Abstract
Neurodegenerative diseases represent a growing public health challenge. Current medications treat symptoms, but none halt or retard neurodegeneration. The recent advent of pluripotent cell biology has opened new avenues for neurodegenerative disease research. The greatest potential for induced pluripotent cells derived from affected individuals is likely to be their utility for modeling and understanding the mechanisms underlying neurodegenerative processes, and for searching for new treatments, including cell replacement therapies. However, much work remains to be done before pluripotent cells can be used for preclinical and clinical applications. Here we discuss the challenges of generating specific neural cell subtypes from pluripotent stem cells, the use of pluripotent stem cells to model both cell-autonomous and non-cell-autonomous mechanisms of neurodegeneration, whether adult-onset neurodegeneration can be emulated in short-term cultures and the hurdles of cell replacement therapy. Progress in these four areas will substantially accelerate effective application of pluripotent stem cells.
Neurodegenerative diseases represent a large group of heterogeneous disorders in which specific neuronal subtypes degenerate and die1. In most cases, these disorders arise for unknown reasons and progress relentlessly1. According to the 2005 report from the World Health Organization (WHO), neurodegenerative disorders such as Alzheimer’s disease, other dementias and Parkinson’s disease made up more than 14% of the global burden of neurological illnesses2, and their incidence is expected to rise as the life expectancy in industrialized countries rises. Current drugs provide only limited benefit by alleviating certain symptoms. Moreover, their chronic use is often associated with serious side effects, and none seems to modify the natural course of these diseases. Clearly, the development of efficacious preventative or protective therapies is impeded by our limited knowledge of the neurobiology of these grave conditions and the lack of more faithful assay systems.
Recent advances in cell biology have fueled the prospect that perhaps the difficulty in unraveling the disease mechanisms that underpin neurodegenerative disorders—due in part to the limited access to human nerve cells—could finally be overcome thanks to the availability of pluripotent stem cells. Indeed, these kinds of human cells can, in principle, be differentiated into any fetal or adult cell types, including degenerating neural cells. To date, only two methods have been validated for the generation of human pluripotent cells: the derivation of human embryonic stem (ES) cells from the inner cell mass of preimplantation embryos that have been discarded from in vitro fertilization clinics, and the conversion of somatic cells, such as skin fibroblasts, into induced pluripotent stem (iPS) cells by genetic manipulations3,4. Routinely, iPS cells are generated using a cocktail of overexpressed transcription factors transferred to the recipient cells by transfection or viral vector infection; improved methods that avoid genetic modification of donor cells are under development5–7.
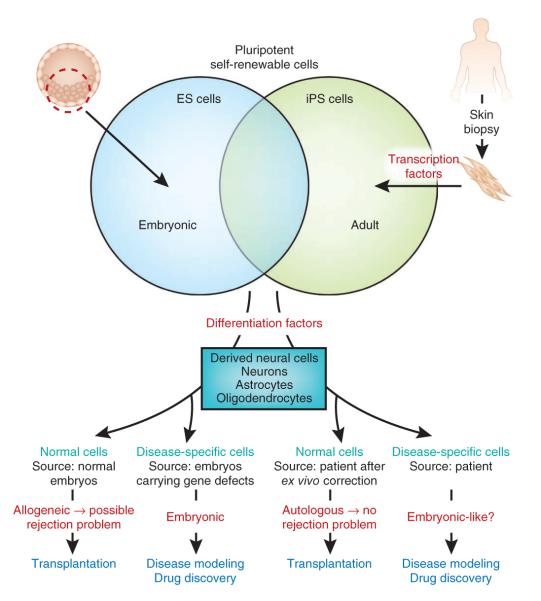
Comparisons of ES and iPS cells (see ref. 8 for detailed discussion) demonstrate that these cell types are genetically and epigenetically similar albeit not identical. They share the ability to self renew indefinitely while maintaining the potential to differentiate into cells from lineages of all germ layers (Fig. 1). The undisputed advantage of iPS cells over ES cells is the possibility of modeling neurological diseases by deriving pluripotent cells directly from somatic cells of affected individuals (Fig. 1). Pluripotent stem cells have already been derived from individuals carrying inherited defects9–12, as well as from those with other common neurological disorders such as sporadic Parkinson’s disease13,14, thus offering the unprecedented opportunity to gain insights into disease mechanisms and to search for new drugs using human disease-specific cell lines (Fig. 1). iPS cells may also provide the chance to obtain a renewable source of healthy cells to treat neurological disorders (Fig. 1), a view that is supported by the demonstration that human pluripotent cell–derived dopaminergic neurons alleviate some of the locomotor abnormalities seen in a rat model of Parkinson’s disease13,15. Below, we will discuss areas in which pluripotent cells—especially iPS cells—show the greatest promise for therapy and consider the challenges that need to be addressed before the full clinical potential of these cells can be realized.
