Abstract
The time-to-maximum of the tissue residue function (Tmax) perfusion index has proven very predictive of infarct growth in large clinical trials, yet its dependency on simple tracer delays remains unknown. Here, we determine the dependency of computed tomography (CT) perfusion (CTP) Tmax estimates on tracer delay using a range of deconvolution techniques and digital phantoms. Digital phantom data sets simulating the tracer delay were created from CTP data of six healthy individuals, in which time frames of the left cerebral hemisphere were shifted forward and backward by up to ±5 seconds. These phantoms were postprocessed with three common singular value decomposition (SVD) deconvolution algorithms—standard SVD (sSVD), block-circulant SVD (bSVD), and delay-corrected SVD (dSVD)—with an arterial input function (AIF) obtained from the right middle cerebral artery (MCA). The Tmax values of the left hemisphere were compared among different tracer delays and algorithms by a region of interest-based analysis. The Tmax values by sSVD were positively correlated with ‘positive shifts' but unchanged with ‘negative shifts,' those by bSVD had an excellent positive linear correlation with both positive and negative shifts, and those by dSVD were relatively constant, although slightly increased with the positive shifts. The Tmax is a parameter highly dependent on tracer delays and deconvolution algorithm.
Keywords: digital phantom, perfusion, Tmax
Introduction
Over the past decade, the development of computed tomography (CT) perfusion (CTP) and magnetic resonance perfusion has provided powerful paradigms for the scientific study and clinical evaluation of acute stroke patients. Meanwhile, there is an increasing need to critically evaluate the available perfusion postprocessing techniques and resulting perfusion metrics (Butcher et al, 2005) to identify imaging paradigms and perfusion indices that provide efficient targets for treatment (Wintermark et al, 2008a, 2008b). The time-to-maximum of the tissue residue function (Tmax) has been established as an accurate perfusion metric to discriminate the infarct core and penumbra (Shih et al, 2003), and has been applied for the definition of the diffusion–perfusion mismatch in recent multicenter trials such as Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (Albers et al, 2006) and Echoplanar Imaging Thrombolytic Evaluation Trial (Butcher et al, 2005; Davis et al, 2008).
Perfusion metrics are obtained by deconvolution analysis of raw CTP and magnetic resonance perfusion data (Cenic et al, 1999; Ostergaard et al, 1996). By deconvolution, the brain tissue time–concentration curves are corrected for the shape of the arterial input function (AIF) (the time–concentration curve recorded over a major artery), yielding the tissue impulse response function, the (theoretical) tissue curve resulting from an infinitely sharp bolus injection into the voxel. This curve is given by CBF × R(t), where R(t) is the residue function (the fraction of contrast still residing in the voxel at time t after an infinitely sharp bolus injection) and CBF is the cerebral blood flow. The peak of the impulse response function is hence defined as CBF, and the corresponding time-to-peak of impulse response function and R(t) as Tmax (Shih et al, 2003). While ideally, Tmax=0, physiological parameters such as bolus arrival time (BAT) and mean transit time (MTT) of brain tissue affect Tmax in a complex manner that depend on the mathematical properties of the deconvolution algorithm (Bang et al, 2008; Butcher et al, 2005; Mlynash et al, 2005; Prosser et al, 2005; Shih et al, 2003; Takasawa and Jones, 2008). Here, we determine the dependency of CTP Tmax estimates on tracer delay using a range of deconvolution techniques and digital phantoms.
Materials and methods
We retrospectively collected CTP data set on six healthy individuals (male, aged 29 to 35 years, mean age=30.7 years), without neurologic deficits or past history of brain disorders. Written informed consent and approval of the institutional review board were obtained. A 4-detector-row CT scanner (Aquilion; Toshiba Medical Systems, Tokyo, Japan) was used for obtaining the data. Nonionic iodine contrast medium (40-mL iopamidol at 370 mgI/mL; Bayer HealthCare, Berlin, Germany) was injected into the right antecubital vein by a power injector at a rate of 5 mL/s. At 5 seconds after initiation of the injection, dynamic scans of four slices with a thickness of 8 mm were started. Scan parameters used were a 22-cm field of view, 1-second gantry rotation speed, 80-kVp tube voltage, 200-mA tube current, and 40 rotations (the total scan time was 40 seconds).
