Abstract
Alpha-synuclein oligomerization and aggregation are considered to have a role in the pathogenesis of neurodegenerative diseases. However, despite numerous in vitro studies, the impact of aggregates in the intact brain is unclear. In vitro, oxidative/nitrative stress and acidity induce α-synuclein oligomerization. These conditions favoring α-synuclein fibrillization are present in the ischemic brain, which may serve as an in vivo model to study α-synuclein aggregation. In this study, we show that 30-minute proximal middle cerebral artery (MCA) occlusion and 72 hours reperfusion induce oligomerization of wild-type α-synuclein in the ischemic mouse brain. The nonamyloidogenic isoform β-synuclein did not form oligomers. Alpha-synuclein aggregates were confined to neurons and colocalized with ubiquitin immunoreactivity. We also found that 30 minutes proximal MCA occlusion and 24 hours reperfusion induced larger infarcts in C57BL/6(Thy1)-h[A30P]alphaSYN transgenic mice, which have an increased tendency to form synuclein fibrils. Trangenics also developed more selective neuronal necrosis when subjected to 20 minutes distal MCA occlusion and 72 hours reperfusion. Enhanced 3-nitrotyrosine immunoreactivity in transgenic mice suggests that oxidative/nitrative stress may be one of the mechanisms mediating aggregate toxicity. Thus, the increased vulnerability of transgenic mice to ischemia suggests that α-synuclein aggregates not only form during ischemia but also negatively impact neuronal survival, supporting the idea that α-synuclein misfolding may be neurotoxic.
Keywords: alpha-synuclein, [A30P]alpha-synuclein mutation, focal cerebral ischemia, neurodegeneration, protein aggregation, selective neuronal necrosis
Introduction
Alpha-synuclein is a protein of 140 amino acids that is predominantly expressed in presynaptic terminals throughout the central nervous system (Jakes et al, 1994; Maroteaux and Scheller, 1991). Under pathologic conditions, α-synuclein aggregates to form neuronal inclusions in several neurodegenerative diseases (Soto, 2003; Spillantini et al, 1997). Alpha-synuclein is the major component of Lewy bodies found in Parkinson's disease, dementia with Lewy bodies, and the Lewy body variant of Alzheimer's disease, as well as in glial cytoplasmic inclusions in multiple system atrophy (Baba et al, 1998; Spillantini et al, 1997; Tu et al, 1998; Ueda et al, 1993). Alpha-synuclein in these inclusions is reported to be nitrated, suggesting a role for oxidative and nitrative damage in α-synuclein modification and aggregation (Giasson et al, 2000). Fibril formation is facilitated by factors such as acidic pH and divalent metals. In vivo, the mechanisms of synuclein oligomerization are more challenging to elucidate. Alpha-synuclein overexpression per se was not essentially toxic (Manning-Bog et al, 2003). In the ischemic brain tissue, conditions favoring α-synuclein fibrillization and aggregation such as strong oxidative and nitrative stress, acidity and liberated divalent ions are present. Therefore, focal cerebral ischemia may serve as an in vivo model to investigate α-synuclein aggregation in the intact brain. In this study, we first show that brief middle cerebral artery (MCA) occlusion induces oligomerization of wild-type α- but not of β-synuclein, the synuclein isoform known to be resistant to fibril formation (Giasson et al, 2001; Kahle et al, 2001) and that these aggregates are proteinase-K resistant, a characteristic feature of insoluble protein aggregates (Kramer and Schulz-Schaeffer, 2007).
To gain further insight whether α-synuclein aggregates formed during ischemia are toxic, we used transgenic mice expressing a human mutation (A30P) identified in a familial form of Parkinson's disease (Kruger et al, 1998). Expression in transgenic mice of human synuclein mutations A53T or A30P induces abnormal cellular accumulations in the central nervous system (Kahle et al, 2000; Masliah et al, 2000; van der Putten et al, 2000). In vitro studies have shown that the recombinant mutant A53T or A30P α-synuclein protein fibrillizes faster than does the wild type (Conway et al, 1998; Giasson et al, 1999; Narhi et al, 1999). Therefore, α-synuclein mutations may render transgenic mice more susceptible to brain injury induced by oxidative and nitrative stress under ischemic conditions as well. These transgenic mice can provide an opportunity to test the potential toxicity of α-synuclein oligomerization in ischemia. In this study, we used two models of focal cerebral ischemia: a 30-minute proximal MCA occlusion model to induce MCA infarct and a 20-minute compression model of the distal MCA. The 20-minute model does not produce an infarct but selective neuronal necrosis (SNN) in the cortex and, hence, provides an opportunity to study α-synuclein oligomerization at the level of individual neurons. Both models showed that transgenic mice expressing the human α-synuclein with the A30P mutation developed larger lesions than did wild-type mice, strongly suggesting that α-synuclein aggregates not only form during ischemia but also negatively impact neuronal survival.
Materials and methods
Proximal Middle Cerebral Artery Occlusion
Animal housing, care, and application of experimental procedures were carried in accordance with the regulations of the Ethics Committee of Hacettepe University (2002/44-2). In the first part of experiments, Swiss Albino mice (weighing 35 to 40 g) were anesthetized with chloral hydrate (400 mg/kg intraperitoneal), and proximal occlusion of the right MCA was performed using a nylon filament as described previously (Gursoy-Ozdemir et al, 2000) to investigate α-synuclein aggregation in wild-type animals. In brief, a silicon-coated nylon filament (8/0) was inserted into the right common carotid artery through a small incision proximal to the bifurcation and advanced into the internal carotid artery up to the origin of the MCA (10 mm from the bifurcation). Mice received 10 IU of heparin before ischemia, and blood pressure was monitored using a catheter placed in the common carotid artery. Regional cerebral blood flow (rCBF) was monitored by laser-Doppler flowmetry (Periflux PF 2B, Perimed, Jarfalla, Sweden). After obtaining a stable 10-minute epoch of preischemic rCBF, the MCA was occluded and rCBF was continuously monitored during ischemia and the first 20 minutes of reperfusion. Reperfusion was accomplished by pulling the filament back. After 30 minutes of MCA occlusion and 72 hours of reperfusion, animals were anesthetized with a high dose (1 g/kg) of chloral hydrate and cardiovascularly perfused with 4% formaldehyde. Mice were decapitated and their brains were either cryoprotected or paraffin embedded.
