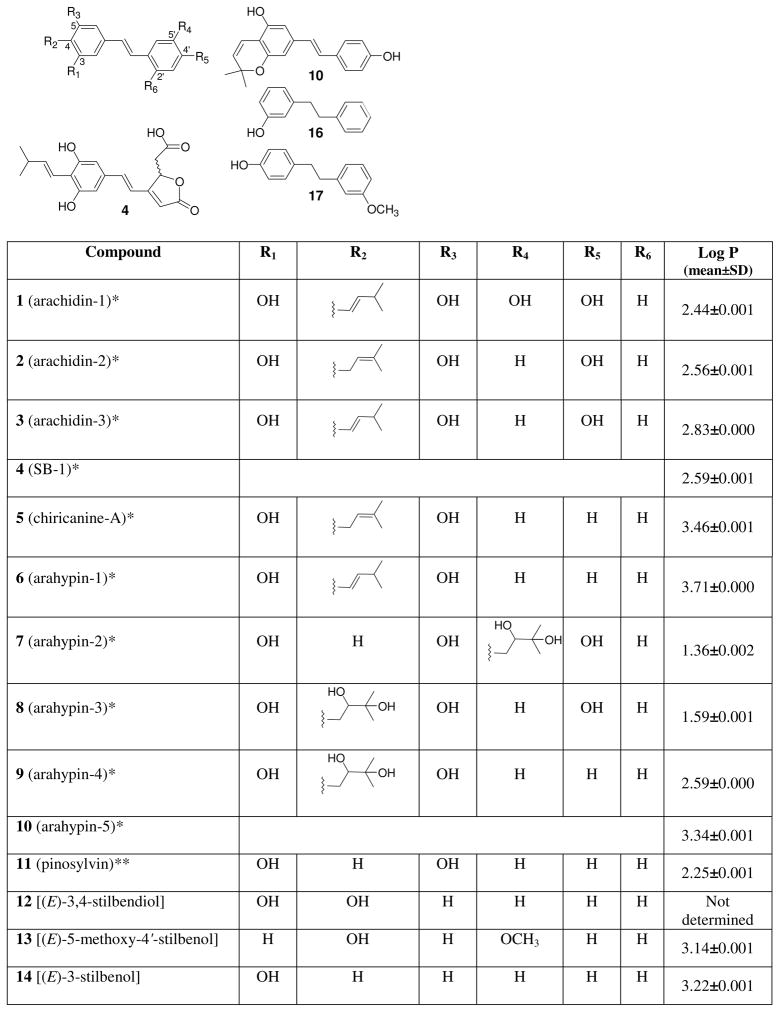
Structures of tested compounds. *Peanut phytoalexins. **Other natural plant stilbenoids. O-Glc refers to a glucosyl moiety.
Peanut stilbenoids are polyphenolic compounds that are isolated from peanut plant materials strictly in the trans olefinic configuration. The trans-olefin structure of the parent stilbene skeleton is an important determinant of bioactivity (1). The major stilbenoids bear isopentenyl, isopentyl, or isopentadienyl moieties arising from prenylation (3–5). Prenylation plays a major role in the diversification of many natural aromatic compounds, including those from peanuts. For example, many prenylated flavonoids have been identified as constituents in plants, and display biological activities, such as anti-cancer, anti-androgen, anti-Leishmania, and anti-nitric oxide production (7). Prenylation also provides greater lipophilicity to molecules, allowing them to readily penetrate through cell membranes. An increase in lipophilicity (commonly expressed as a log P constant) often correlates positively with increased biological activity within groups of compounds of similar structure (8).
As reported by several researchers (8, 9–12), the number and positions of hydroxy groups in stilbenoids tend to be crucial factors in their biological activities. These groups may serve as essential, regulating, or enhancing moieties in such compounds (8, 10–13). For example, a 4′-hydroxy group was demonstrated to be essential for the cytogenetic and estrogenic activities of stilbenoids (1, 11). The higher reactivity of compounds with a 4′-hydroxy group compared to those having 3′- and 5′-hydroxy groups can be attributed to resonance effects (1).
Other aspects of the structure-activity relationship in substituted stilbenes have been investigated. The olefinic double bond in the stilbene skeleton was found to be necessary for tyrosinase inhibitory activity (14). The stilbene moiety also displayed higher antifungal activity against Gloeophyllum trabeum and Poria placenta compared to the corresponding bibenzyl moiety within a group of otherwise structurally similar compounds (8, 10, 13). Similar results were later reported for the inhibition of Botrytis cinerea (15).
While the bioactivity of trans-resveratrol has been extensively studied (1, 2, 6), the effects of prenylated peanut stilbenoids have been only minimally explored (16–19). Anticancer properties of selected peanut stilbenoids, arachidin-1 (1), arachidin-3 (3), trans-3′-isopentadienyl-3,5,4′-trihydroxystilbene, and resveratrol (24) were investigated (19); compopunds 1, 3, and 24 showed concentration-dependent growth inhibitory effects on HL-60 cells. Arachidin-1 (1) appreciably induced mitochondrion-mediated apoptosis at low concentrations and was demonstrated to be an effective anticancer agent that was capable of inducing caspase-independent death of cancer cell with mutations in apoptotic genes (19). These data indicate the need for screening of recently discovered peanut stilbenoids (4, 5) for anticancer and/or other beneficial or adverse effects on other organisms. Modern medicine still demands intensive research on health-beneficial properties of natural stilbenoids and their synthetic analogs (1, 2, 12). Publications (18, 20) on the bioproduction and isolation of 1, 3, and 24 from hairy root cultures of the peanut plant encourage in vivo study of peanut stilbenoids.
The purpose of this investigation was to study biological activities of important natural peanut stilbenoids and compare their biological activities to those of some natural stilbenoids from different plant sources as well as selected synthetic stilbene derivatives. Evaluation of antifungal, anti-inflammatory, cytotoxic, and antioxidant activities was performed. In addition, mosquito adult and larval mortality assays were conducted, and the affinity of these stilbenoids to opioid receptors was examined.
MATERIALS AND METHODS
General experimental procedures
HPLC-grade solvents used in the preparation of mobile phases were obtained from Fisher (Suwanee, GA). HPLC-grade H2O was prepared with a ZD20 four-bowl Milli-Q water system (Millipore). Deuterium oxide (99.9 atom% D) as well as HPLC-grade benzene (99.8%), toluene (99.8%), ethylbenzene (99.8%), propylbenzene (98%), and butylbenzene (99+%) were purchased from Sigma-Aldrich. All chemicals used for opioid receptor tests were also from Sigma-Aldrich with the following exceptions. For the binding experiments, [3H]DAMGO (53.4 Ci/mmol), [3H]U-69,593 (42.7 Ci/mmol), [3H]Enkephalin (45 Ci/mmol), were obtained from Perkin-Elmer Life Sciences Inc. (Boston, MA). DAMGO ([D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin), DPDPE ([D-Pen2,5]-enkephalin), and nor-binaltorphimine were obtained from Tocris Bioscience (Ellisville, Missouri).