Digital phantom data were created by using a free software package (perfusion mismatch analyzer; available at http://asist.umin.jp/index-e.htm), as described previously (Kudo et al, 2009). In brief, we divided source CTP images into halves at the midline of the cerebral hemispheres, shifted the time frames of the left hemisphere forward or backward by up to 5 seconds, and then exported these data sets (11 in total) with a digital imaging and communication in medicine (DICOM) format, in which the contrast bolus of the left hemisphere was −5, −4, −3, −2, −1, 0, 1, 2, 3, 4, or 5 seconds behind that of the right hemisphere.
The digital phantoms were postprocessed by using the same software. Quantitative Tmax maps were created by using three kinds of singular value decomposition (SVD) deconvolution techniques: sSVD (Ostergaard et al, 1996), bSVD (Wu et al, 2003), and delay-corrected SVD by BAT (dSVD) (Kudo et al, 2009). We used a cutoff threshold of 20% in both sSVD and dSVD to minimize oscillation of the tissue residue function, and used an oscillation index of 0.095 in bSVD (Wu et al, 2003). In all the phantom analyses, identical AIF and venous output function were obtained from the insular segment of the right middle cerebral artery (MCA) and the right sigmoid sinus, respectively. After vascular pixels were eliminated by using a cerebral blood volume value of 8 mL/100 g as the threshold (Kudo et al, 2003), quantitative color maps were generated.
For quantitative analysis, we measured the Tmax values using the spherical region-of-interest with a diameter of 15.5 mm (36 pixels). Sixteen regions-of-interest were automatically placed along the entire parenchymal surface of each cerebral hemisphere by using the same software. The absolute Tmax values of the left hemisphere were averaged and compared. One-way repeated-measures analysis of variance was used to test whether a significant difference was observable in the Tmax values among the various frame shifts. Further, a post hoc Dunnett test was used to determine which shifts had significantly different values compared with the original data. The α level was set at 0.05.
Results
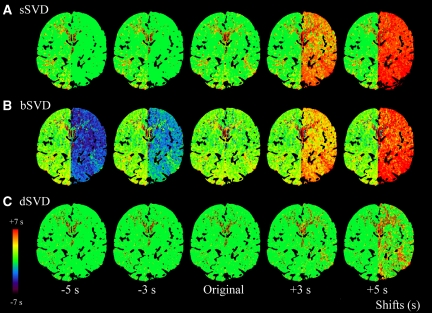
We obtained Tmax color maps of the digital CTP phantoms with various bolus arrival timings by three kinds of SVD algorithms (Figure 1). With ‘positive shifts,' in which the bolus arrival in the left hemisphere was behind that in the AIF of the right MCA, an evident increase in the Tmax was observed by both sSVD and bSVD, whereas the Tmax was almost constant by dSVD. With ‘negative shifts,' in which the bolus arrival in the left hemisphere was ahead of that in the AIF of the right MCA, the Tmax decreased and became negative only by bSVD.
Figure 1.
Color maps of time-to-maximum of the tissue residue function (Tmax). The Tmax color maps of digital computed tomography (CT) perfusion (CTP) phantoms, obtained by different deconvolution algorithms, are shown. Time frames of the left hemisphere were shifted from −5 to +5 seconds compared with the original data. Arterial input function (AIF) was obtained from the identical location at the insular segment of the right middle cerebral artery (MCA). Three kinds of the singular value decomposition (SVD) algorithm, standard SVD (sSVD), block-circulant SVD (bSVD), and delay-corrected SVD (dSVD), were used to obtain the Tmax color maps. In sSVD, the Tmax is apparently increased with the positive delays (+3 and +5 seconds) but is nearly constant with the negative delays (−3 and −5 seconds) compared with the original image (A). In bSVD, the Tmax is increased and decreased with the positive and negative delays, respectively (B). The Tmax is almost stable in dSVD, regardless of the positive or negative delays (C).
