Introduction
Organophosphate (OP) agents are used globally as insecticides and related compounds have been weaponized as nerve agents. Intentional exposures are responsible for a tremendous burden to the health care system in many developing countries[1-4]. OP agents exert their clinical effects largely by binding to peripheral and central acetylcholinesterase, resulting in a rise in the level of post-synaptic acetylcholine (ACh). The increased levels of post-synaptic ACh induce a range of central effects including seizure, apnea and long-term cognitive deficits[5-7].
OP-induced central apnea is well documented in animal models of OP-toxicity and is postulated to be an important cause of fatality following human exposure[5]. The neural substrate underlying normal automatic respiratory rhythm generation (i.e, the central respiratory oscillator or CRO) is a topic of investigation and debate [8, 9]. There is substantial evidence pointing to the importance of specific neuronal groups within the ventral medulla. The pre-Bötzinger complex (preBötC) contains cholinergic neurons[10] and is integral to normal respiratory function[11]. Other sites in the ventral region of the medulla are involved in respiratory control and contain cholinergic synapses, including the ventral surface of the medulla[12], the nucleus ambiguus[13] and the retrotrapezoid nucleus[14]. These and other brainstem sites may be involved in OP-induced central apnea, for example by affecting chemo-responsive or rhythm generating respiratory neurons or by inhibiting inspiratory drive through pontine cholinergic circuits[15].
The hypothesized role of the brainstem in OP-induced central apnea has not been tested directly using validated models of OP poisoning, but the idea is supported indirectly by observations that perturbations of synaptic ACh in circuits involved in breathing cause changes in the pattern of respiratory activity. Some regions of the brainstem demonstrate increased respiratory activity when exposed to elevated ACh levels[10, 16], while others demonstrate respiratory depression but not apnea[17]. However, it is unclear how these findings relate to OP poisoning as they stem from studies designed to mimic the cholinergic perturbations that occur during normal circuit function. Toxic levels of synaptic ACh following acute OP injestion could have different effects compared to the levels achieved during physiological activity. For example, increasing synaptic ACh levels within a physiological range might stimulate rhythmic respiratory activity due to a depolarizing effect on inspiratory neurons, whereas toxic levels of ACh in the same circuit might abolish rhythmic activity through depolarization blockade of the post-synaptic membrane or by other mechanisms involving the network as a whole. Pathological excitation of components of the network involved in respiration could result in a loss of pattern generating capacity at a network level. A direct experimental test of the brainstem hypothesis for OP-induced central apnea would require selective application of the OP to the brainstem or specific sites within the brainstem at levels that would be achieved during fatal apnea following systemic poisoning in vivo[5]. Furthermore, the effects of selective poisoning should be studied in the intact brainstem. Isolating a cholinergic circuit using brain slices might remove or weaken important cholinergic synaptic activity within the slice due to either damage to neurons within the slice (cell bodies or cholinergic end plates) or loss of afferent input from neurons located outside the slice.
The present study was designed to evaluate the hypothesis that selective OP exposure to the brainstem is sufficient for OP-induced apnea. We used the isolated heart-brainstem preparation of the juvenile rat, in which the entire infracollicular brainstem was exposed to dichlorvos. We also tested the effects of selective exposure of the ventral medullary region to dichlorvos using microdialysis in intact anesthetized rats.
2.0 Materials and methods
2.1 Working Heart-Brainstem Preparation
The methodology used for establishing a working heart-brainstem preparation was described by Paton[18]. Wistar rats (85-120 grams) were acquired and cared for in accordance with NIH published guidelines and this research was conducted in accordance with the Guiding Principles in the Use of Animals in Toxicology. The Institutional Animal Care and Use Committee of the University of Massachusetts approved this study protocol. Animals were housed in pairs and maintained on a 12-hour light dark cycle with food and water provided ad libitum.
Rats (n=10) were exposed to anesthetic gas (isoflurane, Sigma Aldrich, St Louis, MO) in an anesthetic chamber until respiratory activity was depressed and there was no response to foot pinch. The animals were removed from the anesthetic chamber, hemisected immediately below the diaphragm, and the head, neck and thorax were immersed in iced artificial cerebrospinal fluid (125Mm NaCl, 5mM KCl, 1.25 mM MgSO4, 24 mM NaHCO3, 1.25 mM KH2PO4, 2.5 mM CaCl and 10mM D-glucose). The skull bone overlying the cerebral hemispheres and cerebellum was removed and the animal was decerebrated at the precollicular level, removing all brain tissue rostral to this transection with suction. The diaphragm was dissected from its attachments to the ribs and spine, and the anterior portion of the rib cage and sternum were removed, exposing the thoracic cavity. The left phrenic nerve was identified and isolated from its surrounding connective tissue. The lungs were removed and the animal was transferred to a recording chamber where the descending aorta was cannulated with a double lumen catheter. The animal was then artificially perfused through the catheter with warmed artificial CSF plus 1.25% ficoll (Sigma Aldrich, St Louis, MO) equilibrated with a 5% CO2/95% O2 gas mixture. The second lumen of the catheter was connected to a pressure transducer to monitor perfusion pressure.
Perfusion was provided by a peristaltic pump (Watson Marlow, Falmouth, Cornwall) circulating artificial perfusate in a circuit from an open reservoir, through the animal and back to the reservoir. The perfusate was warmed by passing through a heated water jacket (Neslab Instruments, Portsmouth, NH) and filtered by passing through a bubble trap and filter paper (Millipore, Billerica, MA). Perfusate seeped out of the open surgical sites of the animal before being collected in the basin of the recording chamber and pumped back to the reservoir of artificial CSF. The reservoir was continuously bubbled with a 5%CO2/95%O2 gas mixture.
Sustained respiratory activity was obtained after achieving appropriate perfusion pressure and temperature. The pump speed was increased gradually to achieve a target perfusion pressure between 40 and 60 mmHg. Once the perfusion pressure was within the target range, temperature was increased by elevating the temperature in the water jacket to a target temperature of 29°C at the aortic catheter. These settings have been shown in previous experiments to reliably produce rhythmic phrenic nerve activity in the preparation[19]. The phrenic nerve was draped over a bipolar electrode and encased in petroleum jelly. Respiratory rate and perfusion pressure were allowed to reach near-constant levels for 10 minutes prior to initiating the experiment. In this model, we defined apnea as the absence of phrenic burst activity for 20 seconds or longer.