Proximal and Distal Middle Cerebral Artery Ischemia in Transgenic Mice
For studying the impact of transgenic α-synuclein on brain infarct, 8-week-old [C57BL/6(Thy1)-h[A30P]alphaSYN, line31H] transgenic mice (Kahle et al, 2000; Neumann et al, 2002) and 8-to-9-week-old wild-type C57BL/6 mice (TUBITAK Marmara Research Center, Gebze, Turkey) were subjected to 30 minutes of proximal MCA occlusion and 24 hours reperfusion as described above under ketamine (50 mg/kg, intraperitoneal) and xylasine (10 mg/kg, intraperitoneal) anesthesia. As synuclein transgenic mice had a C57B/6 background, we used wild-type C57BL/6 mice for this part of the study, whose sensitivity to MCA ischemia is comparable with that of Swiss mice routinely used in our laboratory (Gursoy-Ozdemir et al, 2000; Kaya et al, 2005). C57BL/6(Thy1)-h[A30P]alphaSYN, line31H transgenics were originally generated by injecting the human [A30P] alpha-synuclein transgene to hybrid B6/DBA oocytes (Kahle et al, 2000; Neumann et al, 2002). Founders were then bred over 10 generations back into the C57/BL6 background. In these animals, the [A30P]alphaSYN protein expression results in a two- to three-fold increase in the total α-synuclein protein load. These mice have a salient neuronal phenotype characterized by somatodendritic α-synuclein accumulation and detergent-insoluble α-synuclein oligomers starting within the first postnatal month, but no vascular involvement in line with the use of a selective neuronal promoter (Thy1). Although integration sites were not exactly mapped, C57BL/6(Thy1)-h[A30P] alphaSYN line31H mice were shown to develop a mild phenotype confined to α-synuclein overexpression, such that proteinase-K-resistant aggregates and extrapyramidal clinical phenotypic changes appear only at advanced age, suggesting that the deletion of other genes was rather unlikely.
In another set of transgenic and wild-type C57BL/6 mice, SNN was induced by compressing the distal MCA for 20 minutes with a glass pipette inserted through a cranial window using a micromanipulator without opening the duramater. The pipette tip was thinned to 50 to 70 μm and blunted. The success of the compression was verified by observing a decrease in rCBF to ischemic values in the distal MCA territory with laser-Doppler flowmetry. After this brief ischemia, 72 hours of reperfusion was required for adequate number of SNNs to appear. At the end of reperfusion, mice were perfused with 4% paraformaldehyde under ketamine anesthesia. To avoid dark neurons in SNN experiments, the decapitated head was kept in 4% paraformaldehyde for 12 hours before the brain was taken out of the skull. The brains were then fixed in 4% paraformaldehyde for an additional 12 hours.
In these mice, blood pressure was monitored noninvasively (NIBP Controller, AD Instruments Pty Ltd., Bella Vista, NSW, Australia) from the tail.
Evaluation of Ischemia Outcome
Infarct volumes and the number of selectively necrotic neurons were detected on hematoxylin and eosin-stained 5-μm-thick brain sections. The infarct area was measured using image-analysis software (NIH Image; http://rsb.info.nih.gov/nih-image/) on the posterior surface of each section, and infarct volumes were calculated by summing the areas of sequential 2-mm-thick sections.
For evaluation of SNN, the number of neurons showing necrotic changes such as pyknosis, karyorhexis, karyolysis, and eosinophilia was counted on 6 microscopic fields randomly selected from the distal MCA territory at × 1,000. Neurons were identified by light microscopic criteria (a large cell body, a large nucleus with a single, prominent, centrally located nucleolus) because the experiments performed to develop the SNN model (Arsava et al, 2009) unambiguously showed that degenerating cells were NeuN-positive neurons.
Cells with apoptotic nuclei were counted manually under dark-field microscopy (Lange et al, 1999) at × 400 magnification in 3 selected brain regions (namely, the frontal cortex, the parietal cortex, and the lateral striatum) in the proximal MCA occlusion model and, in 3 adjacent microscopic fields in the frontoparietal cortex in the distal occlusion model, respectively. To reliably detect cells with apoptotic nuclei, dark and bright field images of the same area were captured and superimposed.
Mean values of infarct volumes, number of SNNs, bright nuclei in dark field and immunopositive neurons were compared with Mann–Whitney U-test, and P<0.05 was considered to be significant. Mean values in the text were given with their s.d.
Immunohistochemistry
Coronal brain sections were stained with anti-α-synuclein (sheep polyclonal antibody-AB5334P, Chemicon, Temecula, CA, USA), β-synuclein (rabbit polyclonal antibody-6485 (Kahle et al, 2000), ubiquitin (rabbit polyclonal antibody, RDI-UBIQUITabR, Research Diagnostics, Concord, MA, USA), antinitrotyrosine (anti-NT) (rabbit immunoaffinity purified IgG-06-284, Upstate, Lake Placid, NY, USA), caspase-3-p20 (rabbit polyclonal antibody-9661S, Cell Signaling, Beverly, MA, USA) or glial fibrillary acidic protein (mouse monoclonal, Sigma, St Louis, MO, USA) antibodies.
