Abstract
The normal intestinal microbiota inhabits the colon mucus without triggering an inflammatory response. The reason for this and how the intestinal mucus of the colon is organized have begun to be unraveled. The mucus is organized in two layers: an inner, stratified mucus layer that is firmly adherent to the epithelial cells and approximately 50 μm thick; and an outer, nonattached layer that is usually approximately 100 μm thick as measured in mouse. These mucus layers are organized around the highly glycosylated MUC2 mucin, forming a large, net-like polymer that is secreted by the goblet cells. The inner mucus layer is dense and does not allow bacteria to penetrate, thus keeping the epithelial cell surface free from bacteria. The inner mucus layer is converted into the outer layer, which is the habitat of the commensal flora. The outer mucus layer has an expanded volume due to proteolytic activities provided by the host but probably also caused by commensal bacterial proteases and glycosidases. The numerous O-glycans on the MUC2 mucin not only serve as nutrients for the bacteria but also as attachment sites and, as such, probably contribute to the selection of the species-specific colon flora. This observation that normal human individuals carry a uniform MUC2 mucin glycan array in colon may indicate such a specific selection.
Keywords: bacteria, intestine, goblet cell, glycoprotein, colitis
The number of commensal bacteria in the adult intestine is estimated at 1013–1014, a number well exceeding the number of cells in the human body (1). The intestine contains nutrients that can be used by the bacteria, and the body temperature is optimal for the microbes. With such favorable conditions, it is remarkable that the host is not taken over by the fast-growing bacteria. To handle this, the host has a number of mechanisms, including the adaptive immune system and its production and secretion of secretory IgA (2, 3). Even more important are components belonging to the innate immune system, some of which, like lysozyme and the antibacterial peptides, have antibacterial properties (4, 5). The majority of the intestinal immune system is active in the small intestine, which is also more exposed to the intestinal bacteria (3, 6). Passage through the small intestine is relatively fast (3–5 h), which gives limited time for bacteria to increase in number. This is in contrast to the colon, where bacteria reside for a much longer time.
In colon, the high number of commensal bacteria lives in symbiosis with their host as they help to extract energy by digesting indigestible glycoconjugates and contribute vitamins to the host (7). This microbial community is diverse and complex and contains about 1,000 bacterial species. Each individual has at least 160 species, of which many are shared (8). The bacterial gene pool well exceeds the number of human genes by a factor of 150. Many of these bacterial genes are expected not only to have an impact on the local conditions in the colon but also to have more general effects on the host metabolism (7). Although these bacteria live in a symbiotic relation, they still pose a serious threat to their host. However, under normal conditions these bacteria are well tolerated. How this occurs is not fully understood, but two major principles are involved: (i) minimizing bacterial–epithelial cell contact, and (ii) the immune system having a tolerant way of handling bacteria (9). The separation of bacteria and host is based on the physical network making up the colon mucus. The mucus also contains a number of proteins that limit bacterial growth and penetration, such as the antibacterial proteins and IgA. Both the innate and adaptive immune systems are responsible for eliminating the bacteria that reach the epithelial cells and that pass the epithelial cell barrier. The number of bacteria that penetrate is probably relatively high under normal conditions, but the immune system manages to handle this without an overt reaction. Important components in this process are the phagocytic cells with their capacity to engulf the bacteria, the dendritic cells that are presenting antigens, and especially the T cells repertoire with the regulatory T cell population. This important part of the intestinal protective system has been well covered in a number of recent reviews (9–12) and will not be discussed further. The discussion here will focus instead on the physical separation of bacteria and epithelial cells because this has been less well covered in recent reviews.
Organization of the Gastrointestinal Mucus
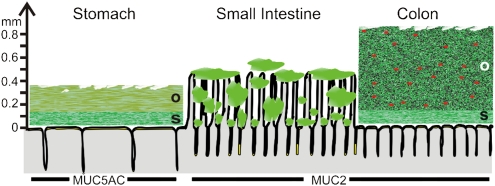
Mucus is important for the protection of the gastrointestinal tract; this has been understood for a long time. However, a deeper understanding of the organization of the mucus and the molecular details regarding the major components has only recently started to emerge. An important step forward was the development of a method for in vivo measurement of mucus thickness in live animals (13, 14). The studies by Holm and colleagues showed that there are two mucus layers in the stomach and colon: an outer “loose” layer that was easy to aspirate and an inner mucus layer that was “firmly” attached to the epithelium (13). These observations are illustrated in Fig. 1, in which the mucus thicknesses shown are from rat. The stomach and colon have relatively well-defined mucus layers. This is in contrast to the small intestine, where the mucus is discontinuous and less well defined. The mucus is secreted at the top of the crypts and then moves upward between the villi. Thus, the tips of the villi are not always covered with mucus.