2.2 Microdialysis into the Ventral Region of the Medulla
The experimental protocol used in this experiment was an adaptation of a previously published OP poisoning model[5]. Anesthesia was induced with 2.2% isoflurane (Abbott Labs, North Chicago, IL) in seven adult male Wistar rats (250-300 grams) and subsequently titrated to maintain a respiratory rate of 40-60 bpm with sufficient anesthesia monitored via foot pinch. Animals underwent a tracheostomy and breathed 100% oxygen spontaneously at all times. 100% oxygen was used to maximize the oxygenation during the poisoning period when respiratory rate decreased. During a baseline period the animals were anesthetized with a constant level of isoflurane (range 1.6-1.8%) that was not changed once the experiment began. Physiologic recordings included respiratory (airflow, respiratory rate, end-tidal Pco2, oxyhemoglobin saturation) and cardiovascular (blood pressure, pulse rate) parameters. All animals served as their own control with comparisons made between baseline values and values during the experiment. Data were recorded using an A-D converter and PC data acquisition system (ADI instruments, Colorado Springs, CO).
Animals were placed prone in a stereotactic apparatus with ear bars and a bite block (Kopf Instruments, Tugunga, Ca). The skin overlying the skull was incised and retracted from midline. Bilateral 2mm trephination holes were drilled in the skull over the posterior fossa using stereotactic coordinates (-3.3 intra-aural, 2 mm lateral). A microdialysis guide cannula (CMA Microdialysis, North Chelmsford, MA) was introduced into the holes to a depth of 6 mm. A 2 mm long, 100 kDa cut-off microdialysis probe was inserted into the guide cannula. If placement of the catheter induced changes in the respiratory or cardiovascular parameters, the animal was allowed to return to baseline values prior to proceeding. The animal was maintained at a baseline level for at least 20 minutes prior to initiating the experimental procedure.
2.3 Organophosphate Exposure and Controls
In the working heart-brainstem preparation 2,2-dichlorovinyl dimethyl phosphate (dichlorvos, C4H7Cl2O4P, Sigma-Aldritch, St Louis, MO) and control solutions were introduced into the reservoir of perfusate, exposing the brainstem and spinal cord to the OP via perfusion through the aorta. As an experimental control for each animal, 1 cc of vehicle alone (35% isopropyl alcohol) was introduced into the artificial perfusate reservoir. Ten minutes later, dichlorvos (50 mg in 1cc of 35% isopropyl alcohol) was introduced into the artificial perfusate reservoir. This dose of dichlorvos was chosen following a dose response curve in 3 animals. Briefly, animals underwent identical surgical procedures as described in the working heart-brainstem section above prior to exposure to escalating doses of dichlorvos. Individual animals were exposed to 1.2mg (animal 1), 12 mg, 24mg, 36mg (animal 2) and 50 mg (animal 3) to identify the lowest dose of dichlorvos that resulted in apnea. Artificial perfusion pressure, fictive respiratory rate and tidal volume (calculated from phrenic nerve activity) were monitored for changes. Physiologic variables were allowed to return to baseline between control and experimental exposures.
In the microdialysis experiments the dichlorvos was microdialyzed directly into the ventral region of the medulla. Dialysis catheters inserted into bilateral guide cannulae were attached to a syringe pump filled with the dialysis solution. The dialysis solution contained 12.5 mg/cc of dichlorvos mixed from a stock solution of 25 mg of dichlorvos dissolved in 1 cc of 35% isopropyl alcohol and 1cc of CNS perfusion fluid (CMA Microdialysis, North Chelmsford, MA). The dichlorvos solution was dialyzed at 5 μl per minute for a total of 12 minutes for a cumulative dose of 1.5 mg.
The primary endpoint of the microdialysis study was the interval from the onset of infusion of dichlorvos to the onset of apnea. Secondary endpoints included changes of respiratory and cardiovascular parameters from baseline. Apnea was defined as a cessation of airflow through the pneumotachometer for more than 20 seconds. Physiologic recordings continued for 10 minutes after the completion of microdialysis or until death occurred.
Confirmation of the probe placement was accomplished using KMnO4 to mark the location of the center of the microdialysis probe membrane[20]. Following the termination of the experiment, the dialysis probes were removed from the guide cannulae. The bilateral guide cannulae remained clamped in stereotactic holders and in place during the removal of the microdialysis catheter. Needles extending 1mm beyond the tip of the guide cannulae were connected via tubing to a syringe pump (CMA Microdialysis, North Chelmsford, MA) and inserted into the guide cannulae. KMnO4 (40mg/cc) was micro-injected at 8.3 μl/min for 5 min resulting in a direct oxidation of cells at the tip of the needle and a precipitation of manganese dioxide visible to the naked eye in brain tissue sliced in a coronal plane. The animal was perfused with 4% paraformaldehyde, and the brain was removed for sectioning and identification of probe tip location. The brain was cut into 2mm thick coronal sections and the location of the preBötC was identified using identification of the obex[21]. Thinner slices were not feasible due to integrity of the slice during handling.
Control experiments for the microdialysis preparation were performed on 6 additional rats prior to dichlorvos exposure. To control for possible effects of the vehicle, vehicle alone (1:1 mixture of 35% isopropyl alcohol and CNS perfusion fluid) was dialyzed for 12 minutes at 5 μl per minute through dialysis catheters placed in the medulla. Respiratory and cardiovascular parameters were compared to the animal's baseline values. To minimize the number of experimental animals as requested by our Institutional Animal Care and Use Committee, the minimum number of animals that allowed a statistically significant comparison was used as controls.
2.4 Measurement of Dichlorvos Concentration in Brain Tissue
Dichlorvos exposure post-dialysis is related to a number of unknown factors such as the permeability of the dialysis membrane to dichlorvos and diffusion constant of dichlorvos in brain tissue. Direct measurements of tissue dichlorvos levels in the brainstem were performed to determine the extent to which microdialysis caused significant dichlorvos exposure to areas of the brainstem remote from the microdialysis membrane. A single animal (n=1) underwent dialysis of dichlorvos into the ventral medulla as described above and was decapitated immediately after the dialysis protocol was completed. The physiologic data from this animal was not included in the results. The brainstem was removed and frozen at -20°C, sectioned into 2mm slices, cut into 1mm by 1mm square sections, weighed and refrozen at -20°C and sent for analysis. All samples of the brainstem, as well as samples of a similar size from the forebrain, were sent for determination of dichlorvos levels using a gas chromatography mass spectrometer (GCMS). As a control, samples of forebrain tissue from a naive animal (no intervention) were also sent for a determination of dichlorvos level.