Fifteen to 20-μm-thick frozen sections were washed in phosphate-buffered saline and permeabilized with 0.2% Triton-X. The tissues were then incubated with 3% bovine serum albumin and 10% host serum of the secondary antibody to eliminate nonspecific binding. The sections were incubated with primary antibodies at 1:100 (synucleins) or 1:500 (ubiquitin) dilution for 12 hours at +4°C and then with secondary antibodies (1:100 dilution, Jackson ImmunoResearch, West Grove, PA, USA) for 1 hour at 37°C. Sections were mounted with 50% glycerol. The immunolabeled frozen sections were examined under a Nikon Eclipse E600 upright microscope (Nikon, Tokyo, Japan) using appropriate filter sets and the images were captured through a TV lens (C-0.6 ×), digital camera and processed using the ACT-1 image processing system (Nikon). Specimens were further analyzed using a Zeiss LSM-510 confocal laser-scanning microscope (Jena, Germany). Digitized images were pseudo-colored according to their original fluorochromes.
Paraffin-embedded sections of 5-μm thickness were deparaffinized at 56°C overnight and rehydrated by graded alcohol. Anti-3-NT and caspase-3-p20 antibodies were diluted (1:200) in phosphate-buffered saline. For staining with antiubiquitin antibody, endogen peroxidase activation was blocked with H2O2, and sections were then microwaved in 1 mmol/L EDTA buffer for antigen retrieval. Antiubiquitin antibody was diluted (1:500) in 3% bovine serum albumin. After incubation with primary antibodies (ubiquitin for 2 hours and the others, overnight) serial sections were stained with conventional avidin–biotin–peroxidase technique and diaminobenzidine was used as the chromogen. Hematoxylin was used as the counterstain. Sections were examined under bright-field microscope (Nikon Eclipse E600, Nikon) and images were captured and analyzed as described above.
The number of mice used for immunohistochemistry studies is given in parentheses in the ‘Results' section. Several coronal brain sections were studied from each mouse.
Paraffin-Embedded Tissue Blotting
To test whether anti-α-synuclein-immunostained aggregates were proteinase-K resistant, paraffin-embedded tissue blotting method (Kramer and Schulz-Schaeffer, 2007; Neumann et al, 2002; Schulz-Schaeffer, 2002) was used. Paraffin-embedded 5-μm-thick sections passing through the anterior commissure were deparaffinized at 56°C overnight, and rehydrated by graded alcohol and xylol. Dried tissue sections layered on nitrocellulose membranes were prewetted and digested with 0.2 mg/mL proteinase-K (Chemicon) for 8 hours at 55°C. After washing, the membranes were treated for 10 minutes with 3 mol/L guanidine isothiocyanate in 10 mmol/L Tris-HCl, pH 7.8, for optimal epitope retrieval. The tissues were then incubated with anti-α-synuclein (1:200, overnight, 4°C, sheep polyclonal antibody-AB5334P, Chemicon), and immunodetection of α-synuclein aggregates was performed using the conventional streptavidin–horseradish peroxidase technique (Dako cytomation kit, Glostrup, Denmark). Diaminobenzidine was used as the chromogen. Proteinase-K step was omitted for conventional α-synuclein immunohistochemistry of simultaneously processed control paraffin sections.
Thioflavin-S Staining
To test whether anti-α-synuclein immunostained accumulations were protein aggregates, we used thioflavin-S (TS-T1892, Sigma) staining. Deparaffinized and rehydrated sections were incubated for 8 minutes with filtered 1% thioflavin-S in 50% ethanol in the dark at room temperature. Sections were then washed under light protection with 80% and 95% ethanol and then with double-distilled water 3 times for 1 minute to remove ethanol. The sections were finally incubated for 3 minutes in phosphate-buffered saline under light protection and mounted with DPX (4458, Sigma).
Detection of Insoluble Protein Aggregates with Western Blotting
Mice subjected to 30 minutes MCA ischemia and 72 hours of reperfusion (n=10) and naive mice (n=8) were decapitated under terminal anesthesia. The specimens obtained by wedge-shaped incisions of both ipsilateral ischemic and contralateral MCA regions were frozen in liquid nitrogen. Half a gram weighing brain specimens from the MCA territory were homogenized in 10 volumes of TBS+ (complete protease inhibitor in Tris-buffered saline; Roche Diagnostics, Mannheim, Germany) and sonicated. After 5 minutes of 1,000 × g centrifugation, the supernatant was ultracentrifuged at 130,000 × g at 4°C for 1 hour and the ‘buffer-soluble' part was extracted. The pellet was washed twice in TBS+ and extracted by 500 μL 5% SDS in TBS+. All subsequent steps were carried out at 24°C. The pellet was ultracentrifuged at 130,000 × g for 30 minutes and the supernatant of the ‘SDS-soluble' part was extracted. The SDS-insoluble pellet was washed and was incubated for 10 minutes at room temperature in 100 μL 8 mol/L urea 5% SDS in TBS+. In all, 80 μL of the suspension and 20 μL of 100% trichloroacetic acid were incubated overnight at 4°C. The protein precipitates were centrifuged, washed with acetone, and resuspended with protein gel loading buffer containing 6 mol/L urea. Denaturating PAGE, western blotting, and probing was carried out as described before (Neumann et al, 2002). Equal volume of loading was confirmed by Coomassie blue staining after the gel transfer. The membranes were stained with primary antibodies against α-synuclein (monoclonal MC42, Transduction Lab, Lexington, KY, USA), β-synuclein (polyclonal antiserum 6485), and ubiquitin (mouse monoclonal Ubi-1, Zymed Lab, San Francisco, CA, USA).