Fig. 1.
Schematic proposal of how mucus is organized in the gut. The thicknesses given are from rat and adapted from the work of Holm and colleagues (13). The red dots in the outer mucus layer of colon illustrate bacteria. The genes encoding the gel-forming mucins (green) expressed by the surface goblet cells in the different parts of the intestine are marked by name. o, outer loose mucus layer; s, inner stratified, firmly attached mucus layer. That the mucus thickness and length of the villi vary along the length of the gut is not illustrated.
Two Mucus Layers in Colon
The thickness of the inner mucus layer in distal colon has been estimated to be approximately 50 μm in mice and 100 μm in rat (13, 15, 16). A gradient with an increased thickness along the length of colon has been observed. To date, it has not been possible to measure the human inner mucus layer, but it is most likely even thicker. Although we do not have the full picture, it is obvious that the thickness is controlled and can vary by unknown mechanisms. This is suggested, for example, from results in mice lacking the Muc1 transmembrane mucin, in which the inner firm mucus layer was found to be slightly thinner (15). The outer loose and easily removed mucus has a less-well-defined outer limit. This layer is degraded by the commensal flora and transported distally together with the intestinal content. In general, the outer layer is estimated to be twice as thick as the inner layer, but this thickness is highly variable. This is dependent on the bacteria present, as suggested by results from germ-free mice that have a relatively thick loose layer in relation to the inner mucus layer (16).
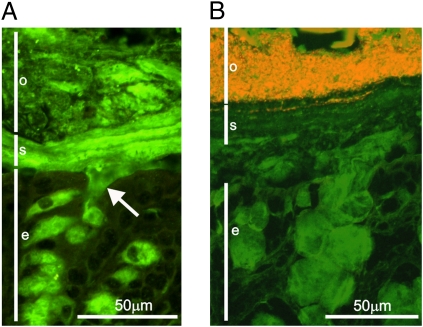
When colon tissue was fixed in Carnoy fixative and stained for the major constituent, Muc2 mucin, the mucus was confirmed to be organized as revealed by in vivo experiments (Fig. 2A). The inner mucus layer (Fig. 2, “s”) had a stratified appearance, with a dark green color. The outer loose layer (Fig. 2, “o”) facing the lumen has a more disorganized appearance and a lighter green color (16). The difference in immunostaining of the MUC2 mucin varies depending on the handling of the tissue sections but probably also reflects the density difference and organization of the Muc2 mucin.
Fig. 2.
Muc2 mucin builds up a stratified mucus layer devoid of bacteria in the distal mouse colon. (A) Immunostaining of Muc2 (green) in the distal mouse colon reveals mucus-filled goblet cells in the epithelium (e) and secreted mucus. The secreted mucus forms two layers: a stratified inner, firmly attached mucus layer (s) and an outer, nonattached mucus (o). Arrow indicates release of Muc2 mucin from a goblet cell. (B) FISH using a general bacterial probe visualizes the bacteria (red) in combination with staining for Muc2 (green). The inner mucus layer (s) forms a barrier impervious to bacteria and thus protects the colonic epithelium.
No Bacteria Are Found in the Inner Mucus Layer
The observation of this two-layered organization in the colon mucus raised the possibility that the colon bacteria are not distributed uniformly in these layers. When this was analyzed by 16S rRNA FISH staining for bacteria, the bacteria were present only in the outer mucus layer and were totally absent in the inner layer (Fig. 2B) (16). The bacteria in the outer loose mucus layer were separated from the inner layer by the same sharp border as between the two Muc2 layers (Fig. 2 A and B). Thus, one can conclude that the colon bacteria normally do not reach the epithelial cells and are well separated from the immune system.
Why Has the Colon Mucus Organization Remained Unrecognized Until Now?
There have been previous observations indicating an organization of the colon mucus layers (17). However, there has not been any description of the two mucus layers or that the inner layer has such an important role in maintaining a bacteria-free epithelial cell surface. There are many reasons why this has been undiscovered, but the two major reasons are directly linked to the very special properties of mucus. When a mouse colon is cut open and studied under a dissection microscope, the epithelial cell surface is easily revealed. When charcoal is sprinkled over the surface, the black powder is observed high above the epithelial surface. This surprising observation is maintained as long as the tissue is covered by a buffer, but as soon as it is not covered, the charcoal falls down onto the epithelial surface. Normal mucus is thus totally transparent, and it quickly collapses when it desiccates.