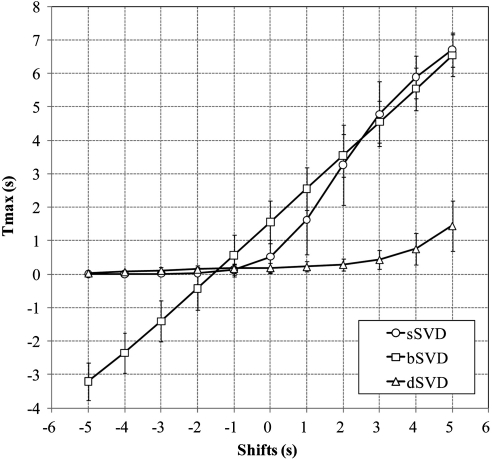
Quantitative analysis demonstrated that the Tmax of the left hemisphere by sSVD was significantly increased in a nearly sigmoid manner under the positive shifts but was nearly zero under the negative shifts (Figure 2). On the other hand, the Tmax by bSVD was significantly increased and decreased in the positive and negative shifts, respectively, having a linear relationship with the amount of frame shift. The regression line was y=0.98x+1.58, and the correlation coefficient of linear regression was remarkably high (r=0.9999) compared with sSVD (r=0.9012). Correlation coefficient became larger in sSVD with third order polynomial fitting (y=−0.05x3+0.29x2+0.96x+0.50; r=0.9996). In contrast, the Tmax by dSVD was relatively constant, although slightly but significantly increased with the positive shifts.
Figure 2.
Relationship between time-to-maximum of the tissue residue function (Tmax) and time shifts. Relationship between the Tmax and positive and negative shifts of the time frame in the left hemisphere is shown. In standard singular value decomposition (sSVD), the Tmax is increased in a sigmoidal manner with the positive delays but is constant with the negative delays. In block-circulant SVD (bSVD), the Tmax is perfectly linear with the positive and negative delays. In delay-corrected SVD (dSVD), the Tmax is mostly constant with both the delays, but is slightly increased with the positive delays. Plots and error bars represent the average and s.d. of the Tmax values in the six subjects.
In all three algorithms, there were significant differences in the Tmax values among the various frame shifts. Further, the post hoc Dunnett test revealed that the Tmax values in sSVD and bSVD with any time shift had significant differences compared with the original data (Table 1). In dSVD, the Tmax values with time shifts less than −4 seconds and more than +2 seconds were significantly different from the original data.
Table 1. P values for post hoc Dunnett test (control=shift 0 seconds).
| Algorithm |
Shifts (seconds) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −5 | −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | |
| sSVD | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | n.a. | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| bSVD | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | n.a. | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| dSVD | <0.001 | 0.002 | 0.091 | 0.623 | 0.99 | n.a. | 0.974 | 0.034 | <0.001 | <0.001 | <0.001 |
bSVD, block-circulant SVD; dSVD, delay-corrected SVD; n.a., not applicable; sSVD, standard SVD; SVD, singular value decomposition.
Discussion
Although the importance of Tmax as a perfusion metric has been emphasized in major multicenter trials such as Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (Albers et al, 2006) and Echoplanar Imaging Thrombolytic Evaluation Trial (Davis et al, 2008), the extent to which these results can be generalized and supported by studies using other perfusion metrics is far from trivial. In particular, the extent to which Tmax depends on study-specific software or tracer-delay effects caused by collateral supply remain crucial to the convergence of methodology and knowledge. Here, we demonstrate the behavior of Tmax as a function of tracer arrival delay by three common SVD algorithms using digital CTP phantoms simulating positive and negative shifts of the arrival timing. We found that the Tmax was positively correlated with the BAT in brain tissue by both sSVD and bSVD in the positive shift domain, suggesting that the Tmax is a tracer delay-sensitive parameter. Consequently, the behavior of the Tmax against the tracer delay appears to be similar to that of TTP, although interindividual variation in terms of the bolus of the contrast agents can be normalized in the Tmax because of the deconvolution. We also noted the difference in dependence on the tracer delay between sSVD and bSVD. The Tmax by sSVD had a sigmoidal correlation with the positive shifts, whereas that by bSVD had a completely linear correlation. The reason for this difference cannot be completely explained; however, it can be related to the different behavior of CBF against delay. The CBF remains constant against delay, because bSVD has been developed as delay-insensitive deconvolution (Wu et al, 2003), and Tmax was linear. In sSVD, CBF decreases with delay, but the degree of decrease was not constant (Kudo et al, 2009). These differences can affect the different sensitivity on Tmax against delay. Further studies will be needed to determine which deconvolution algorithm is suitable to generate the Tmax for estimating the tissue at risk, because all of the previous articles reporting the usefulness of the Tmax used sSVD, but the use of bSVD has been recommended for precise CBF quantification (Wu et al, 2003).