Tissue samples were stored at -80° C prior to extraction. Dichlorvos was extracted from the brain samples with ethyl acetate containing d6-dichlorvos internal standard and the extract was directly analyzed by selected ion monitoring electron impact GCMS using a modification of the method described by Ma et al [22]. Brain samples were extracted by maceration and 5 min of sonication in a bath sonicator with 100 μl of ethyl acetate containing 7.5 ng of d6- dichlorvos internal standard. The extract was cleared by centrifugation for 5 min and the clear supernatant transferred to autosampler vials for GCMS analysis. One μl of each sample was injected onto a 30 mm × 0.25 mm ID capillary column that was connected directly to the ion source of a Waters Quattro-II triple quadrupole mass spectrometer (Milford, MA). Helium was used as the carrier gas at a constant flow of 1 ml/min and the column temperature was programmed from 75° C to 130° C at 30° C/min and held at 130° for 3.7 min. Electron impact ionization (EI) was used at 70 eV with the source at 225° C. Selected ion monitoring of m/z (mass to charge ratio of the ion) 108.9 (dwell 0.3 sec) for dichlorvos and m/z 114.9 (dwell 0.03 sec) for the internal standard. External calibration standards containing 0.25, 0.5 and 1.0 μg/ml of dichlorvos and 0.5 μg/ml internal standard were also analyzed before and after each 10 samples. The amount of dichlorvos in each tissue sample was calculated from the ratio of the dichlorvos peak area to that of the internal standard multiplied by 7.5 ng.
2.5 Data Analysis
2.5.1 Working heart-brainstem Preparation
Data were obtained using an A-D hardware and data acquisition software system (ADI Instruments, Colorado Springs, CO). In this manuscript we define fictive respiratory rate and fictive tidal volume as the neural equivalent of respiratory rate and volume using previously published calculations of phrenic activity[23]. Raw phrenic activity was filtered (Humbug Noise Eliminator, Vancouver, BC) and amplified (Cyberamp 320, Molecular Devices, Sunnyvale, CA) prior to display. Phrenic nerve activity was whole wave rectified and step-wise integrated over 50 ms intervals. The onset of the inspiratory phase of activity was defined when the integrated signal exceeded 3 standard deviations above the mean of the signal during the mid- to late-expiratory phase. Fictive tidal volume was calculated as a percentage of baseline, with 100% defined as peak integrated phrenic activity and 0% as zero. Fictive respiratory rate was calculated as phrenic bursts per minute. Results are presented as mean (± SD) unless otherwise noted. Data were examined for normal distribution using a Lilliefors test. Comparisons of values between baseline and post-exposure were performed using a repeated-measures ANOVA (comparing effects over time), Student t-test or Wilcoxon signed-rank test (comparing final results at endpoint of study) where appropriate.
2.5.2 Microdialysis
Respiratory and cardiovascular data during dialysis were normalized to pre-dialysis baseline and are presented as mean (±SD) unless otherwise noted. Comparisons between baseline and post-exposure for respiratory and cardiovascular parameters were performed using Wilcoxon Signed Rank Test. A comparison of effect of the vehicle (isopropyl alcohol) and dichlorvos was performed using repeated-measures ANOVA.
3.0 Results
3.1 Working heart-brainstem Preparation
Respiratory activity started after reaching an average perfusion pressure of 44.6 mmHg (±8.7) and stabilized at a respiratory rate of 17.8 bpm (± 3.0) during the baseline period. During the baseline period the respiratory activity was characterized by an inspiratory duration and time between phrenic bursts of 1.42 (±0.36) sec and 2.02 (± 0.47) sec respectively. The phrenic burst demonstrated a time to peak discharge of 0.57 (±0.3) sec.
There was no statistically significant change in fictive respiratory rate or fictive tidal volume with exposure to vehicle alone. Respiratory activity began falling around 2 minutes after dichlorvos was introduced into the reservoir (figure 1). Nine of 10 animals demonstrated a steady decline in respiratory rate and eventual apnea an average of 3 min 42 sec (±2 min 48 sec) post dichlorvos exposure. Respiratory rate and fictive tidal volume decreased in all animals 33% (±44.9) and 29% (±42.4) respectively within two minutes of poisoning.
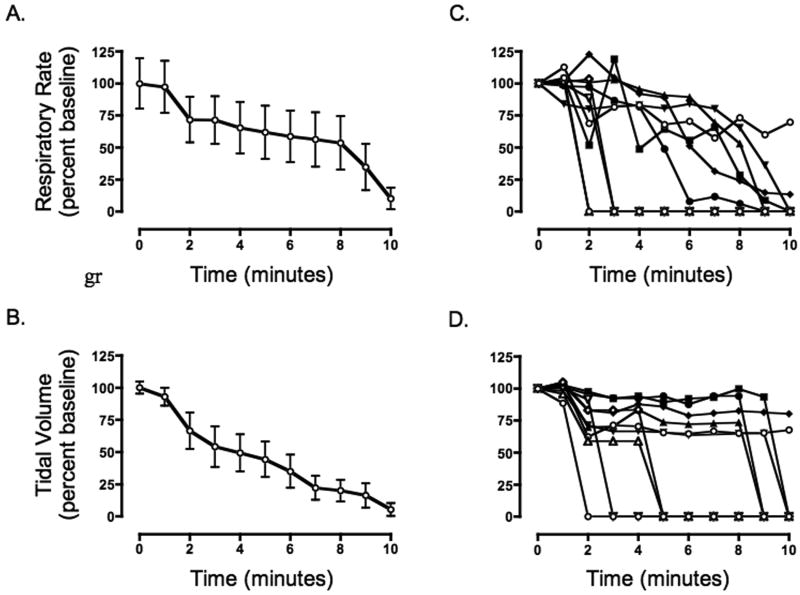
Figure 1. A-E - Fictive respiratory activity post dichlorvos in the working heart brainstem preparation.