Why then have the mucus layers not been observed on tissue sections? Conventional fixation of tissues by formaldehyde or other cross-linking agents causes the mucus layers of colon to collapse and shrink to a very thin film lining the epithelia. The mucus thus only appears as a thin structure on a stained tissue section. The only way to preserve the mucus layers is to use a fixative that does not cross-link proteins and that does not contain any water. Carnoy is such a fixative when it is made from chloroform, dry methanol, and glacial acetic acid. This fixative gives results that are compatible with in vivo mucus measurements, as shown in Fig. 2. Just as important is to collect a whole piece of colon with fecal content, especially a fecal pellet, because this protects the mucus from being washed off. Thus, the physico-chemical properties of mucus explain why the organization of the mucus layer in colon has been overlooked.
Mucus Composition
Studies of the protein composition of the two mucus layers by proteomics show almost identical protein profiles (16). The major component is the only gel-forming mucin found, Muc2 mucin. Other components are Fcgbp, Clca3, Zg16, Agr2, immunoglobulins, and many more proteins. The mucus also contains cellular proteins because cells are continuously shed out into the lumen and trapped in the mucus. We also detected plasma proteins, but these are more likely due to contamination acquired during opening of the intestine.
The Fcgbp protein or Fc Ig binding protein was originally suggested to bind IgG (18). Although it might also do this, its main function is probably different and reflected in its domain structure, with 13 von Willebrand D domains. Eleven of these domains have the Gly-Asp-Pro-His (GDPH) sequence, similar to the D4 domain of MUC2 and MUC5AC mucins (18–21). In the two mucins this sequence is cleaved between aspartate and proline by an autocatalytic mechanism that generates a new C-terminal end with an internal aspartate anhydride. This is a very reactive group that can link to both primary amines and hydroxyl groups. Our observation that Fcgbp is covalently attached to Muc2 mucin suggests that Fcgbp can act as a mucus cross-linker (21).
Structure of MUC2 Mucin
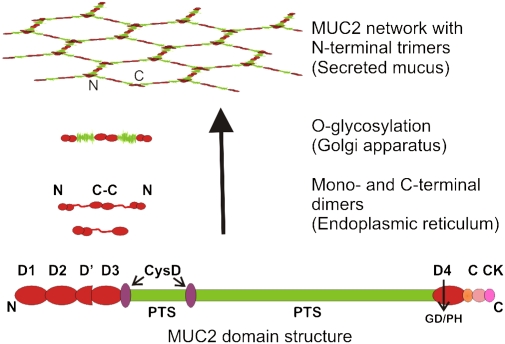
How then can Muc2 mucin build such a stable mucus layer? Human MUC2 mucin has a protein core of approximately 5,179 amino acids, with a domain structure as shown in Fig. 3 (22, 23). Typical for mucins is the PTS domain with a high frequency of the amino acids proline, threonine, and serine (24). These domains are often made up by repetitive sequences ordered in tandem and thus referred to as tandem repeats. The large PTS domain of human MUC2 has not been fully sequenced, but the known parts are built of an almost perfectly tandemly repeated sequence of 23 amino acids. The small PTS domain has, on the other hand, more degenerate repeats, and the PTS sequences known for mouse and rat Muc2 also suggest less perfect repeats. Studies on the evolution of mucins show that there is no amino acid sequence conservation of the PTS domains between species, and it was not possible to use these domains for mucin ortholog mining (24). There is, thus, a low selection pressure for a specific amino acid sequence when comparing the PTS domain of the same mucin between species. It seems instead that it is the frequency of the PTS amino acids clustered in sufficiently long sequences that is conserved. In fact, the frequency of these three amino acids concentrated in long sequences can be used to search for mucin genes (25). The PTS domains become densely O-glycosylated by the glycosylation machinery of the Golgi apparatus (Fig. 3). When the PTS domains become decorated with glycans, these form “mucin domains” that have a long and extended rod appearance that resembles a bottle brush, with a central protein core and the O-glycans extending in all directions like brush bristles. These typical mucin domains give mucins their extended conformation and a high capacity to bind water, something that highly contributes to their gel-forming properties.
Fig. 3.
Domain structure and biosynthesis of MUC2 mucin. MUC2 has cysteine-rich N- and C-terminal parts with four complete von Willebrand D domains in total. The central PTS domains are rich in serine, threonine, and proline, and these domains become heavily O-glycosylated to generate mucin domains. MUC2 forms dimers in the endoplasmic reticulum by disulfide bonds between the C termini. The dimer is translocated into the Golgi apparatus, where it is O-glycosylated, resulting in a size of ≈5 MDa. The MUC2 network is formed by disulfide-linked trimers connecting the N termini. The large polymers are stored in mucin granulae in the goblet cells before being secreted.
