Abstract
Iodine is a vital micronutrient required at all stages of life; fetal life and early childhood being the most critical phases of requirement. Diet is the sole source of iodine, which in turn is dependent upon the iodine content of water and soil. Iodine is metabolized in the human body through a series of stages involving the hypothalamus, pituitary, thyroid gland and blood. Recent advances in physiology and molecular science have revolutionized our understanding of iodine metabolism at the cellular and sub-cellular level. This in turn has improved our knowledge of Iodine Deficiency Disorders (IDD), their prevention, management and control. This article makes an attempt to revisit this important topic in light of recent advances and provides a comprehensive account of the subject.
Keywords: Iodine, iodine metabolism, iodine deficiency, IDD
INTRODUCTION
Iodine is a micronutrient of crucial importance for the health and well-being of all individuals. It is a trace element, just 5 gm of which are sufficient to meet the life-time needs of an individual with a life-span of 70 years(1). Iodine is mostly concentrated in thyroid gland(2). A healthy adult body contains 15-20 mg of iodine, 70-80% of which is stored in the thyroid gland. Daily intake of iodine by an individual amounts to 500 micrograms; daily physiological requirement during adult life is 150 micrograms; during pregnancy and lactation period is 200 micrograms; and during neonatal period is 40 micrograms(3). Normally about 120 micrograms of iodide are taken up by the thyroid gland for the synthesis of thyroid hormones(4).
Oceans are the world's main repositories of iodine and very little of earths iodine is actually found in the soil. The deposition of iodine in the soil occurs due to volatilization from ocean water, a process aided by ultraviolet radiation. The coastal regions of the world are much richer in iodine content than the soils further inland; here the problem gets more compounded by continuous leeching of iodine from the soil(5). Therefore, the crops grown in such soil remain iodine deficient; even ground water in these areas is deficient in iodine(2). This explains the endemic distribution of Iodine Deficiency Disorders (IDD) in the world.
Iodine metabolism
Iodine is mostly obtained from food sources particularly vegetables grown on iodine-rich soil; the remaining requirement is met from drinking water(1). Seaweeds such as wakame, nori or mekabu, which are widely used in some Asian cultures for making soups, salads and condiments, are rich sources of iodine. Iodine is found in nature in various forms: inorganic sodium and potassium salts (iodides and iodates); inorganic diatomic iodine (molecular iodine or I), and organic monoatomic iodine(5). (Table 1)
Table 1.
Source of Iodine*
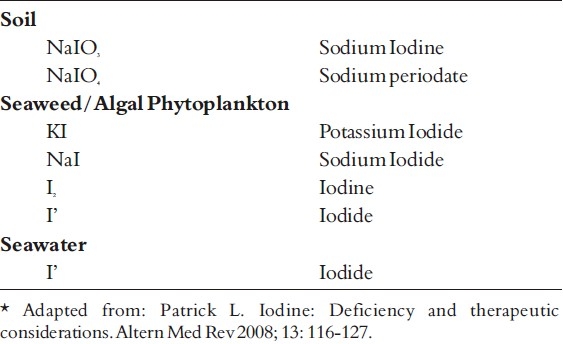
Thyroid gland plays a central role in the metabolism of iodine. The gland comprises multiple follicles lined by follicular cells resting on a basement membrane. The follicles are filled by a clear viscous material called colloid. The colloid is a gycoprotein called thyroglobulin (4).
Iodine trapping is the first step in the metabolism of iodine (Fig. 1). The process commences with the uptake of iodide from the capillary into the follicular cell of the gland by an active transport system. This occurs against chemical and electrical gradients by sodium / iodine symported protein (NIS) found in the basolateral membrane of the follicular cell; the energy required by this process is linked to the ATPase dependent Na+;- K pump (3).
Figure 1.
Synthesis and Release of thyroid hormones
Synthesis and secretion of thyroglobulin is the second step. It occurs by another independent process within the follicular cell; the synthesis starts on the rough endoplasmic reticulum as peptide units of molecular weight 330,000 (the primary translation product of its messenger RNA). Later these units combine into a dimer, followed by addition of carbohydrate moieties, after which the molecule moves to the Golgi apparatus. The completed thyroglobulin molecule contains about 140 tyrosine residues, which serve as substrate for the synthesis of thyroid hormones (3,4). The thyroglobulin is contained within small vesicles which then move towards the apical surface of the plasma membrane before being released into the follicular lumen.