Figure 1.
Pluripotent cells are obtained either directly from ES cells or indirectly from somatic cells such as skin fibroblasts transformed into iPS cells by exposure to transcription factors. Both ES and iPS cells can be coaxed into a variety of neural cell types. Once produced and, if necessary, purified, these neural cells could be used for disease modeling if the ES cells originate from embryos that carry the disease-causing defect, or if the iPS cells derive from an individual with the disease. These neural cells could also be used for reparative therapies. However, in this case, neural cells deriving from normal ES cells may trigger a rejection reaction (owing to their allogeneic nature with respect to the host), and neural cells deriving from patient iPS cells may have to undergo ex vivo reprogramming of the disease-causing defect before being used.
Modeling neurodegenerative disease
The discovery of causal genes in neurodegenerative diseases has triggered much excitement because it offers the opportunity to generate animal models of specific forms of these human diseases (Fig. 1). As stressed before16, the rationale for studying these rare genetic forms of neurological disorders is the expectation that the phenotypic similarity between the familial and sporadic forms of neurological disorders reflects shared pathogenic mechanisms and biochemical pathways. However, major gaps remain in our understanding of the molecular pathways that provoke neuronal degeneration, and the question remains whether therapeutically significant insights will be gained from the study of these animal models.
In this context, iPS cells derived from humans with inherited neurological disorders may provide invaluable tools for elucidating the mechanisms by which the disease-causing gene products kill neurons in the human cellular context and at endogenous levels of expression (Fig. 1). iPS cells derived from affected individuals may also provide a unique opportunity to gain insights into the neurobiology of the more frequent sporadic (or complex multigenic) forms of neurodegenerative diseases (Fig. 1). However, our ability to effectively use iPS cell technology depends on two important conditions. First, we need to be able to direct the differentiation of iPS cells into clinically relevant populations of neural cells. This includes not only the neurons that degenerate, but also non-neuronal cells that contribute to the pathogenesis in a non-cell-autonomous manner, as discussed below. Second, we need to recapitulate in vitro key aspects of neuronal degeneration in a time frame that is conducive to pathophysiological studies and, eventually, to drug screening. Neither of these goals is trivial.
Unraveling the complexity of neurological disorders
The CNS is an organ of unparalleled cellular complexity. Developmental studies have revealed that neuronal identity is specified by a series of time-dependent patterning signals that activate transcriptional programs progressively restricting developmental potential of neural progenitors and maturing neurons. These programs control not only the principal neuronal properties such as neurotransmitter phenotype, but also migratory behavior, dendritic arborization patterns, axonal pathfinding preferences, electrophysiological properties and synaptic specificity17. Such a complex stepwise process of neuronal identity specification is exemplified by spinal motor neuron development. After neural induction, nascent neuro-ectoderm is diversified into forebrain, midbrain, hindbrain and spinal cord territories. Spinal cells exposed to the ventralizing signal Sonic hedgehog acquire a generic motor neuron progenitor identity18 that is further refined in response to rostro-caudal patterning signals, retinoic acid, fibroblast growth factor (FGF) and growth differentiation factor-11 (refs. 19–21). The result is specification of hundreds of distinct motor neuron subtypes along the rostro-caudal aspect of the spinal cord, each showing a unique cell body position and axon pathfinding trajectory to innervate appropriate muscle group and to establish the functional motor system17,18.