Tested compounds
Peanut phytoalexins 1–10 were obtained as described (4, 5, 21). Compounds 11–21 (Figure 1) were a gift from Dr. T.P. Schultz (Department of Forest Products, Mississippi State University, MS). Chlorophorin (22) was prepared as described (22). Reference samples of rhapontin (23) and resveratrol (24) were purchased from Sigma-Aldrich. Identities and purity of all compounds were confirmed by means of UV, NMR and mass spectrometry (4, 5, 10, 13). MS analysis of individual compounds tested did not reveal any unrepresentative mass units and therefore the compounds were evaluated as “MS-pure.” Chromatographic purity of all tested compounds was >98.0% based on HPLC area percent measurements that were performed at the light absorption maxima for each individual compound.
HPLC-DAD-MS analyses
Analysis of compounds tested was performed using a tandem HPLC-MS Surveyor HPLC system equipped with MS Pump Plus, Autosampler Plus, a PDA Plus Detector (Thermo Electron Corp., San Jose, CA), and a 50 mm × 3.0 mm i.d., 2.5-μm, XBridge C18 analytical column. H2O (A), MeOH (B), and 2%-HCOOH in H2O (C) were mixed in the following gradient: initial conditions, 68% A/ 30% B/ 2% C, changed linearly to 0% A/ 98% B/ 2% C in 12 min, held isocratic for 5 min, then changed to initial conditions in 0.01 min. The flow rate was 0.5 mL/min. The column was maintained at 40° C in a model 105 column heater (Timberline Instruments, Boulder, CO).
Mass Spectrometric measurements
ESI-MS/MSn data were obtained on a Finnigan LCQ Advantage MAX ion trap mass spectrometer equipped with APCI interface and operated with Xcalibur™ version 1.4 software (Thermo Electron Corporation, San Jose, CA). All data were acquired in the full-scan positive and negative polarity modes from m/z 100 to 2000. Capillary temp. was 270° C, sheath gas flow 60 units, auxiliary/sweep gas flow 20 units, source voltage 4.5 kV. In MS2 analyses, the [M+H]+ and [M-H]− ions observed for each chromatographic peak in full-scan analyses were isolated and subjected to source collision-induced dissociation (CID) using He buffer gas. In all CID analyses, the isolation width, relative fragmentation energy, relative activation Q, and activation time were m/z = 2.4, 35–40%, 0.25, and 30 ms, respectively.
Estimation of octanol-water partition constants
Octanol-water partition constants (log P or KOW) of all tested compounds were deduced using an HPLC method. HPLC equipment and solvents were the same as listed above. A Gemini C18 analytical column (5-μm particle size, 4.6 × 150 mm; Phenomenex) and an isocratic mobile phase composed of MeOH, H2O and 2% HCOOH in H2O (55:43:2, w/w) were used for the experiments. The flow rate was 1.0 mL/min and the column temperature was 37 °C. Before injecting into the system, the compounds were dissolved in the mobile phase. At least 5 injections of standards and tested compounds were performed. The following homologous compounds with known log P constants (given in parentheses) were used as standards: benzene (2.13), toluene (2.73), ethylbenzene (3.15), propylbenzene (3.69), and butylbenzene (4.26) (23, 24). A linear regression that was used for calculation of log P values was determined using the SigmaStat software, version 3.5 (Systat Software, Inc., Chicago, IL) and was characterized by high R2 = 0.995. Capacity factor, K, was calculated based on the equation, K = (t1- t0) / t0, where t1 is the retention time of the tested compound, and t0 is the void volume of the analytical column. The void volume of the column was determined by injecting unretained neat D2O into the HPLC system.
Antifungal assay
A modified method of the National Committee for Clinical Laboratory Standards (NCCLS) M27-A reference method for broth dilution antifungal susceptibility testing of yeast was adapted (25) for evaluation of antifungal compounds against agriculturally important conidia-forming filamentous fungal plant pathogens to natural compounds in comparison to known fungicidal standards. The method (25) was used to evaluate the antifungal activity of test compounds towards Botrytis cinerea, Colletotrichum acutatum, C. fragariae, C. gloeosporioides, Phomopsisviticola, P. obscurans, and Fusarium oxysporum. The experimental procedures and the sources of fungal isolates were described (25). The commercial fungicide captan was used as an internal fungicide standard in all assays. The SAS system analysis of variance procedure (Statistical Analysis System, Cary, NC) was used to identify significant factors, and Fisher’s protected LSD was used to separate means.
Cytotoxicity assay
Cytotoxicity was determined against a panel of four human tumor cell lines [SK-MEL (malignant melanoma); KB (oral epidermal carcinoma); BT-549 (breast ductal carcinoma); and SK-OV-3 (ovary carcinoma)] ; and two noncancerous cell lines [Vero (African green monkey kidney fibroblasts) and LLC-PK11 (pig kidney epithelial cells)] as described earlier (26). Doxorubicin was used as a positive control.
Anti-inflammatory assays
Anti-inflammatory activity was determined in terms of the inhibition of NF-κB -mediated transcription and inhibition of intracellular generation of reactive oxygen species (ROS) and nitric oxide (NO). Inhibition of NF-κB mediated transcription was determined in human chondrosarcoma (SW1353) cells by a reporter gene assay as described earlier (28). Sp-1 was used as a control transcription factor to evaluate the toxicity of tested compounds in the same assay. Parthenolide was used as the positive control. Inhibition of intracellular NO production as a result of iNOS activity was assayed in mouse macrophages (RAW 264.7 cells) as described (29). Parthenolide was included in each assay as the positive control. Inhibition of intracellular ROS generation (antioxidant activity) was assayed in human promyelocytic leukemia (HL-60) cells by using DCFH-DA as described previously (30). Trolox was used as a positive control.
Cell culture for opioid receptor assay
Cho-K1 cells stably transfected with opioid receptor subtypes μ, Δ, and κ were a generous gift from Dr. Brian Roth (University of North Carolina at Chapel Hill, Chapel Hill, NC). These cells were maintained at 37 °C and 5% CO2 in a DMEM nutrient mixture supplemented with 2mM L-glutamine, 10% fetal bovine serum, 0.5% penicillin–streptomycin, and either G418 (600 mg/mL) or hygromycin B (300 mg/mL). Membranes were prepared by scraping the cells in a 50 mM Tris buffer, homogenized via sonication and centrifuged for 40 min at 13650 rpm at 4 °C. These were kept at −80 °C. Protein concentration was found via Bio-Rad Protein Assay (Hercules, CA).