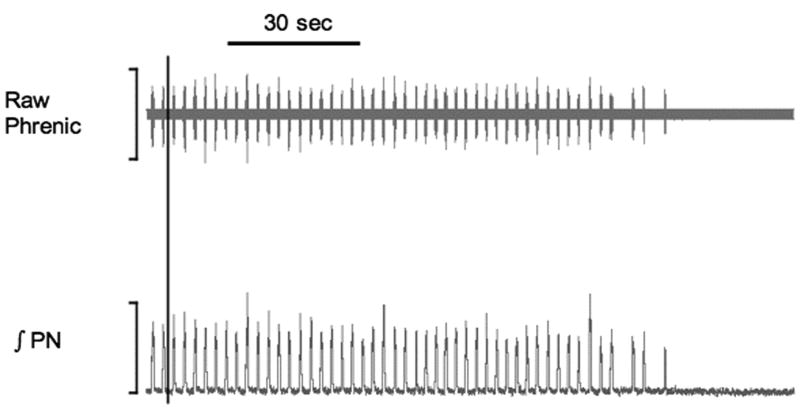
Fictive respiratory rate and tidal volume were calculated from an analysis of phrenic nerve activity. Dichlorvos was introduced into the reservoir at time zero. Values are presented at a percentage of baseline activity with 100% equal to peak phrenic discharge (tidal volume) or rate of phrenic discharge (respiratory rate) during baseline period. A,B. Both fictive respiration and fictive tidal volume drop significantly at 10 min post exposure to dichlorvos, (n=10, p=0.0001 and p=0.0002, one sample t-test). C,D. Plots for each animal demonstrate the heterogeneity of the poisoning model. E. A sample tracing of single animal phrenic nerve activity is included (raw (above), integrated (below)) both before poisoning and post-poisoning. Vertical black line represents dichlorvos exposure.
Respiratory activity resumed post apnea in 8 of the 9 animals that demonstrated apnea. Respiratory activity resumed an average of 11 min (±11 min 54 sec) after the point of apnea with some animals demonstrating a sustained respiratory activity (n=5) and others a short burst of respiratory activity followed by a resumption of apnea (n=3). Animals that demonstrated sustained post-apneic respiratory activity had phrenic bursts at a regular rate of 12.8 (±4.3) breaths per minute. The resumption of respiratory activity started regular but slow and the rate increased gradually over time until the termination of the experiment one-hour post dichlorvos exposure.
Following dichlorvos exposure we noted a rise in perfusion pressure despite constant pump speed. Perfusion pressure increased 34% over baseline in all animals (10 of 10), peaking 1 min 43 sec (±17 sec) post exposure. The perfusion pressure showed no change during exposure to vehicle alone.
3.2 Microdialysis into the PreBötC Region
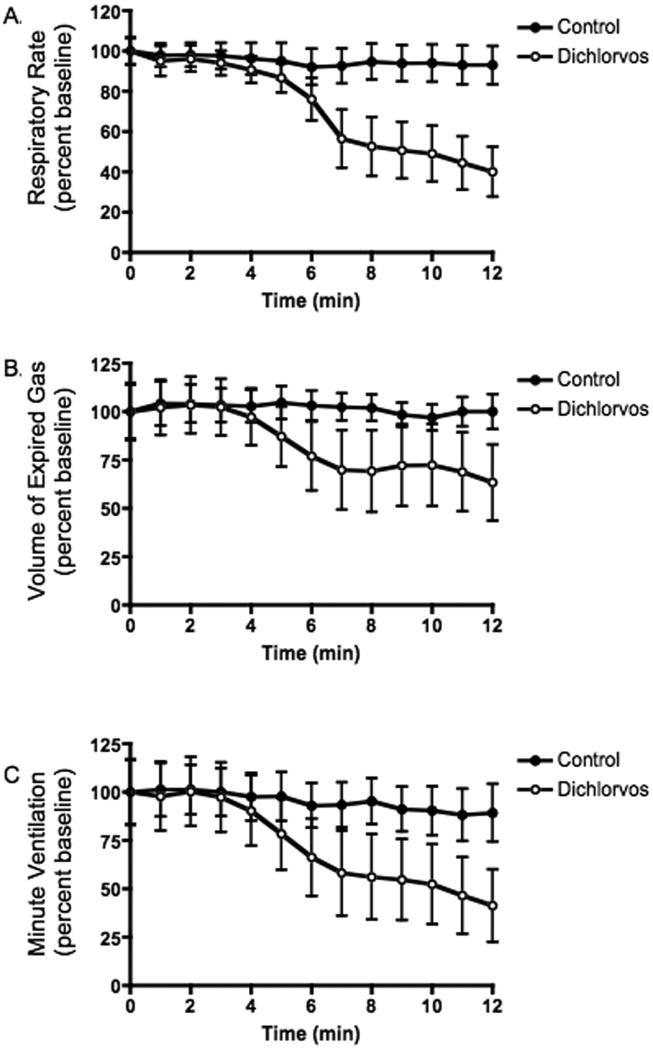
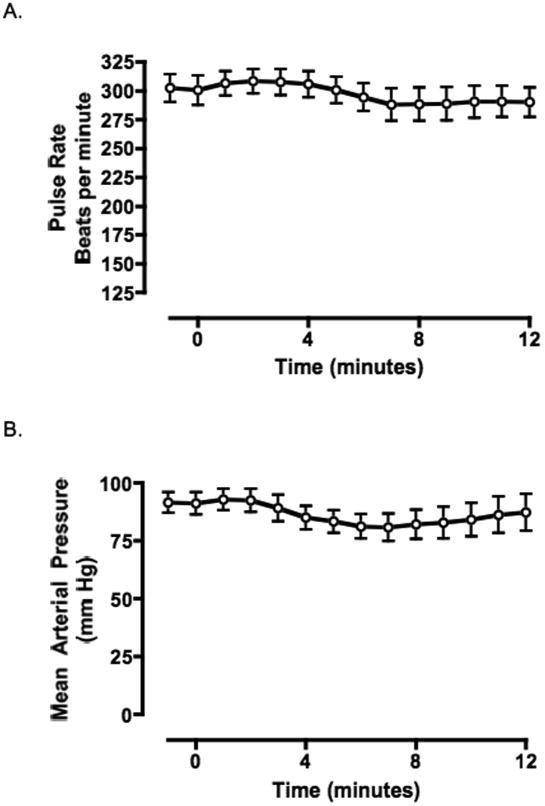
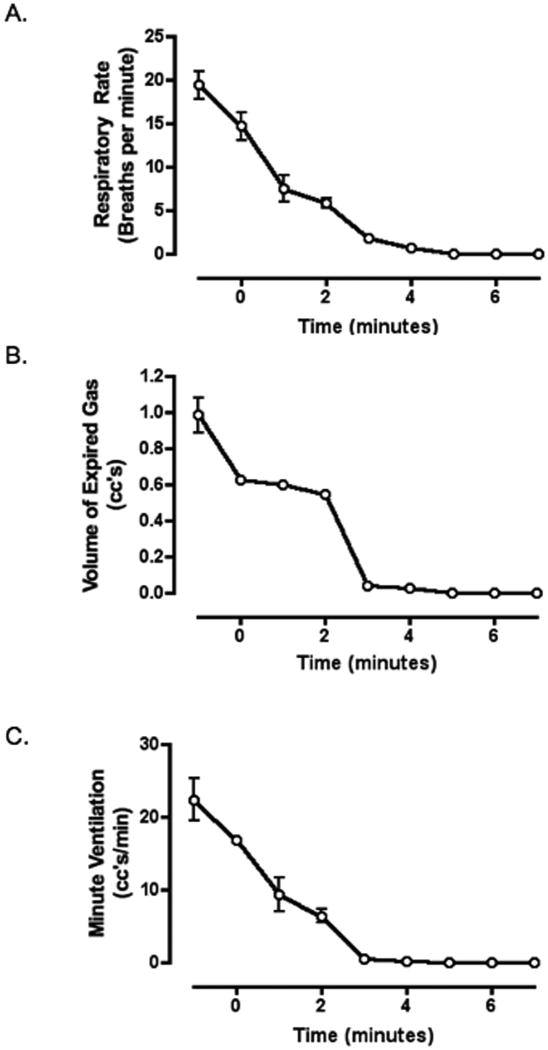
Microdialysis of dichlorvos into the ventral region of the medulla resulted in a progressive decrease in respiratory rate, volume of expired gas and minute ventilation (figure 2) but no change in mean arterial pressure or pulse rate (figure 3). For a sample recording of physiologic parameters see figure 4. Apnea was seen in 28.6 percent of the animals (2 of 7) an average of 9 min 52 sec after the initiation of dialysis. In all animals, average respiratory rate, volume of expired gas and minute ventilation each declined 45% or more by the end of the study.