Considering the iterative developmental process that underlies specification of neuronal identity in vivo, in vitro recapitulation of programs that accurately reflect normal brain development may be challenging. Yet protocols for the specification of dopaminergic neurons and cholinergic spinal motor neurons, the main neuronal subtypes affected in Parkinson’s disease and ALS, respectively, have been successfully applied to the differentiation of iPS cells10,13,22. Neuralized stem cells were shown to respond correctly to rostro-caudal patterning signals, including FGF, Wnt proteins and retinoic acid, to acquire the identity of forebrain, midbrain or spinal tissue23,24. Similarly, neural cells can be patterned along the dorso-ventral axis using bone morphogenetic proteins and Sonic hedgehog25. But although in vitro generated motor neurons and dopaminergic neurons adopt characteristic molecular and functional properties, it remains unclear whether they also acquire ultimate neuronal subtype identities and whether they can be considered faithful replicas of endogenous neurons. Only detailed characterization of nerve cells implanted into the developing nervous system will reveal whether these in vitro–generated cells can functionally replace their in vivo neural counterparts. Nevertheless, the above studies indicate that application of patterning factors to pluripotent cells in vitro is sufficient to trigger the execution of developmental genetic programs that control the progression of dividing neural progenitors into defined postmitotic neurons and glia in a manner indistinguishable from normal embryonic development. Although we are far from understanding all signals and genetic programs controlling differentiation of each individual CNS cell type, these initial studies provide impetus for believing that differentiation of pluripotent stem cells can be programmed by rational application of developmentally relevant signals.
It is therefore reasonable to expect in the near future that the methods to derive the most clinically relevant neural cell types from pluripotent stem cells in vitro will improve significantly. But it is unlikely that the differentiation methods will yield pure populations of the desirable cells, thus necessitating the development of methods for the purification of the cells of interest. These methods will rely on our ability to manipulate human pluripotent cells genetically to express selectable markers under the control of cell type–specific promoters, or on identification of unique combinations of cell surface epitopes that would facilitate fluorescent or magnetic cell sorting26,27. Alternatively, the efficiency of the specific cell derivation might be improved by rational programming of cell differentiation with a combination of extrinsic patterning signals and overexpression of endogenous transcription factors specifying progenitor or postmitotic neuronal identity. Such approach was successfully applied to improve the efficiency of dopaminergic neuron production from both human and mouse pluripotent stem cells28,29. From a clinical perspective, being able to generate large number of purified neurons of particular identity may have far-reaching implications. For instance, significant neuronal subtype diversity and differential sensitivity to degeneration are characteristic of neurodegenerative disorders1. In ALS, for example, motor neurons from the anterior horn of the lumbar spinal cord degenerate more than those innervating the extraocular muscles or belonging to Onuf’s nucleus30. Likewise, in Parkinson’s disease, the substantia nigra dopaminergic neurons die to a greater extent than those in the neighboring ventral tegmental area16. Thus, generation of individual, well characterized neuronal subtypes from pluripotent stem cells may be a prerequisite for our attempts to unravel the molecular determinants of differential neuronal vulnerability to a disease process and to devise effective disease-modifying drug therapies.
The non-cell-autonomous contribution to neuronal death
Initial studies of neurodegenerative diseases were consistently ‘neuron-centrist’ in that they all focused on the cell-autonomous changes in neurons that lead to a selective neurodegeneration. These studies yielded valuable insights into our understanding of the neurobiology of diseases by showing, for example, an increased sensitivity of specific neurons to several stressors, including oxidative stress, nitric oxide exposure and apoptotic signaling pathways30. However, the involvement of non-neuronal cells, such as astrocytes or microglia, in the overall neurodegenerative process is increasingly recognized. Studies on chimeras, composed of mutant and wild-type cells or animals in which genetic defects were genetically attenuated in subsets of neurons or non-neuronal cells, argue for a significant non-autonomous contribution to the disease processes31. Subsequently, a series of in vivo and in vitro studies has provided compelling evidence that mutant non-neuronal cells can transmit the neurodegenerative phenotype to wild-type neighboring neurons32–34.
Such non-cell-autonomous pathogenic scenarios can be challenging to investigate from a molecular standpoint, in part because it is difficult to model them in vitro, as reconstruction of glial-neuronal interactions or entire neuronal networks may be necessary. Nonetheless, this has been achieved in mouse models of ALS, whereby astrocytes carrying a mutated form of the superoxide dismutase-1 (SOD1) gene were shown to mediate a toxic activity that kills both wild-type and mutant SOD1 mouse motor neurons as well as human ES cell–derived motor neurons30. But although overexpression of mutant SOD1 in human astrocytes results in motor neuron toxicity35, it has not been established whether astrocytes derived from humans with ALS carrying only a single copy of the mutated gene would show a similar degree of toxicity. Here, for example, is where iPS cells derived from individuals with ALS carrying a SOD1 mutation10 could be decisive in resolving this outstanding issue through their differentiation into astrocytes by adapting protocols for astrocytes derivation from mouse ES cells36. Thus, the pluripotency of iPS cells derived from affected individuals may enable us to tease out the nature and the sequence of the non-cell-autonomous insults that lead to neuronal degeneration.
