Abstract
Imbalance in the regulatory immune mechanisms that control intestinal cellular and bacterial homeostasis may lead to induction of the detrimental inflammatory signals characterized in humans as inflammatory bowel disease. Induction of proinflammatory cytokines (i.e., IL-12) induced by dendritic cells (DCs) expressing pattern recognition receptors may skew naive T cells to T helper 1 polarization, which is strongly implicated in mucosal autoimmunity. Recent studies show the ability of probiotic microbes to treat and prevent numerous intestinal disorders, including Clostridium difficile-induced colitis. To study the molecular mechanisms involved in the induction and repression of intestinal inflammation, the phosphoglycerol transferase gene that plays a key role in lipoteichoic acid (LTA) biosynthesis in Lactobacillus acidophilus NCFM (NCK56) was deleted. The data show that the L. acidophilus LTA-negative in LTA (NCK2025) not only down-regulated IL-12 and TNFα but also significantly enhanced IL-10 in DCs and controlled the regulation of costimulatory DC functions, resulting in their inability to induce CD4+ T-cell activation. Moreover, treatment of mice with NCK2025 compared with NCK56 significantly mitigated dextran sulfate sodium and CD4+CD45RBhighT cell-induced colitis and effectively ameliorated dextran sulfate sodium-established colitis through a mechanism that involves IL-10 and CD4+FoxP3+ T regulatory cells to dampen exaggerated mucosal inflammation. Directed alteration of cell surface components of L. acidophilus NCFM establishes a potential strategy for the treatment of inflammatory intestinal disorders.
Keywords: antiinflammatory, lactobacilli, Toll-like receptor 2, innate immunity
The maintenance of intestinal immune homeostasis involves the balanced interaction of bacterial microflora, gut epithelium, and host immune cells. Deregulation of these immunological interactions can result in immune dysfunction and lead to overt inflammation typical of human inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease (1). Although the cellular and molecular mechanisms of IBD are not fully understood, data indicate that chronic intestinal inflammation induced by inflammatory cytokines (i.e., IL-12) plays a pivotal role. These cytokines initiate the differentiation of pathogenic CD4+CD45RBhighT cells that are strongly involved in disease progression (2). Studies show that regulation of these cells mitigates experimental colitis (3, 4). Additionally, like IL-12, secreted IL-23 from activated dendritic cells (DCs) that use the IL-12p40 subunit is also implicated in the development of various autoimmune diseases, including IBD (5). The inflammatory nature of IL-23 has been attributed to induce T helper (Th) 17 (6, 7). Furthermore, IL-23 also activates the production of TNFα and IL-6 in DCs (8). Together, studies show that blocking the IL-12p40 subunit signaling significantly reduces inflammation and indicate that both IL-12 and IL-23 are strongly involved in IBD. In contrast to both of these cytokines, IL-10 exerts regulatory effects on the inflammatory signals (9), highlighting its potential ability in controlling pathogenic CD4+ T-cell immune responses in IBD.
Lactobacillus species are normal inhabitants of the human gastrointestinal tract and major components of the natural microbiota in the small bowel (10). These bacteria are considered beneficial commensals, and some species are generally recognized as safe because of a long history of human consumption (11). Notably, their successful use in the prevention of IBD has been highlighted (11). We and others recently showed that specific Lactobacillus species stimulated DCs to produce inflammatory cytokines (i.e., IL-12) and regulatory IL-10 (12, 13). The precise mechanisms by which lactobacilli exert such effector signals are not clearly known. However, recent data indicate that components of these bacteria, such as surface-layer proteins (13–16) and lipoteichoic acid (LTA), stimulate DCs through specific pattern recognition receptors, including Toll-like receptor 2 (TLR2), resulting in such a cytokine release (17, 18). Accordingly, the quality and levels of D-alanine (D-Ala) on LTA are critical for cytokine production, as shown by the synthesis of LTA-deficient in D-Ala (19–21). LTA may also mediate Lactobacillus adhesion to epithelial cells (ECs) through the negative charge that it confers to the bacterial surface, which facilitates the electrostatic binding to surface molecules. To investigate the potential role of L. acidophilus LTA in inducing inflammatory signals, we deleted the phosphoglycerol transferase gene that primes the synthesis of bacterial LTA. The data show that disruption of LTA synthesis resulted in an L. acidophilus derivative that acted on intestinal immune cells to augment the production of IL-10, down-regulate IL-12 levels, and significantly mitigate induced dextran sulfate sodium (DSS) and CD4+CD45RBhighT cell-mediated colitis in mice.
Results
Generation of NCK2025-Deficient in LTA.
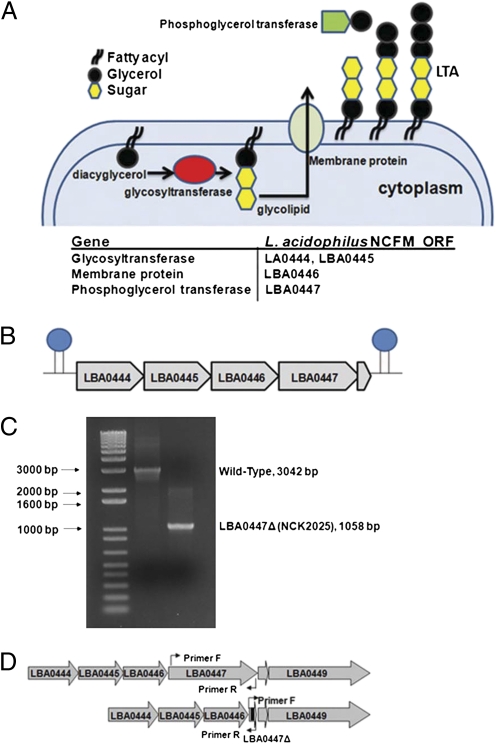
One of the constituent molecules of Gram-positive bacteria is LTA, consisting of a membrane-anchored glycolipid and a polyglycerophosphate chain with covalently linked D-Ala residues (22). The current model of LTA biosynthesis suggests three distinct stages in the expression of LTA, indicating that a glycolipid anchor unit is initially synthesized by action of a glycosyltransferase. Subsequently, the glycolipid is translocated to the exterior of the bacterium by a membrane-associated protein followed by extracellular addition of polyglycerolphosphate to the glycolipid anchor by a phosphoglycerol transferase (Fig. 1A). In L. acidophilus NCFM (NCK56), the genes LBA0444-LBA0447 were identified and annotated for their putative roles in LTA biosynthesis (Fig. 1B). The genes LBA0444 and LBA0448 are flanked by putative ρ-independent terminators (23), indicating that these five genes are cotranscribed and function as an operon. Although LBA0444–LBA0447 genes have a putative role in LTA biosynthesis, LBA0448 is a small hypothetical protein that does not seem to play a role in LTA biosynthesis (24).