Flanking the PTS domains are regions that, on average, have an amino acid composition with nearly 10% cysteines. The MUC2 N terminus has three complete von Willebrand D domains (D1–D3) and one incomplete (D′), whereas the MUC2 C terminus has one such domain (D4), an overall organization similar to the related von Willebrand factor (Fig. 3) (26). As for the von Willebrand factor, the N- and C-terminal parts of MUC2 mucin form disulfide bond–stabilized polymer networks, although the von Willebrand factor forms a linear polymer. The first event in the MUC2 polymerization process is the formation of dimers in the far C-terminal end, by the cysteine-knot domain (27). The folding and dimerization of the MUC2 mucin seems to be a demanding process owing to the large number of disulfide bonds. We have found that, when expressing recombinant and truncated forms of MUC2, CHO cells easily accumulate large amounts of unfolded proteins. Cells that normally express mucins are more efficient in expressing and secreting recombinant MUC2. Mice with a mutation in the endoplasmic reticulum stress response key regulator XBP1 are more susceptible to induced colitis (28), but how this affects Muc2 has not been studied. The Agr2 protein, which has a special thioredoxin-like domain, is essential for the in vivo production of Muc2, and Agr2-null mice are highly susceptible to colitis (29, 30). Finally, it has also been observed that several mouse strains with mutations causing a dysfunctional Muc2 mucin show a strong unfolded response (31). All these observations suggest that the folding of Muc2 is a demanding process and that problems in these processes may cause defects in the colon mucus.
After the endoplasmic reticulum, the MUC2 dimers pass into the Golgi apparatus, where the O-glycans are attached and the dimers reach molecular masses of approximately 5 MDa (32). In the trans-Golgi network, the MUC2 mucin is sorted to the regulated secretory pathway by properties in its N-terminal part. The low pH and high calcium concentration in the secretory vesicles triggers the formation of disulfide-bonded N-terminal trimers (33). When the N and C termini intermolecular disulfide bonds have been formed, a net-like structure is generated, as depicted in Fig. 3. There is at present no experimental proof of such an organized net, and most probably it is not as symmetrical in vivo. In addition to these disulfide-mediated intermolecular bonds, the MUC2 mucin is also cross-linked by another covalent bond that is formed after MUC2 has passed the Golgi apparatus (32). The nature of this is still not deciphered, but its formation seems to be related to the property of insolubility, a typical feature for MUC2 mucin (32, 34).
Secretion of MUC2 Mucin
MUC2 is stored in a condensed way in the goblet cell mucin granulae. When the granulae are released, MUC2 mucin expands dramatically in volume. The release can often be observed on sections fixed in Carnoy, as for example in Fig. 2A (arrow), where Muc2 mucin is shown streaming out from one of the goblet cells. It is sometimes possible to observe how the released mucins spread out and organize a sheet underneath the inner mucus layer (16). This process is probably facilitated by the net-like structure of the MUC2 polymer, and it is possible that MUC2 can self-organize in this way. The formation of MUC2 organized sheets could be responsible for the lamellar stratified appearance of the inner mucus layer (16).
Properties of the Mucus Layers
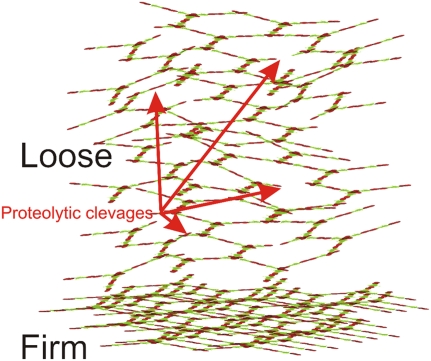
Muc2 of the inner firm mucus layer is insoluble in the chaotropic salt guanidinium chloride, as is the Muc2 that is stored in the secretory vesicles (16). On the other hand, the outer loose mucus layer is readily soluble. The transformation of Muc2 from an insoluble to a soluble form is probably a reflection of proteolytic cleavages that take place in Muc2 of the outer mucus layer (16). These cleavages do not disrupt the Muc2 polymer but allow the mucus to expand approximately four times in volume (16). This expansion is probably driven by the capacity of the mucin glycans to bind water. We do not know the molecular details of this process, but it is regulated by the host because germ-free mice also have an outer loose mucus layer (16). Proteolytic cleavages in the cysteine-rich parts of Muc2, especially the C terminus, are probably a major explanation (16). Cleavages in the Muc2 mucin do not necessarily dissolve the polymeric network because it can still be held together by the abundant disulfide bonds in these parts of Muc2, as illustrated by trypsin treatment of the Muc2 gel. This allows Muc2 to expand, but it does not fall apart (16). Thus disulfide bonds can keep the Muc2 polymeric network together even after a cleavage in its peptide backbone. The net-like organization of the Muc2 polymer will also help to maintain the gel. The expansion in volume is illustrated in Fig. 4.