The third step is the oxidation of iodide. The iodide within the follicular cell moves towards the apical surface of the plasma membrane, to enter into the follicular lumen; this transport by a sodium independent iodide/chloride transporter called pendrin. The iodide (I') is then immediately oxidized to Iodine by (I) (3,4) .
This is followed by organification of the thyroglobulin, wherein iodination of the tyrosine residues present within the thyroglobulin molecule occurs. Iodination first occurs at position 3 to form monoiodotyrosine (MIT) and then at position 5 to form diiodityrosine (DIT). Iodination of tyrosine is followed by coupling reaction, whereby, two molecules of DIT couple to form thyroxine (T4) hormone; and one molecule of MIT couples with one molecule of DIT to form Triiodothyronine (T3) hormone (3,4) . The reaction is catalyzed by thyroid peroxidase (TPO) (5,7). The thyroid hormones are stored inside the thyroid follicles as colloid for several months. The stored hormones can meet the body requirements for up to 3 months (3,4).
The colloid containing iodinated thyroglobulin undergoes endocytosis, whereby it is salvaged from the follicular lumen by the epithelial cells; this is facilitated by TG receptor megalin which is present on the apical membrane. The colloid now enters the cytoplasm in the form of colloid droplets, which move towards the basal membrane possibly by way of microtubule and microfilament function. The colloid droplets next fuse with lysosome vesicles which contain proteolytic enzymes. The proteases help digest the thyroglobulin molecule releasing T4, T3, DIT and MIT into the cytoplasm. While T4 and T3 diffuse via the basal surface into the blood stream, the MIT and DIT get rapidly deiodinated by enzyme deiodinase. This mechanism helps retrieve iodide for recycling along with tyrosine for recycling (3,4).
In the blood stream, T4 and T3 may circulate in the bound or free form; whereas 99 percent of T4 and T3 circulate in the bound form, less than 1 percent circulates in an unbound form. The binding proteins include thyroxine binding globulin (TBG), thyroxine binding prealbumin (TBPA) and thyroxine binding albumin (TBA). Binding of hormones apart from serving as a reservoir also helps to prevent urinary loss of hormones. The unbound hormones are biologically active. About 80 percent of circulating T3, the most active thyroid hormone is derived from peripheral deiodination of T4 hormone (3).
Thyroid secretion is regulated by pituitary gland through TSH which operates on a feed-back mechanism tuned to T4 level in blood. A fall in T4 level stimulates the pituitary to increase its TSH secretion which in turn stimulates the thyroid gland to release T4 in circulation to maintain normal level of the hormone in blood (4).
Thyroid gland secretes 80 micrograms of iodine in the form of T3 and T4 hormones per day; 40 micrograms of iodine secreted appear in extracellular fluid (ECF) per day. T3 and T4 are metabolized in liver which releases about 60 micrograms of iodine into ECF and 20 micrograms of iodine into the bile to be excreted in stools. On an average, 480 micrograms of iodine get excreted in urine and 20 micrograms in stools per day (4).
Since the thyroid possesses a remarkably efficient iodine-trapping mechanism, it normally maintains a gradient of 100:1 between the iodine content of thyroid cells and extra-cellular iodine (2). The effectiveness of iodide trapping is assessed by thyroid / serum (T/S) ratio. The T/S [I] is measured with radioactive iodide. Thyroid stimulating hormone (TSH) regulates the T/S ration for iodide. After hypophysectomy the drop in TSH level, leads to decrease in T/S ration. High levels of TSH as in secondary (pituitary) hyperthyroidism increase T/S ratio (7).