Fig. 1.
(A and B) LTA synthesis and the genes involved in LTA biosynthesis. (C and D) Two noncontiguous fragments flanking an internal region of the target ORF were amplified and joined using splicing by overlap extension (SOEing) PCR (60). The resulting fragments were cloned into pORI28 and transformed into L. acidophilus NCK1392 containing the temperature-sensitive helper plasmid pTRK669. Erythromycin-sensitive cells were screened for a deletion mutation using PCR with primers flanking the targeted region and confirmed by sequencing the region containing the deletion.
We have shown previously that specific Lactobacillus species can effectively activate various signals in DCs that, in turn, induce T-cell immune responses (12, 13). To further investigate the molecular mechanisms involved in modifying DC function, we specifically deleted the phosphoglycerol transferase gene (LBA0447) in L. acidophilus NCK56. PCR analysis of this genomic region showed that the deletion mutant, NCK2025, lost 2 kbp (Fig. 1 C and D). Sequencing over this region confirmed the elimination of LBA0447. Chromatographic analysis of cell wall extracts of the parent NCK56 and NCK2025 showed the absence of LTA in NCK2025 where the phosphoglycerol transferase gene had been deleted (Fig. S1A). No difference in the growth rate of NCK56 or NCK2025 was observed in broth culture (Fig. S1B). To evaluate the potential physiological impact of the LTA deletion, NCK2025 was challenged in both gastric juice and small intestinal juice in vitro (Fig. S1 C and D). NCK2025 was more sensitive to both challenge conditions and displayed an approximate 1-log reduction in survival compared with the parent, but the overall rate of inactivation seemed similar over the time course of the challenges.
Regulation of DCs by NCK2025.
Coculturing DCs with NCK56 or NCK2025 showed that NCK2025 down-regulated MHC II, CD40, CD80, and CD86 on the surface of DCs (Fig. 2A). Moreover, treatment of DCs with NCK56 or LTA from Staphylococcus aureus induced the transcription of TLR2, whereas this pattern recognition receptor was not activated in NCK2025-treated DCs (Fig. 2B). It was documented that LTA derived from S. aureus or L. plantarum NCIMB8826 signals through TLR2, inducing TNFα in DCs (25). We, thus, generated bone-marrow DCs from C57BL/6 TLR2+/+, TLR2−/−, Balb/C MyD88+/+, or MyD88−/− mice to determine TNFα production in these cells. Data show that NCK56 and LTA from S. aureus enhanced the production of TNFα in the mouse DCs, whereas NCK2025 was weaker in the induction of TNFα in these cells derived from C57BL/6 TLR2+/+ mice and Balb/C MyD88+/+ mice, indicating that LTA is strongly involved in TLR2/MyD88 (26) signaling of DCs (Fig. S1 E and F).
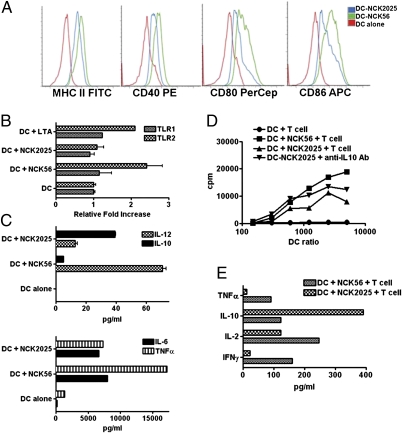
Fig. 2.
(A) Phenotype of DCs treated with NCK56 or NCK2025. Bone marrow-derived DCs were cocultured with NCK56 or NCK2025 for 24 h. Cells were stained with corresponding antibodies, fixed, and subsequently, analyzed by a FACSCalibur. Experiments were repeated at least three times with the same outcome. (B) DCs were cultured alone or 1:1 with either NCK56, NCK2025, or LTA from Staphylococcus aureus (100 ng/mL) for 1 h. RNA was extracted and reverse-transcribed, and real-time PCR was performed using primers for TLR1 and TLR2. Data shown represents the 1-h DC alone or cocultures of these cells with NCK56, NCK2025, or LTA. (C) Cytokine analysis. Cytokines released in the supernatants of NCK56- or NCK2025-treated and untreated DCs were assayed by ELISA. (D) T-cell proliferation. Groups of C57BL/6 mice (five per group) were orally treated with NCK56 or NCK2025 for 4 consecutive d. Mesenteric LN-T cells were derived and cocultured with NCK56- or NCK2025-treated and untreated DCs for 4 d to assay T-cell proliferation using thymidine incorporation. In some experiments, to restore the suppressed T-cell proliferation, anti–IL-10 antibodies were added to supernatants derived from DCs that were cocultured with NCK2025. (E) Mesenteric LN-T cells derived from each group of mice were cocultured with DCs that were treated or untreated with L. acidophilus strains to assay cytokines.
Both strains induced IL-10 production in DCs; however, the level of this cytokine was significantly increased in DCs cocultured with NCK2025 (Fig. 2C). Concomitantly, IL-12 and TNFα were significantly reduced in NCK2025-treated DCs (Fig. 2C). T-cell proliferation was determined by coculturing mesenteric lymph node (LN)-derived T cells with bone marrow-derived DCs treated with NCK56 or NCK2025. T-cell proliferation was significantly abrogated in NCK2025-treated DCs cocultured with T cells relative to NCK56 (Fig. 2D). Interestingly, adding anti–IL-10 antibody to the supernatant of NCK2025-treated DCs partially restored the proliferation of T cells, indicating that IL-10 may be a pivotal factor that regulates T-cell proliferation in the DC:T cell cocultures (Fig. 2D). Analysis of harvested supernatants from DC:T cell cocultures showed that IL-10 was highly induced in T cells, whereas IFNγ, IL-2, and TNFα were minimally released from T cells of mice that were treated with NCK2025 (Fig. 2E).