Fig. 4.
The firmly adherent mucus is converted to the loose mucus and expanded in volume. The secreted mucus is organized into the firmly adherent mucus that shows a stratified appearance. This mucus layer contains densely packed Muc2 that is insoluble in the chaotropic salt guanidinium chloride. At a certain distance from the epithelium it is converted into a nonattached, soluble mucus layer (loose). This is expanded in volume four times owing to proteolysis within the cysteine-rich parts of MUC2, in such a way that the polymeric network is maintained. The net-like structure will also allow limited proteolysis, disrupting the polymeric network without dissolving the gel. Arrows indicate both type of cleavages.
MUC2 Glycosylation in Human Sigmoid Colon
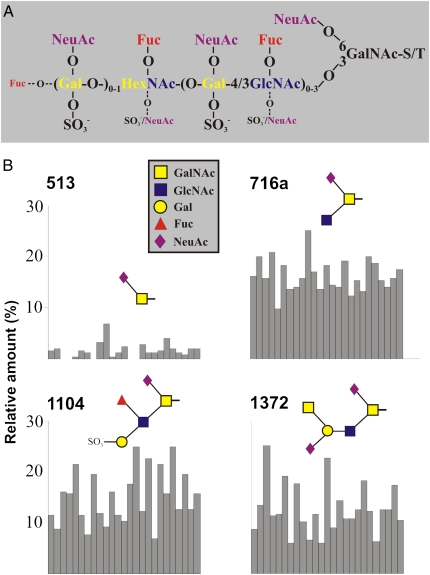
Glycans make up approximately 80% of the MUC2 mucin mass. As discussed above, mucins are dominated by the O-glycans that are concentrated in the two central mucin domains. We recently performed a detailed study of the O-linked glycans on MUC2 from sigmoid colon of 25 control patients (35). As illustrated in Fig. 5A, a majority of the glycans were of the core 3 type, and most glycans had a sialic acid on the C-6 of the GalNAc attached to the protein core. More than 100 different glycan variants were discovered, and a majority of them were permutations of the composite structure shown in Fig. 5A. When the structures and their amounts were compared between these individuals, a relatively uniform pattern was observed. Fig. 5B shows an example of this, with the relative amounts of four different components. The uniform repertoire of glycans is highly interesting because mucins from other organs, such as the small intestine, stomach, and lungs, show a polymorphic profile reflecting the blood group status of the host. Why distal colon is different is not understood, but one could speculate that this has to do with selection of the commensal flora. Bacteria often carry adhesins, usually at the tip of their fimbrie, that bind mucins (36). This was recently illustrated by the isolation of an adhesin from the commensal bacteria Lactobaccilus rhamnosus (37). Rawls et al. (38) also illustrated that the host has the capacity to select for a species-specific commensal gut flora. Interestingly, the O-glycans on MUC2 mucin from different species show a very variable glycan repertoire (39, 40). Because the core bacteria also differ substantially between species, it is possible that the bacteria and host use glycan attachment for mutually selecting each other, to maintain an optimal symbiotic relation specific for each combination of host and bacteria.
Fig. 5.
MUC2 mucin from the human sigmoid colon of different individuals has, in contrast to mucins from other organs, a relatively uniform O-glycan composition. (A) Schematic composite structure describing a majority of the O-linked MUC2 oligosaccharides found in human sigmoid colon. This general composite structure is never observed with all substituents present at the same time. The structure is based on a core 3 structure and a repeated polylactosamine chain with up to three repeats on the C-3 position of the innermost N-acetylgalactosamine (GalNAc), as previously described (35). The most used positions observed for different substituents are indicated by large letters, and the ones used less frequently are indicated by small letters. The residue marked HexNAc (N-acetylhexosamine) is most often a GlcNAc, but it can also be a terminal GalNAc. (B) Four examples showing the relative amounts of O-glycans present on MUC2 mucin from sigmoid colon in 25 individual control patients (each individual is represented by one bar). The relative amounts of glycans are in the same range for all patients. Overall, the MUC2 O-glycan repertoire and their relative amounts were uniform among healthy individuals.