Figure 2. ABC- Respiratory rate, volume of expired gas and minute ventilation post dialysis of dichlorvos and vehicle into the ventral medulla.
Microdialysis of dichlorvos was initiated at time zero. Data represent an average of all experimental (dichlorvos) animals (open circles, n=7) and control (vehicle only) animals (filled circles, n=6). All respiratory measurements decreased significantly post exposure (p<0.0001, repeated measures ANOVA). The respiratory rate (A) and volume of expired gas (B) were significantly different, comparing dichlorvos with vehicle alone (p<0.04, ANOVA). There was no significant difference in minute ventilation (C) between groups (p=0.12, ANOVA).
Figure 3. AB - Pulse rate and mean arterial pressure post dialysis of dichlorvos into ventral medulla.
Microdialysis of dichlorvos was initiated at time zero. Data represent an average of all animals (n=7). Blood pressure and pulse showed no significant change from baseline (p=0.46, repeated measures ANOVA).
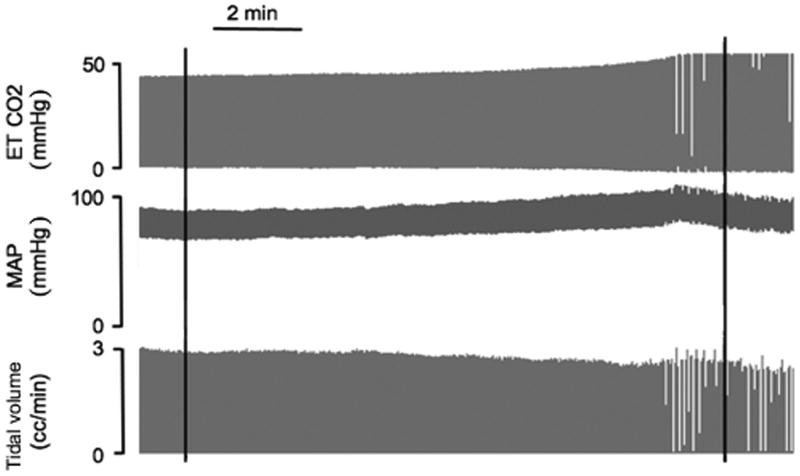
Figure 4. Sample tracing of single animal recording in animal exposed to dichlorvos via microdialysis into the ventral medulla.
Physiologic recordings from a single animal during microdialysis of dichlorvos into the ventral medulla with end-tidal carbon dioxide (ETCO2), Mean arterial pressure (MAP) and calculated tidal volume. ETCO2 and MAP are presented as millimeters of mercury (mmHg). Tidal volume was calculated as cc's. Dichlorvos microdialysis was initiated at the first black line and completed at the second black line. For this animal, respiratory rate declined during the microdialysis from a rate of 44 breaths per minute during the baseline period to 18 breaths per minute post microdialysis.
Microdialysis of vehicle alone (35% isopropyl alcohol in artificial CNS fluid, n=6) caused a change in respiratory output, but did not cause apnea. Animals in the control group showed no statistical change in volume of expired gas or minute ventilation but they did show an average 9% decrease in respiratory rate (figure 2). The respiratory depressant effects (volume of expired gas and respiratory rate) of dichlorvos dialysis were significantly greater than vehicle alone. (p=0.036, ANOVA).
