Abstract
The degree of cellular injury within the stroke ischaemic penumbra is controversial. Clinical and experimental studies using the hypoxia tracer fluoromisonidazole (FMISO) have shown retention of this tracer in the penumbra, but cellular outcome has not been well characterised. We hypothesised that macroscopically intact FMISO-retaining penumbral tissues would show evidence of microscopic injury, and that no FMISO retention would be seen in the infarct core. To determine the distribution of FMISO retention, a tritium-labelled tracer (hydrogen-3 FMISO ([3H]FMISO)) was administered 5 minutes after induction of 2-hour temporary middle cerebral artery occlusion. Coregistered brain histology and autoradiography at 24 hours revealed marked retention of FMISO within the infarct. However, 48% of the FMISO-retaining tissue was not infarcted. Within this noninfarcted tissue, only 27% (17 of 64) of sampled regions showed no evidence of neuronal loss, whereas 44% (28 of 64) showed injury to >50% of neurons within the sample. To determine whether FMISO retention occurred after the tissue was already committed to infarction, FMISO was administered 4 to 6 hours after the onset of permanent vessel occlusion. Intense FMISO retention was consistently seen throughout the infarct core. In conclusion, FMISO retention occurs both within the ischaemic penumbra and within the early infarct core. Most penumbral tissues show evidence of selective cellular injury.
Keywords: hypoxia, brain; infarction, middle cerebral artery; misonidazole/analogue and derivative/diagnostic use; radionuclide imaging; rat, inbred SHR; stroke
Introduction
Fluorine-18 fluoromisonidazole [18F]FMISO has been used to image the hypoxic tissue in human stroke and is a putative marker of the ischaemic penumbra. The peri-infarct distribution and timing of [18F]FMISO retention are consistent with retention in the potentially salvageable hypoxic tissue, and regions that showed [18F]FMISO retention during the acute phase of stroke have been shown on follow-up imaging to have a duality of potential tissue outcomes—tissue salvage or infarction (Read et al, 2000). Retention of FMISO has been observed to be absent from central infarct regions in patients with stroke, suggesting that FMISO retention may define the ischaemic penumbra without labelling either the hypoperfused but unthreatened oligaemic zone or the already irreversibly injured infarct core (Markus et al, 2003; Read et al, 1998). Such a marker would have enormous potential for both clinical and research use; however, confirmation that the FMISO-retaining tissue is both threatened and potentially salvageable is required. This is difficult to achieve in human studies because of several factors, including difficulties in determining the time of vessel reperfusion in patients. Without this, it is not possible to determine the accuracy of the technique, because variability of tissue outcome could equally be caused by variability in the timing of reperfusion.
We have recently developed a method for determining FMISO retention and histologic outcome in the same histologic sections using hydrogen-3 FMISO ([3H]FMISO) in a rat stroke model (Spratt et al, 2006a, 2009). We showed that the volume of [3H]FMISO retention after 2-hour temporary middle cerebral artery occlusion (MCAo) was the same as that of infarction after permanent vessel occlusion, indicating that most of the noninfarcted FMISO-retaining areas detected early would have progressed to infarction in the absence of reperfusion. However, there is evidence from animal and human pathologic and human benzodiazepine receptor labelling studies that there may be areas of significant cellular loss within penumbral regions that are macroscopically intact (Garcia et al, 1997; Giffard et al, 2008; Guadagno et al, 2008; Hughes et al, 2009; Jones et al, 1981; Lassen et al, 1983; Marcoux et al, 1982; Nakagawara et al, 1997; Saur et al, 2006; Weiller et al, 1993). To date, microscopic histologic outcome in the macroscopically or radiologically intact FMISO-retaining tissue salvaged by reperfusion is unknown. Therefore, demonstration of a degree of microscopic injury in the noninfarcted FMISO-retaining tissue seen after temporary MCAo would provide further confirmation that this tissue is indeed penumbral, rather than the unthreatened oligaemic tissue. Second, demonstration that FMISO is not retained in the infarct core would also provide evidence that the tracer is specific to the penumbra.
Therefore, the aims of this study were twofold: first, to help confirm the penumbral nature of the noninfarcted FMISO-retaining tissue after reperfusion by characterising both its spatial distribution and its histologic outcome and second, to determine whether FMISO retention occurs within the infarct core.