In contrast to the well recognized diversity of neuronal subtypes, less is known about the molecular and functional diversity of glial cells. Are only particular subtypes of glial cell involved in the neurodegenerative process? We believe that in vitro coculture model systems made of different disease-relevant cell types derived from iPS cells will be at the forefront of the research in neurodegeneration in the coming years, as they hold the promise to produce important insights into the disease mechanisms of neurodegeneration and to provide new biomarkers and targets for therapeutic intervention.
Cell-based models for identification and testing of therapies
Derivation of iPS cells from people with neurodegenerative diseases and their differentiation into clinically relevant cell types are only the first steps on the road to successful therapy. As many neurodegenerative disorders are adult onset, identification of disease-related phenotypes in short-term in vitro settings might be a particular challenge, unless it turns out that the ontogenic age of the iPS cell derivatives matches that of the donor rather than that of embryonic cells (Fig. 1). Of possible relevance to this issue is the observation that, thus far, disease-related cellular phenotypes have been observed in iPS models only of developmental neuropathologies: spinal muscular atrophy and familial dysautonomia11,12. iPS-derived motor neurons from a child with spinal muscular atrophy recapitulated some of the hallmarks of this fatal pediatric paralytic disorder, including selective motor neuron deficit and a reduced expression of the survival motor neuron (SMN) protein12. Familial dysautonomia is a severe genetic disorder of the autonomic and sensory nervous systems due to a tissue-specific splicing defect that leads to a decreased amount of the transcript encoding IκB kinase complex–associated protein. This defect was recapitulated in neural crest precursor cells derived from iPS cells of three people with familial dysautonomia11. Although these results are encouraging, confirmation that the observed phenotypes are truly related to the disease requires a repair of the gene defects, and, in both studies, this key rescue experiment was missing. We argue that, in future studies of human disease models using iPS cells, such rescue experiments should become the norm, as genetic differences among the human population and, hence, among affected and control iPS cell lines, are enormous.
In contrast to the models of neurodevelopmental disorders, no disease-related phenotypes have been thus far reported in iPS cells from adult-onset diseases such as ALS or Parkinson’s disease10,37. Perhaps here the disease phenotype may never manifest itself under basal cell culture conditions, but it may be revealed by challenging the neural cells with stressors such as nitrogen or oxygen reactive species, proinflammatory factors or even toxins. Identification of new and more effective and relevant stressors eliciting early neuronal phenotypes in models of adult-onset neurologic diseases will therefore be an important goal for future research.
Once disease-specific phenotypes are identified and translated into robust cell-based assays, the most consequential use of iPS cells derived from affected individuals will be the screening of candidate drugs by, for example, high-throughput platforms (Fig. 1). Such efforts at drug discovery will be greatly facilitated by the virtually unlimited supply of pluripotent cells and their derivatives. Another potential advantage in using iPS technology is that the new drugs will have already been tested on human cells, which may facilitate the identification of better therapies and accelerate their translation to the clinic.
In the past, even the most promising disease-modifying therapies discovered using animal models of neurological diseases have failed in clinical trials. Perhaps here is where iPS cells also have a major and immediate medical role. Cohorts of human subjects are markedly heterogeneous both clinically and genetically (for example, in age of onset, types and loci of mutation, type and body distribution of the manifestations, and disease progression). Furthermore, the response to treatment can be highly variable from patient to patient. This makes it hard to detect beneficial effects unless their magnitude is large. As such, we can imagine patient selection being performed on the basis of in vitro testing using disease-relevant cell types derived from iPS cells of diverse genetic backgrounds. This may allow the selection of patients whose iPS-derived cells have shown a beneficial response to a drug before moving forward in a clinical trial or having the drug prescribed. Alternatively, patient-derived iPS cells may be used to develop patient-specific drugs tailored for a particular genetic and clinical profile. A similar predictive approach could apply to the issue of adverse effects. Serious but often patient-specific hematologic, liver or cardiac toxicity is often the cause of participant dropout from clinical trials and of discontinuing treatment in patients. In this case, disease-relevant cell types derived from patients could be used to predict, in a dish, toxicity of a given drug—a bit like the use of antibiograms in infectious medicine, which is a method of testing the sensitivity of an isolated bacterial strain to different antibiotics.