Commensal Bacteria Live in the Outer Mucus Layer
The commensal bacteria in colon live and thrive in the outer loose mucus layer. This is possible after the Muc2 mucin network has expanded in volume, such that it allows the bacteria to penetrate into the mucin network. Once inside the mucus gel, the commensal bacteria can use its large number of glycan-degrading enzymes that release one monosaccharide at a time from the mucin glycans. In this way, it will take some time for the bacterial enzymes to reach and expose the mucin protein core for proteolysis that will degrade the mucin protein core. In this way the mucin polymeric network of the loose mucus is maintained for some time to give a relatively thick outer mucus layer. Although difficult to visualize, the outer loose mucus will probably be increasingly degraded the closer it comes to the luminal content. It is further indicated that bacteria contribute to dissolving the outer loose mucus as germ-free mice have, relative to the inner layer, a thick outer loose mucus layer (16). As discussed, the volume expansion of the mucus network of the outer loose mucus layer is a process that involves endogenous proteases of the host that degrade Muc2 in such a way that the polymeric network remains largely intact (16). However, it is not unlikely that bacteria can also contribute to this, because this should increase the amount of glycans available for the bacteria. This type of activity would be beneficial for the bacteria because it could facilitate bacteria reaching the energy-rich mucin glycans. However, such bacteria and enzymes have not been described to date.
Glycans are a very important energy source for bacteria. That commensal bacteria have the opportunity to degrade indigestible glycans has been crucial for the coevolution of host and its microbiota, because this increases the energy extraction from food significantly. The bacteria use its high number of glycan hydrolytic enzymes to degrade polysaccharides and other glycoconjugates that come to the colon from food via the small intestine (41, 42). However, it is not only the extra energy that comes from the nondigestible food that is important: the bacterial degradation of the mucins and their glycans will also provide significant energy. This will allow the host to recover some of the energy spent to produce the protective mucus. The bacteria use the energy recovered for their own use, but a significant amount of carbon is converted into short fatty acids. These small molecules can easily diffuse through the inner mucus layer and are taken up by the colon epithelial cells. In fact, a major part of the energy used by the colonocytes is derived from the short fatty acids (acetate and butyrate) (41). The discovery of the G protein–coupled receptors GPR41 and GPR43 in colon epithelial cells and that these are binding and gets activated by short fatty acids suggests that glycan metabolites can provide a close coupling between bacteria and the host (43). Such a connection has been implied for long time because, for example, butyrate is known to affect differentiation and growth of epithelial cells.
We have shown that the parasite Entamoeba histolytica can secrete a protease that is able to disrupt the polymeric network of MUC2 mucin (44). This parasite secretes a cysteine protease that is able to cleave human MUC2 mucin at a specific site between two threonines (the sequence RT/T), an odd specificity for a protease. This sequence is positioned in a part of MUC2 mucin that is not stabilized by any disulfide bonds. As expected, this enzyme was able to dissolve the guanidinium chloride–insoluble mucus gel that we now know is the major constituent of the inner firm mucus layer. Thus, the protease secreted by the Entamoeba parasite disrupts the MUC2 polymer network, and by this it is able to penetrate the inner mucus layer and reach the epithelial cell surface and invade the host. Although not described yet, there might be bacteria that also secrete enzymes that can dissolve the MUC2 polymeric network. If there are such bacteria, these might potentially be harmful for the host.
In the Absence of Muc2, Bacteria Are in Contact with the Epithelium
How important is MUC2 mucin for the mucus organization of colon? This was addressed by studies on Muc2−/− mice (45). Depending on environmental (animal house) conditions, these mice develop more or less severe inflammation, diarrhea, colon prolapsis, and failure to thrive, and have an increased risk of developing colon cancer (16, 45, 46). When tissue sections of colon from Muc2−/− mice were analyzed with FISH for bacterial 16S rRNA, the bacteria were found in direct contact with the epithelial cells and deep down in the crypts, something not observed in wild-type animals (16). Some epithelial cells also had intracellular bacteria. Lack of Muc2 mucin thus means that there is no polymeric network that keeps the mucus layer together. This results in the loss of an inner mucus layer that can protect the epithelial cells. The observations in Muc2-null mice also show that the immune system of colon responds with inflammation when the bacteria constantly come in close contact with the epithelial cells.