Radio-ligand binding for opioid receptor subtypes
Opioid binding took place under the following conditions: 10 μM of each compound was incubated with [3H]DAMGO (μ), [3H]U-69,593 (κ), or [3H]enkephalin (Δ) for 60 min in a 96-well plate. Tritium and membrane concentration for each cell line was determined by saturation experiments performed after each batch of membrane was scraped. The reaction was terminated via rapid vacuum filtration through GF/B filters presoaked with 0.3% BSA using a Perkin Elmer 96-well Unifilter followed by 10 washes with 50 mM Tris. Plates were read using a Perkin Elmer Topcount. Total binding was defined as binding in the presence of 0.1% DMSO. Non-specific binding was defined as binding observed in the presence of 10μM DAMGO (μ), nor-binaltorphimine (κ), or DPDPE (Δ). Specific binding was the difference between total and non-specific binding. Percent binding was found with the following formula: 100-(binding of compound- non-specific binding) × 100 / specific binding. Compound GC-143-8 was run in competition binding against all three opioid subtypes (μ, κ, and Δ). In short, concentrations of compound ranging from 100 μM to 48 nM were incubated for 60 min in a 96-well plate with a predetermined amount of [3H] specific to each membrane type. Optimal membrane concentration was also predetermined by a saturation experiment. The reaction was terminated via rapid vacuum filtration through GF/B filters presoaked in 0.3% BSA using a Perkin Elmer 96-well Unifilter followed by 10 washes of 50 mM Tris. Plates were read using a Perkin Elmer Topcount. Total binding was defined as binding in the presence of 0.1% DMSO. Non-specific binding was defined as binding observed in the presence of 10μM CP55,940. Specific binding was the difference between total and non-specific binding. Ki and IC50 values were calculated using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
Mosquito larvae and adult mosquito assays
Larval bioassays were performed as described (31). For assays against mosquito adults, stock chemical solutions prepared as described (31), were diluted into acetone to a final concentration of 6.25 μg/μL. Ten adult A. aegypti female mosquitoes, 3–5 d post-eclosion, were cold-anaesthetized and placed on a BioQuip chill table (Rancho Dominguez, CA) set at 4°C. One-half of a microliter (0.5 μL) of the test chemical was applied to the dorsal thorax of each insect using a #1702 gas-tight Hamilton syringe mounted onto a Hamilton PB600 repeating dispenser (Reno, NV), with a final dose of 3.12 μg per insect. For any chemical producing 50% or greater mortality, a second assay was performed using 1.56 μg per insect. After treatment, mosquitoes were placed in 3.5-oz plastic cups, containing 10% sucrose solution, and maintained at 28°C and 80% relative humidity. All mosquito larvae and adult mosquito assays were performed in triplicate. Controls included negative (untreated), carrier (DMSO-acetone), and positive (permethrin).
RESULTS AND DISCUSSION
Evaluation of antifungal activity
Since peanut phytoalexins appear to play a role in plant defense mechanisms (3), the stilbenoids (Figure 1) were evaluated first for their antifungal effects against plant pathogenic fungi. The fungal species tested are economically important worldwide (25) for many crops, including peanut. Botrytis blight of peanuts is caused by Botrytis cinerea. Phomopsis spp. frequently produce fruiting structures in the peanut necrotic tissue of leaf scorch lesions (secondary invasion of leaf tissue). Phomopsis spp. were commonly associated with Colletotrichum spp. in marginal necrotic peanut leaf and stem lesions. Several Colletotrichum spp. have been reported as casual agents of anthracnose on peanut (32). Fusarium oxysporum together with F. solani generally are the causal agents of Fusarium wilt of peanuts; these species also can dominate the pod microflora. Many Fusarium spp. that are normally soil saprophytes can become pathogenic to peanuts.
In order to possess biological activity, compounds must be able to move through cell membranes. The octanol/water partition constant (log P) is an important physical parameter that characterizes the lipophilicity of individual compounds. Log P values for all tested compounds were estimated using an HPLC method, which was characterized by low error values (Figure 1).
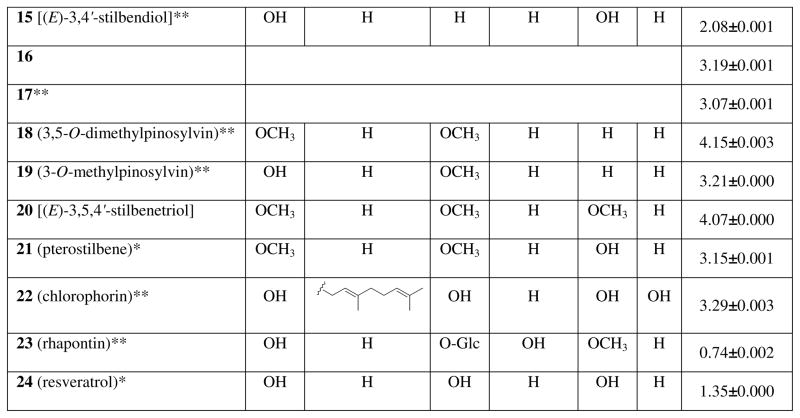
A standard procedure (25) was chosen for the antifungal study, so that the results obtained for the stilbenoids could be directly compared to those of other compounds that were tested using the same method. Lipophilicity–structure–antifungal activity relationships were estimated in a series of assays with significant fungi of the genera Colletotrichum, Botrytis, Fusarium, and Phomopsis (25). Compounds tested were found to be active only against three species: Phomopsis obscurans, P. viticola, and Botrytis cinerea (Figure 2). Chiricanine A (5), arahypin-1 (6), and arahypin-5 (10), with log P values 3.46, 3.71, and 3.34, respectively, when tested against P. viticola and P. obscurans after 144 h of incubation, were more fungicidal than compounds with lower (< 3) log P values (Figures 1 and 2A-C). However, no reliable lipophilicity–bioactivity correlation was deduced with compounds 11–24 (Figures 1 and 2D-G). For example, 23 (log P = 0.74) demonstrated activity similar to that of 22 (log P = 3.29), when assayed against B. cinerea at 72 h (Figure 2G). Apparently, the means by which compounds tested inhibit fungal growth cannot necessarily be directly linked to stilbenoid lipophilicity.
Figure 2.
Antifungal properties of peanut phytoalexinsagainst Phomopsis viticola (A) and P. obscurans (B and C). Antifungal properties of tested natural and synthetic stilbenoidsagainst Phomopsis viticola (D), P. obscurans (E), and Botrytis cinerea (F and G).
The importance of the olefinic double bond in bioactivity of such compounds has been recognized (8, 10, 13, 15). To investigate whether some of tested compounds follow the same pattern observed with other such compounds, we tested two pairs of compounds, the stilbenoids 13 and 14, and their bibenzyl analogues 17 and 16, respectively. Surprisingly, no significant difference in the activity of these compounds was observed in any of the experiments. Moreover, 16 demonstrated statistically higher activity compared to 14 against P. viticola at 144 h at the highest tested concentration of 30 μM. Schultz et al (8, 10) investigated the activity of 13 and 17, and 14 and 16, respectively, among other stilbenoids against Coriolus versicolor, Gloeophyllum trabeum, and Poria placenta. The results were mixed for 14 and 16 and were not significantly different. At the same time, 13 was significantly more active against G. trabeum, and P. placenta compared with 17. The inhibitory effect of pinosylvin (11) was significantly greater than that of dihydropinosylvin (15). In contrast to our data, these results indicate the importance of the olefinic double bond for the activity against G. trabeum, and P. placenta.