While major portion of iodine is concentrated in the thyroid gland, the non hormonal iodine is found in a variety of body tissues including mammary glands, eye, gastric mucosa, cervix and salivary glands (7). With the exception of mammary tissue the function of iodine in these tissues is still not clear (8). Accumulation of iodine in the breast plays an important role during breast feeding in fetal and neonatal development; however such iodine has also proven to have antioxidant function. In the presence of hydrogen peroxide and peroxidase, iodide acts as an electron donor, thereby decreasing damage by free oxygen radicals (9,10). On the contrary, breasts with inadequate iodine stores are prone to get damaged by accumulating high levels of malondialdehyde, a product of lipid peroxidation (11). Much alike ascorbic acid, iodine concentrations as low as 15 micromole, can have significant antioxidant effects (12). This antioxidant effect of iodine could explain the therapeutic effects of seaweed baths or iodine rich solutions that were historically used to treat many diseases (12).
Animal studies have proven that iodine normalizes elevated adrenal corticosteroid hormone secretion related to stress and reverses the effects of hypothyroidism on the ovaries, testicles and thymus in thyroidectmized rats (13,14). Iodine may also have a role in immune function; when placed in a medium containing 10-6 M iodide, human leukocytes synthesize thyroxine (15).
Iodine deficiency disorders
Iodine Deficiency Disorder (IDD) is the most common endocrinopathy in the world and also the most preventable cause of mental retardation (5). In 1998, one-third of the worlds′ population lived in iodine deficient areas (16). The two major factors responsible for IDD are inadequate iodine intake and inadequate iodine utilization. The inadequate iodine intake may be secondary to low iodine content of the soil and consequently of the consumed food or low consumption of sea food dictated by its high cost and low availability On the other hand the presence of goitrogens in certain foods may lead to inadequate iodine utilization (17)(Fig. 2).
Figure 2.
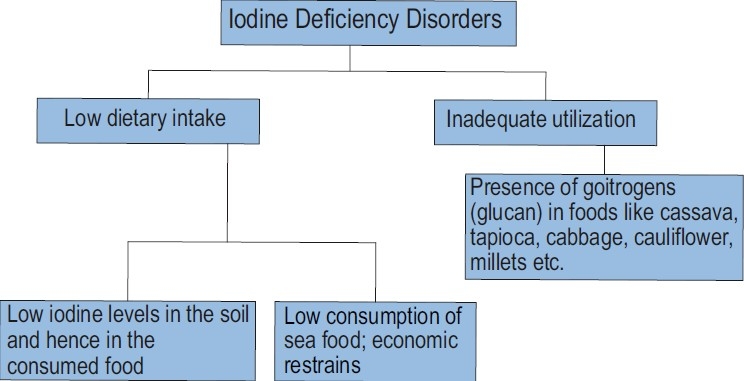
Causes of iodine deficiency disorders
IDD is a term that collectively reflects the clinical and sub-clinical manifestations of iodine deficiency. Iodine being an indispensable component of T3 and T4 hormones, its deficiency interferes seriously with the synthesis of these hormones. For a time thyroid responds by releasing the hormones stored as components of thyroglobulin molecules. But when the stores are exhausted and blood level of T4 start declining, pituitary intervenes by increasing TSH output which stimulates the thyroid to increase the uptake of iodide and ensure the release of thyroid hormones in adequate strength. However, in the state of deficiency when iodide uptake of thyroid is seriously hampered TSH fails to promote the release of T4 and only ends up with the hyperplasia of follicular cells. In a situation of severe iodine deficiency, while the level of T4 remains low, the level of TSH remains high (3,4). Under continuing TSH stimulation in endemic areas, thyroid gland undergoes hypertrophy and hyperplasia of follicular cells and in the process, enlarges in size and appears as a goiter which may in certain cases attain an enormous size.
The damage caused to the human body due to iodine deficiency is in fact the result of deficiency of thyroid hormones. The effects of IDD in humans at different stages of live are presented in Table 2(6,17). The deficiency not only leads to goiter formation but also to severe retardation of growth, development and maturation of nearly all the tissues of the body, especially those that are fast developing. There is large variation between the sensitivity of fast developing organs to the deficiency of thyroid hormones; brain is the most sensitive organ. The critical period in endemic areas extend from second trimester to second year of life. Deficient intake of iodine during this period can lead to devastating consequences resulting from permanent damage to brain. Administration of iodine during second trimester of pregnancy reverses the damage caused by iodine deficiency. However, the damage sustained after the end of the second trimester of pregnancy is permanent (18). Owing to maternal IDD, it is estimated that about one-fifth of pregnant women in India will give birth to children who will not reach their optimum physical and mental potential (19).