Amelioration of DSS and CD4+CD45RBhighT Cell-Induced Colitis by NCK2025.
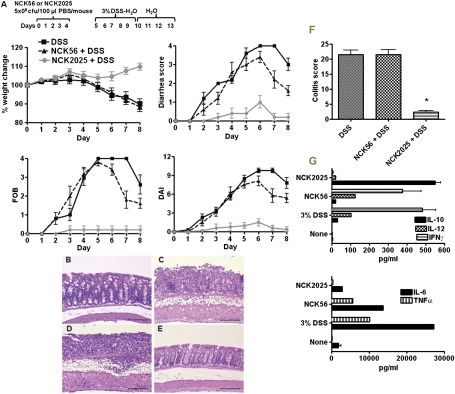
To determine the immunomodulatory properties of NCK2025 in vivo, we analyzed DSS-induced colitis in mice that were treated with NCK56 or NCK2025 for 4 consecutive d before exposure to 3% DSS. Data show that DSS induced clinical and histological colitis in untreated C57BL/6 mice (Fig. 3 A and C) relative to baseline (Fig. 3B). Clinically, mice began to lose weight after day 9, with ∼10% overall weight loss by day 13, and developed severe bloody diarrhea around days 10–11 (Fig. 3A). By contrast, oral inoculation of the mice with NCK2025 significantly prevented weight loss, reduced diarrhea, and reduced hemoccult positivity (Fig. 3A). Overall, the disease activity index (DAI) was significantly reduced from day 7. Moreover, pretreatment of the mice with NCK2025 reduced histological colitis scores up to 90% (21.5 ± 1.4 to 2.3 ± 0.5) (Fig. 3 B–F). Accordingly, Fig. 3E represents intact, nonulcerated epithelium with limited inflammation confined to the mucosa. To specifically address the role of LTA deletion in the prevention of colitis by NCK2025, a third group of mice was treated with NCK56. NCK56 did not prevent the onset of colitis (Fig. 3 A and D), and the mice developed similar clinical and histological colitis to nontreated mice. To elucidate the cytokines expressed by colonic tissues, the colons were extracted from each group of mice and cultured overnight. Colonic cytokine analysis shows the levels of IL-6, IL-12, TNFα, and IFNγ were higher in colons derived from NCK56- and DSS-treated mice (Fig. 3G). By contrast, IL-10 production was significantly elevated in colonic cultures derived from NCK2025-treated mice. IL-12, IL-6, IFNγ, and TNFα were significantly reduced in the colons of the mice treated with NCK2025 (Fig. 3G).
Fig. 3.
Amelioration of DSS-induced colitis by NCK2025. (A) C57BL/6 mice (n = 10) were orally inoculated with NCK56, NCK2025 (both 5 × 108 cfu·100 μL·mouse), or PBS for 4 consecutive d. These groups of mice were exposed to 3% DSS dissolved in the drinking water for 5 d followed by 7 d of plain water and assessed over time for colitis progression, including hematoxylin & eosin (H&E) staining, weight lost, diarrhea, and hemoccult positivity. FOB, fecal hemoccult blood positivity; DAI, disease activity index. (B–E) Colonic H&E staining. (B) Untreated mice. (C) DSS-treated mice. (D) NCK56-DSS–treated mice. (E) NCK2025-DSS–treated mice. (F) Colitis score. Data are representative of at least three independent experiments. (G) Colonic cytokine analysis. Colons of the mice (five per group) that were treated with NCK56, NCK2025, or DSS were cleaned with cold PBS, cut into pieces, and cultured for 18 h at 37 °C. Cytokines were assayed by ELISA.
Additionally, colitis was induced by transferring CD4+CD45RBhighT cells to Rag1−/− mice (27) to test whether treatment with NCK2025 prevents T cell-induced colitis. All groups that received CD4+CD45RBhighT cells gained weight, had negative occult blood testing, and did not have diarrhea through day 37. Transfer of CD4+CD45RBhighT cells resulted in weight loss beginning 5 wk after transfer, with expected infiltration of inflammatory cells into the submucosa and lamina propria (LP), crypt destruction, and goblet-cell depletion in Rag1−/− mice (27) and mice treated with NCK56 (Fig. S2 A–E). By contrast, treatment with NCK2025 mitigated CD4+CD45RBhighT cell-induced colitis in Rag1−/− mice (Fig. S2 A–F).
Investigation of genes in distal and proximal regions of mice that were inoculated with NCK56 vs. NCK2025 in DSS-induced colitis revealed the up-regulation of immune stimulatory (i.e., CD40, TNFα, tumor necrosis factor receptor [TNFR], interleukin [IL]-6, IL-1β, chemokine (C-C motif) ligand 11 [CCL11], and nitric oxide synthase 2 [NOS2]), signaling (i.e., tissue inhibitor of metalloproteinases [TIMP]1 and CD45), and proliferation/apoptosis/angiogenesis/adhesion (i.e., FAS ligand [FASL], inter-cellular adhesion molecule [ICAM]1, vasopressin activated calcium-mobilizing receptor [VACM]1, and cyclooxygenase [COX]-2) genes, suggesting active inflammatory responses in NCK56- but not NCK2025-treated mice (Fig. S3). This implies that such genes may become significantly activated to exert their inflammatory functions in the distal colon where DSS-induced colitis is more severe. Nonetheless, further studies are required to elucidate the role of these and other unknown stimulatory or regulatory genes that become activated through NCK56 or NCK2025 interaction with intestinal immune cells (i.e., ECs and DCs).
Therapeutic Effects of NCK2025 on DSS-Induced Colitis.