The overall activity of peanut phytoalexins (Figure 1), particularly at lower concentrations, against P. viticola (Figure 2A) was higher in comparison with the activity of compounds 11–24 (Figure 2D). The antifungal properties of 5, 6, and 10 were similar to those of the standard, captan (Figure 2A) at 144 h. Captan is a multisite inhibitor fungicide with no systemic activity and is commonly used as an internal standard. The antifungal activity of all tested peanut stilbene derivatives at 120 h virtually matched their bioactivity at 144 h. Among compounds 11–24, chlorophorin (22), 13, 16, and 19 (Figure 2D) demonstrated appreciable activity at the highest concentrations, while resveratrol (24) as well as 11 and 12 were essentially inactive.
On the other hand, stronger inhibition of P. obscurans was evident with compounds 11–24 (Figure 2E) relative to the natural peanut metabolites (Figure 2B-C). The majority of the compounds (Figure 2E), with the exception of 11, 12, 18, and 24, exhibited high selective activity at 30 μM against P. obscurans at 144 h. Three of the peanut metabolites, 5, 6, and 7 (Figure 1) demonstrated the highest levels of inhibition from 120 to 144 h. While the activity of 6 within this period of time statistically remained at the same level, the activity of 5 changed from low to very high, and the activity of 7 changed from very high to extremely low (Figure 2B-C). Since the other compounds retained their activity within the assay time, and since there was no sign of compound precipitation, this phenomenon could probably be attributed to an inducible detoxification mechanism in the fungal test strain. Interestingly, compounds 1, 2, 3, and 4, the stilbenoids that are often found in fungal-challenged peanuts at significantly higher levels than other stilbenoids (5), did not exhibit any activity against P. obscurans at 120 and 144 h (Figure 2B-C).
All tested peanut metabolites were inactive against the Botrytis cinerea strain at the levels tested. It is important to note that B. cinerea is an important pathogen of peanut, and there are no peanut cultivars known to be resistant to it( 32). B. cinerea may be capable of effectively detoxifying the plant’s stilbene-derived phytoalexins, or the fungus might send a signal to the host that does not allow to recognize the pathogen invasion.
In contrast to peanut stilbenoids, the majority of compounds 11–23 exhibited selective activity against the same B. cinerea strain (Figure 2F-G). Chlorophorin (22), at 48 and 72 h, and rhapontin (23), at 72 h, demonstrated very high growth inhibition values comparable with those of captan. Resveratrol (24), as well as 11 and 12, showed minimal activity at 48 h, but showed no activity after 72 h of incubation, and actually appear to have promoted growth of B. cinerea (Figure 2F-G). The lack of influence of 24 on germination and growth of P. viticola and B. cinerea has been previously reported (33). In our experiments, pterostilbene (21), a dimethylated analogue of resveratrol (24), exhibited significantly stronger fungal growth-inhibiting properties than resveratrol (Figure 2D-G). Similar results have been published for the bioactivity of these compounds against B. cinerea (9).
In all the assays for fungal growth inhibition, neither 11, 12, nor 24 showed appreciable signs of activity (Figure 2D-G). The inactivity of resveratrol (24) may indicate a role in fungal challenged peanut seeds as a precursor of more active prenylated stilbenoids (4). Stilbene derivatives 11–24 (with the exception of 20 and 23) were previously studied in assays against the wood-decaying fungi, C. versicolor, G. trabeum, and P. placenta (8, 10). Although the results cannot be compared directly with our data, we note that, in both studies, resveratrol (24) demonstrated negligible activity against the tested fungal species.
Analysis of the antifungal activity of the limited number of compounds 11–24 did not lead to conclusions regarding their structure-activity relationship that could help to explain the bioactivity of natural peanut stilbene derivatives. However, this research revealed that the new peanut metabolites 4, 5, 6–10 are capable inhibitors of P. viticola and/or P. obscurans (Figure 2A-C ), and that some simpler stilbene derivatives also possess strong fungal growth inhibition properties (Figure 2D-G) against economically important fungi.
Evaluation of anti-inflammatory, antioxidant and cytotoxic activities
We further investigated biological activity of peanut phytoalexins together with some natural and synthetic stilbenoids in a panel of target oriented bioassays to explore their anti-inflammatory and antioxidant effects in mammalian cells (Table 1). The anti-cell proliferative activity was also evaluated in a panel of cell lines to determine their cytotoxic potential.
Table 1.
Cytotoxic, Anti-inflammatory, and Antioxidant Activities of Tested Compounds.
| Cytotoxicity (anti cell proliferation activity) in a panel of cell lines | Inhibition of NF-κ B activity in SW1353 cells | Antioxidant Activity (inhibition of ROS generation) in HL-60 cells | Inhibition of iNOS activity (NO production) in RAW 264.7 cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | |||||||||
| Compound | SK- MEL1 | KB1 | BT- 5491 | SK- OV-31 | VERO2 | LLC-PK112 | NF-κB | SP-1 | Antioxidant activity | Cytotoxicity | iNOS | Cytotoxicity |
| 1 (arachidin-1) | 15.0 | 4.8 | 4.8 | 14.5 | 12.0 | 2.8 | 12.0 | - | 0.45 | 5.2 | 1.9 | 8.3 |
| 2 (arachidin-2) | 20.0 | 22.5 | 7.5 | - | 13.5 | 13.5 | 0.025 | <0.025 | 0.04 | 16.0 | 4.1 | - |
| 3 (arachidin-3) | - | - | - | - | - | - | 18.0 | - | 0.17 | - | 6.0 | - |
| 4 (SB-1) | - | - | - | - | - | - | - | - | 0.3 | - | >25 | - |
| 5 (chiricanine A) | 20.0 | - | - | - | - | 19.5 | - | - | - | 19.0 | 6.5 | 13.0 |
| 6 (arahypin-1) | >25 | - | - | - | - | - | - | - | 0.35 | >31.3 | - | - |
| 7 (arahypin-2) | >25 | - | - | - | - | - | - | - | 0.19 | - | - | - |
| 8 (arahypin-3) | - | - | - | - | - | - | - | - | 0.4 | - | - | - |
| 9 (arahypin-4) | - | - | - | - | - | - | - | >25 | 9.5 | >31.3 | - | - |
| 10 (arahypin-5) | 17.5 | >25 | >25 | - | >25 | 19.5 | - | - | 1.3 | 4.5 | 7.0 | 16.0 |
| 11 | 12.5 | 13.8 | 13.0 | 17.0 | 13.0 | 10.5 | 14.5 | 12.5 | - | 18.0 | 6.9 | 10.5 |
| 12 | - | - | - | - | - | - | - | - | 20.0 | >25 | - | - |
| 13 | - | - | - | - | - | - | 19.5 | 25.0 | - | 4.0 | 8.0 | 19.5 |
| 14 | - | - | - | - | - | - | >25 | >25 | - | 19.0 | 11.4 | 22.0 |
| 15 | - | - | - | - | 19.5 | 12.0 | - | - | - | 4.0 | 10.3 | - |
| 16 | - | - | - | - | - | >25 | - | - | 0.012 | 17.0 | 19.5 | - |
| 17 | - | - | - | - | - | - | - | - | 0.027 | 9.0 | 20.0 | - |
| 18 | - | - | - | - | - | - | - | - | - | >25 | >25 | - |
| 19 | - | - | - | - | - | - | 17.0 | 23.0 | - | 18.0 | 8.5 | 12.0 |
| 21 (pterostilbene) | - | - | - | - | - | - | - | - | 0.3 | 21.0 | 9.9 | - |
| 22 | - | >25 | 20.0 | >25 | 15.5 | 14.5 | 22.5 | - | 0.8 | 25.0 | 4.8 | 9.0 |
| 23 | - | - | - | - | - | - | - | - | 0.55 | 20.0 | - | - |
| 24 (resveratrol) | - | - | - | - | - | >25 | 18.5 | >25 | 0.28 | 8.0 | 10.0 | - |
| Doxorubicin3 | 0.8 | 1.45 | 1.35 | 1.3 | >5 | 0.9 | 0.24 | |||||
| Parthenolide3 | 0.8 | 8.35 | 0.42 | 13.5 | ||||||||
| Trolox3 | 0.11 | |||||||||||
Cancer cells.