Table 2.
Effect of Iodine Deficiency Disorder*

A growing fetus in the womb of an iodine deficient mother is at high risk. The pregnancy may end in abortion, still birth, congenital anomalies or low birth weight outcome. Infants born to iodine deficient mothers, who survive the critical postnatal phase, may develop endemic cretinism. The neurological form of endemic cretinism is characterized by severe mental retardation and is usually associated with cerebral diplegia and deaf-mutism (2). Children in endemic areas show retarded physical and mental development, low I.Q. levels and impaired school performance. The grave implications of iodine deficiency on child's learning capacity and the quality of life of child population is thus evident in the erosion of quality of our human resources (20).
Brain development depends on adequate supply of thyroxine. Neonatal Chemical Hypothyroidism (NCH) is a forerunner to mental sub normality in childhood. T4 and TSH levels are measured to diagnose NCH. T4 levels less than 3 mcg per dl and TSH value greater than 50 micro units per ml indicate NCH. The incidence of NCH in endemic regions of India and its neighboring countries range from 6 to 130 per thousand births (21). Areas with high incidence of NCH have shown marked reduction in I.Q. scores and increased cases of nerve deafness. It has been estimated that about 10% or more of newborns in severe goiter endemic regions are estimated to be at risk of neonatal hypothyroidism and resultant mental and physical development (22).
Goiter incidence increases with age reaching maximum frequency at adolescence with girls showing higher frequency than boys. Adult population inhabiting the iodine deficient areas is characterized by a high degree of apathy, reduced mental functioning, lack of physical energy and reduced work output, all contributing to poor quality of life. Iodine deficiency has emerged as a socio-medical problem of vast dimensions associated with physical and mental retardation, neurological disorders, feeble mindedness, low educability, poor performance, social handicaps, dependability and disfigurement (1).
Traditional food of Japanese contains significant amounts of dietary iodine, possibly consuming at least 7000 mcg of iodine daily from kombu alone (23). It has been estimated that the Japanese consumption of dietary iodine exceeds the upper safety limit of 1 mg by approximately 5-14 times 5). These higher levels appear to have no suppressive effect on the thyroid function in normal individuals, yet intake of excess of iodine could cause problems in patients with thyroid nodules, hyperthyroidism and autoimmune thyroid disease (5). On the contrary it has been interestingly observed that Japanese women who consume a high iodine content diet have a low incidence of benign and malignant breast disease; however, this protective advantage is lost in the same ethnic group once they immigrate to other countries (24–26). Japan also has a low incidence of autoimmune thyroiditis (27). Stadel has postulated that given the geographical distribution of iodine deficiency, there is low incidence of cancers of the prostate, endometrium, ovary and breast in populations consuming diets with high iodine content (28).
Iodine supplementation strategies
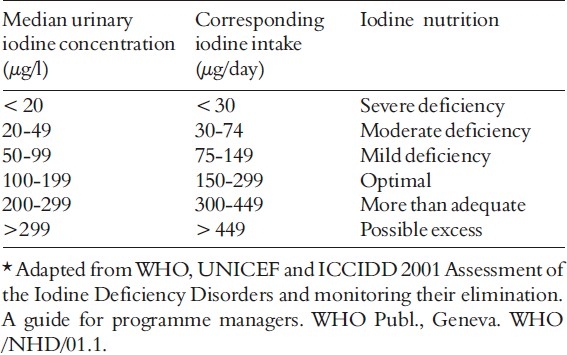
Since iodine is released from the body through urine, the best way to determine iodine deficiency across a large population is to measure the amounts of iodine in urine samples. The WHO defines iodine deficiency as a median urinary iodine concentration less than 50 μg/L in a population (6). (Table 3)
Table 3.
