Abstract
Rationale
Using biological markers to objectively measure addiction severity or to identify individuals who might benefit most from pro-cognitive treatment could potentially revolutionize neuropsychopharmacology. We investigated the use of dopamine receptor mRNA levels in circulating blood cells as predictors of cognitive response following dopamine agonist treatment, and as biomarkers of the severity of stimulant drug dependence.
Methodology
We employed a double-blind, placebo-controlled cross-over design, administering a single dose of the selective dopamine D2/3 receptor agonist pramipexole (0.5 mg) to increase dopamine transmission in one session and a placebo treatment in another session in 36 volunteers. Half the volunteers had a formal diagnosis of stimulant dependence, while half had no psychiatric history. Participants performed neurocognitive tests from the CANTAB battery on both occasions, and stimulant-dependent individuals rated drug craving using visual analog scales. Whole-blood mRNA levels were measured for three dopamine-related genes: DRD3 and DRD4 (dopamine receptors), and catechol-O-methyltransferase (COMT; a dopamine catabolic enzyme).
Results
Stimulant users performed worse than healthy volunteers on the cognitive tests. The variation in peripheral dopamine D3 receptor mRNA expression explained over one quarter of the variation in response to pramipexole on the spatial working memory test across all participants. The severity of stimulant dependence was also significantly associated with peripheral COMT mRNA expression in stimulant users.
Conclusions
Peripheral expression of dopamine-related genes may be useful as a biomarker of cognitive response to dopamine agonist drugs and of severity of addiction to dopamine-releasing stimulant drugs.
Electronic supplementary material
The online version of this article (doi:10.1007/s00213-010-2087-1) contains supplementary material, which is available to authorized users.
Keywords: Peripheral biomarker, mRNA, Dopamine, DRD3, COMT, Spatial working memory, Stimulant dependence, Pramipexole, Personalized medicine
Introduction
Cognitive impairments are a central feature of many neuropsychiatric conditions, including stimulant dependence, attention deficit hyperactivity disorder, and schizophrenia; they are also a feature of normal aging, prompting recent therapeutic focus on cognitive enhancement. Disease- and age-related impairments are often particularly pronounced on prefrontal cortex-dependent tests involving working memory. Performance on such tests is amenable to dopaminergic modulation (Mehta et al. 2000). However, not everybody benefits equally from boosting dopamine transmission, as the effects of dopaminergic agents depend on the nature of the task and baseline dopamine neurotransmission in the brain (Arnsten 1998). Being able to predict pharmacological effects on tests of cognitive function could offer a promising opportunity for neuropsychiatry, as cognitive performance is strongly related to psychosocial functioning in patients (Green 1996) and facilitates their retention in treatment (Aharonovich et al. 2003).
Besides its effects on cognitive function, dopamine is also an important modulator of motivated behavior for natural and drug rewards (Berridge and Robinson 1998; Everitt and Robbins 2005; Kelley and Berridge 2002; Schultz 1997). Dopamine receptors are implicated in the reinforcing effects of stimulant drugs such as cocaine or amphetamines (Koob 1992; Wise and Bozarth 1987). Despite the important role dopamine plays in addictive behavior, it has been difficult to delineate the exact roles of the various dopamine receptor types in drug addiction (Foll et al. 2009). Research in experimental monkeys has shown that chronic abuse of stimulant drugs reduces dopamine D2 receptor levels throughout the striatum (Nader et al. 2002). Indeed, reduced D2 and D3 receptor densities, as measured with positron emission tomography (PET), have been documented in abstinent stimulant-dependent individuals (SDIs). Importantly, this reduction in D2/3 receptors in the midbrain appears to be associated with hypo-metabolism in prefrontal cortex (PFC; Volkow et al. 1993; Volkow et al. 2001), which is likely to contribute to cognitive dysfunction and the maladaptive behavior patterns seen in individuals with stimulant dependence (Goldstein and Volkow 2002). One possible pharmacological strategy would be to increase dopamine transmission in the striatum and to reduce symptoms of stimulant withdrawal and craving. However, the therapeutic efficacy of dopamine agonists for this indication has been inconsistent (Grabowski et al. 2004; Shearer et al. 2002; Soares et al. 2004).
Dopaminergic neurotransmission in the PFC is itself influenced by the activity of the enzyme catechol-O-methyltransferase (COMT), which is implicated in dopamine catabolism (Axelrod and Tomchick 1958) and PFC-dependent cognition (Tunbridge et al. 2004). One association study has suggested that an interaction between COMT and dopamine D4 receptor polymorphisms may influence the risk for the development of stimulant abuse (Li et al. 2004). The D4 receptor, in contrast to D2 and D3 receptors, is mainly located in brain areas innervated by mesocortical dopamine pathways, such as the PFC (Meador-Woodruff et al. 1996; Tarazi and Baldessarini 1999). Since dopamine provides an important modulatory influence on cognitive functions subserved by prefrontal networks, such as working memory (Robbins and Arnsten 2009), markers of dopamine neurotransmission may be able to identify individuals who might experience the greatest cognitive benefits from dopaminergic treatment. Corroborating this idea, it was recently demonstrated that D2/3 receptor availability in the striatum, assessed with PET, was associated with cognitive performance following methylphenidate administration (Clatworthy et al. 2009). These data suggest that central dopamine receptor expression may be predictive of response to dopaminergic drugs, but clearly, the cost, invasiveness, and radiation risk of PET effectively preclude this technique as a routine clinical tool for assessment of dopamine receptor status.