Noncancer cells. SK-MEL – Human malignant melanoma; KB – Human oral epidermal carcinoma; BT-549 – Human breast ductal carcinoma; SK-OV-3 – Human ovary carcinoma; Vero – Monkey kidney fibroblasts; LLC-PK11 – Pig kidney epithelial cells.
Standard compounds with known biological activities. Hyphen (-) means no activity up to 25 μg/mL.
NF-κB is a protein complex that controls the transcription of DNA. NF-κB is found in animal cells and is involved in cellular responses to various exogenous and endogenous stimuli. Abnormal activation of NF-κB is frequently observed in many cancers; suppression of NF-κB limits the proliferation of cancer cells. NF-κB also is an active participant in the inflammatory response. Therefore, compounds inhibiting NF-κB signaling have potential therapeutic application in treatment of cancer and inflammatory diseases.
Peanut stilbenoids 1, 3, and 24, and non-peanut stilbenoids, 11, 13, 19, and 22 (Figure 1) demonstrated inhibition of NF-κB-dependent transcription in SW1353 cells induced by phorbol myristate acetate (PMA) with IC50 values in the range of 12 – 22.5 μg/mL, as shown in Table 1. Unexpectedly, arachidin-2 (2) inhibited both NF-kB and Sp-1 at significantly lower concentrations (0.025 and < 0.025 μg/mL, respectively). These results are indicative of extremely high cytotoxicity or general nonspecific inhibition of transcriptional activity of 2 in this assay. Stilbenoids 11, 13, and 19 inhibited both NF-κB and Sp-1 to a similar extent indicating that inhibition of NF-κB was not specific and the effect could be due to general toxicity to the cells. At the same time, inhibition of NF-κB activity by 1 and 3 seems to be specific (Table 1).
Most of the stilbenoids inhibited intracellular generation of reactive oxygen species (ROS) in PMA induced HL-60 cells. The strongest antioxidant effect was demonstrated by 2, 16 and 17 which was significantly higher than the standard Trolox (Table 1). Several other stilbenoids (1, 3, 4, 6–10, 21–24) also demonstrated significantly high antioxidant properties which were comparable to Trolox. In the same assay, the majority of stilbenoids demonstrated moderate cytoxicity towards HL-60 cells but the antioxidant effect was observed at much lower concentrations confirming that antioxidant effect was not related to cytotoxic effect. Among the stilbenoids tested, 1, 10, 13, 15, 17 and 24, were more cytotoxic than others towards HL-60 cells (Table 1). A remarkable structure-activity relationship was observed while testing two pairs of compounds, the stilbenoids 13 and 14, and their bibenzyl analogues 17 and 16, respectively. While compounds 17 and 16 demonstrated significantly higher antioxidant activity compared to Trolox, corresponding stilbenoids 13 and 14 were inactive (Table 1). The same compounds demonstrated reversed structure-activity relationship when tested for inhibition of iNOS activity as well as the inhibition of NF-κB-dependent transcription assay and cytotoxicity to corresponding cell lines (Table 1). In these tests stilbenoids 13 and 14 were moderately active, while bibenzyls 17 and 16 were inactive. Supposedly, the lack of conjugation between the benzyl rings is responsible for the observed effects.
More than half of the peanut stilbenoids and most of other stilbenoids tested inhibited the activity of inducible nitric oxide synthase (iNOS) in LPS-induced macrophages resulting in a decrease of nitric oxide (NO) levels. Arachidin-1 (1) was the most potent inhibitor (IC50 = 1.9 μg/mL) in this test. Many other compounds also demonstrated considerable inhibition of iNOS activity (Table 1). Although cytotoxicity was also observed for some of the compounds but the concentration responsible for cytotoxicity was higher than the concentration responsible for inhibition of iNOS activity in the same assay which indicates that the inhibition of iNOS was not due to the cytotoxic effect. However, no cytotoxicity was observed for 2, 3, 15–17, 21 and 24 (Table 1). The majority of stilbenoids did not demonstrate appreciable selective anticancer properties in human solid tumor cell lines. The fact that they are not cytotoxic, and at the same time possess strong antioxidative properties and inhibition of iNOS activities that results into a decrease in the cellular nitrite level, allows to suggest their chemopreventive health benefits and warrants further tests (including in vivo effects).
Moderate cytotoxicity was observed among limited number of peanut stilbenoids and stilbene derivatives 11–24 (Figure 1) in a panel of mammalian kidney cells (Vero and LLC-PK11) and cancer cells (SK-MEL, KB, BT-549, and SK-OV-3) up to the peak concentration tested (25 μg/mL). The highest, but moderate cytotoxicity was exhibited in all cell lines by arachidin-1 (1). At the same time its close 3′-dehydroxy analog 3 (Figure 1) was not cytotoxic (Table 1). Arachidin-2 (2) demonstrated comparable, but significantly lower cytotoxicity than that of 1 (Table 1). The only difference between moderately cytotoxic 2 and non-toxic 3 is the position of the double bond in the prenyl chain at C-4 in the stilbene skeleton (Figure 1). It seems that the interrupted conjugation in 2 provides cytotoxic properties to this molecule compared to 3, which bears a longer conjugation system of alternating single and double bonds. In addition to arachidins 1–3 (1–3) only 5, 10, 11, 15, 22, and 24 were moderately toxic at higher concentrations (up to 25 μg/mL). Compounds 5, 10, 11, and 22 were also moderately toxic to other cell lines tested (Table 1). The rest of stilbenoids did not demonstrate appreciable selective anticancer properties in human solid tumor cell lines.
In our experiments overall activity of 3 and 24 was about equal in cytotoxic, anti-inflamatory, and antioxidant assays, while 1 demonstrated overall higher activity (Table 1). Similar results (19) were obtained for these stilbenoids. All three stilbenoids showed concentration-dependent growth inhibitory effects on HL-60 cells. Arachidin-1 (1) was about 4-fold more active compared to arachidin-3 (3) and resveratrol (24). However, in addition to growth inhibition, 1 was found to be cytotoxic (19). The above compounds were also investigated for their antioxidant activity and cytotoxicity (18) and for antioxidant and anti-inflamatory activities (16). Both research groups reported that all three stilbenoids did not exhibit significant cytotoxicity to RAW 264.7 cells. At the same time, these compounds were found to be potent antioxidants (16, 18) and anti- inflamatory agents (16). It has been demonstrated (17) that 1 and 24 were effective inhibitors of PGE2- or NO-mediated inflammation. The results of present research as well as findings of other research groups indicate the merits of systematic investigation of natural stilbenoids for their anticancer activities.