Results
Ischemia Induces Insoluble Alpha-Synuclein Aggregates
Western Blotting
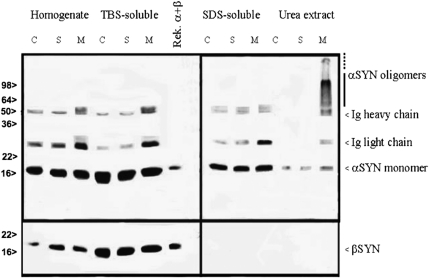
We observed significant α-synuclein oligomerization in western blots prepared from the ischemic MCA territory of wild-type mice subjected to 30 minutes of ischemia and 72 hours of reperfusion (n=10). Insoluble α-synuclein oligomers were abundant in urea extracts. The monoclonal α-synuclein antibody detected detergent-insoluble α-synuclein monomers (19 kDa) and considerable amounts of oligomers (producing a smear between 57 and 100 kDa) in the ischemic MCA territory but not in the homologous area in the contralateral nonischemic hemisphere or in naive control brains (n=8) (Figure 1). We did not observe any β-synuclein bands or oligomers in the urea extract, whereas the typical β-synuclein band in the homogenate or TBS-soluble extract was obtained when the same membranes were stained with anti-β-synuclein antibody. Western blotting analyses were performed in two independent experiments with the same results.
Figure 1.
Detergent-insoluble α-synuclein monomers and oligomers were detected in the ischemic MCA area (M) but not in the nonischemic contralateral hemisphere (C) or in the sham-operated brains (S). Beta-synuclein bands were found in the soluble fraction but no bands or aggregates were detected in the urea extract of the ischemic MCA area. MCA, middle cerebral artery.
Immunohistochemistry
We also searched for α-synuclein aggregates in the ischemic brain by immunohistochemistry to detect their cellular distribution. We used an antibody developed against human α-synuclein because it shows high affinity to aggregated α-synuclein (e.g., to Lewy bodies) (Jakes et al, 1999). In line with previous reports (Kahle et al, 2000, 2001), we observed a diffuse, neuropillic α-synuclein staining in the nonischemic brain (n=11), as expected from primarily presynaptic location of α-synuclein (Figure 2a). Cell bodies and nuclei were not stained. In the ischemic hemispheres prepared from 5 mice subjected to 30 minutes ischemia and 72 hours reperfusion, neuron somas were also labeled with the anti-α-synuclein antibody. Glial fibrillary acidic protein-positive astrocytes did not show any α-synuclein immunostaining (Figure 2b). Thin (1 μm) confocal images of these frozen sections disclosed a course, granular staining pattern, suggestive of clumps of α-synuclein in the cytoplasm (Figures 2c and 2e). A similar but less evident pattern was observed in sections obtained 6 (n=3) and 24 hours (n=3) after reperfusion on initial screening experiments.
Figure 2.
Ischemia induces neuronal α- but not β-synuclein aggregation. (A) A diffuse neuropillic immunostaining was observed on frozen brain sections (15-μm thick) with antibodies against (a) α-synuclein and (b) β-synuclein, in accordance with their primarily presynaptic location. The soma and nuclei were not stained. In the ischemic hemispheres of mice subjected to 30 minutes ischemia and 72 hours reperfusion, cell somas were also labeled with (c, arrows) α-synuclein but not with (d) β-synuclein antibody. Confocal images of 1-μm-thick sections disclosed that α-synuclein immunoreactivity exhibited a course, granular pattern throughout the soma (red, e), which was frequently colocalized with ubiquitin immunostaining (f, yellow clumps in the merged image). (B) In line with α-synuclein's primarily neuronal expression, α-synuclein immunoreactive clumps (red) were confined to neurons (identified with their large nuclei and thin perinuclear cytoplasm in the fifth layer of the cortex, also see the first panel in the middle row in Figure 4), but not observed in astrocytes (GFAP positive, green), including the reactive (hyperthrophic) ones as illustrated in panel B (the second row). Alpha-synuclein aggregates were usually perinuclear in thin confocal sections (panel B, upper row) as long as the nucleus was not left out of the section as seen in panels e and f. The bottom row illustrates the control brain sections stained after omission of the primary antibody. All images were taken from the cortex. Scale bar: 10 μm. GFAP, glial fibrillary acidic protein. The color reproduction of this figure is available on the html full text version of the manuscript.
We also stained the sections with β-synuclein to compare its staining pattern in the ischemic hemisphere with that of α-synuclein (n=5). In line with previous reports (Kahle et al, 2000, 2001), β-synuclein staining paralleled the neuropillic labeling of α-synuclein in the nonischemic brain (Figure 2b). However, in contrast to α-synuclein, the nonamyloidogenic (nonaggregating) β-synuclein did not display granular somatic immunoreactivity except faint, diffuse, perinuclear staining in some neurons (Figure 2d).
Aggregates Colocalize with Ubiquitin
Alpha-synuclein aggregates are ubiquitinated in inclusion bodies seen in several neurodegenerative diseases (Goedert, 2001; Matsuzaki et al, 2004; Soto, 2003; Spillantini et al, 1997, 1998). We therefore examined colocalization of the α-synuclein aggregates with ubiquitin immunoreactivity in ischemic brain sections. The diffuse ubiquitin immunoreactivity observed in nonischemic cells was replaced by a coarse granular pattern after ischemia–reperfusion on paraffin-embedded brain sections (n=5) as reported previously (Hu et al, 2001) (data not shown). Accordingly, we investigated whether these ubiquitin-immunopositive granules in ischemic neurons were colocalized with α-synuclein immunoreactivity. For this, we stained frozen brain sections with a low (1/500 dilution) antiubiquitin antibody concentration, which did not exhibit appreciable immunoreactivity in the nonischemic brain but disclosed granular immunostaining in ischemic cells (n=5). Confocal microscopy showed that ubiquitin-immunopositive granules were indeed highly colocalized with α-synuclein immunoreactive clumps within ischemic cells (Figure 2f). Conversely, we could not detect ubiquitin immunoreactivity associated with α-synuclein oligomers on western blots. However, it is generally difficult to show pathologic poly-ubiquitinylation of α-synuclein on western blots (Nonaka et al, 2005); therefore, this failure might simply be owing to a methodological limitation.