However, markers of dopamine receptor gene expression in the D2-receptor family (D3 and D4 receptor subtypes) are not only detectable in the brain but also expressed in peripheral blood, where dopamine plays a pivotal role in mediating communication between the nervous and immune systems (Basu and Dasgupta 2000; Eskandari and Sternberg 2002). Based on observations showing altered dopamine receptor expression in peripheral blood cells in patients with known abnormalities in the central dopamine transmission (Ilani et al. 2001; Nagai et al. 1996), it has been suggested that dopamine receptor mRNA expression in circulating blood might reflect the dopamine receptor level in the brain (Ilani et al. 2001) and might serve as a useful surrogate marker for more direct measurements of central receptor status in the brain (Gladkevich et al. 2004).
In the present study, we investigated the effects on cognitive performance of a single dose of the selective dopamine D2/3 agonist pramipexole (0.5 mg) in 36 volunteers, half with a diagnosis of cocaine or amphetamine dependence and half with no history of substance abuse/dependence. Specifically, we hypothesized that peripheral dopamine markers (i.e., DRD3, DRD4, and COMT mRNA levels in whole blood) would be predictive of individual differences in performance on PFC-dependent cognitive tests following pramipexole treatment. In individuals with stimulant dependence, we also predicted that peripheral dopamine markers might be associated with the severity of addictive symptoms.
Methods and materials
Participants
Thirty-six volunteers participated in the study: 18 individuals with a chronic history of stimulant drug abuse (SDIs), meeting the Diagnostic and Statistical Manual of Mental Disorders-Text Revision (DSM-IV-TR; American Psychiatric Association 2000) criteria for dependence on either cocaine/crack-cocaine (n = 10) or amphetamines (n = 8), and 18 matched healthy controls without a history of substance abuse/dependence. Diagnosis was ascertained using the Structured Clinical Interview for DSM-IV (First et al. 2002). Sixteen SDIs also met DSM-IV criteria for nicotine dependence, two for cannabis dependence, and five for alcohol abuse. Half the SDIs smoked cannabis regularly (50%) and consumed other drugs sporadically (ecstasy 33%, hallucinogens 22%, benzodiazepines 6%, and opiates 6%). The non-drug-using volunteers were recruited from the GlaxoSmithKline Clinical Unit Cambridge panel. Eleven percent smoked cannabis sporadically, 5% were occasional tobacco smokers, and 28% had previously smoked tobacco. Urine samples provided by SDIs tested positive for stimulants on each testing visit; all healthy volunteer urine samples were negative. At a baseline assessment, participants completed the National Adult Reading Test (NART, Nelson 1982) to provide an estimate of verbal IQ, the Beck Depression Inventory (BDI-II, Beck et al. 1996) to record dysphoric mood, and the Barratt Impulsiveness Scale (BIS-11, Patton et al. 1995) to assess trait-impulsivity. The protocol was approved by the Cambridge Research Ethics Committee (REC06/Q0108/130; principal investigator: TWR). All volunteers provided written informed consent prior to study enrolment.
Experimental procedures
We used a randomized, double-blind, placebo-controlled, cross-over design. On each session, a single dose of placebo or pramipexole was administered in counterbalanced order. Pramipexole was chosen on the basis of its selective profile for D2/3 receptors, particularly in mesolimbic brain areas (Catnacho-Ochoa et al. 1995; Suzuki et al. 1998). The study also included a dopamine D2/3 antagonist intervention using amisulpride, (data reported elsewhere: Ersche et al. 2010).
We administered pramipexole at a dose of 1.5 mg to the first six volunteers (three SDIs and three controls). While the SDIs tolerated this dose, this was not the case for the controls, who were unable to perform any cognitive tests due to nausea, vomiting, sweating, and tiredness. These controls were subsequently administered 0.5 mg pramipexole on a separate testing session, which was tolerated well. Thereafter, all remaining participants received 0.5 mg pramipexole. In total, we included data from 18 controls and 18 SDIs (three of whom were administered the 1.5-mg dose).
On each visit, participants took domperidone tablets (30 mg) as a pre-treatment to prevent emetic side effects. Domperidone is a peripheral D2 antagonist that does not cross the blood–brain barrier (Champion 1988). At 2.5 and 4 h after dosing, blood samples were drawn for assessment of drug plasma concentrations. The time elapsed between administration of dopaminergic treatment and SDIs’ last use of illicit stimulants was similar between the two treatment conditions [placebo, 8.5 h (SD ± 5.6) and pramipexole 8.6 h (SD ± 5.7); F < 1]. Approximately 180 min after dosing, participants performed four tests from the computerized Cambridge Neuropsychological Test Automated Battery (CANTAB; www.camcog.com) and the digit-span subtest of the Wechsler Adult Intelligence Scale (WAIS, Wechsler 1981).
The testing sessions were separated by at least 1 week to prevent carry-over effects, verified by the analysis of prolactin levels in blood drawn immediately before dosing on each testing session.
Cognitive measures
The Spatial Working Memory (SWM, Robbins et al. 1994) test is a self-ordered search task in which participants have to search through a spatial array of colored boxes for tokens, without returning to a box which had already contained a token. The number of errors was measured, including returning to a box in which a token was already found and returning to a box that was already found to be empty.
Rapid Visual Information Processing (RVIP, Sahakian et al. 1988) is a test of sustained attention, where a series of single digits (ranging from 2 to 9) is presented one at a time (100 digits/min) in a random order. Following a training sequence, participants detect three-digit target sequences by pressing the response pad. Sustained attention ability was measured by the metric A′, a signal detection measure of sensitivity to targets independent of the general overall tendency to respond, calculated using both the probability of hits and the probability of false alarms.