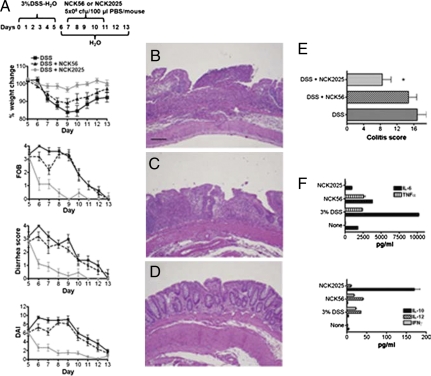
To address the effects of NCK2025 exposure in mice with established colitis, mice were first exposed to 3% DSS before treatment with NCK56 or NCK2025. After disease symptoms occurred, mice received NCK56 or NCK2025 for 4 consecutive d (Fig. 4A). Data show that both NCK56 and NCK2025 attenuated established colitis but to a significantly different degree. Treatment of mice with NCK2025 resulted in stabilization of body weight and rapid resolution of diarrhea and blood loss, whereas NCK56-treated mice continued to lose weight, albeit at a slower rate, and were significantly slower to resolve diarrhea and blood loss (Fig. 4A). Histological analysis revealed ongoing, active colitis and ulceration with a colitis score of 16.8 ± 2.2 in DSS-treated mice at day 13 (Fig. 4 B and E). Mice treated with NCK2025 had significantly improved colitis scores (8.4 ± 2.2; P = 0.01), showing accelerated healing, including rare ulceration, regenerated crypt structures, and inflammation primarily limited to the mucosa (Fig. 4 D and E). NCK56 treatment mildly improved histological colitis scores (14.7 ± 2.0; P was not significant), with restitution of the epithelium but limited crypt regeneration and ongoing active inflammation within the mucosa and submucosa (Fig. 4 C and E). Interestingly, cytokine analysis of the mice treated with NCK2025 shows up-regulation of IL-10 and minimal release of IL-12, TNFα, IL-6, and IFNγ in the colons of these mice (Fig. 4F). By contrast, IL-12, TNFα, IFNγ, and IL-6 were highly induced in the colons of mice treated with NCK56 or DSS alone, and IL-10 was minimally released in these groups of mice (Fig. 4F).
Fig. 4.
Mitigation of established colitis by NCK2025. (A–D) Three groups of C57BL/6 mice (10 per group) first received a 5-d cycle of 3% DSS dissolved in sterile water to initiate colitis, and two of the groups were subsequently treated orally with NCK56 or NCK2025 for 4 consecutive d. Disease progression was monitored to day 13 of the protocol when mice were killed, and colons were assessed. FOB, fecal hemoccult blood positivity; DAI, disease activity index. (B–D) Colonic H&E staining. (B) DSS-treated mice. (C) DSS-NCK56–treated mice. (D) DSS-NCK025–treated mice. (E) Colitis score. Data are representative of at least three independent experiments. (F) Colonic cytokine analysis. Colons of each group of mice were washed, cut into pieces, and cultured for 18 h at 37 °C. Cytokines were assayed by ELISA.
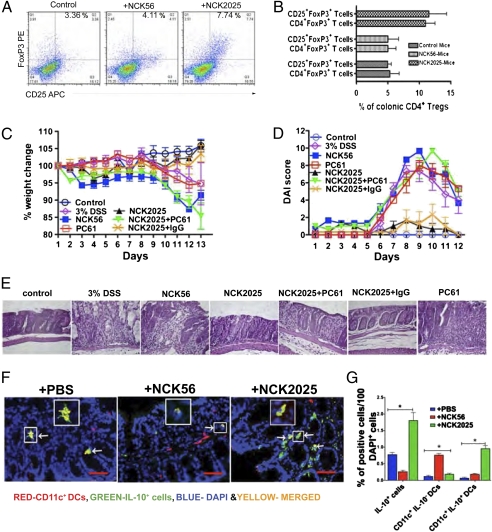
Induction of Colonic CD4+Foxp3+T Cells by NCK2025.
The role of CD4+ T regulatory cells (Tregs) in suppressing deregulated immune responses to self and commensal microbiota has recently been highlighted (28). Accordingly, we investigated whether NCK2025 enhances colonic CD4+Foxp3+ Tregs in C57BL/6 mice compared with NCK56. Indeed, Fig. 5 A and B shows that colonic Tregs were significantly induced in NCK2025- compared with NCK56-treated mice, suggesting that the suppressor effects of these cells may impact exaggerated intestinal inflammation induced by DSS or CD4+CD45RBhighT cells. To address the role of Tregs in DSS-induced colitis, Treg function was inactivated using anti-CD25 antibody (29, 30). The inactivation of CD25+T cells using PC61 antibody in mice that were treated with NCK2025 resulted in enhanced DSS disease severity compared with animals that received NCK2025 alone or NCK2025 plus control IgG (Fig. 5 C–E). Moreover, to investigate the source of IL-10 specifically in colonic CD11c+DCs, colonic sections of the mice that were treated with NCK56, NCK2025, or PBS were studied. As seen in Fig. 5 F and G, NCK2025 significantly induced IL-10 in CD11c+DCs. IL-10 secretion is not limited to DCs but probably other colonic cell types, including macrophages, or Tregs (27). Thus, these findings suggest that the regulation of active inflammatory immune responses might be elicited by colonic CD11c+IL-10+DCs and Tregs (31).
Fig. 5.
Induction of Treg cells by NCK2025. (A and B) C57BL/6 mice (five per group) were orally inoculated with NCK56, NCK2025 (5 × 108 cfu·100 μL·mouse), or PBS for 4 consecutive d. On day 5, mice were killed, and isolated colons were cleaned. Colonic single-cell suspensions were prepared from each group of mice and enriched by Percol gradient. Lymphocytes were stained with corresponding or isotype-match antibodies and analyzed by FACSCalibur. Experiments were repeated at least seven times. (C–E) Exacerbation of DSS-induced colitis by Treg inactivation using PC61 antibody. NCK2025-treated mouse groups were given i.p. PC61 mAb or control rat IgG (500 g/100 L) on days 3 and 5 pre- and post-DSS treatment. Mice and control mice received one 5-d cycle of 3% DSS in drinking water followed by 3 d of regular drinking water and were then killed on day 13. (C and D) Weight changes and DAI. (E) H&E staining with 400× magnification. (F and G) Induction of IL-10 in colonic CD11c+DCs. NCK56- or NCK2025-treated and untreated (PBS) mouse colons were stained with CD11c (red dots) and IL-10 (green dots) antibodies and visualized by TissueGnostics Tissue/Cell High-Throughput Imaging and Analysis System. F Insets show a representation of CD11c+/IL-10+ cells (yellow, merged red/green/blue). Red bar represents 50 m. White arrows point to CD11c+IL-10+ cells, colocalized in yellow dots. Data are representative of three experiments.