Alpha-Synuclein Aggregates are Proteinase Resistant
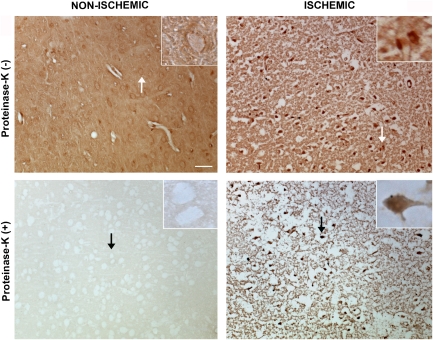
The paraffin-embedded tissue blotting method (Kramer and Schulz-Schaeffer, 2007; Neumann et al, 2002; Schulz-Schaeffer, 2002) allows selective detection of protein aggregates because nonaggregated proteins are readily degraded by proteinase-K treatment. In untreated paraffin-embedded sections, we detected a diffuse neuropilic α-synuclein immunostaining in the nonischemic hemisphere, whereas somatic α-synuclein clumps were seen in ischemic neurons similar to our observations on frozen sections. After proteinase-K treatment, neuropilic α-synuclein immunoreactivity in the nonischemic areas completely disappeared, whereas somatic α-synuclein clumps and part of the neuropilic immunostaining in the ischemic MCA area persisted (n=4) (Figure 3).
Figure 3.
Paraffin-embedded tissue (PET) blotting method illustrated that α-synuclein clumps observed with immunohistochemistry were proteinase-K resistant aggregates. In sections untreated with proteinase-K (upper row), a diffuse neuropilic α-synuclein immunostaining was detected in the nonischemic hemisphere, whereas somatic α-synuclein clumps were seen in neurons in the ischemic area 72 hours after 30 minutes MCA occlusion. After proteinase-K treatment, neuropilic α-synuclein immunoreactivity in the nonischemic areas completely disappeared, whereas somatic α-synuclein clumps and part of the neuropilic immunostaining in the ischemic MCA area persisted (lower row). Ischemia-induced neuropilic sponginess looks exaggerated than expected for this time point because of proteinase-K-induced tissue degradation and PET blotting procedures. Microphotographs were taken from the frontoparietal cortex and insets are enlarged views of the areas marked with arrows. Scale bar: 20 μm. MCA, middle cerebral artery.
Alpha-Synuclein Transgenic Mice are More Sensitive to Cerebral Ischemia
We subjected wild-type and [A30P]α-synuclein transgenic C57BL/6 mice to 30 minutes proximal MCA occlusion or to 20 minutes distal MCA compression to compare their vulnerability with ischemic brain injury. We chose the 30-minute ischemia plus 24-hour reperfusion model because it produces a fairly consistent infarct size providing satisfactory statistical power with a small number of animals (n=6 in each group) and it has no mortality (contrary to the 72-hour reperfusion model), both of which are important features when working with valuable transgenic animals. We used the 20-minute distal ischemia model to study the impact of α-synuclein aggregation at a neuronal level without confounding effects of the associated pannecrosis. We found that α-synuclein transgenic mice developed larger infarcts (16±3 mm3 versus 9±2 mm3, P<0.05, n=6 in each group) and more selective neuronal death (79%±14% versus 38%±5% of neurons in the MCA-supplied cortex) than did controls (P<0.05, n=6 in each group) (Figure 4). We found that ischemic neurons but not those in the nonischemic hemisphere in transgenic mice exhibited intraneuronal thioflavin-S-positive inclusions, a pathologic hallmark of protein aggregates in tissue sections (Figure 4). Ischemia–reperfusion induced more intense oxidative/nitrative stress in transgenics. In wild-type mice, 16%±9% of the morphologically intact neurons located among selectively necrotic neurons exhibited strong 3-NT immunoreactivity (a marker of oxidative/nitrative stress) in addition to necrotic neurons, whereas this ratio increased to 35%±15% in the transgenic group (P<0.05) (Figure 4). Similarly, the ratio of morphologically intact neurons displaying 3-NT immunopositivity at the periphery of the infarct in the proximal MCA occlusion groups was significantly higher in transgenics (35%±11% versus 10%±5%, P<0.05).
Figure 4.