Paired Associates Learning (PAL, Sahakian et al. 1988) assesses episodic memory and learning of geometric patterns and spatial locations. Participants are shown six boxes, and in the last trial, eight boxes, which briefly disclose an abstract pattern. Once all the patterns have been briefly displayed, participants are shown the patterns one at a time and asked to select the location where they were previously displayed. The total number of errors was recorded.
The Information Sampling Task (IST, Clark et al. 2006) measures the tendency to gather information prior to making a simple probabilistic decision about which color of a hidden two-colored five-by-five matrix is in the majority. Prior to making the decision, participants have the opportunity to gather as much information about the color of the matrix as they feel they need for making a correct choice. The number of boxes opened, i.e., the number of elements in the matrix which are known to the participant at the time of decision-making, was recorded.
In the digit-span test (Wechsler 1981), participants have to listen to an increasingly long list of digits presented for immediate recall in either the exact order presented (forward span) or in the reverse order (backward span). The score reflects the maximum number of digits participants can successfully retain in working memory. Since the backward span has been considered to be more demanding on working memory resources than the forward span (Silver et al. 2003), we focused on the backward condition.
mRNA extraction and analysis
On the second testing visit, prior to drug administration, a peripheral blood sample (2.5 ml) was drawn into PAXgene tubes (PreAnalytiX GmbH), and total mRNA was isolated using the PAXgene Blood RNA System (PreAnalytiX GmbH). The use of whole blood collected into PAXgene tubes was chosen to maximize the stability of the dopamine receptor mRNA and to avoid potential artifactual changes from isolating individual peripheral cell types. RNA purity and concentration was determined by UV spectrophotometry. Total RNA (400 ng in 100 μl) was reverse-transcribed into first-strand cDNA using MultiscribeTM Reverse Transcriptase (Applied Biosystems) and Oligod(T)16 primers. Amplification of the DRD3, DRD4, and COMT target genes was carried out using a 7900 ABI Prism Sequence Detection System (Applied Biosystems) using 20 ng of cDNA in 25 μl of reaction mixture by gene-specific primers using the following Assay-on-Demand Gene Expression Assays (Applied Biosystems); DRD3 (Hs00364455_M1), DRD4 (Hs00609526_M1), and COMT (Hs00241349_M1), β-actin (4333762F), GAPDH (4333764F), and cyclophilin A (4333763F). Amplification efficiency was guaranteed for all the aforementioned TaqMan Gene Expression assays by Applied Biosystems (www.appliedbiosystems), and therefore, we did not further investigate gene expression relative to a standard curve, which might have provided us a more precise estimate of amplification efficacy. The PCR cycling parameters were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, and 60°C for 1 min. Relative DRD3, DRD4, and COMT mRNA levels were calculated using the threshold cycle for target amplification, Ct (target gene) − average Ct value (GAPDH, β-actin and cyclophiln A) to generate ∆Ct values. The average of the three reference genes was used for normalization since the resulting values should be less noisy than using any individual reference gene. However, correlations between the single housekeeper genes suggest that our key analyses would have been largely the same irrespective of which control gene was used for analysis, in particular for DRD3 and DRD4 (see Supplemental Material). Correlations of the ∆Ct values with those calculated with each of the individual reference genes were generally very high. The 2-ΔΔCt method (Livak and Schmittgen 2001) is a convenient way to analyze the relative changes in gene expression from real-time quantitative PCR experiments and was calculated for use in the analyses.
Dopamine receptor mRNA expression was not detectable in five samples (two SDIs, three controls); in one control volunteer, the DRD3 level was deviated more than eight standard deviations from the sample mean, and this sample was therefore excluded from the analysis. We initially wanted to extract all dopamine receptors of the D2 family from the blood, but mRNA of the DRD2 was not identified in our blood samples, which is consistent with previous reports showing that DRD2 expression in the blood is lower compared with the expression of DRD3 or DRD4 (Kirillova et al. 2008).
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences 13 (SPSS Inc.). In preparation for parametric analyses, digit-span and PAL data were square-root transformed to reduce skew (Howell 1997), but untransformed values are displayed in the figures and tables. First, we fitted repeated-measures analysis of co-variance models to examine group differences in cognitive performance, the main effect of pramipexole administration compared with placebo, and the interaction between group and pramipexole treatment. Mean BDI scores and years of education were included as covariates.
We then focused on the associations between levels of each of the three mRNA transcripts measured (DRD3, DRD4, and COMT) and each of the five cognitive measures. To summarize the influence of pramipexole administration on cognitive performance, we calculated change scores for each individual on each test by subtracting the cognitive test scores following pramipexole from the scores following placebo. We then examined the effects of dopamine markers on change scores, as well as group-by-dopamine marker interactions; these analyses also included the effect of group. Where significant effects or interactions of dopamine markers were identified, we then calculated the Pearson’s correlation co-efficient r for descriptive purposes.
Finally, we explored effects of drug treatment on symptom severity and possible associations between dopamine markers and treatment-related changes in symptom severity in SDIs. We calculated symptom change scores as the change in craving severity rating following pramipexole relative to placebo, 4 h following administration, corrected for baseline craving level. Symptom change scores were correlated with dopamine markers. We also estimated correlations between dopamine marker expression levels and baseline measures of symptom severity in the SDI group.
Correction for multiple comparisons was performed by dividing the initial P threshold (0.05) by 10, resulting in a threshold of P < 0.005. However, since this was an exploratory analysis, results reaching the P < 0.05 threshold are also reported, though not commented on in the discussion.
Results
Demographic and clinical variables
As shown in Table 1, the groups were well-matched on age, verbal IQ, gender, and ethnicity, but SDIs had spent less time in full-time education, and as expected from the clinical literature (Booth et al. 2006), SDIs also scored higher on the BDI-II than controls (Buckley et al. 2001). Consequently, both variables were included as covariates in the first set of analyses, though not in the analyses of dopamine markers.