Evaluation of affinity of compounds tested to opioid receptors
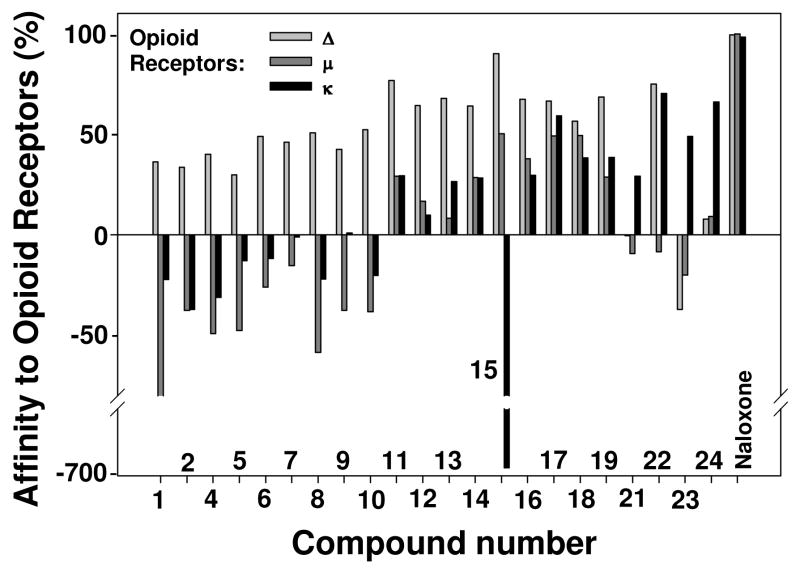
All data were obtained from three experiments using three replicate plates per concentration; standard deviations in all experiments were equal to zero. No correlation between affinity to the opioid receptor subtypes and log P for all tested compounds was observed (Figure 3). Such behavior can be explained by the nature of the specific mechanisms of receptor activation with corresponding compounds.
Figure 3.
Affinity of compounds tested to opioid receptors. Compounds 3 and 20 were not tested in this assay due to their insufficient quantity.
The assay that was used in this study is an important pharmacological tool that measures the relationship of binding between the radiolabeled ligand and the test sample. If a compound has activities on sites remote from the actual binding site, then the ability of the radioligand to bind to the active site may be increased. As such, a control value for the radioligand may be close or equal to 100%, while a test compound may cause this value to increase to a higher than 100% level, which results in a negative value for that compound. Among all tested compounds, the most striking effect on κ opioid receptor systems was exhibited by trans-3,4′-dihydroxystilbene 15;the effect is reflected in Figure 3 as a negative 697% value. This effect cannot be reasonably defined in terms of structure – activity association. However, it is likely that the specific position and number of hydroxy groups in the structure of the simple stilbenoid 15 may be responsible for such a distinctive response. Stilbenoid 15 might have usefulness as an allosteric modulator, which could be employed to enhance the effects of known analgesic agents such as morphine. The low cytotoxicity of 15 (Table 1) allows the suggestion that this compound could be used in vivo. Combined use of 15 and analgesic agents may result in lower amounts of the latter needed to block pain, which is a benefit to the patient since it would reduce the potential for addiction. Compared with other compounds, 15 also demonstrated the highest degree (64.2%) of affinity to Δ-opioid receptors and moderate affinity (16.7%) to μ-receptors (Figure 3). Binding of the standard, naloxone, an extremely potent μ-opioid (and to a lesser degree κ- and Δ-opioid) receptor competitive antagonist, was close to 100% (Figure 3). Compounds 11–24 also showed some degree of activity as well (Figure 3). All of these compounds, with the exception of 21, 23, and 24, demonstrated over 50% competitive binding to μ-receptors. In contrast, rhapontin (23) showed appreciable negative values for Δ- and μ-opioid receptors (Figure 3). Chlorophorin (22) had equally high affinity to both δ- and κ-opioid receptors, but no affinity to μ-receptors. Several of the other compounds demonstrated considerably high competitive antagonism at μ- and κ-sites (Figure 3). All of the peanut metabolites (Figure 1) were satisfactory Δ-opioid receptor competitive blockers (Figure 3) as well as agonists at μ- and κ-receptor subtypes. Two of the nine stilbenoids, 1 and 3, exhibited high (−80.0 and −58.3%, respectively) non-competitive binding to μ-opioid receptor. Taking into account their low cytotoxicity and selective affinity to different types of receptors, some of the compounds tested may be of interest as prospective candidates for in vivo assays.
Evaluation of toxicity of tested compounds to mosquito larvae and adults
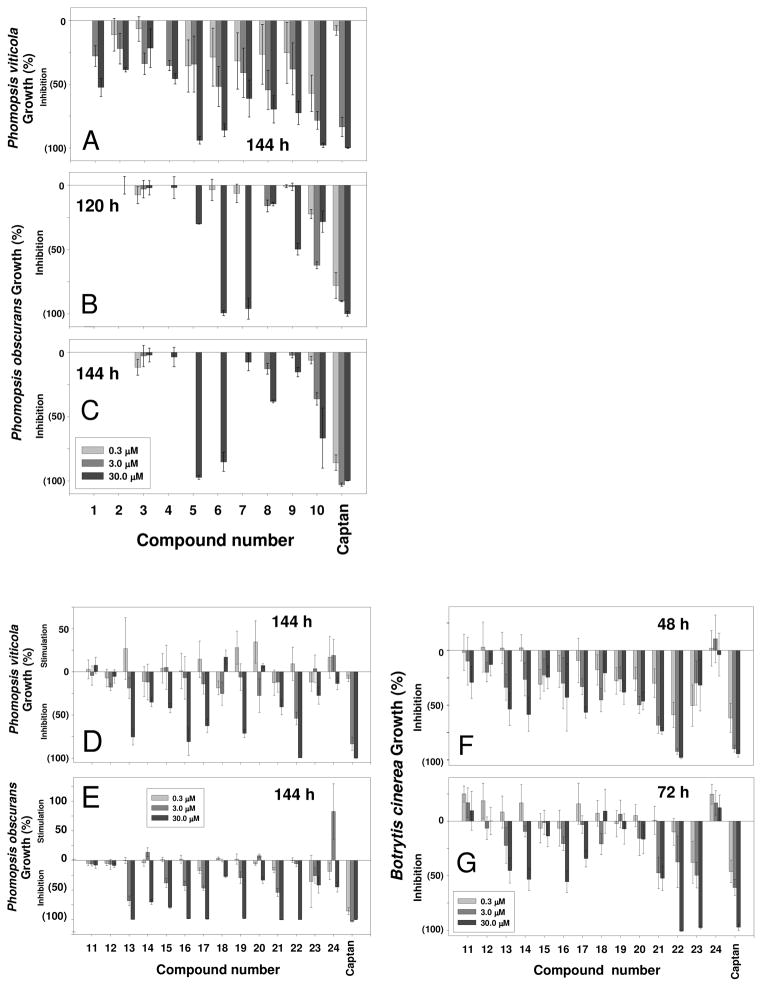
As an extension of our quest to explore the beneficial properties of peanut stilbene-derived compounds, we evaluated their toxicity to adult mosquito and mosquito larvae. While a lack of log P–affinity to opioid receptors in compounds tested was not surprising, some degree of lipophilicity–activity association in the mosquito larvae and adult mosquito assays was anticipated. Indeed, compounds with log P >3.14 (13, 14, 16, 18, 19, and 21) showed the highest activity (Figure 4B-C) with the exception of chlorophorin (22), which was less active despite a high log P value of 3.29 (Figure 1). Compounds with lower lipophilicity, such as 11, 24, 23, and 5 (Figure 1), demonstrated significantly lower toxicity (Figure 4A). There was a positive correlation between the lipophilicity of tested stilbene derivatives and their toxicity to mosquito larvae.