An important finding in this study is that the Tmax calculated by sSVD was constant during the negative shifts and that by bSVD was linearly correlated to these shifts. This result suggests that the Tmax by sSVD is insensitive to the tracer arrival timing when the AIF precedes tracer arrival in brain tissue. Therefore, the AIF should be obtained from the arteries in the contralateral, nonaffected side when using sSVD to obtain the Tmax. This issue is one of the potential pitfalls when adopting the Tmax to determine the tissue at risk.
Theoretically, the Tmax by dSVD should be unchanged regardless of any positive and negative shifts because of the correction of the tracer arrival timing. In dSVD, BAT was estimated in each pixel, and tissue curve of the pixel was shifted to correct arrival time delay. If the estimation of arrival time was perfect, deconvolution was performed without any delay between AIF and tissue, and Tmax should be zero. In this study, however, the Tmax by dSVD was slightly but significantly increased with the positive shifts, suggesting incomplete correction of the delay when using BAT. This phenomenon may be caused by errors in calculating BAT due to image noise, and could become significant in ischemic brain tissue in which the magnitude in the time–density curve is smaller than that in healthy tissue. We speculate that the Tmax by bSVD can be applied for correcting the tracer delay instead of BAT, because the Tmax by bSVD has a nearly ideal linear correlation with positive and negative shifts, and is expected to have robustness to image noise because it is obtained after deconvolution.
There are several limitations in this study. First, we used digital phantoms derived from young healthy subjects who have normal CBF and MTT. Characteristics of the Tmax with regard to the tracer arrival timing might be slightly different between healthy subjects and stroke patients who tend to have low CBF and/or high MTT. Second, we could not investigate the MTT and dispersion effect (Calamante et al, 2000), another source of error in deconvolution analyses, because the tissue curves of our simulation data were derived from real data sets. We found that the tracer delay strongly affects the Tmax values, but MTT and dispersion of the tissue bolus can also affect these values in sSVD and bSVD (Calamante et al, 2010). Further investigation is needed to clarify the contribution of the MTT and dispersion effects on the Tmax in various algorithms. Third, although the Tmax was originally introduced in magnetic resonance perfusion analysis, we used CTP data for evaluating the characteristics of the Tmax, because there are several advantages in CTP analysis for accurate evaluation: (1) the AIF is easily obtained; (2) a linear relationship exists between the CT number and the tracer concentration; and (3) the source data are ‘isophasic' (i.e., all the slice data are obtained simultaneously). Among these advantages, the last appears to be crucial for the precision of time-dependent parameters such as the Tmax. We speculate that our results are applicable to the Tmax in magnetic resonance perfusion analysis because identical theories and postprocessing techniques are used in both the perfusion analyses.
This study revealed that the Tmax is tracer delay dependent and varies among the deconvolution algorithms. Further investigations are needed to determine the potential advantages and limitations of the Tmax in comparison with other perfusion parameters before applying it to clinical practice and future clinical trials concerning acute stroke management.
The authors declare no conflict of interest.