Table 1.
Demographic and baseline measures for the groups of stimulant-dependent individuals (n = 18) and non-drug-taking comparison volunteers (n = 18)
| Group | Healthy control | Stimulant-dependent | F | df | P values |
|---|---|---|---|---|---|
| Age (years) | 32.7 (±6.9) | 34.3 (±7.2) | 0.47 | 1, 34 | 0.498 |
| Gender ratio (male/female) | 15:3 | 15:3 | Fisher′s exact | 1.000 | |
| Ethnic ratio (Caucasian/Afro-Caribbean) | 17:1 | 16:2 | Fisher′s exact | 1.000 | |
| Verbal intelligence quotient (NART) | 108.4 (±6.0) | 109.0 (±8.1) | 0.55 | 1, 34 | 0.816 |
| Years of education | 12.4 (±1.8) | 11.2 (±1.0) | 6.85 | 1, 34 | 0.013 |
| Depressive mood (BDI-II total score) | 1.1 (±2.4) | 9.3 (±11.1) | 9.50 | 1, 34 | 0.004 |
| Impulsivity (BIS-11 total score) | 62.0 (±7.2) | 82.0 (±9.5) | 50.4 | 1, 34 | <0.001 |
| Duration of stimulant abuse (years) | – | 11.7 (±7,4) | – | – | – |
| Compulsivity of stimulant abuse (OCDUS score) | – | 26.5 (±7.9) | – | – | – |
| Frequency of stimulant use (times per week) | – | 5.4 (±2.0) | – | – | – |
NART National Adult Reading Test, BIS-11 Barratt Impulsiveness Scale, BDI-II Beck Depression Inventory, OCDUS Obsessive-Compulsive Drug Use Scale
Plasma pramipexole, prolactin, and mRNA levels
There was no significant difference in plasma levels of pramipexole between the two groups (t 18.6 = 1.78, P > 0.05), and pramipexole plasma levels did not correlate with mean cognitive performance following pramipexole treatment. Prolactin levels decreased following administration of pramipexole in both groups. At baseline (i.e., following the pre-treatment with domperidone and prior to dosing), there was no difference in prolactin levels between the groups (F 1,34 = 0.01, P = 0.922) or between sessions (F 1,34 = 0.04, P = 0.834). The groups did not differ in terms of peripheral DRD3 (t 28 = 0.23, P > 0.05), DRD4 (t 29 = 0.08, P > 0.05), or COMT (t 29 = 0.49, P > 0.05) mRNA levels. Peripheral dopamine markers were unrelated to demographic or personality variables, including years of education, impulsivity, and depressive mood. Furthermore, we did not identify any associations between mRNA expression and baseline performance that survived correction for multiple comparisons.
Effects of pramipexole and group on cognitive performance
Cognitive data are shown in Table 1. SDIs performed significantly less accurately than controls on the SWM (F 1,32 = 10.14 P = 0.003), digit-span (F 1,31 = 5.00, P = 0.033), RVIP (F 1,32 = 15.76, P < 0.001), and PAL (F1,32 = 12.04, P = 0.002) tests and were marginally more impulsive on the IST (F 1,32 = 3.71, P = 0.063), replicating our previous findings (Clark et al. 2006; Ersche et al. 2006). No main effect of pramipexole was identified on any of the measures. The only significant drug-by-group interaction was observed on the IST test (F 1,31 = 6.67, P = 0.015). This interaction was driven by effects in the control participants, who performed less impulsively following pramipexole compared with placebo (t 17 = −2.29, P = 0.035), while performance in the SDI group was unaffected by pramipexole (t 16 = 0.53, P = 0.604). Stimulant craving increased significantly in SDIs over the course of the testing sessions (F 1,17 = 12.2, P = 0.003), but the main effect of drug treatment (F 1,17 = 1.33, P > 0.1) and drug-by-time interaction (F 1,17 = 0.15, P > 0.5) were non-significant (see also Table 2).
Table 2.
Performance data (mean and standard deviation, SD) on placebo and pramipexole in controls (n = 18) and stimulant-dependent individuals (n = 18)
| Group | Healthy control | Stimulant-dependent | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F (drug) | P (drug) | F (group) | P (group) | F (drug × group) | P (drug × group) | |
| SWM-placebo | 17.67 | 21.40 | 31.94 | 16.21 | 0.50 | 0.484 | 10.14 | 0.003 | 0.06 | 0.811 |
| SWM- pramipexole | 15.56 | 13.05 | 37.06 | 17.33 | ||||||
| DS-placebo | 7.72 | 2.52 | 5.61 | 1.79 | 0.89 | 0.353 | 5.00 | 0.033 | 1.07 | 0.308 |
| DS-pramipexole | 8.06 | 2.75 | 5.67 | 2.47 | ||||||
| RVIP-placebo | 0.96 | 0.03 | 0.91 | 0.04 | 2.66 | 0.113 | 11.57 | 0.002 | 0.18 | 0.672 |
| RVIP-pramipexole | 0.95 | 0.03 | 0.90 | 0.05 | ||||||
| PAL-placebo | 4.94 | 4.17 | 11.56 | 10.40 | 3.31 | 0.078 | 12.04 | 0.002 | 0.47 | 0.499 |
| PAL-pramipexole | 6.94 | 8.23 | 19.44 | 15.52 | ||||||
| IST-placebo | 10.58 | 3.28 | 9.27 | 4.50 | 0.35 | 0.560 | 3.71 | 0.063 | 6.67 | 0.015 |
| IST-pramipexole | 11.79 | 3.21 | 8.62 | 4.48 | ||||||
Task (dependent variable): SWM (total errors), DS digit-span (number), RVIP (A’), PAL (total errors), IST (number of boxes opened)
Associations between peripheral dopamine markers and pramipexole effects on cognitive test performance
Results of all regression analyses relating dopamine biomarkers to pramipexole-induced changes in cognition are presented in Table 3.