Summary of Goblet Cell and Mucus Turnover
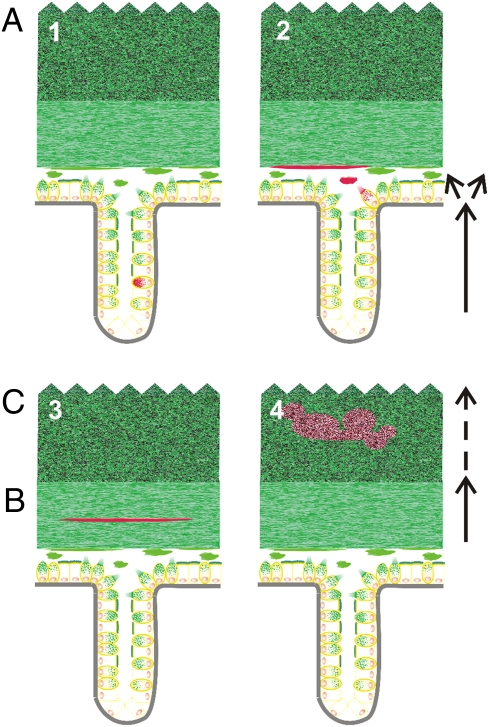
Our current understanding of the turnover of goblet cells, the formation of the inner mucus layer, and the transformation to the outer layer in the colon is summarized schematically in Fig. 6. The crypt stem cells are dividing in the crypt bottom, and the Wnt-signaling pathway drives the goblet cell lineage differentiation (47). To obtain fully mature goblet cells with well-filled granulae, the Spdef transcription factor is necessary (48). The goblet cells are filling up their granulae with Muc2 mucin during their migration from the crypt bottom (Fig. 6, Upper Left, arrow). Most goblet cells are secreting their content when they reach the crypt opening. Goblet cells are responding to certain receptor agonists like carbachol, but we do not have the full picture of how colon goblet cell secretion is controlled. Once the mucins are released, they expand 100–1,000-fold in volume (Fig. 6, Upper Right) by the capacity of the mucin glycans to bind water (49). MUC2 mucin forms a polymeric net-like mucus layer that is anchored to the epithelial cells. The secreted Muc2 mucin is continuously refilling the inner mucus layer from underneath. Because the inner mucus layer is impervious to bacteria, it keeps the bacteria at a distance from the epithelial cell. Concomitant with the newly secreted mucins forming the inner mucus layer, this is transformed into the outer loose mucus on the luminal side. The molecular nature of this sharp transition from the inner to the outer mucus layer is not known, but it is followed by an expansion in volume. The loose mucus seems to be less organized, is degraded by the bacteria, and is finally passed distally with the stool. In the outer loose layer, the commensal bacteria can bind to MUC2 mucin and use its abundant glycans as an energy source.
Fig. 6.
Schematic view of the turnover of goblet cells and mucus in the colon. The subsequent steps in this process are marked by red MUC2. Schematic explanation of how (i) goblet cells are formed in the crypt and MUC2 mucin is formed in the crypt goblet cells and during transportation to the surface (Upper Left); (ii) MUC2 is released and expanded in volume to form a sheet that forms the inner mucus layer from below (A) (Upper Right); (iii) MUC2 are transported with the inner mucus layer (B) (Lower Left); and (iv) MUC2 is converted to the outer loose mucus layer at a sharp border and then expanded in volume in the outer loose mucus layer (C) (Lower Right). Finally, MUC2 mucin is dissolved by bacterial enzymes and transported away with the fecal stream (not illustrated).
The two-layered mucus organization is probably instrumental for a balanced and symbiotic relation between host and bacteria (3, 50). This concept, with an inner mucus layer shielding the colon epithelial cells from bacteria, is new, and as usual in science, new concepts raise new questions. What is the explanation for the difference in the mucus organization between small and large intestine, given that the same MUC2 mucin is produced in both parts? How is the mucus layer attached to the epithelium in colon? How does the transition from the inner to outer layer take place, and how is it regulated? Are the MUC2 glycans involved in the selection of commensal flora? Finally, how is the inner mucus layer of colon related to colitis and the human disease ulcerative colitis? All these questions demand further detailed molecular understanding of the structure and processing of MUC2 mucin and its associated proteins that build the mucus layers of colon.