Regulatory Effect of IL-10.
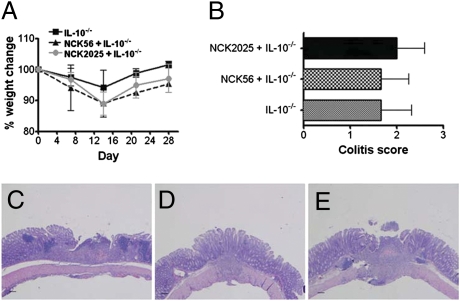
To highlight the role of IL-10, we performed preventative studies in IL-10−/− mice that develop spontaneous Th1-mediated colitis (32). Before the onset of colitis, mice were inoculated with NCK56, NCK2025, or PBS for 4 consecutive d. Colitis was then induced by treatment with piroxicam. Clinically, weight loss was monitored over 28 d, and colons were isolated after sacrifice to assess the degree of histologic colitis. There was no protection from expected weight loss seen during the first 2 wk of induced colitis by either NCK56 or NCK2025 (Fig. 6A). Collectively, all three groups developed similar histological colitis and colitis scores (Fig. 6 B–E), suggesting that endogenous IL-10 is required for NCK2025s immunomodulatory properties. Additionally, transferring pathogenic CD4+CD45RBhighT cells induced severe colitis in untreated, NCK56-treated, and NCK2025-treated Rag1−/−IL-10−/− mice (Fig. S4A). All three groups showed significant weight loss and worsening DAI on CD4+CD45RBhighT cell transfer, highlighting the critical effects of host IL-10 in regulating intestinal inflammation (Fig. S4B).
Fig. 6.
Induction of colitis in IL-10−/− mice. (A and B) C57BL/6 IL-10−/− mouse groups (10 per group) were housed conventionally for 1 wk. Mice were inoculated with NCK56 or NCK2025 for 4 consecutive d and then fed low-dose piroxicam for 1 wk followed by high-dose piroxicam for 1 wk to accelerate and induce the onset of colitis. Weight lost was determined; 2 wk later, mice were killed, and colon cross-sectional Swiss rolls and tissue sections were prepared for H&E staining. (C) Piroxicam only-treated mice. (D) NCK56-piroxicam–treated mice. (E) NCK2025-piroxicam–treated mice. The scores were blindly determined on a scale from 0 to 4.
Discussion
Breakdown in the immune mechanisms controlling intestinal immune tolerance leads to chronic IBD (1, 33). Both CD8+ (34, 35) and CD4+ T cells (36, 37) trigger intestinal inflammation when peripherally activated by inflammatory cytokines (i.e., IL-12) released from highly activated DCs. Thus, an efficacious immunotherapy requires a deep understanding of the immune-signaling mechanisms that underlie the immune tolerance breakdown generally sustained by regulatory signals (38).
Recently, sequencing and annotation of the L. acidophilus NCFM genome have allowed genetic manipulation of this bacterium's cell surface proteins (Slps) that may affect mucosal cellular and molecular events, ultimately leading to therapeutic applications (13, 39). The Slps of L. acidophilus are critical for the maintenance of cell shape and activation of immune cells, including DCs (13). To study the complex cross-talk between bacteria and DCs and its relevance to colitis, we deleted the gene encoding the phosphoglycerol transferase that synthesizes the glycerol chain of LTA in L. acidophilus NCFM. Data show that the L. acidophilus-negative in LTA, NCK2025, induced regulatory signals (i.e., IL-10) and down-regulated costimulatory molecules (CD40 and CD86) in mouse DCs, in effect converting these cells to regulatory DCs (40). Subsequent interaction of such regulatory DCs with CD4+ T cells significantly altered T-cell activation. Furthermore, NCK2025 treatment ameliorated DSS and pathogenic CD4+T cell-induced colitis, indicating that pretreatment of the animal with this bacterial strain induced regulatory immunity that resists DSS- or T cell-induced colitis as shown by colonic histology, weight loss, reduced diarrhea, and hemoccult positivity.
IL-10 regulates overt inflammation within the mucosa (41, 42). The data showed that IL-10 was highly secreted in DCs of NCK2025-treated mice, which might play a critical role to tune down induced inflammation seen in both preventive and therapeutic strategies for treatment of DSS colitis as shown previously (43, 44). This cytokine functionally suppresses T cells by down-regulating MHC II, costimulatory molecules, and production of IL-12 in DCs (43, 45). Additionally, IL-10 also regulates CD8+ cytotoxic T lymphocytes, B cells, and Th1 polarization (43) through its activated IL-10 receptors that initiate multiple signaling cascades, including Jak1, Tyk2, and Stat3 pathways in lymphocytes (46). These observations show that the regulation and maintenance of intestinal tolerance are critically governed by IL-10–producing cells, including DCs that profoundly modify DSS- or pathogenic CD4+CD45RBhighT cell-induced colitis. In addition, the pivotal role of IL-10 in response to innocuous antigens was recently shown using IL-10−/− mice that develop severe colitis (47). As seen above, the immunomodulatory effects of NCK2025 were not sufficient to completely reverse established colitis in IL-10−/− or Rag1−/−IL-10−/− mice; however, it did abrogate colitis when given in a DSS preventative/therapeutic manner or by T cell transfer into Rag1−/− mice, highlighting the critical role of IL-10 in regulating the onset of inflammation.
Importantly, mounting evidence supports the notion that IL-10–secreting Tregs control the inflammatory properties of DCs (48) that, in turn, regulate T-cell responses to control collateral tissue damage (49). In this regard, NCK2025 treatment increased CD4+Foxp3+ Treg frequency. Inactivating their function in vivo resulted in severe colitis, suggesting that Tregs play a critical role in inflammatory disorders where mutations in their Foxp3 gene resulted in uncontrolled T cell repertoire (28). Our data are in agreement with previously reported studies using charge modification of LTA in L. plantarum to alleviate colitis in mice (21). Deletion of the entire gene involved in LTA synthesis in L. acidophilus NCFM significantly impacted the intestinal microenvironment, inducing regulatory signals (i.e., IL-10, Tregs, and regulatory DCs) without neglecting the required costimulatory signals for efficacious immune activation in diseases such as infection.