Materials and methods
Experimental Protocols
Characterisation of the spatial distribution and histologic outcome of the noninfarcted FMISO-retaining tissue was conducted on sections collected from animals with 2-hour temporary MCAo and 22-hour reperfusion (n=6). These experiments were designed to try to obtain autoradiographic data that would as closely as possible replicate the timing of image acquisition (poststroke) in human [18F]FMISO positron emission tomography (PET) studies, yet allow quantification of histologic outcome. A 2-hour temporary MCAo with reperfusion was used. Retention of [3H]FMISO has previously been shown to cease after reperfusion (Spratt et al, 2006a; Takasawa et al, 2007), and delaying animal killing until 24 hours allows the development of histologic changes. This delay does not alter the volume or relative intensity of [3H]FMISO retention within the hypoxic region (Spratt et al, 2006a). Hydrogen-3 FMISO was administered 5 minutes after the induction of MCAo. The FMISO-retaining volumes for this cohort were previously reported as one point in a series characterising the timing of FMISO retention (Spratt et al, 2009).
To determine whether any FMISO retention occurs in the infarct core, [3H]FMISO was administered 4 to 6 hours after the onset of 24-hour permanent MCAo (pMCAo) (4 to 5 hours n=5, 6 hours n=4). Evidence indicates that the central infarct core is already irreversibly injured in this model at these time points, on the basis of available reperfusion and neuroprotection studies (Memezawa et al, 1992; O'Collins et al, 2006; van der Worp et al, 2007). That is not to indicate that there is no potentially salvageable tissue present at this time, but rather that the available data do not support salvage of the central core at this time in this model.
Experimental Stroke Model
All animal experimentations were performed with the approval of the Austin Health Animal Ethics Committee and in accordance with guidelines of the National Health and Medical Research Council. A total of 16 Sprague–Dawley rats weighing 280 to 320 g were used. The MCAo model was used as described previously (Spratt et al, 2006b, 2009). In brief, under isoflurane anaesthesia, and using temperature regulation and physiologic monitoring throughout the surgical procedure, thread occlusion of the MCA was achieved using a silicone-tipped 3/0 monofilament. Physiologic monitoring was performed throughout surgery, including pulse oximetry (Nellcor, Boulder, CO, USA). Blood flow over the ischaemic hemisphere was monitored by continuous laser Doppler flowmetry (Moor Instruments, Axminster, Devon, UK). A laboratory-manufactured rubber probe holder was affixed to the thinned skull over the MCA–anterior cerebral artery watershed territory, allowing subsequent monitoring of reperfusion at the same location. For reperfusion experiments, 150 μCi [3H]FMISO in 150 μL physiologic saline was administered 5 minutes after MCAo. Reperfusion was performed after 2 hours of MCAo. Animals were then briefly reanaesthetised, and the MCA-occluding suture was retracted into the external carotid stump. Successful occlusion was confirmed by a step-wise decrease in laser Doppler flow (LDF) of ⩾60%, and reperfusion by a step-wise increase in ⩾60% of prereperfusion baseline; otherwise animals were excluded.
Tissue Processing and Histology
Cardiac perfusion fixation was performed with 4% paraformaldehyde and 40-μm-thick cryostat sections were prepared as described previously (Spratt et al, 2006a). Sections were stained with haematoxylin and eosin (H&E). Slides were scanned on an Epson expression 1600 flatbed scanner (Seiko Epson, Sydney, NSW, Australia), and 1,000-d.p.i. red–green–blue colour images were analysed using the MCID M2 software package (Imaging Research, Brock University, Ontario, Canada). This pixel density was chosen to match that from the autoradiographs; hence, no scaling of images was required for subsequent alignment. The area of infarction was assessed by drawing an outline of the area of H&E pallor on scanned images of sections by an assessor blinded to ischaemia duration, and the volume of infarction was calculated without oedema correction, to enable comparison with the autoradiographic volumes. Histologic sections were examined under high-power light microscopy at specific locations within the penumbra for analysis of selective neuronal loss (see below).
Hydrogen-3 Fluoromisonidazole Autoradiography Image Analysis and Cellular Morphology
Tritiated FMISO (1-(3-fluoro-2-oxopropyl)-2-nitro-1H-imidazole) synthesis and autoradiography were performed as described previously (Spratt et al, 2006a). Sections were stained with H&E before autoradiography, a sequence that minimises contamination of phosphor-imaging plates (Liberatore et al, 1999). Subsequently, the same sections were coverslipped, digital photographs were taken, and the infarct area at 30 tissue planes 0.24 mm apart was quantified by outlining the area of pallor, using the MCID M2 image analysis software (Imaging Research). Autoradiography was performed using phosphor-imaging plates and a BAS-5000 plate reader (Fuji, Tokyo, Japan), and analysed using the MCID software. Fiducial markers visible on both autoradiographs and histologic slides were used for accurate alignment of autoradiographs with the corresponding histologic sections (Spratt et al, 2006a). As these were images of the same sections, at the same resolution, no transformation of any images was required and the only limiting factor was a slight ‘overlabelling' with radiotracer of the combined autoradiograph/optical fiducials on some sections. The corresponding fiducial marks (three per section) from autoradiographs and histology were centred one inside the other in case of any such minor size discrepancy to minimise error. Areas of increased FMISO retention were assessed using a previously reported objective thresholding method (Spratt et al, 2006a, 2009), by an assessor blind to the results of the histologic analysis. Intensities greater than the mean+4 s.d. of the contralateral hemisphere were defined as hypoxic. The 4-s.d. threshold was derived empirically by testing different thresholds against manual outlines of areas of increased FMISO retention. The 3-s.d. threshold, as used in human FMISO PET studies (Read et al, 1998), resulted in excessive labelling of the contralateral hemisphere, and the 4-s.d. threshold provided the closest approximation to manual outlines.