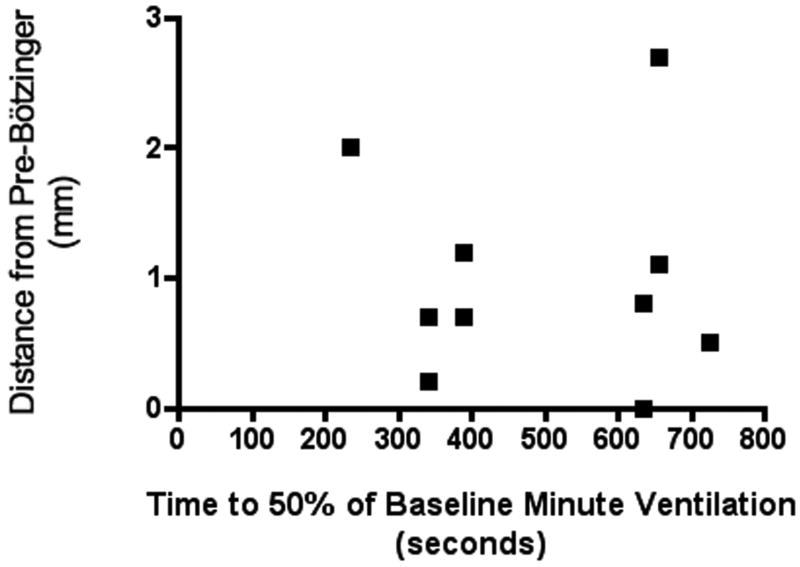
KMnO4 injection successfully marked the location of the microdialysis catheter tip in 72% of animals. In the remaining cases injection of the KMnO4 failed due to obstruction within the guide cannulae. The distribution of microdialysis catheter tip placements is depicted in figure 5. A scatter plot of time to 50% reduction of minute ventilation vs distance of the catheter tip from preBötC (figure 6) shows no correlation between the two data sets. KMnO4 injection caused localized tissue coagulation and resulted in apnea in 83% of the animals that were spontaneously breathing prior to the injection of KMnO4 (figure 7). In animals that were spontaneously breathing prior to the KMnO4, apnea occurred an average of 1 min 34 sec post-initiation of injection, with three of the animals becoming apneic within 6 seconds of initiating the KMnO4 injection.
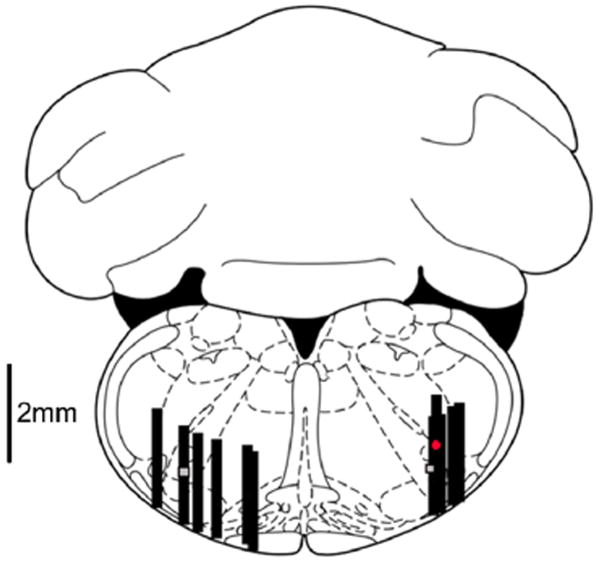
Figure 5. Microdialysis probe locations.
Dialysis probe tip locations (n=10) were marked using microinjection of KMnO4 at the midpoint of the 2mm × 0.4mm dialysis membrane. The level of the brainstem slice depicted in the drawing is 3.3 mm caudal to intra-aural. The grey squares represent the region of the preBötC. Black rectangles represent the 2mm dialysis membrane. The red circle represents an injection site 2mm cephalad to the preBötC. Animals with inadequate KMnO4 injection resulting in an insufficient lesion on the histological specimen were not included. The figure is adapted from a digital rat brain atlas (Paxinos & Watson, 2008).
Figure 6. Relationship of changes in minute ventilation and average distance from preBötC.
The distance of the probe location from the preBötC was measured in three dimensions and plotted against time to 50% of baseline minute ventilation. Each dot represents an average of both sites for a single animal. Animals with inadequate KMnO4 injection resulting in an insufficient lesion on the histological specimen were not included.
Figure 7. ABC – Respiratory rate, volume of expired gas and minute ventilation post injection of KMnO4 into the ventral medulla.
Microinjection of KMnO4 was initiated at time zero. Data represents an average for vehicle alone (2 of 2) and experimental (3 of 4) animals that were breathing at the end of the experiment. Animals that were not breathing at the beginning of the KMnO4 injection were not included in this data set. Respiratory parameters showed a significant change from baseline (p<0.05, Wilcoxon Signed Rank Test)
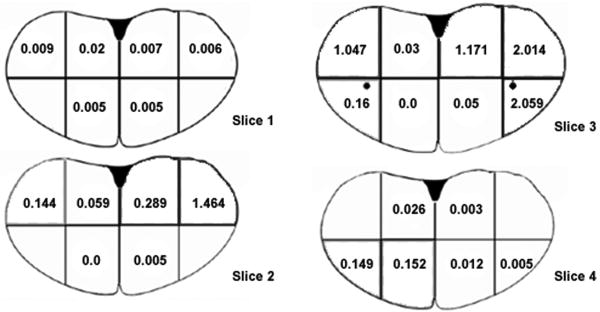
Dichlorvos levels in the brainstem are depicted in figure 8. The areas of the brainstem closest to the dialysis catheter showed elevated levels of dichlorvos, but dichlorvos was detected throughout the brainstem. Two samples taken from the forebrain of a naïve animal showed levels of dichlorvos of 0 and 0.02 μg/g.
Figure 8. Amount of dichlorvos in brainstem following microdialysis of dichlorvos into the ventral medulla.
Microdialysis of dichlorvos (bilateral 750 μg over 12 minutes) was performed prior to sectioning the brainstem of a single animal along solid lines. The dichlorvos in each sample (1mm × 1mm × 2mm) was extracted and measured via gas chromatography-mass spectrometer. The results are presented as μg of dichlorvos per gram of brain tissue. Brain slices are number from rostral (slice 1) to caudal (slice 4). The region of the preBötC is depicted by a black circle.
4.0 Discussion
Dichlorvos exposure of the brainstem and spinal cord in the working heart-brainstem preparation resulted in a cessation of respiratory activity. The working heart-brainstem preparation contains all respiratory-related brainstem sites as well as the thoracic and cervical spinal cord. This animal preparation retains the connections within the brainstem, but lacks descending input from the cerebral hemispheres (decerebration) or afferent input from the lungs (bilateral pneumonectomy). Our findings imply that feedforward respiratory inhibition secondary to cerebral hemispheric ictal activity[24] is not a necessary component of dichlorvos-induced central apnea. It is possible that spinal circuits are involved in OP-induced central apnea[25] as they remain present in this poisoning model, but the involvement of brainstem circuits seems more likely because of our observation that apnea was preceded by progressive slowing of rhythmic activity. The brainstem contains many sites involved in respiratory control but it remains unclear which specific site in the brainstem is both necessary and sufficient for OP-induced central apnea and the role of specific sub-regions within the brainstem remains to be defined.