Table 3.
Pramipexole-induced performance change in controls (n = 15) and stimulant-dependent individuals (n = 16)
| Marker | Marker × group | |||
|---|---|---|---|---|
| SWM | ||||
| DRD3 | F1, 26 = 9.86 | P = 0.004 | F1, 26 = 0.71 | P = 0.406 |
| DRD4 | F1, 27 = 0.06 | P = 0.802 | F1, 27 = 1.03 | P = 0.320 |
| COMT | F1, 27 = 4.21 | P = 0.050 | F1, 27 = 0.20 | P = 0.658 |
| Digit-span | ||||
| DRD3 | F1, 25 = 0.45 | P = 0.510 | F1, 25 = 4.32 | P = 0.048 |
| DRD4 | F1, 26 = 0.001 | P = 0.996 | F1, 26 = 11.12 | P = 0.003 |
| COMT | F1, 26 = 2.43 | P = 0.131 | F1, 26 = 6.19 | P = 0.020 |
| RVIP | ||||
| DRD3 | F1, 26 = 4.24 | P = 0.050 | F1, 26 = 1.79 | P = 0.192 |
| DRD4 | F1, 27 = 0.67 | P = 0.419 | F1, 27 = 0.39 | P = 0.539 |
| COMT | F1, 27 = 1.03 | P = 0.319 | F1, 27 = 0.02 | P = 0.880 |
| PAL | ||||
| DRD3 | F1, 26 = 0.07 | P = 0.788 | F1, 26 = 0.001 | P = 0.991 |
| DRD4 | F1, 27 = 2.55 | P = 0.122 | F1, 27 = 0.01 | P = 0.910 |
| COMT | F1, 27 = 1.76 | P = 0.196 | F1, 27 = 1.98 | P = 0.171 |
| IST | ||||
| DRD3 | F1, 25 = 0.02 | P = 0.901 | F1, 25 = 0.18 | P = 0.672 |
| DRD4 | F1, 26 = 0.20 | P = 0.657 | F1, 26 = 0.02 | P = 0.884 |
| COMT | F1, 26 = 0.96 | P = 0.337 | F1, 26 = 0.21 | P = 0.652 |
DRD3
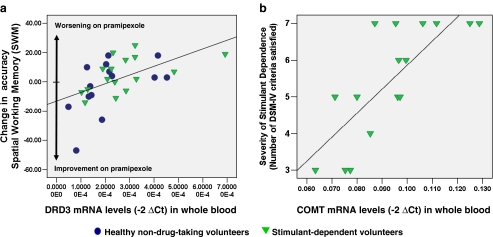
We identified a significant main effect of DRD3 mRNA levels on the pramipexole-induced change in SWM performance (F 1,26 = 9.86, P < 0.005). The group-by-DRD3 interaction was non-significant. Figure 1a illustrates this result: individuals with lower peripheral DRD3 mRNA levels exhibited improved accuracy on SWM performance following pramipexole administration. For the digit-span test, the main effect of DRD3 was non-significant, and the group-by-DRD3 interaction did not survive correction for multiple comparisons (F 1,25 = 4.32, P = 0.048), as shown in Fig. S1a. For the RVIP test, the main effect of DRD3 did not survive correction for multiple comparisons (F 1,26 = 4.24, P = 0.05), and the group-by-DRD3 interaction was non-significant (see Fig. S1b). On PAL and IST, no main effects of DRD3 or interactions were identified at a threshold of P < 0.05.
Fig. 1.
Relationships between peripheral biomarkers and cognitive/clinical measures. a Associations between peripheral DRD3 mRNA levels in blood cells and pramipexole-induced changes in accuracy in working memory; b Association between peripheral COMT mRNA levels in blood cells and severity of stimulant dependence, as reflected by the number of DSM-IV criteria satisfied for the diagnosis of dependence on stimulant drugs. Levels of mRNA were not detectable in five blood samples (two stimulant users, three controls)
DRD4
For digit-span, although the main effect of DRD4 was non-significant, there was a significant group-by-DRD4 interaction (F 1,26 = 11.12, P = 0.003) shown in Fig. S2a. Change in digit-span performance was negatively correlated with DRD4 in SDIs (r = −0.57, P = 0.017) and positively correlated in healthy volunteers (r = 0.52, P = 0.057). On the other cognitive tests, no main effects of DRD4 or interactions were detected at a threshold of P < 0.05.
COMT
For SWM, the main effect of COMT (F 1,27 = 4.21, P = 0.050) did not survive correction for multiple comparisons, and the group-by-COMT interaction was non-significant (see Fig. S2c). For digit-span, the main effect of COMT was non-significant and the group-by-COMT interaction, as shown in Fig. S2b, but did not survive correction for multiple comparisons (F 1,26 = 6.19, P = 0.020). No main effects of COMT or interactions were identified on RVIP and IST at a threshold of P < 0.05.
Associations between peripheral dopamine markers, pramipexole effects on symptom severity, and baseline symptom severity
The correlation between DRD4 mRNA levels and pramipexole-induced changes in craving in the SDI group (r = 0.63, P = 0.009) did not survive correction for multiple comparisons, and no correlations with DRD3 or COMT were identified at a threshold of P < 0.05.