To determine the proportion of the tissue showing FMISO retention that was infarcted and vice versa, and to map the spatial distribution of the salvaged FMISO-labelled penumbra, a concurrent sampling methodology was used. Using tissue planes 0.96 mm apart (8 planes per animal), traces of areas of infarction and fiducial markers from previously analysed histologic sections were aligned with the corresponding autoradiographic image using the MCID system. Linked analysis was performed permitting superimposition of macroscopic infarction from histologic sections with the region of increased FMISO retention from the autoradiograph of the same tissue section. The topography of three defined tissue regions could then be outlined and the volumes of each quantified. These volumes were infarcted+[3H]FMISO retention, noninfarcted+[3H]FMISO retention, and infarcted without [3H]FMISO retention. Confirmation of the validity of this sampling restricted to eight tissue planes per animal was performed by comparing the volumes calculated for total [3H]FMISO retention and infarct using this linked analysis with those calculated independently on autoradiographs and histologic sections using 30 tissue planes per animal.
To determine the histologic outcome in the ‘salvaged' [3H]FMISO-retaining tissues, morphologic sampling was performed of [3H]FMISO-retaining, noninfarcted regions. Each eligible image from the linked analysis overlay had three regions of interest placed within the noninfarcted [3H]FMISO-retaining region. The sampling region of interest was sized so that a high-power field centred on the marker would sit just inside the outer edge of the FMISO-retention area. Matching regions just outside the area of [3H]FMISO retention were also marked. The resultant tracing was projected through an Olympus BX-60 microscope (Olympus Corporation, Tokyo, Japan) using a Lucivid MR1-103 cathode ray tube display (MicroBrightField Inc., Williston, VT, USA). The projected images were aligned with the corresponding histologic sections using fiducial markers. As the sampling marks were placed blind to histology, some of the marks outside the area of [3H]FMISO retention were in fact placed within the ventricle. In this case, the next closest point outside the FMISO retention area but within the brain substance was chosen for sampling. Similarly, some of the regions marked for cell counting within the [3H]FMISO-retaining regions contained predominantly white matter with few neuronal cells. Owing to the small numbers of eligible cells, the reliability of scoring in these regions was uncertain. Therefore, analyses excluding the predominantly white matter-containing regions were also performed. A semiquantitative scoring system was used to estimate the degree of cellular injury in noninfarcted tissues exhibiting FMISO retention. Using the × 40 objective lens, necrotic neurons were identified as those with characteristic neuronal morphology exhibiting one or more of the following features: pyknosis, karryorhexis, karryolysis, cytoplasmic eosinophilia, or loss of affinity for haematoxylin (Garcia et al, 1997). Each high-power field identified was scored as 0% necrotic, <10% necrotic (if >90% of neurons within the field had normal or near-normal appearance), 10% to 50%, 50% to 90%, >90% necrotic, or all necrotic (if there were no normal or near-normal neurons within the field).
Statistical Analysis
Statistical analyses were performed using SigmaStat version 2.03 for Windows (Systat Software, San Jose, CA, USA). All parametric data were tested for normality and equal variance before analysis. One-way ANOVA (analysis of variance) was used for multiple group comparisons. Significant results were analysed using an all pairwise multiple comparisons procedure (Bonferroni's t-test) owing to the small numbers of groups compared. Paired t-test was used for comparison of differences in infarct and FMISO-retention volumes within individual experimental cohorts. Pearson's product–moment correlation was used to determine the correlation coefficient of volumetric measurements made using the two different methods. All results are presented as mean±s.e.m.
Results
There were no deaths or exclusions in temporary MCAo animals. In pMCAo animals with delayed administration of FMISO, one animal died 2 hours after surgery, with a large subarachnoid haemorrhage found at postmortem. Oxygen saturations were maintained >92% throughout the course of surgery in all animals.