Previous studies have shown that bilateral ablation of the preBötC results in prolonged apnea[26] and unilateral ablation of the preBötC results in transient apnea[11]. Shao and Feldman demonstrated respiratory excitation after exposure of the preBötC to an acetylcholinesterase inhibitor[27], but these findings are the reverse of what is seen following a clinical exposure. This difference in findings may reflect differences in experimental models. Their studies involved a medullary slice preparation without an intact ponto-medullary respiratory neural network, and the perturbations in ACh levels were designed to mimic changes within a physiological range rather than the acute toxic levels achieved in OP poisoning. Another consideration is that OP exposure to other respiratory-related neurons in the medulla may result in a modulation of afferent input, resulting in respiratory depression. For example, stimulation of peripheral muscle afferent fibers (as would occur during muscle fasciculation) has an inhibitory effect on respiratory activity[25] and it is unknown how these inputs are modulated centrally by the poison.
Microdialysis of dichlorvos into the ventral aspect of the medulla in the region of the preBötC did not result in apnea. Following microdialysis, animals demonstrated modest respiratory depression; less than a third of the animals became apneic after prolonged exposure, in contrast to the rapid and uniformly fatal development of central apnea following parenteral OP exposure[5]. This implies that the dichlorvos exposure in this study did not silence either preBötCs. This lack of apnea may be explained functionally (dichlorvos exposure of the preBötC is not required for OP-induced apnea) or methodologically (methodology did not provide a sufficient level of OP exposure to the preBötC).
It is possible that methodological limitations of microdialysis affected preBötC exposure to dichlorvos. The exact brain tissue levels of dichlorvos associated with a lethal poisoning is not known, so we used an extrapolation of data from a previous study that measured brain tissue levels following sub-lethal doses.[24] This assumes a linear correlation between exposure (dose) and tissue levels that has not been verified. In addition, the microdialysis probes were stereotactically placed at varying distances to the preBötC and there was a lack of histological staining for the preBötC to corroborate the accuracy of probe placement. Also, the methodology involved in measuring tissue dichlorvos levels is technically challenging. The clearance of dichlorvos from brain tissue is rapid (with a half-life of ∼15 min[28]); therefore our measurements of tissue levels may be significantly lower than actual peak levels achieved during in vivo microdialysis because of the inherent delays in euthanizing the animals and preparing the tissue blocks prior to freezing. Questions concerning the accuracy of probe placement, lethality of dichlorvos levels and challenges in accurately measuring dichlorvos levels limit our ability to draw firm conclusions concerning the role of the preBötC in OP-induced apnea.
A number of lines of evidence support the possibility that a wider exposure of the brainstem, and not an isolated exposure of the preBötC, is required for OP-induced apnea. First, there was no relationship between respiratory effects of dichlorvos and the distance from the preBötC to the dialysis-membrane, implying the preBötC is not the area within the brainstem that is necessary for OP-induced apnea. In addition, respiratory depression was delayed from the initiation of microdialysis, implying a diffuse exposure of the brainstem, or exposure to an area remote from the dialysis membrane. Microdialysis produces a diffusion gradient extending away from the surface of the dialysis membrane that expands as the volume of dialysis (and time of dialysis) increases. Third, microdialysis of dichlorvos through bilateral catheter sites within the ventral region of the medulla in the area of the preBötC resulted in measurable levels of dichlorvos throughout the brainstem. If our assumptions used to extrapolate a lethal dichlorvos level are valid (see above) then we would conclude that toxic levels of dichlorvos were achieved throughout the lower brainstem. It is difficult to determine which respiratory-related sites within the brainstem were exposed, as direct histological identification of subnuclei was not performed in this study, but it is likely that regions of the ventral respiratory group and regions of the dorsal respiratory group were exposed. It then follows that our findings of only modest and delayed respiratory depression suggest that the region of the ventral medulla, including the preBötC is not the necessary toxic site for dichlorvos-induced central apnea.
Limitations of microdialysis
Microdialysis is an inherently imprecise method for introducing a compound into a biological tissue as evidenced by the higher dorsal levels of dichlorvos despite ventral placement of the catheter membranes. Our findings suggest that microdialysis may not be the optimal technique to use when addressing questions concerning specific brainstem respiratory sites and OP-induced apnea, as it does not allow sufficient and reliable targeting of specific areas such as the preBötC. The large catheter size (2mm) and the reliance on diffusion for dichlorvos delivery are major limitations.
In summary, dichlorvos exposure of the entire rat brainstem is sufficient for OP-induced central apnea. Our preliminary conclusion from the microdialysis experiments is that a wider exposure of the brainstem beyond the region of the preBötC may be required for OP-induced apnea. However we cannot exclude the possibility that dichlorvos dialyzed into the region of the preBötC did not reach toxic levels.
5.0 Future Directions
Microinjection studies might allow more targeted exposure of discrete regions of the brainstem to determine which sites are both necessary and sufficient for OP-induced apnea. Alternative sites include areas in the medulla and pons involved in respiratory control. The pontine reticular formation is involved in the modulation of respiratory activity during sleep [29] and respiratory depression occurs when stimulated by elevated levels of acetylcholine[30]. Other sites in the ventral medulla produce an increase in respiration when stimulated with acetylcholine[12] but a exposure to a combination of sites may be responsible for apnea.
Acknowledgments
We would like to thank Drs. Julian Paton and Walter St. John, and Ms. Allison Rudkin for their advice and assistance in the artificial perfusion techniques. Dr. Barbara Evans in the UMMS Proteomic & Mass Spectrometry Core Facility performed the dichlorvos measurements. Dr. James Evans helped determine the appropriate technique for measuring dichlorvos levels in the tissue samples. Ian Zuarte helped in conditioning and filtering phrenic nerve recordings.
Funding: This work was supported by the National Institutes of Neurological Diseases and Stroke at the National Institutes of Health [grant number K08-NS48857] and the Millennium Program at the University of Massachusetts Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Romolo J Gaspari, Department of Emergency Medicine, University of Massachusetts, Worcester, MA 01655.
David Paydarfar, Email: David.Paydarfar@umassmed.edu, Department of Neurology, University of Massachusetts, Worcester, MA 01655.