With regard to baseline clinical variability in the SDI group, dopamine markers were unrelated to the duration, frequency, or compulsivity of stimulant abuse. However, peripheral levels of COMT mRNA were significantly correlated with the severity of stimulant dependence, as reflected by the number of criteria met according the DSM-IV (r = 0.78, P < 0.001); see Fig. 1b.
Discussion
The results of our study provide direct experimental evidence in support of the potential utility of peripheral dopamine biomarkers to predict treatment response to dopamine agonist drugs and to provide objective correlates of variability in clinical severity of addiction to dopamine-releasing drugs.
Peripheral dopamine markers to predict drug response
We found that peripheral D3 mRNA levels predict changes in working memory performance following a single dose of pramipexole. Although stimulant dependence has been associated with cognitive impairments (Ersche and Sahakian 2007; Rogers and Robbins 2003), the prediction of the pramipexole-induced performance change was independent of baseline performance or drug-taking history. Our findings are, however, in keeping with an inverted U-shaped function relating basal dopamine function with performance, i.e., those individuals with relatively low DRD3 mRNA expression should improve, while participants with relatively high peripheral expression levels should perform worse following dopamine agonist treatment. Our findings are also consistent with recent PET studies in which dopamine D2/3 receptor binding in the ventral striatum predicted methylphenidate-induced change in working memory performance in healthy volunteers (Clatworthy et al. 2009) and dopamine synthesis capacity predicted the cognitive response to the dopamine agonist bromocriptine (Cools et al. 2009). Given that the procedure for obtaining peripheral dopamine markers is minimally invasive and orders of magnitude less expensive than a PET scan, peripheral biomarkers could be attractive for future use in clinical practice.
The notion of predicting inter-individual variation in the effects of dopaminergic agents on cognition and behavior is not completely novel, although previous attempts have depended on the use of behavioral measures rather than peripheral biomarkers. Thus, trait-impulsivity (Cools et al. 2007) or baseline working memory performance (Kimberg et al. 2001) has previously been suggested to serve as indicators for cognitive change following dopamine agonist administration. However, the utility of these personality or performance indicators in psychiatric patients is questionable given that impulsivity is a key feature of several psychiatric disorders (Moeller et al. 2001) and cognitive impairment is common in psychiatric conditions (Harrison and Owen 2001). By contrast, the prediction of cognitive change by peripheral DRD3 mRNA expression was independent of self-reported impulsivity, mood, baseline cognitive performance, or psychiatric status. This implies that the use of dopamine mRNA level in circulating blood might be particularly suitable for mental health patients.
Our findings do not support the view that abnormalities of peripheral dopamine markers are related to the diagnosis of stimulant dependence per se (Czermak et al. 2004), but suggest that these markers could be used to predict individual variability addiction severity. We found that the higher the levels of peripheral COMT mRNA in the blood, the more severely dependent the SDIs were. It has been suggested that the genetic polymorphism that regulates COMT activity in the brain may also regulate the levels of COMT activity in lymphocytes (Sladek-Chelgren and Weinshilboum 1981). Higher COMT activity has repeatedly been suggested to be associated with increased vulnerability to the development of drug abuse (Beuten et al. 2005; Li et al. 2004; Vandenbergh et al. 1997), consistent with our data. However, the relationship between COMT polymorphism and cocaine dependence has been inconsistent (Lohoff et al. 2008). For other peripheral dopamine markers such as the DRD3 receptor a similar relationship with symptom severity has been identified in Parkinson’s disease, i.e., a decrease in DRD3 mRNA levels was associated with the degree of clinical severity in these patients (Nagai et al. 1996). Longitudinal studies would be needed to investigate the stability of these measures over time, given that the severity of substance dependence may change following therapeutic interventions.
Does expression of peripheral dopamine markers reflect levels in the brain?
The mechanisms underlying the expression of dopamine receptor mRNA in the brain and in the periphery are not fully understood. It has been suggested that mRNA levels detectable peripherally in the blood reflect levels of expression in the brain (Caronti et al. 1998; Ilani et al. 2001; Pacheco-Lopez et al. 2003), but evidence for this direct relationship is still lacking. While there are clear similarities in gene expression profiles in whole blood and brain tissue, variation in expression between tissue types also exists (Sullivan et al. 2006). Between-subjects variation in gene expression may be driven by genetic polymorphisms, some of which regulate mRNA transcription, but this regulation might also vary across different tissues. Post-mortem analyses of stimulant users who died of a cocaine overdose showed a significant increase in both D3 receptors (Staley and Mash 1996) and DRD3 mRNA levels (Segal et al. 1997) in the nucleus accumbens compared with age-matched, drug-free control brains. Unfortunately, dopamine markers in peripheral blood in these samples were not analyzed, limiting direct comparison with the present study. The current uncertainties suggest that speculations about the comparability of gene expression peripherally and centrally must remain tentative.
PET studies in SDIs who had been drug abstinent for at least two weeks also showed a significant reduction in D2/3 receptor density in the striatum compared with non-drug-taking controls (Martinez et al. 2004; Volkow et al. 1993). Accordingly, a separate study in a different sample showed that peripheral dopamine receptor mRNA expression in lymphocytes was reduced in abstinent drug users (Czermak et al. 2004). Our SDIs had been actively using stimulant drugs for an average of 11.7 years (SD ± 7.4) and did not differ from controls in terms of peripheral dopamine markers. One may speculate whether active drug use increases dopamine receptor mRNA, masking a pre-morbid down-regulation similarly to what reported for D4 and D5 mRNA expression in opiate-dependent individuals on methadone maintenance therapy (Goodarzi et al. 2009). Support for this view comes from de novo patients with Parkinson’s disease, whose dopamine receptor expression in lymphocytes normalized following three months treatment with l-DOPA or a dopamine agonist (Barbanti et al. 1999).