Restorative neurology and stem cells
Use of pluripotent or multipotent cells for restoration of damaged neural networks in a broad range of neurological disorders is one avenue that holds tremendous promise from a translational medicine perspective and is one of the strongest forces driving research in stem cell biology (Fig. 1). Several preclinical studies have generated encouraging data showing that stem cells and their derivatives can improve function and mitigate neurodegeneration in a variety of experimental models of neurological disorders38. How close, however, are we with these types of stem cell replacement therapy to an actual clinical application?
There are several ways in which pluripotent cells may contribute to tissue repair not only in neurological diseases such as Parkinson’s disease or ALS, but also in ischemic and traumatic injuries of the CNS. First, there is the possibility that the replacement of damaged neurons, astrocytes or oligodendrocytes in the adult mammalian CNS could be achieved by endogenous neurogenesis, as self-renewable neural cells are found in discrete regions of the adult CNS39. In the intact adult CNS, neurogenesis outside the subventricular zone of the lateral ventricle wall or the subgranular zone of the hippocampal dentate gyrus seems to be extremely limited, if not nonexistent40. However, in a model of forebrain transient ischemia in adult rats, neural progenitor cells were found to proliferate in response to CNS injury and then to migrate to the damaged hippocampus and give rise to new mature neurons41. Although this neurogenic activity, even in response to injury, was meager, it was successfully enhanced by the infusion of FGF41. Thus, finding ways to modulate adult neurogenesis may be pivotal to any efforts aimed at repairing damaged neural networks with endogenous stem cells. Perhaps the greatest challenge for clinical use of endogenous stem cells is their limited developmental potential. Although potency of endogenous stem cells can be broadened in response to injuries42, it is unlikely that all principal degenerating nerve cells causing disorders such as Alzheimer’s disease, Parkinson’s disease or ALS can be generated without resorting to genetic reprogramming of endogenous progenitors3,43.
An alternative possibility is to attempt to repair damaged CNS networks by using exogenous pluripotent cells and their derivatives for transplantation. For the past few decades, a large body of literature on grafting fetal neural cells or tissues into the adult CNS, especially for Parkinson’s disease, has been published44. Having said this, one should not underestimate either the risks or the complexities of such reparative strategies45, including the much-discussed risk of tumor formation. For instance, among the host of outstanding questions regarding the use of pluripotent cell derivatives here is the fact that both ES- and iPS-derived neural cells will be placed inside the damaged or injured CNS. The diseased parenchyma may thus constitute a hostile environment, which can challenge the proper integration and even survival of the transplant within the host CNS. Even in sporadic neurological disorders, the disease phenotype may be communicated to neighboring wild-type cells, as suggested by the report that pathological proteinaceous inclusions can be observed in wild-type, healthy fetal ventral midbrain neurons after transplantation into the affected striatum of patients with Parkinson’s disease46. Furthermore, in the case of known genetic defects, patient-specific iPS cells and their derivatives may not be usable off the shelf in absence of ex vivo gene therapy to restore a normal cellular function. This prerequisite may be accomplished using homologous recombination47, human artificial chromosomes48 or zinc-finger nucleases26, but, if this is even technically feasible, it seems very labor intensive, raising concerns about whether iPS-based autologous cell replacement therapy could ever be offered to patients on a large scale.
Assuming that pluripotent cell derivatives, once engrafted, maintain their fate and their required functional phenotypes, some goals for such cell therapies still may be more realistic than others. For instance, as shown in experimental models of Parkinson’s disease13, if the goal is merely to restore the local concentration of the lost production of a neurotransmitter, then implantation of any kind of pluripotent cell derivatives with the capacity to release the missing neurotransmitter may suffice to provide clinical benefit, in absence of a genuine circuitry restoration. If the goal is to actually repair the neural circuit with either endogenous or exogenous stem cells, then the most significant hurdle will be the regrowth of projections to the proper target structure in a manner that respects the somatotopic organization of the network. The mechanical barrier caused by disease- or injury-related gliosis and the presence of inhibitors of axonal regrowth49 can also undermine the restoration of damaged neuronal networks in the adult CNS. These are some, but certainly not the only, pressing issues that must be overcome before cell replacement therapy is attempted in neurodegenerative, ischemic and traumatic CNS disorders to avoid the harmful results that have plagued other therapeutic strategies that were, at times, hailed as breakthroughs of modern medicine.