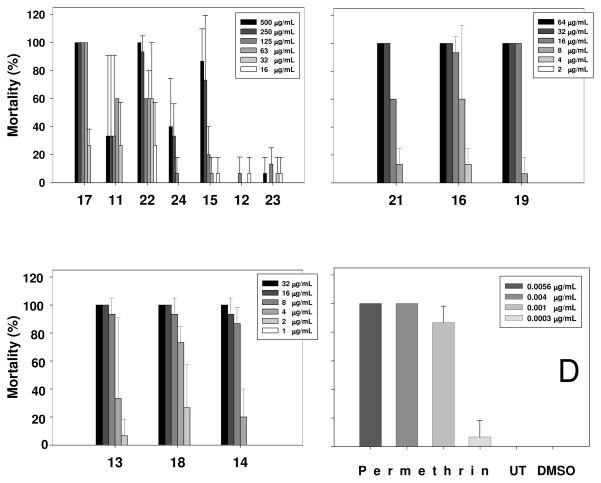
Figure 4.
Toxicity of compounds tested to mosquito larvae; panel D represents toxicity data for positive and negative standards. UT means Untreated; DMSO means dimethyl sulfoxide. Compound 20 was not tested in this assay due to its insufficient quantity.
Although a structure–activity relationship cannot be suggested for the examined compounds, we note that stilbenoid 13 was significantly more active than its bibenzyl analogue 17. At the same time, stilbenol 14 showed about equal toxicity to that of hydroxybibenzyl 16. These two pairs of compounds provide a good model for estimation of the importance of the olefinic double bond for the toxicity of the stilbenoids in this assay. While compounds with virtually matching activities, 14 and 16 have close log P values (3.22 and 3.19, respectively), considerably more active stilbenoid 13 is characterized by a significantly higher lipophilicity (log P = 3.14) than its bibenzyl derivative 17 (log P = 3.07). Although the number of examined samples was limited, a suggestion can be made that the olefinic double bond in stilbenes is not essential for toxicity against mosquito larvae.
The activities of compounds 13, 14, 16, and 18 are very low compared to the positive control permethrin. Stilbenoids 19, 21, and 22 display somewhat greater activity, but the effects were still relatively modest. The remaining compounds were inactive in these assays. At first glance, the lipophilicity data seems to play a role in the toxicity of natural peanut stilbenoids to adult mosquito as well (data not shown). Peanut stilbenoids with higher lipophilicity (log P values are given in parenthesis followed by mortality rate ± SD, %), including 5 (3.46; 6.7±5.8), 9 (2.59; 10±0.0), and 10 (3.34; 5.8±1.8), demonstrated slightly higher toxicity than the less lipophilic 1 (2.44), 7 (1.36), and 8 (1.59), which were marginally active. However, similar results were obtained with acetone/DMSO control that gave 5.8±1.8% mortality, while untreated control was inactive and permethrin standard (6.25 μg/mosquito) caused 100% mortality. On the basis of these results, stilbenoids tested (Figure 1) do not seem to be insecticidal in the adult mosquito assay.
A systematic study of biological activity of new and known peanut stilbenoids was performed. This research revealed diverse biological activity of peanut stilbenoids as well as other natural and synthetic stilbene derivatives. Despite close structural similarities, individual stilbenoids demonstrated significantly different activities in the various assays employed. The results of the present research are consistent with published data on the bioactivity of some natural stilbenoids from peanut and other plant sources (1, 8, 10, 18, 19).
Acknowledgments
The authors thank Dr. Tor P. Schultz for samples of some of the stilbenoids. We also thank Ms. J. Linda Robertson, Ms. Ramona Pace and Ms. Xiaoning Wang for assistance with the antifungal assay, Mr. John Trott, Mr. Paul Bates, and Ms. Katherine Martin for bioassays for cytotoxic and anti-inflammatory activities, Ms. Olivia Dale for assistance with the opioid receptor affinity assay, Ms. Milbra Schweikert for assistance with the determination of octanol-water partition constants, Ms. Ada Larkin for her help with the isolation of peanut stilbenoids, and Ms. Katelyn Chalaire for the mosquito bioassays. The authors express their gratitude for USDA, ARS, NPURU financial support. Support for the University of Iowa group from the National Science Foundation (CHE-0718315) is gratefully appreciated. Part of this study was supported by a grant from the Deployed War-Fighter Protection (DWFP) Research Program and the U.S. Department of Defense through the Armed Forces Pest Management Board (AFPMB). One of the projects was also supported by Grant Number 5P20RR021929 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
LITERATURE CITED
- 1.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 2.Roupe KA, Remsberg CM, Yáñez JA, Davies NM. Pharmacometrics of stilbenes: segueing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 3.Subba Rao PV, Strange RN. Chemistry, biology, and role of groundnut phytoalexins in resistance to fungal attack. In: Daniel M, Purkayastha RP, editors. Handbook of Phytoalexin Metabolism and Action. Marcel Dekker, Inc; New York, NY: 1995. pp. 199–227. [Google Scholar]
- 4.Sobolev VS, Deyrup ST, Gloer JB. New peanut (Arachis hypogaea) phytoalexin with prenylated benzenoid and but-2-enolide moieties. J Agric Food Chem. 2006;54:2111–2115. doi: 10.1021/jf052948o. [DOI] [PubMed] [Google Scholar]
- 5.Sobolev VS, Neff SA, Gloer JB. New stilbenoids from peanut (Arachis hypogaea) seeds challenged by an Aspergillus caelatus strain. J Agric Food Chem. 2009;57:62–68. doi: 10.1021/jf802891v. [DOI] [PubMed] [Google Scholar]
- 6.Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 7.Yazaki K, Sasaki K, Tsurumaru Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry. 2009;70:1739–1745. doi: 10.1016/j.phytochem.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Schultz TP, Nicholas DD, Fisher TH. Quantitative structure-activity relationships of stilbenes and related derivatives against wood-destroying fungi. Recent Res Devel Agric Food Chem. 1997;1:289–299. [Google Scholar]
- 9.Pont V, Pezet R. Relation between the chemical structure and the biological activity of hydroxystilbenes against Botrytis cinerea. J Phytopathol. 1990;130:1–8. [Google Scholar]