Gene modification in an allochthonous bacterium such as L. acidophilus NCFM that does not permanently colonize the gut may offer more therapeutic options when immune responses are regulated (i.e., colitis) or left unperturbed (i.e., intestinal infections) by administering such a bacterial strain. This would facilitate development of therapeutic vehicles that may optimize the regulation of oral immune responses. Together, targeted preventive or therapeutic microbial strategies may be effective when cellular interactions are understood in depth and critical structural molecules delivered by the bacterium to the intestinal mucosa are identified that culminate in autoimmunity, inflammation, or antiinflammatory responses.
Material and Methods
Mice.
Six- to eight-week-old C57BL/6, C57BL/6 recombination-activation gene 1-deficient (Rag1−/−), C57B/6 Rag1−/−IL-10−/−, C57BL/6 TLR2−/−, C57BL/6 IL-10−/−, and Balb/C MyD88−/− mice were purchased from Jackson Laboratories. Mice were maintained in microisolator cages under specific pathogen-free, Helicobacter-free conditions at the animal care facility at the Northwestern University. We did not observe any spontaneous signs of inflammation in the colons of IL-10−/− mice. Experiments were performed in an accredited establishment according to National Institutes of Health (NIH) guidelines in the Guide for Care and Use of Laboratory Animals (NIH-72-23), and animal protocols were approved by the local ethics committee.
Reagents.
Piroxicam and Sulindac were obtained from Sigma. DSS was obtained from MP Biochemicals. NS-398 was obtained from Cayman Chemical. Monoclonal antibodies for CD4, CD25, FoxP3, CD3, CD11c, CD11b, CD40, CD80, CD86, IL-10, and mouse GM-CSF were purchased from Invitrogen and eBioscience. LTA from S. aureus and LTA from Bacillus subtilis were purchased from Sigma.
Bacterial Strains.
L. acidophilus NCFM (NCK56) and NCK2025 were inoculated at 1% and propagated in de Man, Rogosa, and Sharpe broth (MRS; Difco) at 37 °C for 15 h. Subsequently, 1 mL of each culture was transferred to 50 mL fresh MRS and incubated at 37 °C for 18 h. The number of cfu of L. acidophilus strains was determined by measuring the OD at 600 nm (50). Cells were harvested by centrifugation, washed two times with sterile PBS, resuspended at 5 × 108 cfu/mL PBS containing 20% glycerol, and subsequently stored at −80 °C until used to stimulate immature DCs (1:1) in vitro. For oral inoculation of the mice, bacteria grown for 48 h were washed two times with sterile PBS, resuspended at 5 × 109/mL PBS, and used for oral inoculation of mice (5 × 108 cfu·100 μL PBS·mouse). Methods for assessing bacterial survival in simulated gastric and small intestinal fluids are provided in SI Materials and Methods.
Phosphoglycerol Transferase Targeting.
A mutant strain of L. acidophilus NCK56 was constructed with a deletion of phosphoglycerol transferase (LBA0447) using standard integration and excision methods, tools, and strains (51, 52). A pORI28 deletion vector was constructed containing two targeting fragments, Del1_SphI and Del2_BglII, that flank LBA0447. After a double cross-over integration and excision event, NCK2025 was recovered that harbored a 1,984-bp deletion of LBA0447 in the genome. PCR amplicons and DNA sequencing over the LBA0447 region in NCK2025 confirmed the loss of ∼2 kbp and revealed no additional mutations in the genes surrounding the deletion.
Cell Culture.
Mice femurs were removed and mechanically purified from surrounding tissues, and bone marrow was flushed using cold PBS. Cells were treated with Tris-buffered ammonium chloride to lyse erythrocytes. Subsequently, B cells, T cells, MHC II+ cells, and Gr-1+ granulocytes were removed positively by specific antibodies against CD19, CD3, MHC II, and Gr-1 (PharMingen). The remaining cells were MHC II− and were cultured in RPMI 1640 complete medium plus 10% FBS with mouse GM-CSF alone (25 ng/mL) in six-well plates for 6 d. Every other day, cultures were fed with fresh media containing GM-CSF. On day 6, cells were harvested and used for different experiments. To study T-cell activation and proliferation, NCK56 or NCK2025 was administrated (5 × 108 cfu·100 μL·mouse) to C57BL/6 mice for 4 consecutive d; 1 wk later, mice were killed to isolate mesenteric LNs of each group of mice. Mesenteric T cells were enriched by negative magnetic bead depletion. To assay T-cell activation and proliferation, NCK56- or NCK20250-treated and untreated DCs (104 per well of 96-well plate) were cultured at graded doses with isolated mesenteric LN CD4+ T cells (105 per well) for 5 d in serum-free media. Afterward, 25 μL of each well of 96-well plates were harvested and frozen for cytokine analysis. Cells were then pulsed for the last 16 h with thymidine (0.5 μCi/well; New England Nuclear) (53). In some experiments, anti–IL-10 antibody (final concentration = 100 ng/mL) was used in DC:T cell cocultures, respectively.
DSS-Induced Colitis.
For vaccination/prevention studies, groups of C57BL/6 mice (10 mice/group) were inoculated orally with NCK56 or NCK2025 (5 × 108 cfu·100 μL PBS·mouse) for 4 consecutive d. To deplete/inactivate CD4+ CD25+T cells (29), the same mice were given i.p. anti-CD25 monoclonal antibody (PC61; BioXcell) or control rat IgG (500 μg/100 μL) on days 3 and 5 pre- and post-DSS treatment. These groups of mice and control mice received one 5-d cycle of 3% DSS in drinking water followed by 3 d of regular drinking water; then, they were killed on day 11 or 13. Acute colitis was observed after the first cycle of DSS in the noninoculated group. Disease progression, including weight lost, diarrhea, and fecal hemoccult blood positivity (FOB), was monitored throughout the study. Thereafter, mice were killed, and colon cross-sectional Swiss rolls were fixed in 10% formaldehyde and embedded in paraffin. Tissue sections (4 μm) were stained with H&E and blindly scored as described previously (54, 55). The grading based on a scale from 0 to 28 takes into account the degree of inflammatory infiltrate, the presence of erosion, ulceration, or necrosis, and the depth and surface extension of the lesion. For treatment studies, three groups of C57BL/6 mice (10 per group) first received a 5-d cycle of 3% DSS to initiate colitis, and two of the groups were subsequently treated through oral gavage with NCK56 or NCK2025 (5 × 108 cfu·100 μL PBS·mouse) for 4 consecutive d. Disease progression was monitored to day 13 of the protocol when mice were killed, and colons were assessed as above.