Looking forward
Despite the need for a more detailed characterization of iPS cells and for improvement in the methodologies for iPS cell derivation without stably integrated exogenous genes, iPS cells are already greatly influencing the way in which we approach the preclinical study of neurological disorders. Although cell replacement therapy might be theoretically the most direct therapeutic application of pluripotent cells, the time is not yet ripe for this type of reparative neurology. It is our opinion that the possibility of using human pluripotent cells, especially iPS cells, for in vitro applications of clinical importance may be a near-term attainable goal. We believe that the greatest potential for iPS technology resides in its sensible application toward the modeling and studying of neurodegenerative diseases, leading eventually to the development of new therapeutic approaches.
ACKNOWLEDGMENTS
The authors are supported by US National Institutes of Health grants AG021617, NS042269, NS062180, NS064191, NS38370, NS058502 and NS055923; US Department of Defense grants W81XWH-08-1-0522, W81XWH-08-1-0465 and DAMD 17-03-1; the Parkinson Disease Foundation (New York); the Thomas Hartman Foundation For Parkinson’s Research; the Project A.L.S. foundation; and the Muscular Dystrophy Association/Wings-over-Wall Street.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J. Clin. Invest. 2003;111:3–10. doi: 10.1172/JCI17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Neurological Disorders: Public Health Challenges. World Health Organization; Geneva: 2006. [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Lin T, et al. A chemical platform for improved induction of human iPSCs. Nat. Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abujarour R, Ding S. Induced pluripotent stem cells free of exogenous reprogramming factors. Genome Biol. 2009;10:220. doi: 10.1186/gb-2009-10-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maherali N, Hochedlinger K. Tgfβ signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr. Biol. 2009;19:1718–1723. doi: 10.1016/j.cub.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol. Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Sermon KD, et al. Creation of a registry for human embryonic stem cells carrying an inherited defect: joint collaboration between ESHRE and hESCreg. Hum. Reprod. 2009;24:1556–1560. doi: 10.1093/humrep/dep062. [DOI] [PubMed] [Google Scholar]
- 10.Dimos JT, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebert AD, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wernig M, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Gomez JA, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25:918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 17.di Sanguinetto S.A. Dalla Torre, Dasen JS, Arber S. Transcriptional mechanisms controlling motor neuron diversity and connectivity. Curr. Opin. Neurobiol. 2008;18:36–43. doi: 10.1016/j.conb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 19.Bel-Vialar S, Itasaki N, Krumlauf R. Initiating Hox gene expression: in the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development. 2002;129:5103–5115. doi: 10.1242/dev.129.22.5103. [DOI] [PubMed] [Google Scholar]
- 20.Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- 21.Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- 22.Karumbayaram S, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irioka T, Watanabe K, Mizusawa H, Mizuseki K, Sasai Y. Distinct effects of caudalizing factors on regional specification of embryonic stem cell-derived neural precursors. Brain Res. Dev. Brain Res. 2005;154:63–70. doi: 10.1016/j.devbrainres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 25.Mizuseki K, et al. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2003;100:5828–5833. doi: 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hockemeyer D, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Placantonakis DG, et al. BAC transgenesis in human embryonic stem cells as a novel tool to define the human neural lineage. Stem Cells. 2009;27:521–532. doi: 10.1634/stemcells.2008-0884. [DOI] [PubMed] [Google Scholar]
- 28.Friling S, et al. Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2009;106:7613–7618. doi: 10.1073/pnas.0902396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson E, et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 30.Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu. Rev. Neurosci. 2010;49:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 31.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat. Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Custer SK, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat. Neurosci. 2006;9:1302–1311. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- 34.Nagai M, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchetto MC, et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Fraichard A, et al. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J. Cell Sci. 1995;108:3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- 37.Soldner F, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders–time for clinical translation? J. Clin. Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kempermann G, Gage FH. New nerve cells for the adult brain. Sci. Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- 40.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 41.Nakatomi H, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 42.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deierborg T, Soulet D, Roybon L, Hall V, Brundin P. Emerging restorative treatments for Parkinson’s disease. Prog. Neurobiol. 2008;85:407–432. doi: 10.1016/j.pneurobio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Lau D, et al. Stem cell clinics online: the direct-to-consumer portrayal of stem cell medicine. Cell Stem Cell. 2008;3:591–594. doi: 10.1016/j.stem.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 47.Hanna J, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 48.Kazuki Y, et al. Complete genetic correction of iPS cells from Duchenne muscular dystrophy. Mol. Ther. 2010;18:386–393. doi: 10.1038/mt.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J. Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]