Median population urinary iodine values and iodine nutrition*
With increasing awareness of the wide spectrum of iodine deficiency disorders, a steady increase has been noticed in the estimation of the magnitude of the problem in the world. In 1990, WHO reported that the total population at risk of iodine deficiency in developing countries was 1 billion, of which 200 million suffered from goiter; over 5 million were cretins with gross mental retardation; and 15 million had less degrees of mental defect. In India, 150 million people are estimated to be exposed to the risk of iodine deficiency disorders of which 54 million have goiter, 2.2 million are cretins and 6.6 million have neurological defects 29) . Still births and neonatal deaths attributable to iodine deficiency exceed 90,000. The world's most intense endemic belt lies in India which runs along the southern slopes of Himalayas, extending from Kashmir in the west to the Naga Hills in the east. With increasing awareness of the problem, “extra-Himalayan” foci of iodine deficiency have been discovered in the country (1). As of today, not a single state in India can be absolved of the iodine deficiency problem.
Iodine supplementation in areas deprived of iodine rich food is viewed as the most cost effective solution to address the problem of IDD. Iodine deficiency can be corrected by adding iodine to dietary media like salt, oil, water, sauces etc. The methods of proven value for mass use are iodized salt and iodized oil. To this end, fortification of salt with iodine has been identified and considered to be the most suitable method of fortification. Being not only technically feasible, this food item is consumed worldwide in standard amounts by all sections of the population (1,17). In India, the main stay of National Goiter Control Programme (NIDDCP) is fortification of the common salt with potassium iodate (17). The sale of non-iodated salt has been banned. Monitoring of the salt quality is continuously done and recent analysis of 18,011 salt samples revealed that 73.15% conformed to the prescribed standards (1).
References
- 1.Dhaar GM, Robbani I. Foundations of Community Medicine. India: Reed Elsevier; 2008. Nutritional problems of mothers and children. pp. 272–280. [Google Scholar]
- 2.Detels R, Holland WW, Mc Ewen HJ, Omenn GS. Oxford Textbook of Public Health. 3. Oxford University Press; 1977. Endocrine and metabolic disorders; pp. 1114–1115. [Google Scholar]
- 3.Khurana I. Textbook of Medical Physiology. India: Reed Elsevier; 2006. Endocrinal System; pp. 710–715. [Google Scholar]
- 4.Pal GK. Textbook of Medical Physiology. India: Ahuja Publishing House; 2007. Endocrine Physiology; p. 346. [Google Scholar]
- 5.Patrick L. Iodine: Deficiency and therapeutic considerations. Altern MedRev. 2008;13:116–127. [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) ICCIDD, UNICEF, WHO. 2. ICCIDD, UNICEF, WHO: 2001. Assessment of iodine deficiency disorders and monitoring their elimination: A guide for programme managers. [Google Scholar]
- 7.Porterfield SP. Endocrine Physiology. 2. 2001. Thyroid gland; p. The Mosby physiology monograph series. [Google Scholar]
- 8.Dunn JT. What's happening to our iodine? J Clin Endocrin Metab. 1998;83:3398–3400. doi: 10.1210/jcem.83.10.5209. [DOI] [PubMed] [Google Scholar]
- 9.Venturi S. Is there a role for iodine in breast diseases? Breast. 2001;10:379–382. doi: 10.1054/brst.2000.0267. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi M, Venturi S. Iodide, antioxidant function and omega-6 and omega-3 fatty acids: a new hypothesis of biochemical cooperation? Prog Nutr. 2000;2:15–19. [Google Scholar]
- 11.Many MC, Papadopolos C, Martin I, et al. Iodine induced cell damage in mouse hyperplastic thyroid is associated with lipid peroxidation. In: Gardon A, Gross J, Hennemann G, editors. Progress in thyroid research. New York : NY; 1991. pp. 213–215. [Google Scholar]