Colonic Tissue Cultures.
Colonic tissue cultures were performed as previously described (56). Briefly, colonic tissues of each mouse group treated with L. acidophilus strains before or after 3% DSS application were thoroughly cleaned with cold PBS. Tissues were cut into 1-cm pieces and shaken in complete RPMI 1640 containing gentamicin (50 μg/mL) for 30 min at 16 × g. Colonic tissues were cultured in RPMI medium 1640 supplemented with 5% FCS, 50 μg/mL gentamicin, and 1% penicillin/streptomycin/amphotericin B for 18 h at 37 °C. Supernatants were then collected and stored at −80 °C before use for cytokine analysis.
IL-10−/− Colitis.
Groups of C57BL/6 IL-10−/− (n = 10 per group) were transferred from pathogen-free housing to conventional housing and allowed to acclimate for 1 wk. Mice were then inoculated with NCK56 or NCK2025 (5 × 108 cfu·100 μL PBS·mouse) for 4 consecutive d and fed low-dose piroxicam for 1 wk followed by high-dose piroxicam for 1 wk to accelerate and synchronize the onset of colitis, as previously described (57). After 2 wk on standard chow (day 28), mice were killed, and colon cross-sectional Swiss rolls were fixed in 10% formaldehyde and embedded in paraffin. Tissue sections (4 μm) were stained with H&E and blindly scored on a scale from 0 to 4, as described previously (57).
Flow Cytometry.
L. acidophilus-treated and untreated DCs (5 × 105) were incubated with surface marker monoclonal antibodies for 30 min at 4 °C, washed extensively with PBS plus 0.1% FCS, fixed with 0.1% paraformaldehyde, and analyzed by a FACSCalibur four-laser cytometry by using standard CELLQUEST acquisition analysis software (Becton Dickinson). At least 104 gated events per condition were acquired. In some experiments to derive colonic lymphocytes, groups of mice (five mice per group) were inoculated with NCK56 or NCK2025 (5 × 108 cfu·100 μL sterile PBS·mouse) for 4 consecutive d. Mice were killed, colons were cleaned, and single cells were isolated from the lamina propria as previously described (58). Lymphocytes were enriched using Percol and stained with anti-CD4 FITC, CD25 allophycocynin antibodies, and 7AAD. Subsequently, stained cells were fixed, permeabilized, stained with anti-FoxP3 phycoerythrin or isotype antibodies, and analyzed by FACSCalibur.
Immunoflourescence.
Colons of mice treated with NCK56, NCK2025, or PBS alone were fillet-opened, rolled, and snap-frozen at −80 °C in optimal cutting temperature. Sections (5 μm) were cut, fixed in ice-cold methanol (−20 °C) for 15 min, and blocked with 1% BSA. Subsequently, sections were incubated overnight at 4 °C with purified hamster anti-mouse CD11c (BD Biosciences) and rat anti-mouse IL-10 (BioLegend), washed two times with PBS, and incubated with anti-hamster AlexaFluor 594 and anti-rat AlexaFluor 488 (Invitrogen) for 1 h. Sections were then washed two times with PBS, incubated with DAPI (Invitrogen) for 10 min, washed with PBS two times, and mounted with antifade mounting medium (59). Images were acquired using TissueGnostics Tissue/Cell High-Throughput Imaging and Analysis System and analyzed using ImageJ software.
Real-Time PCR.
Total RNA was isolated from bone-marrow DCs using the RNeasy Mini Kit (Qiagen). The high-capacity cDNA reverse transcription kit was used to synthesize cDNA from 5 μg RNA and expression of TLR1 and TLR2 genes determined by real-time semiquantitative PCR using the ABI 7500 real-time PCR system with Power Syber green 2× PCR master mix (Applied Biosystems). Primers for TLR1 (Forward-TTAATGAGTGTTTGTGAATGCAGTTG; Reverse-GAGCATTGCCACATGGGTATAG) and TLR2 (Forward-CAAAGCGTCAAATCTCAGAGGAT; Reverse-ACACCCCAGAAGCATCACATG) were selected for regions spanning intron junctions to exclude amplification of genomic DNA. Results reflect the fold increase relative to the control sample using the ddCT method using GAPDH as the endogenous control (Forward-GTCGTGGATCTGACGTGCC; Reverse-TGCCTGCTTCACCACCTTC).
Supplementary Material
Acknowledgments
We thank Dr. E. Gounaris, Dr. P. Grippo, Dr. S. Miller, and S. H. Ryu for technical assistance and fruitful scientific discussions. This work was supported by the Northwestern University Feinberg School of Medicine (Chicago, IL), the North Carolina Dairy Foundation, and Danisco USA, Inc.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Microbes and Health” held November 2–3, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at http://www.nasonline.org/SACKLER_Microbes_and_Health.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005066107/-/DCSupplemental.