Methodological issues and limitations
Our findings may differ from biomarker results reported by other groups (Czermak et al. 2004; Goodarzi et al. 2009) as we extracted mRNA markers from whole blood not only from lymphocytes. We used whole blood to maximize the stability of the dopamine receptor mRNA and to avoid potential artifactual changes from isolating individual peripheral cell types. We cannot rule out that the pre-treatment with domperidone for the prevention of emetic side effects may have affected the levels of mRNA expression in the blood. This seems unlikely, however, because all our participants took the same dose of domperidone prior to drug treatment administration, and prolactin levels, assessed immediately before dosing of each drug treatment session, did not differ between the groups or between the treatment sessions.
Conclusions
Our findings that peripheral D3 receptor mRNA levels predicted individual variability in working memory following dopamine agonist treatment suggest that such metrics might be usefully employed in clinical practice. The ability of peripheral biomarkers to predict response to dopamine agonist administration could have important implications for personalizing the treatment for psychiatric patients, allowing clinicians to predict which patients will receive greatest benefit from dopaminergic medications. It would be of great interest to examine the relationship between peripheral mRNA levels relating to neurotransmitter function and response to treatment in other neuropsychiatric disorders. Our data also speak to the ongoing debate about the potential utility of cognitive enhancing drugs to optimize performance in the context of normal cognitive variability over the lifecycle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Correlations between the single housekeeper genes and the target genes (DOC 29 kb)
Associations between peripheral dopamine receptor DRD3 expression and pramipexole effects on digit-span performance (a) and sustained attention (b) (DOC 1570 kb)
Associations between peripheral dopamine receptor DRD4 expression and pramipexole effects on digit-span performance (a), and between peripheral COMT expression and digit-span (b) and spatial working memory (c) performance (DOC 2370 kb)
Acknowledgments
We thank all volunteers for their participation in this study and staff at the GlaxoSmithKline Clinical Unit Cambridge for their dedicated support. Special thanks go to Sam Miller, Joanna Ward, and Jonathan Mill for their expert advice and practical assistance. The authors also thank Kevin Craig for his support with the data collection and the provision of medical cover. This work was funded and sponsored by GlaxoSmithKline and conducted within the GlaxoSmithKline Clinical Unit Cambridge, UK; Jonathan Roiser and Trevor Robbins both consult for Cambridge Cognition. Mark Lucas and Enrico Domenici were previously employed by GlaxoSmithKline Ltd (GSK). Ed Bullmore is a part-time employee at GSK and part-time at the University of Cambridge. Karen Ersche is supported by grant from the Medical Research Council (MRC) and declares that she has no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/S0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders-4th Edition. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Arnsten AFT. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci. 1998;2:436–447. doi: 10.1016/S1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- Barbanti P, Fabbrini G, Ricci A, Cerbo R, Bronzetti E, Caronti B, Calderaro C, Felici L, Stocchi F, Meco G, Amenta F, Lenzi GL. Increased expression of dopamine receptors on lymphocytes in Parkinson’s disease. Mov Disord. 1999;14:764–771. doi: 10.1002/1531-8257(199909)14:5<764::AID-MDS1008>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 2000;102:113–124. doi: 10.1016/S0165-5728(99)00176-9. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Beuten J, Payne TJ, Ma JZ, Li MD. Significant association of catechol-O-methyltransferase (COMT) haplotypes with nicotine dependence in male and female smokers of two ethnic populations. Neuropsychopharmacology. 2005;31:675–684. doi: 10.1038/sj.npp.1300997. [DOI] [PubMed] [Google Scholar]
- Booth BM, Leukefeld C, Falck R, Wang JC, Carlson R. Correlates of rural methamphetamine and cocaine users: results from a multistate community study. J Stud Alcohol. 2006;67:493–501. doi: 10.15288/jsa.2006.67.493. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Parker JD, Heggie J. A psychometric evaluation of the BDI-II in treatment-seeking substance abusers. J Subst Abuse Treat. 2001;20:197–204. doi: 10.1016/S0740-5472(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Caronti B, Calderaro C, Passarelli F, Palladini G, Pontieri FE. Dopamine receptor mRNAs in the rat lymphocytes. Life Sci. 1998;62:1919–1925. doi: 10.1016/S0024-3205(98)00160-X. [DOI] [PubMed] [Google Scholar]
- Catnacho-Ochoa M, Walker EL, Evans DL, Piercey MF. Rat brain binding sites for pramipexole, a clinically useful D3-preferring dopamine agonist. Neurosci Lett. 1995;196:97–100. doi: 10.1016/0304-3940(95)11857-S. [DOI] [PubMed] [Google Scholar]
- Champion MC. Domperidone. Gen Pharmacol Vasc Syst. 1988;19:499–505. doi: 10.1016/0306-3623(88)90153-X. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in chronic and former substance users. Biol Psychiatry. 2006;60:512–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJG, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron JC, Fryer TD, Robbins TW. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermak C, Lehofer M, Wagner EM, Prietl B, Lemonis L, Rohrhofer A, Schauenstein K, Liebmann PM. Reduced dopamine D4 receptor mRNA expression in lymphocytes of long-term abstinent alcohol and heroin addicts. Addiction. 2004;99:251–257. doi: 10.1111/j.1360-0443.2003.00621.x. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychology Review. 2007;17(3):317–336. doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, Ooi C, Suckling J, Barnes A, Sahakian BJ, Merlo-Pich EV, Robbins TW. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch Gen Psychiatry. 2010;67:632–644. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Neuroendocrine Immune Basis Rheum Dis Ii. 2002;966:20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). Biometrics Research, New York State Psychiatric Institute, New York
