Abstract
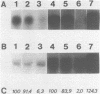
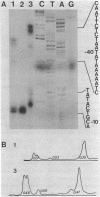
We have generated mutations in the promoter region of the octopine type cytokinin gene of Agrobacterium tumefaciens, and studied their effects on mRNA formation in different plant species. The promoter region of this gene contains several putative TATA boxes. Phenotypic expression and Northern blot hybridization showed that TATA boxes are essential for expression, but that one TATA box leads to wild-type transcript levels. Analysis of the 5' ends of T-cyt transcripts by primer extension using RNA from T-cyt gene transformed tobacco shoots revealed two major cap site clusters and one minor cap site. TATA box consensus sequences can be found approximately 30 bp upstream from each cap site cluster. Deletion of a TATA box results in loss of the corresponding cap sites. An insertion of 7 bp between the right TATA box and corresponding cap sites results in a shift of the position of the cap sites, so that the original distance of TATA box to cap sites is conserved as much as possible.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Bruce W. B., Gurley W. B. Functional domains of a T-DNA promoter active in crown gall tumors. Mol Cell Biol. 1987 Jan;7(1):59–67. doi: 10.1128/mcb.7.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann I., Marner F. J., Schröder G., Waffenschmidt S., Schröder J. Tumour genes in plants: T-DNA encoded cytokinin biosynthesis. EMBO J. 1985 Apr;4(4):853–859. doi: 10.1002/j.1460-2075.1985.tb03710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidekamp F., Dirkse W. G., Hille J., van Ormondt H. Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid-encoded tmr gene. Nucleic Acids Res. 1983 Sep 24;11(18):6211–6223. doi: 10.1093/nar/11.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs B. J., Butterworth P. H. The effect of changing the distance between the TATA-box and cap site by up to three base pairs on the selection of the transcriptional start site of a cloned eukaryotic gene in vitro and in vivo. Nucleic Acids Res. 1986 Mar 25;14(6):2429–2442. doi: 10.1093/nar/14.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein C., Klee H., Montoya A., Garfinkel D., Fuller S., Flores C., Nester E., Gordon M. Nucleotide sequence and transcript mapping of the tmr gene of the pTiA6NC octopine Ti-plasmid: a bacterial gene involved in plant tumorigenesis. J Mol Appl Genet. 1984;2(4):354–362. [PubMed] [Google Scholar]
- Nagawa F., Fink G. R. The relationship between the "TATA" sequence and transcription initiation sites at the HIS4 gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8557–8561. doi: 10.1073/pnas.82.24.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Hooykaas P. J., Van Veen R. J., Van Beelen P., Regensburg-Tuïnk T. J., Schilperoort R. A. Octopine Ti-plasmid deletion mutants of agrobacterium tumefaciens with emphasis on the right side of the T-region. Plasmid. 1982 Jan;7(1):15–29. doi: 10.1016/0147-619x(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Ooms G., Klapwijk P. M., Poulis J. A., Schilperoort R. A. Characterization of Tn904 insertions in octopine Ti plasmid mutants of Agrobacterium tumefaciens. J Bacteriol. 1980 Oct;144(1):82–91. doi: 10.1128/jb.144.1.82-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater B. S., Klinkhamer M. P., Amesz P. A., de Kam R. J., Memelink J., Hoge J. H., Schilperoort R. A. Plant expression signals of the Agrobacterium T-cyt gene. Nucleic Acids Res. 1987 Oct 26;15(20):8267–8281. doi: 10.1093/nar/15.20.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]