References
- 1.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 2.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 3.Simpson SJ, et al. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J Exp Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kullberg MC, et al. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: Cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun. 2001;69:4232–4241. doi: 10.1128/IAI.69.7.4232-4241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannon PJ, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 6.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puccetti P, Belladonna ML, Grohmann U. Effects of IL-12 and IL-23 on antigen-presenting cells at the interface between innate and adaptive immunity. Crit Rev Immunol. 2002;22:373–390. [PubMed] [Google Scholar]
- 9.Mohamadzadeh M. Potential factors induced by filoviruses that lead to immune supression. Curr Mol Med. 2009;9:174–185. doi: 10.2174/156652409787581628. [DOI] [PubMed] [Google Scholar]
- 10.Mohamadzadeh M, Duong T, Hoover T, Klaenhammer TR. Targeting mucosal dendritic cells with microbial antigens from probiotic lactic acid bacteria. Expert Rev Vaccines. 2008;7:163–174. doi: 10.1586/14760584.7.2.163. [DOI] [PubMed] [Google Scholar]
- 11.Ouwehand AC, Salminen S, Isolauri E. Probiotics: An overview of beneficial effects. Antonie van Leeuwenhoek. 2002;82:279–289. [PubMed] [Google Scholar]
- 12.Mohamadzadeh M, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;102:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantinov SR, et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamadzadeh M, Duong T, Sandwick SJ, Hoover T, Klaenhammer TR. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc Natl Acad Sci USA. 2009;106:4331–4336. doi: 10.1073/pnas.0900029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh YJ, et al. Development and application of a upp-based counterselective gene replacement system for the study of the S-layer protein SlpX of Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2009;75:3093–3105. doi: 10.1128/AEM.02502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh YJ, Klaenhammer TR. Genomic features of Lactobacillus species. Front Biosci. 2009;14:1362–1386. doi: 10.2741/3313. [DOI] [PubMed] [Google Scholar]
- 17.Mayer ML, Phillips CM, Stadnyk AW, Halperin SA, Lee SF. Synergistic BM-DC activation and immune induction by the oral vaccine vector Streptococcus gordonii and exogenous tumor necrosis factor. Mol Immunol. 2009;46:1883–1891. doi: 10.1016/j.molimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Mayer ML, Phillips CM, Townsend RA, Halperin SA, Lee SF. Differential activation of dendritic cells by Toll-like receptor agonists isolated from the Gram-positive vaccine vector Streptococcus gordonii. Scand J Immunol. 2009;69:351–356. doi: 10.1111/j.1365-3083.2009.02232.x. [DOI] [PubMed] [Google Scholar]
- 19.Palumbo E, et al. D-alanyl ester depletion of teichoic acids in Lactobacillus plantarum results in a major modification of lipoteichoic acid composition and cell wall perforations at the septum mediated by the Acm2 autolysin. J Bacteriol. 2006;188:3709–3715. doi: 10.1128/JB.188.10.3709-3715.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193:393–397. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grangette C, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci USA. 2005;102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuhaus FC, Baddiley J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingsford CL, Ayanbule K, Salzberg SL. Rapid, accurate, computational discovery of Rho-independent transcription terminators illuminates their relationship to DNA uptake. Genome Biol. 2007;8:R22. doi: 10.1186/gb-2007-8-2-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman O, Dover LG, Sutcliffe IC. Lipoteichoic acid biosynthesis: Two steps forwards, one step sideways? Trends Microbiol. 2009;17:219–225. doi: 10.1016/j.tim.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Schröder NW, et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 26.Lee IT, et al. Cooperation of TLR2 with MyD88, PI3K, and Rac1 in lipoteichoic acid-induced cPLA2/COX-2-dependent airway inflammatory responses. Am J Pathol. 2010;176:1671–1684. doi: 10.2353/ajpath.2010.090714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 29.Ochoa-Repáraz J, et al. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol. 2007;178:1791–1799. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 31.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald TT, Gordon JN. Bacterial regulation of intestinal immune responses. Gastroenterol Clin North Am. 2005;34:401–412. doi: 10.1016/j.gtc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Cheroutre H. In IBD eight can come before four. Gastroenterology. 2006;131:667–670. doi: 10.1053/j.gastro.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Vezys V, Lefrançois L. Cutting edge: Inflammatory signals drive organ-specific autoimmunity to normally cross-tolerizing endogenous antigen. J Immunol. 2002;169:6677–6680. doi: 10.4049/jimmunol.169.12.6677. [DOI] [PubMed] [Google Scholar]
- 36.Elson CO, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 37.Wirtz S, Neurath MF. Animal models of intestinal inflammation: New insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int J Colorectal Dis. 2000;15:144–160. doi: 10.1007/s003840000227. [DOI] [PubMed] [Google Scholar]
- 38.Wirtz S, et al. Cutting edge: Chronic intestinal inflammation in STAT-4 transgenic mice: Characterization of disease and adoptive transfer by TNF- plus IFN-gamma-producing CD4+ T cells that respond to bacterial antigens. J Immunol. 1999;162:1884–1888. [PubMed] [Google Scholar]
- 39.Altermann E, et al. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc Natl Acad Sci USA. 2005;102:3906–3912. doi: 10.1073/pnas.0409188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belkaid Y, Oldenhove G. Tuning microenvironments: Induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraus TA, et al. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–2243. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraus TA, Mayer L. Oral tolerance and inflammatory bowel disease. Curr Opin Gastroenterol. 2005;21:692–696. doi: 10.1097/01.mog.0000182862.88798.28. [DOI] [PubMed] [Google Scholar]
- 43.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–1090. [PubMed] [Google Scholar]
- 47.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 48.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matarese G, De Rosa V, La Cava A. Regulatory CD4 T cells: Sensing the environment. Trends Immunol. 2008;29:12–17. doi: 10.1016/j.it.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Greene JD, Klaenhammer TR. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl Environ Microbiol. 1994;60:4487–4494. doi: 10.1128/aem.60.12.4487-4494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeiler EA, Klaenhammer TR. Role of transporter proteins in bile tolerance of Lactobacillus acidophilus. Appl Environ Microbiol. 2009;75:6013–6016. doi: 10.1128/AEM.00495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell WM, Klaenhammer TR. Efficient system for directed integration into the Lactobacillus acidophilus and Lactobacillus gasseri chromosomes via homologous recombination. Appl Environ Microbiol. 2001;67:4361–4364. doi: 10.1128/AEM.67.9.4361-4364.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulendran B, et al. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur J Immunol. 2004;34:66–73. doi: 10.1002/eji.200324567. [DOI] [PubMed] [Google Scholar]
- 54.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 55.Murthy SN, et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 56.Sellon RK, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berg DJ, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 58.Haddad W, et al. P-selectin and P-selectin glycoprotein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J Exp Med. 2003;198:369–377. doi: 10.1084/jem.20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gounaris E, et al. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS ONE. 2008;3:e2916. doi: 10.1371/journal.pone.0002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horton RM. PCR-mediated recombination and mutagenesis. SOEing together tailor-made genes. Mol Biotechnol. 1995;3:93–99. doi: 10.1007/BF02789105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.