- 12.Smyth PA. Role of iodine in antioxidant defense in thyroid and breast disease. Biofactors. 2003;19:121–130. doi: 10.1002/biof.5520190304. [DOI] [PubMed] [Google Scholar]
- 13.Nolan LA, Windle RJ, Wood SA, et al. Chronic iodine deprivation attenuates stress-induced and diurnal variation in corticosterone secretion in female Wistar rats. J Neuroendocrinol. 2000;12:1149–1159. doi: 10.1046/j.1365-2826.2000.00569.x. [DOI] [PubMed] [Google Scholar]
- 14.Thrall KD, Bull RJ. Differences in the distribution of iodine and iodide in the Sprague-Dawley rat. Fundam Appl Toxicol. 1990;15:75–81. doi: 10.1016/0272-0590(90)90164-f. [DOI] [PubMed] [Google Scholar]
- 15.Stolc V. Stimulation of iodoproteins and thyroxine formation in human leucocytes by phagocytosis. Biochem Biophys Res Commun. 1971;45:159–166. doi: 10.1016/0006-291x(71)90064-7. [DOI] [PubMed] [Google Scholar]
- 16.Dunn JT. Seven deadly sins in confronting iodine deficiency and how to avoid them 1996;81:1332-1335. J Clin Endocrin Metab. 1996;81:1332–1335. doi: 10.1210/jcem.81.4.8636328. [DOI] [PubMed] [Google Scholar]
- 17.Hetzel BS. Iodine deficiency disorders (IDD) and their eradication. Lancet. 1983;2:1126–1129. doi: 10.1016/s0140-6736(83)90636-0. [DOI] [PubMed] [Google Scholar]
- 18.Delong GR, Robbins J, Condliffe PG. Iodine and the brain. New York: Plenum. 1989 [Google Scholar]
- 19.Vir SC. Current status of iodine deficiency disorders (IDD) and strategy for its control in India. Ind J Paed. 2002;69:589–596. doi: 10.1007/BF02722687. [DOI] [PubMed] [Google Scholar]
- 20.Gopalan C. Iodisation of common salt for control of IDD: not the time to backtrack. NFI Bulletin. 2000;21 [Google Scholar]
- 21.Kochupillai N. Neonatal chemical hypothiesdism of nutritional origin.A major health problem that impairs growth and developing countries. In: Gopalan C, editor. Recent Trends in nutrition. Recent Trends in nutrition; 1993. pp. 181–193. [Google Scholar]
- 22.Ramji S. Sachdeva HPS, Choadhary Panna, editors. Iodine deficiency disorders.Epidemiology, Clinical Profile and Diagnosis. Nutrition in Children. 1994:245. [Google Scholar]
- 23.Fuse Y, Saito N, Tsuchiya T, et al. Smaller thyroid gland volume with high urinary iodine excretion in Japanese school children: normative reference values in an iodine-sufficient area and comparison with the WHO/ICCIDD reference. Thyroid. 2007;17:145–155. doi: 10.1089/thy.2006.0209. [DOI] [PubMed] [Google Scholar]
- 24.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.LeMarchand L, Kolonel LN, Nomura AM. Breast cancer survival among Hawaii, Japanese and Caucasian women Ten-year rates and survival by place of birth. Am J Epidemiol. 1985;122:571–578. doi: 10.1093/oxfordjournals.aje.a114136. [DOI] [PubMed] [Google Scholar]
- 26.Minami Y, Takano A, Okuno Y, et al. Trends in the incidence of female breast and cervical cancers in Miyagi Prefecture, Japan, 1959-1987. Jpn J Cancer Res. 1996;87:10–17. doi: 10.1111/j.1349-7006.1996.tb00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konno N, Yuri K, Miura K, et al. Clinical evaluation of the iodide / creatinine ratio of casual urine samples as an index of daily iodide excretion in a population study. Endocr J. 1993;40:163–169. doi: 10.1507/endocrj.40.163. [DOI] [PubMed] [Google Scholar]
- 28.Stadel BV, endometrial and ovarian cancer. Dietary iodine and risk of breast cancer. Lancet. 1976;1:890–891. doi: 10.1016/s0140-6736(76)92102-4. [DOI] [PubMed] [Google Scholar]
- 29.Pandav CS. IDD in South East Asia. In: Hetzel BS, Pandav CS, editors. In SOS for a billion: The conquest of Iodine Deficiency Disorders. New Delhi, India: Oxford University Press; 1996. p. 278. [Google Scholar]