Acknowledgments
This work was supported by Swedish Research Council Grants 7461, 21027, and 342-2004-4434; The Swedish Cancer Foundation; Knut and Alice Wallenberg Foundation Grant KAW2007.0118; Inga-Britt and Arne Lundberg Foundation; Sahlgren's University Hospital (LUA-ALF); European Community's Seventh Framework Programme IBDase; Wilhelm and Martina Lundgren's Foundation; The Swedish Foundation for Strategic Research–Innate Immunity Program and The Mucosal Immunobiology and Vaccine Center; and Torsten och Ragnar Söderbergs Stiftelser.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Microbes and Health,” held November 2–3, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at http://www.nasonline.org/SACKLER_Microbes_and_Health.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Xu J, Gordon JI. Inaugural article: Honor thy symbionts. Proc Natl Acad Sci USA. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV. Do symbiotic bacteria subvert host immunity? Nat Rev Microbiol. 2009;7:367–374. doi: 10.1038/nrmicro2114. [DOI] [PubMed] [Google Scholar]
- 4.Boman HG. Innate immunity and the normal microflora. Immunol Rev. 2000;173:5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- 5.Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol. 2007;23:115–120. doi: 10.1097/MOG.0b013e32803cadf4. [DOI] [PubMed] [Google Scholar]
- 6.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 7.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 8.Qin J, et al. MetaHIT Consortium. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 10.Baumgart DC, Carding SR. Inflammatory bowel disease: Cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 11.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 12.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee IA, Havler ME, Dettmar PW, Allen A, Pearson JP. Colonic mucus: Secretion and turnover in relation to dietary fibre intake. Proc Nutr Soc. 2003;62:245–249. doi: 10.1079/pns2003206. [DOI] [PubMed] [Google Scholar]
- 15.Malmberg EK, et al. Increased levels of mucins in the cystic fibrosis mouse small intestine, and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol. 2006;291:G203–G210. doi: 10.1152/ajpgi.00491.2005. [DOI] [PubMed] [Google Scholar]
- 16.Johansson MEV, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swidsinski A, et al. Viscosity gradient within the mucus layer determines the mucosal barrier function and the spatial organization of the intestinal microbiota. Inflamm Bowel Dis. 2007;13:963–970. doi: 10.1002/ibd.20163. [DOI] [PubMed] [Google Scholar]
- 18.Harada N, et al. Human IgGFc binding protein (FcgammaBP) in colonic epithelial cells exhibits mucin-like structure. J Biol Chem. 1997;272:15232–15241. doi: 10.1074/jbc.272.24.15232. [DOI] [PubMed] [Google Scholar]
- 19.Lidell ME, Johansson MEV, Hansson GC. An autocatalytic cleavage in the C terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J Biol Chem. 2003;278:13944–13951. doi: 10.1074/jbc.M210069200. [DOI] [PubMed] [Google Scholar]
- 20.Lidell ME, Hansson GC. Cleavage in the GDPH sequence of the C-terminal cysteine-rich part of the human MUC5AC mucin. Biochem J. 2006;399:121–129. doi: 10.1042/BJ20060443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson MEV, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res. 2009;8:3549–3557. doi: 10.1021/pr9002504. [DOI] [PubMed] [Google Scholar]
- 22.Gum JR, Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 23.Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem. 1999;274:31751–31754. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 24.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang T, Alexandersson M, Hansson GC, Samuelsson T. Bioinformatic identification of polymerizing and transmembrane mucins in the puffer fish Fugu rubripes. Glycobiology. 2004;14:521–527. doi: 10.1093/glycob/cwh066. [DOI] [PubMed] [Google Scholar]
- 26.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 27.Asker N, Axelsson MAB, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- 28.Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SW, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao F, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2-/- mice. Dev Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Axelsson MAB, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273:18864–18870. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- 33.Godl K, et al. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann A, et al. Studies on the “insoluble” glycoprotein complex from human colon. Identification of reduction-insensitive MUC2 oligomers and C-terminal cleavage. J Biol Chem. 1999;274:15828–15836. doi: 10.1074/jbc.274.22.15828. [DOI] [PubMed] [Google Scholar]
- 35.Larsson JM, Karlsson H, Sjövall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19:756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- 36.Kline KA, Fälker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe. 2009;5:580–592. doi: 10.1016/j.chom.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Kankainen M, et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc Natl Acad Sci USA. 2009;106:17193–17198. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlsson NG, et al. The glycosylation of rat intestinal Muc2 mucin varies between rat strains and the small and large intestine. A study of O-linked oligosaccharides by a mass spectrometric approach. J Biol Chem. 1997;272:27025–27034. doi: 10.1074/jbc.272.43.27025. [DOI] [PubMed] [Google Scholar]
- 40.Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 41.Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102:1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 42.Sonnenburg JL, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 43.Brown AJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 44.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci USA. 2006;103:9298–9303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 46.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 47.van Es JH, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 48.Gregorieff A, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. doi: 10.1053/j.gastro.2009.06.044. e1–e3. [DOI] [PubMed] [Google Scholar]
- 49.Verdugo P. Mucin exocytosis. Am Rev Respir Dis. 1991;144:S33–S37. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- 50.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]