[A30P]alpha-synuclein transgenic C57BL/6 mice developed larger lesions compared with wild-type C57BL/6 mice when subjected to transient ischemia. Thirty-minutes proximal MCA occlusion (upper row) caused pannecrosis (microphotograph at × 400, H&E staining). Transgenic mice had significantly larger infarct volumes (graph, * denotes P<0.05, n=6 in each group). Twenty-minutes distal MCA compression (upper row) led to selective neuronal necrosis (SNN), which was significantly more prevalent in transgenic mice compared with nontransgenic wild-type control mice (graph, * denotes P<0.05, n=6 in each group, X axis shows the ratio of SNNs to total number of neurons). H&E-stained microphotographs ( × 400) illustrate several necrotic neurons identified with their pyknotic nuclei and eosinophillic cytoplasm among intact microvessels and nonneuronal cells. Insets are enlarged views (arrowheads and arrows indicate necrotic and morphologically normal neurons, respectively). The fluorescent image in the middle row illustrates α-synuclein accumulation (red immunoreactivity) in neuronal somas (large pyramidal neurons in layers III and V) and processes in the cortex supplied by the distal MCA after 20 minutes ischemia 72 hours reperfusion in contrast to the neighboring nonischemic cortex (Scale bar: 20 μm; scale bars in the other panels; 50 μm). The fluorescent image just below (bottom row) illustrates that accumulations in ischemic neuronal somas and processes in transgenic mice are thioflavin-S (TS) positive, a hallmark of aggregated proteins (green). Dark-field images corresponding to the H&E-stained images in the upper row illustrate the nuclei with apoptotic-like chromatin condensation (bright spots) in transgenic and wild-type cortices. Arrowheads in insets indicate the condensed chromatin (which refracts light) in fragmented or pyknotic nuclei and, arrows point to the normal nuclei. In transgenics, 20-minutes distal MCA occlusion and 72-hours reperfusion not only induced more SNNs but also significantly more prevalent 3-nitrotyrosine (3-NT, a marker of oxidative/nitrative stress) and caspase-3-p20 (active form of caspase-3) immunoreactivity in morphologically intact neurons dispersed among the SNNs. The Y axis in the graph (bottom row) shows the ratio of morphologically intact 3-NT or caspase-3-p20-immunopositive neurons to the total number of morphologically intact neurons among the selectively necrotic neurons. Both * and ** denote P<0.05 compared with the corresponding wild-type controls). Panels illustrate, among the SNNs, several morphologically intact neurons displaying 3-NT or caspase-3-p20 immunoreactivity. SNNs were immunopositive for 3-NT and caspase-3-p20. Arrows in insets point to the neurons displaying 3-NT or caspase-3-p20 immunoreactivity but have an intact morphology, whereas the arrowhead indicates an immunopositive necrotic neuron. H&E, hematoxylin and eosin; MCA, middle cerebral artery. The color reproduction of this figure is available on the html full text version of the manuscript.
To investigate whether ischemic cell death mechanisms were modified in transgenic mice, we counted the nuclei with apoptotic morphology under dark-field microscopy, in which clumped chromatin refracts light and appears as bright objects in a dark background, whereas dispersed chromatin in necrotic cells does not (Lange et al, 1999). We found that transgenic mice had a similar number of apoptotic nuclei compared with wild types in both models; 1,181±589/mm2 versus 1,380±397/mm2 in the proximal and 970±568/mm2 versus 935±297/mm2 in SNN models (P>0.05). Almost all of the morphologically necrotic neurons in both groups exhibited caspase-3-p20 immunoreactivity. Interestingly, significantly (P<0.05) more morphologically intact neurons at the peri-infarct area (31%±11% versus 10%±5%) or among the selectively necrotic neurons (26%±19% versus 7%±6%) (Figure 4) displayed caspase-3-p20 immunoreactivity in transgenics, suggesting that the ischemic lesion will grow even larger in transgenic mice at later time points. Taken together, these observations are in line with mixed morphologic/biochemical neuronal death phenotype (necroapoptosis) seen after ischemia and do not suggest a significant phenotypic change in transgenic mice (Unal-Cevik et al, 2004; Kilinc et al, 2010).
There were no significant differences between the transgenic and wild-type groups regarding the vasculature at macroscopic and microscopic levels, for rCBF recorded during ischemia and the first 20 minutes of reperfusion, as well as for arterial blood pressure (Table 1), suggesting that the observed differences between the groups were not caused by hemodynamic changes but by an increased sensitivity to ischemic injury in transgenic mice.
Table 1. Physiologic parameters.
|
Infarct group |
Selective neuronal necrosis group |
|||
|---|---|---|---|---|
| Wild | Transgenic | Wild | Transgenic | |
| Arterial blood pressure (mm Hg)* | 90±4 | 86±5 | 98±5 | 80±5 |
| rCBF–ischemia (%) | 22±2 | 30±4 | 43±5 | 47±6 |
| rCBF–reperfusion (%) | 92±4 | 99±5 | 90±3 | 96±3 |
rCBF, regional cerebral blood flow; SNN, selective neuronal necrosis.
rCBF was recorded during ischemia and 20 minutes following reperfusion.
*Arterial blood pressure was monitored using an intracarotid catheter in the infarct group and noninvasively from the tail in the SNN group.
No statistically significant differences for any parameter were found between the groups.
Values are mean±s.e.
Discussion
This study shows that transient MCA ischemia induces oligomerization of wild-type α-synuclein in the mouse brain. This effect is isoform specific because β-synuclein, the isoform known to be resistant to fibril formation (Giasson et al, 2001; Kahle et al, 2001), does not form oligomers. Immunohistochemistry illustrated that α-synuclein aggregates in neurons were proteinase-K resistant and colocalized with ubiquitin immunoreactivity as seen in synucleinopathies (Goedert, 2001; Matsuzaki et al, 2004; Soto, 2003; Spillantini et al, 1997, 1998). Importantly, our findings also showed that α-synuclein aggregates not only form during ischemia but also aggravates oxidative/nitrative stress and negatively impact neuronal survival in transgenic mice expressing the human α-synuclein with the [A30P] mutation, supporting the idea that α-synuclein misfolding may be neurotoxic.