- Foll BL, Gallo A, Strat YL, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probe: potential for studying psychiatric disorders. Prog Neuro Psychopharmacol Biol Psychiatry. 2004;28:559–576. doi: 10.1016/j.pnpbp.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi A, Vousooghi N, Sedaghati M, Mokri A, Zarrindast MR. Dopamine receptors in human peripheral blood lymphocytes: changes in mRNA expression in opioid addiction. Eur J Pharmacol. 2009;615:218–222. doi: 10.1016/j.ejphar.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Harrison J, Owen AM. Cognitive deficits in brain disorders. London, U.K: Informa Healthcare; 2001. [Google Scholar]
- Howell DC. Statistical methods for psychology. 4. London: Duxbury Press; 1997. [Google Scholar]
- Ilani T, Ben-Shachar D, Strous RD, Mazor M, Sheinkman A, Kotler M, Fuchs S. A peripheral marker for schizophrenia: increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci USA. 2001;98:625–628. doi: 10.1073/pnas.021535398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, Lease J, D’Esposito M. Cortical effects of bromocriptine, a D-2 dopamine receptor agonist, in human subjects, revealed by fMRI. Hum Brain Mapp. 2001;12:246–257. doi: 10.1002/1097-0193(200104)12:4<246::AID-HBM1019>3.0.CO;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillova GP, Hrutkay RJ, Shurin MR, Shurin GV, Tourkova IL, Vanyukov MM. Dopamine receptors in human lymphocytes: radioligand binding and quantitative RT-PCR assays. J Neurosci Meth. 2008;174:272–280. doi: 10.1016/j.jneumeth.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann NY Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Li T, Chen CK, Hu X, Ball D, Lin SK, Chen W, Sham PC, Loh EW, Murray RM, Collier DA. Association analysis of the DRD4 and COMT genes in methamphetamine abuse. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:120–124. doi: 10.1002/ajmg.b.30024. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-delta delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Weller AE, Bloch PJ, Nall AH, Ferraro TN, Kampman KM, Pettinati HM, Oslin DW, Dackis CA, O’Brien CP, Berrettini WH. Association between the catechol-O-methyltransferase Val158Met polymorphism and cocaine dependence. Neuropsychopharmacology. 2008;33:3078–3084. doi: 10.1038/npp.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang YY, Perez A, Frankel WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and D-2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Wang JC, Haroutunian V, Davis KL, Watson SJ. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15:17–29. doi: 10.1016/0893-133X(95)00150-C. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Ueno S, Saeki Y, Soga F, Hirano M, Yanagihara T. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson’s disease. Neurology. 1996;46:791–795. doi: 10.1212/wnl.46.3.791. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test Manual. Windsor (UK): NFER-Nelson; 1982. [Google Scholar]
- Pacheco-Lopez G, Niemi MB, Kou W, Bildhauser A, Gross CM, Goebel MU, del Rey A, Besedovsky HO, Schedlowski M. Central catecholamine depletion inhibits peripheral lymphocyte responsiveness in spleen and blood. J Neurochem. 2003;86:1024–1031. doi: 10.1046/j.1471-4159.2003.01914.x. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Mcinnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB)—a factor-analytic study of a large-sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. The neuropsychology of chronic drug abuse. In: Ron MA, Robbins TW, editors. Disorders of brain and mind: vols 2. Cambridge: Cambridge University Press; 2003. pp. 447–467. [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative-study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s-disease. Brain. 1988;111:695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/S0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Mol Brain Res. 1997;45:335–339. doi: 10.1016/S0169-328X(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Shearer J, Sherman J, Wodak A, Van Beek I. Substitution therapy for amphetamine users. Drug Alcohol Rev. 2002;21:179–185. doi: 10.1080/09595230220139082. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Sladek-Chelgren S, Weinshilboum RM. Catechol-O-methyltransferase biochemical genetics—human-lymphocyte enzyme. Biochem Genet. 1981;19:1037–1053. doi: 10.1007/BF00484563. [DOI] [PubMed] [Google Scholar]
- Soares BG, Lima MS, Reisser AA, Farrell M (2004) Dopamine agonists for cocaine dependence. Cochane Database Syst.Rev. 1–45
- Staley JK, Mash DC. Adaptive increase in D-3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:261–268. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Gr S. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779:58–74. doi: 10.1016/S0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Dopamine D-4 receptors: significance for molecular psychiatry at the millennium. Mol Psychiatry. 1999;4:529–538. doi: 10.1038/sj.mp.4000674. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Rodriguez LA, Miller IT, Uhl GR, Lachman HM. High-activity catechol-O-methyltransferase allele is more prevalent in polysubstance abusers. Am J Med Genet. 1997;74:439–442. doi: 10.1002/(SICI)1096-8628(19970725)74:4<439::AID-AJMG16>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine-D(2) receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D-2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The psychometric tradition—developing the Wechsler Adult Intelligence Scale. Contemp Educ Psychol. 1981;6:82–85. doi: 10.1016/0361-476X(81)90035-7. [DOI] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. doi: 10.1037/0033-295X.94.4.469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlations between the single housekeeper genes and the target genes (DOC 29 kb)
Associations between peripheral dopamine receptor DRD3 expression and pramipexole effects on digit-span performance (a) and sustained attention (b) (DOC 1570 kb)
Associations between peripheral dopamine receptor DRD4 expression and pramipexole effects on digit-span performance (a), and between peripheral COMT expression and digit-span (b) and spatial working memory (c) performance (DOC 2370 kb)