- 10.Schultz TP, Boldin WD, Fisher TH, Nicholas DD, McMurtrey KD, Pobanz K. Structure-fungicidal properties of some 3- and 4-hydroxylated stilbenes and bibenzyl analogues. Phytochemistry. 1992;31:3801–3806. [Google Scholar]
- 11.Matsuoka A, Takeshita K, Furuta A, Ozaki M, Fukuhara K, Miyata N. The 4′-hydroxy group is responsible for the in vitro cytogenetic activity of resveratrol. Mutation Res. 2002;521:29–35. doi: 10.1016/s1383-5718(02)00211-5. [DOI] [PubMed] [Google Scholar]
- 12.Lappano R, Rosano C, Madeo A, Albanito L, Plastina P, Gabriele B, Forti L, Stivala LA, Iacopetta D, Dolce V, Ando S, Pezzi V, Maggiolini M. Structure-activity relationships of resveratrol and derivatives in breast cancer cells. Mol Nutr Food Res. 2009;53:845–858. doi: 10.1002/mnfr.200800331. [DOI] [PubMed] [Google Scholar]
- 13.Schultz TP, Cheng Q, Boldin WD, Hubbard TF, Jin L, Fisher TH, Nicholas DD. Comparison of the fungicidal activities of (E)-4-hydroxylated stilbenes and related bibenzyls. Phytochemistry. 1991;30:2939–2945. [Google Scholar]
- 14.Ohguchi K, Tanaka T, Ito T, Iinuma M, Matsumoto K, Akao Y, Nozawa Y. Inhibitory effects of resveratrol derivatives from dipterocarpaceae plants on tyrosinase activity. Biosci Biotechnol Biochem. 2003;67:1587–1589. doi: 10.1271/bbb.67.1587. [DOI] [PubMed] [Google Scholar]
- 15.Kostecki K, Engelmeier D, Pacher T, Hofer O, Vajrodaya S, Greger H. Dihydrophenanthrenes and other antifungal stilbenoids from Stemona cf. pierrei Phytochemistry. 2004;65:99–106. doi: 10.1016/j.phytochem.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Chang JC, Lai YH, Djoko B, Wu PL, Liu CD, Liu YW, Chiou RYY. Biosynthesis enhancement and antioxidant and anti-inflammatory activities of peanut (Arachis hypogaea L.) arachidin-1, arachidin-3, and isopentadienylresveratrol. J Agric Food Chem. 2006;54:10281–10287. doi: 10.1021/jf0620766. [DOI] [PubMed] [Google Scholar]
- 17.Djoko B, Chiou RYY, Shee JJ, Liu YW. Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-lnduced inflammation of RAW 264.7 macrophages. J Agric Food Chem. 2007;55:2376–2383. doi: 10.1021/jf062741a. [DOI] [PubMed] [Google Scholar]
- 18.Abbott JA, Medina-Bolivar F, Martin EM, Engelberth AS, Villagarcia H, Clausen EC, Carrier DJ. Purification of resveratrol, arachidin-1, and arachidin-3 from hairy root cultures of peanut (Arachis hypogaea) and determination of their antioxidant activity and cytotoxicity. Biotechnol Prog. 2010;26:1344–1351. doi: 10.1002/btpr.454. [DOI] [PubMed] [Google Scholar]
- 19.Huang CP, Au LC, Chiou RYY, Chung PC, Chen SY, Tang WC, Chang CL, Fang WH, Lin SB. Arachidin-1, a peanut stilbenoid, induces programmed cell death in human leukemia HL-60 cells. J Agric Food Chem. 2010;58:12123–12129. doi: 10.1021/jf102993j. [DOI] [PubMed] [Google Scholar]
- 20.Condori J, Sivakumar G, Hubstenberger J, Dolan MC, Sobolev VS, Medina-Bolivar F. Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol Biochem. 2010;48:310–318. doi: 10.1016/j.plaphy.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Sobolev VS, Cole RJ, Dorner JW, Yagen B. Isolation, purification, and liquid chromatographic determination of stilbene phytoalexins in peanuts. J AOAC Int. 1995;78:1177–1182. [Google Scholar]
- 22.Minn J, Daly WH, Negulescu II, McMurtrey KD, Schultz TP. Antioxidant properties of the hydrophobic stilbenol chlorophorin. J Agric Food Chem. 1996;44:2946–2947. [Google Scholar]
- 23.Leo A, Hansch C, Elkins D. Partition coefficients and their uses. Chem Rev. 1971;71:525– 616. [Google Scholar]
- 24.Sangster J. Octanol-water partition coefficients of simple organic compounds. J Phys Chem Ref Data. 1989;18:1111–1229. [Google Scholar]
- 25.Wedge DE, Kuhajek JM. A microbioassay for fungicide discovery. SAAS Bulletin Biochem Biotech. 1998;11:1–7. [Google Scholar]
- 26.Mustafa J, Khan SI, Ma G, Walker LA, Khan I. Synthesis and anticancer activities of fatty acid analogs of podophyllotoxin. Lipids. 2004;39:162–172. doi: 10.1007/s11745-004-1215-5. [DOI] [PubMed] [Google Scholar]
- 27.De Stefano D, Tommonaro G, Simeon V, Poli A, Nicolaus B, Carnuccio RA. Polysaccharide from tomato (Lycopersicon esculentum) peels affects NF-κB activation in LPS-stimulated J774 macrophages. J Nat Prod. 2007;70:1636–1639. doi: 10.1021/np070168z. [DOI] [PubMed] [Google Scholar]
- 28.Ma G, Khan SI, Benavides G, Schühly W, Fischer NH, Khan IA, Pasco DS. Inhibition of NF-κB mediated transcription and induction of apoptosis by melampolides and repandolides. Cancer Chemother Pharmacol. 2007;60:35–43. doi: 10.1007/s00280-006-0344-0. [DOI] [PubMed] [Google Scholar]
- 29.Quang DN, Harinantenaina L, Nishizawa T, Hashimoto T, Kohchi C, Soma G, Asakawa Y. Inhibition of nitric oxide production in RAW 264.7 cells by azaphilones from Xylariaceous fungi. Biol Pharm Bull. 2006;29:34–37. doi: 10.1248/bpb.29.34. [DOI] [PubMed] [Google Scholar]
- 30.Reddy MK, Gupta SK, Jacob MR, Khan SI, Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum. Planta Med. 2007;73:461–467. doi: 10.1055/s-2007-967167. [DOI] [PubMed] [Google Scholar]
- 31.Pridgeon J, Becnel J, Clark G, Linthicium K. A high-throughput screening method to identify potential pesticides for mosquito control. J Med Entomol. 2009;46:335–341. doi: 10.1603/033.046.0219. [DOI] [PubMed] [Google Scholar]
- 32.Porter DM, Smith DH, Rodriguez-Kabana R. Peanut Diseases. In: Pattee HE, Young CT, editors. Peanut Science and Technology. Am. Peanut Res. Educ. Soc., Inc; Yoakum, TX: 1982. pp. 326–410. [Google Scholar]
- 33.Hoos G, Blaich R. Influence of resveratrol on germination of conidia and mycelial growth of Botrytis cinerea and Phomopsis viticola. J Phytopathol. 1990;129:102–110. [Google Scholar]