Western blots convincingly showed that ischemic wild-type brain contained an abundance of insoluble oligomers of α-synuclein (Kahle et al, 2001; Miake et al, 2002; Neumann et al, 2002). Alpha-synuclein-immunopositive clumps observed with immunohistochemistry, very likely represented the aggregated oligomers because they were resistant to proteinase-K treatment and were also immunopositive for ubiquitin (Goedert, 2001; Matsuzaki et al, 2004; Soto, 2003; Spillantini et al, 1997, 1998). Ischemia may have disrupted axonal transport of α-synuclein and led to its somatic accumulation and predisposed to aggregation in the cytoplasm in addition to the proteinase-K-resistant aggregation in the neuropil. Somal accumulation of α-synuclein was also found in mice subjected to a chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine regimen (Vila et al, 2000). Once oligomerization is triggered during ischemia, initial aggregates may continue to trigger further oligomerization despite reperfusion (Soto, 2003; Wood et al, 1999). Further strengthening the specificity of these observations, the above findings were not observed with β-synuclein, which is not amyloidogenic (Giasson et al, 2001; Kahle et al, 2001).
Aggregates containing ubiquitinated proteins have been reported to form in neurons after experimental cerebral ischemia; Hu et al (2000) showed that transient global cerebral ischemia caused protein aggregation in hippocampal CA1 neurons. Two hours of MCA occlusion was also reported to induce protein aggregation in neocortical neurons (Hu et al, 2001). After cerebral hypoxia–ischemia, misfolded proteins were shown to accumulate in the lumen of the endoplasmic reticulum (DeGracia and Montie, 2004). This accumulation leads to stress responses, inhibition of protein synthesis, and activation of programmed cell death through the mitochondrial apoptotic pathway (Paschen and Mengesdorf, 2005).
Similarly, the α-synuclein oligomers and aggregates that we observed in ischemic neurons may unfavorably impact neuronal survival as suggested by larger lesions seen in A30P transgenic mice subjected to focal cerebral ischemia. A30P α-synuclein has a higher tendency to oligomerize when exposed to oxidizing conditions in vitro (Conway et al, 2000). Transgenic mice expressing the mutant A30P α-synuclein reportedly show reduced performance and cognitive decline when they grew older than 12 months (Freichel et al, 2007). At 4 months, transgenic mice behaved no different than wild-type controls and showed no proteinase-K-resistant α-synuclein aggregates throughout the brain, strongly suggesting that neuronal α-synuclein oligomerization and aggregation in our 2-month-old transgenic mice was induced by ischemia as was in wild-type mice. Several mechanisms of α-synuclein oligomer-induced neurotoxicity including the endoplasmic reticulum stress response, disruption of trafficking between the endoplasmic reticulum and Golgi, ubiquitin–proteosome system dysfunction, mitochondrial dysfunction, synaptic dysfunction, inactivation of survival-promoting factors, and production of reactive oxygen species, have been proposed (Lee and Trojanowski, 2006). Indeed, we observed intensified 3-NT immunoreactivity in A30P transgenic mice when they were subjected to ischemia–reperfusion, supporting the idea that oxidative/nitrative stress has a role in aggregate toxicity. However, it should be noted that mechanisms of α-synuclein-induced toxicity might differ between slowly progressive neurodegenerative disorders and an acute ischemic insult, in which an abundance of oligomers are produced in a short time.
There is still controversy whether inclusion bodies that are considered pathologic hallmarks of various neurodegenerative diseases, are toxic to the affected neurons (Arrasate et al, 2004; Kopito, 2000; Kramer and Schulz-Schaeffer, 2007; Ross and Poirier, 2005; Soto, 2003). In a recent postmortem study, of the 106 brains having α-synuclein-positive inclusions, only 30% had been clinically diagnosed as having a neurodegenerative disorder, whereas 30% did not have any neurologic impairment (Parkkinen et al, 2005). In fact, some experimental data imply that these inclusions may be formed as a defense against cellular stress induced by misfolded proteins (Arrasate et al, 2004; Giasson et al, 2000; Lee et al, 2001). These conflicting observations may be reconciled by assuming that clinical syndromes may result from a ‘critical mass' of aggregated insoluble material, whereas lower amounts do not exert toxicity and may even be protective (Burn, 2006). Our findings from A30P transgenic mice strongly support the idea that misfolded α-synuclein can be neurotoxic in ischemic neurons. In an acute and severe insult such as ischemia, the threshold may be reached in a short time and toxicity induced by α-synuclein aggregates may contribute to cell death. Interestingly, in the above-mentioned postmortem study, more than one-third of patients with α-synuclein inclusions had died of primarily cerebrovascular and cardiovascular diseases, increasing the possibility that the ischemic/anoxic episodes to the brain may have promoted α-synuclein-positive inclusions as we observed in the mouse brain. Hence, further postmortem examination of human brains that had experienced brief ischemic episodes seems warranted to see whether they bore presynaptic α-synuclein aggregates in the absence of Lewy bodies (Kramer and Schulz-Schaeffer, 2007).
The term ‘selective neuronal necrosis (SNN)' denotes the death of neurons with sparing of glial and vascular elements following mild and/or short-term ischemia. The process is different from pannecrosis, which affects all elements of the nervous system. Selective neuronal necrosis is assumed to have a role after transient ischemic attacks, and in the formation of silent brain lesions and vascular dementia (Vermeer et al, 2003). [A30T] transgenic mice developed more extensive SNN after brief, focal ischemia, suggesting that protein misfolding may have a role in the pathogenesis of silent brain lesions and the consequent vascular dementia as recently reported after traumatic brain injury in humans (Uryu et al, 2007). The SNN model we used appears promising to study the pathophysiology of aggregated or fibrillar α-synuclein in future studies because it achieves rapid aggregation in vivo without overexpressing the protein.
In conclusion, brief, transient ischemia induces oligomerization of wild-type α-synuclein. Alpha-synuclein aggregation is toxic to ischemic neurons. The A30P mutation promotes α-synuclein oligomerization and, hence, unfavorably affects neuronal survival when these mice are subjected to brief focal cerebral ischemia. The SNN model used in this study appears as a promising simple technique to investigate neuronal α-synuclein aggregation under in vivo conditions in intact animals.