Footnotes
This work was partly supported by a Research Grant for Cardiovascular Diseases (17C-3) from the Ministry of Health, Labour and Welfare of Japan, and by a Grant-in-Aid for Strategic Medical Science Research Center from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, Levi C, Kimber T, Schultz D, Fink J, Tress B, Donnan G, Davis S. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- Calamante F, Christensen S, Desmond PM, Ostergaard L, Davis SM, Connelly A. The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke. 2010;41:1169–1174. doi: 10.1161/STROKEAHA.110.580670. [DOI] [PubMed] [Google Scholar]
- Calamante F, Gadian DG, Connelly A. Delay and dispersion effects in dynamic susceptibility contrast MRI: simulations using singular value decomposition. Magn Reson Med. 2000;44:466–473. doi: 10.1002/1522-2594(200009)44:3<466::aid-mrm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Cenic A, Nabavi DG, Craen RA, Gelb AW, Lee TY. Dynamic CT measurement of cerebral blood flow: a validation study. AJNR Am J Neuroradiol. 1999;20:63–73. [PubMed] [Google Scholar]
- Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 hours after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- Kudo K, Sasaki M, Ogasawara K, Terae S, Ehara S, Shirato H. Difference in tracer delay-induced effect among deconvolution algorithms in CT perfusion analysis: quantitative evaluation with digital phantoms. Radiology. 2009;251:241–249. doi: 10.1148/radiol.2511080983. [DOI] [PubMed] [Google Scholar]
- Kudo K, Terae S, Katoh C, Oka M, Shiga T, Tamaki N, Miyasaka K. Quantitative cerebral blood flow measurement with dynamic perfusion CT using the vascular-pixel elimination method: comparison with H2(15)O positron emission tomography. AJNR Am J Neuroradiol. 2003;24:419–426. [PMC free article] [PubMed] [Google Scholar]
- Mlynash M, Eyngorn I, Bammer R, Moseley M, Tong DC. Automated method for generating the arterial input function on perfusion-weighted MR imaging: validation in patients with stroke. AJNR Am J Neuroradiol. 2005;26:1479–1486. [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- Prosser J, Butcher K, Allport L, Parsons M, MacGregor L, Desmond P, Tress B, Davis S. Clinical-diffusion mismatch predicts the putative penumbra with high specificity. Stroke. 2005;36:1700–1704. doi: 10.1161/01.STR.0000173407.40773.17. [DOI] [PubMed] [Google Scholar]
- Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Villablanca JP, Vespa PM, Kidwell CS. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003;34:1425–1430. doi: 10.1161/01.STR.0000072998.70087.E9. [DOI] [PubMed] [Google Scholar]
- Takasawa M, Jones PS, Guadagno JV, Christensen S, Fryer TD, Harding S, Gillard JH, Williams GB, Aigbirhio FI, Warburton EA, Ostergaard L, Baron JC. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke. 2008;39:870–877. doi: 10.1161/STROKEAHA.107.500090. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, Davis S, Demaerschalk BM, Derdeyn CP, Donnan GA, Eastwood JD, Fiebach JB, Fisher M, Furie KL, Goldmakher GV, Hacke W, Kidwell CS, Kloska SP, Kohrmann M, Koroshetz W, Lee TY, Lees KR, Lev MH, Liebeskind DS, Ostergaard L, Powers WJ, Provenzale J, Schellinger P, Silbergleit R, Sorensen AG, Wardlaw J, Wu O, Warach S. Acute stroke imaging research roadmap. Stroke. 2008a;39:1621–1628. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, Davis S, Demaerschalk BM, Derdeyn CP, Donnan GA, Eastwood JD, Fiebach JB, Fisher M, Furie KL, Goldmakher GV, Hacke W, Kidwell CS, Kloska SP, Kohrmann M, Koroshetz W, Lee TY, Lees KR, Lev MH, Liebeskind DS, Ostergaard L, Powers WJ, Provenzale J, Schellinger P, Silbergleit R, Sorensen AG, Wardlaw J, Wu O, Warach S. Acute stroke imaging research roadmap. AJNR Am J Neuroradiol. 2008b;29:e23–e30. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu O, Ostergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med. 2003;50:164–174. doi: 10.1002/mrm.10522. [DOI] [PubMed] [Google Scholar]