References
- 1.Khan MM. Suicide on the Indian subcontinent. Crisis. 2002;23(3):104–7. doi: 10.1027//0227-5910.23.3.104. [DOI] [PubMed] [Google Scholar]
- 2.Murali R, Bhalla A, Singh D, et al. Acute pesticide poisoning: 15 years experience of a large North-West Indian hospital. Clin Toxicol (Phila) 2009;47(1):35–8. doi: 10.1080/15563650701885807. [DOI] [PubMed] [Google Scholar]
- 3.Phillips MR, Li X, Zhang Y. Suicide rates in China, 1995-99. Lancet. 2002;359(9309):835–40. doi: 10.1016/S0140-6736(02)07954-0. [DOI] [PubMed] [Google Scholar]
- 4.Tsai JR, Sheu CC, Cheng MH, et al. Organophosphate poisoning: 10 years of experience in southern Taiwan. Kaohsiung J Med Sci. 2007;23(3):112–9. doi: 10.1016/S1607-551X(09)70385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspari RJ, Paydarfar D. Pathophysiology of respiratory failure following acute dichlorvos poisoning in a rodent model. Neurotoxicology. 2007;28(3):664–71. doi: 10.1016/j.neuro.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuovinen K. Organophosphate-induced convulsions and prevention of neuropathological damages. Toxicology. 2004;196(1-2):31–9. doi: 10.1016/j.tox.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Joosen MJ, Jousma E, van den Boom TM, et al. Long-term cognitive deficits accompanied by reduced neurogenesis after soman poisoning. Neurotoxicology. 2009;30(1):72–80. doi: 10.1016/j.neuro.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Feldman JL, Janczewski WA. Point:Counterpoint: The parafacial respiratory group (pFRG)/pre-Bötzinger complex (preBötC) is the primary site of respiratory rhythm generation in the mammal. Counterpoint: the preBötC is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100(6):2096–7. doi: 10.1152/japplphysiol.00119.2006. discussion 2097-9, 2103-8. [DOI] [PubMed] [Google Scholar]
- 9.Onimaru H, Homma I. Point:Counterpoint: The parafacial respiratory group (pFRG)/pre-Bötzinger complex (preBötC) is the primary site of respiratory rhythm generation in the mammal. Point: the PFRG is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100(6):2094–5. doi: 10.1152/japplphysiol.00119.2006. [DOI] [PubMed] [Google Scholar]
- 10.Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J Neurophysiol. 2000;83(3):1243–52. doi: 10.1152/jn.2000.83.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St-Jacques R, St-John WM. Transient, reversible apnoea following ablation of the pre-Bötzinger complex in rats. J Physiol. 1999;520 Pt 1:303–14. doi: 10.1111/j.1469-7793.1999.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nattie EE, Wood J, Mega A, et al. Rostral ventrolateral medulla muscarinic receptor involvement in central ventilatory chemosensitivity. J Appl Physiol. 1989;66(3):1462–70. doi: 10.1152/jappl.1989.66.3.1462. [DOI] [PubMed] [Google Scholar]
- 13.Jordan D, Spyer KM. Effects of acetylcholine on respiratory neurones in the nucleus ambiguus-retroambigualis complex of the cat. J Physiol. 1981;320:103–11. doi: 10.1113/jphysiol.1981.sp013937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499(1):64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- 15.Kok A. REM sleep pathways and anticholinesterase intoxication: a mechanism for nerve agent-induced, central respiratory failure. Med Hypotheses. 1993;41(2):141–9. doi: 10.1016/0306-9877(93)90061-t. [DOI] [PubMed] [Google Scholar]
- 16.Gillis RA, Walton DP, Quest JA, et al. Cardiorespiratory effects produced by activation of cholinergic muscarinic receptors on the ventral surface of the medulla. J Pharmacol Exp Ther. 1988;247(2):765–73. [PubMed] [Google Scholar]
- 17.Taguchi O, Kubin L, Pack AI. Evocation of postural atonia and respiratory depression by pontine carbachol in the decerebrate rat. Brain Res. 1992;595(1):107–15. doi: 10.1016/0006-8993(92)91458-q. [DOI] [PubMed] [Google Scholar]
- 18.Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65(1):63–8. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 19.Pickering AE, Paton JF. A decerebrate, artificially-perfused in situ preparation of rat: Utility for the study of autonomic and nociceptive processing. J Neurosci Methods. 2006;155(2):260–71. doi: 10.1016/j.jneumeth.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Sun M, Hildebrandt L, Filiano J. Potassium permanganate can mark the site of microdialysis in brain sections. J Histotechnology. 2000;23(2):151–154. [Google Scholar]
- 21.Smith JC, Ellenberger HH, Ballanyi K, et al. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254(5032):726–9. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma J, Xiao R, Li J, et al. Determination of organophosphorus pesticides in underground water by SPE-GC-MS. J Chromatogr Sci. 2009;47(2):110–5. doi: 10.1093/chromsci/47.2.110. [DOI] [PubMed] [Google Scholar]
- 23.Eldridge FL. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971;221(2):535–43. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- 24.Paydarfar D, Eldridge FL, Wagner PG, et al. Neural respiratory responses to cortically induced seizures in cats. Respir Physiol. 1992;89(2):225–37. doi: 10.1016/0034-5687(92)90052-x. [DOI] [PubMed] [Google Scholar]
- 25.Eldridge FL, Gill-Kumar P, Millhorn DE, et al. Spinal inhibition of phrenic motoneurones by stimulation of afferents from peripheral muscles. J Physiol. 1981;311:67–79. doi: 10.1113/jphysiol.1981.sp013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray PA, Janczewski WA, Mellen N, et al. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4(9):927–30. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao XM, Feldman JL. Cholinergic neurotransmission in the preBötzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience. 2005;130(4):1069–81. doi: 10.1016/j.neuroscience.2004.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simeon V, Skrinjaric-Spoljar M, Wilhelm K. Reactivation of phosphorylated cholinesterases in vitro and protecting effects in vivo of some pyridinium and quinolinium oximes. Arh Hig Rada Toksikol. 1973;24(1):11–8. [PubMed] [Google Scholar]
- 29.Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol. 2002;131(1-2):135–44. doi: 10.1016/s1569-9048(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 30.Fung ML, St John WM. Pontine cholinergic respiratory depression in neonatal and young rats. Life Sci. 1998;62(24):2249–56. doi: 10.1016/s0024-3205(98)00203-3. [DOI] [PubMed] [Google Scholar]