Acknowledgments
We thank Dr E Murat Arsava for the establishment of SNN method and, Elvan Ciftci and Ahmet Gürcan for their help with cell counting. Dr Turgay Dalkara's work is supported by the Turkish Academy of Sciences.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants from the Hacettepe University Research Fund-0101101004 and TUBITAK-SBAG-AYD-435 and Brain Research Organization.
References
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- Arsava EM, Gurer G, Gursoy-Ozdemir Y, Karatas H, Dalkara T. A new model of transient focal cerebral ischemia for inducing selective neuronal necrosis. Brain Res Bull. 2009;78:226–231. doi: 10.1016/j.brainresbull.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- Burn DJ. Cortical Lewy body disease and Parkinson's disease dementia. Curr Opin Neurol. 2006;19:572–579. doi: 10.1097/01.wco.0000247607.34697.a2. [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- Freichel C, Neumann M, Ballard T, Muller V, Woolley M, Ozmen L, Borroni E, Kretzschmar HA, Haass C, Spooren W, Kahle PJ. Age-dependent cognitive decline and amygdala pathology in alpha-synuclein transgenic mice. Neurobiol Aging. 2007;28:1421–1435. doi: 10.1016/j.neurobiolaging.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- Goedert M. The significance of tau and alpha-synuclein inclusions in neurodegenerative diseases. Curr Opin Genet Dev. 2001;11:343–351. doi: 10.1016/s0959-437x(00)00200-8. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Bolay H, Saribas O, Dalkara T.2000Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia Stroke 311974–1980.discussion 81 [DOI] [PubMed] [Google Scholar]
- Hu BR, Martone ME, Jones YZ, Liu CL. Protein aggregation after transient cerebral ischemia. J Neurosci. 2000;20:3191–3199. doi: 10.1523/JNEUROSCI.20-09-03191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BR, Janelidze S, Ginsberg MD, Busto R, Perez-Pinzon M, Sick TJ, Siesjo BK, Liu CL. Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2001;21:865–875. doi: 10.1097/00004647-200107000-00012. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Jakes R, Crowther RA, Lee VM, Trojanowski JQ, Iwatsubo T, Goedert M. Epitope mapping of LB509, a monoclonal antibody directed against human alpha-synuclein. Neurosci Lett. 1999;269:13–16. doi: 10.1016/s0304-3940(99)00411-5. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Odoy S, Okamoto N, Jacobsen H, Iwatsubo T, Trojanowski JQ, Takahashi H, Wakabayashi K, Bogdanovic N, Riederer P, Kretzschmar HA, Haass C. Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am J Pathol. 2001;159:2215–2225. doi: 10.1016/s0002-9440(10)63072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya D, Gursoy-Ozdemir Y, Yemisci M, Tuncer N, Aktan S, Dalkara T. VEGF protects brain against focal ischemia without increasing blood–brain permeability when administered intracerebroventricularly. J Cereb Blood Flow Metab. 2005;25:1111–1118. doi: 10.1038/sj.jcbfm.9600109. [DOI] [PubMed] [Google Scholar]
- Kilinc M, Gürsoy-Özdemir Y, Gürer G, Erdener SE, Erdemli E, Can A, Dalkara T. Lysosomal rupture, necroapoptotic interactions and potential crosstalk between cysteine proteases in neurons shortly after focal ischemia. Neurobiol Dis. 2010;40:293–302. doi: 10.1016/j.nbd.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lange MS, Johnston MV, Tseng EE, Baumgartner WA, Blue ME. Apoptosis detection in brain using low-magnification dark-field microscopy. Exp Neurol. 1999;158:254–260. doi: 10.1006/exnr.1999.7097. [DOI] [PubMed] [Google Scholar]
- Lee M, Hyun D, Halliwell B, Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001;76:998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Scheller RH. The rat brain synucleins; family of proteins transiently associated with neuronal membrane. Brain Res Mol Brain Res. 1991;11:335–343. doi: 10.1016/0169-328x(91)90043-w. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Hasegawa T, Takeda A, Kikuchi A, Furukawa K, Kato Y, Itoyama Y. Histochemical features of stress-induced aggregates in alpha-synuclein overexpressing cells. Brain Res. 2004;1004:83–90. doi: 10.1016/j.brainres.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem. 2002;277:19213–19219. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Iwatsubo T, Hasegawa M. Ubiquitination of alpha-synuclein. Biochemistry. 2005;44:361–368. doi: 10.1021/bi0485528. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- Paschen W, Mengesdorf T. Cellular abnormalities linked to endoplasmic reticulum dysfunction in cerebrovascular disease–therapeutic potential. Pharmacol Ther. 2005;108:362–375. doi: 10.1016/j.pharmthera.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Opinion: what is the role of protein aggregation in neurodegeneration. Nat Rev Mol Cell Biol. 2005;6:891–898. doi: 10.1038/nrm1742. [DOI] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ. [BSE and variant CJD: about the difficulties to establish a new pathogenetic principle] Dtsch Med Wochenschr. 2002;127:344–346. doi: 10.1055/s-2002-20152. [DOI] [PubMed] [Google Scholar]
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinç M, Can A, Gürsoy-Ozdemir Y, Dalkara T. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke. 2004;35:2189–2194. doi: 10.1161/01.STR.0000136149.81831.c5. [DOI] [PubMed] [Google Scholar]
- Uryu K, Chen XH, Martinez D, Browne KD, Johnson VE, Graham DI, Lee VM, Trojanowski JQ, Smith DH. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the Parkinsonian toxin MPTP. J Neurochem. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. Alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson's disease. J Biol Chem. 1999;274:19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]