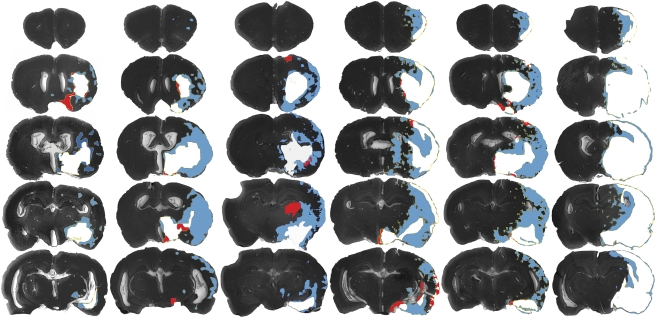
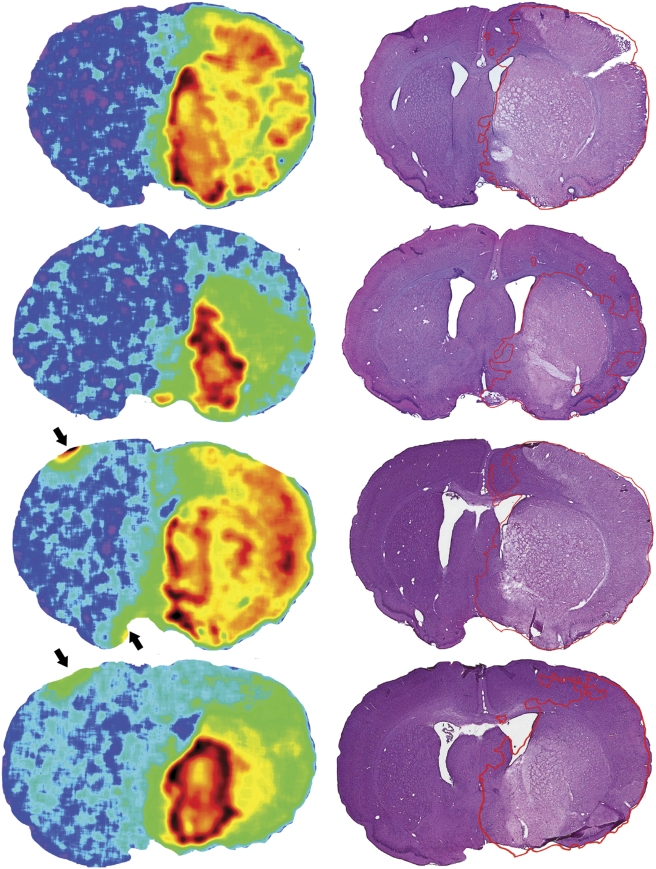
In 2-hour MCAo animals, the mean LDF decreases and increases at vessel occlusion and reperfusion were 78% (range 67% to 84%) of preocclusion baseline and 170% of prereperfusion baseline (range 78% to 313%), respectively. Retention volumes of [3H]FMISO were larger than infarct volumes in all these animals. The pattern of infarction and FMISO labelling for each experimental animal (Figure 1) and individual animal data (Figure 2) are presented. The noninfarcted FMISO-retaining penumbra (blue) was predominantly located lateral and superior to the infarcted regions, and retention was also seen in white-matter tracts (internal capsule and others). The mean volumes of FMISO retention and infarction (from the full 30 sections per animal) were significantly different, ie., 140.1±30.3 mm2 and 77.9±27.3 mm2, respectively (P<0.05). Concurrent sampling (8 sections per animal) showed that the mean proportion of the [3H]FMISO-retention tissue that was also infarcted was 52%±6%. Only 8%±2% of the total infarct volume (4%±1% of the total ischaemic volume, FMISO+infarct) did not show increased [3H]FMISO retention. Validation of FMISO retention and infarct volumes obtained using the concurrent sampling method showed excellent correlation with those obtained from the more extensive analysis of each component separately (r=0.985, P<0.001).
Figure 1.
Pattern of FMISO retention and infarction after 2-hour MCA occlusion with 24-hour survival. The areas of infarction only (red), infarction and [3H]FMISO retention (white), and [3H]FMISO retention without infarction (FMISO-defined penumbra, blue) are superimposed over images of the histologic sections. Coronal sections at five tissue planes are shown for experimental animals 1 to 6 in the corresponding columns, arranged by infarct volume. A concurrent sampling methodology was used to superimpose the areas of infarction and those of FMISO retention from autoradiographs of the same tissue slices. [3H]FMISO, hydrogen-3 fluoromisonidazole; MCA, middle cerebral artery.
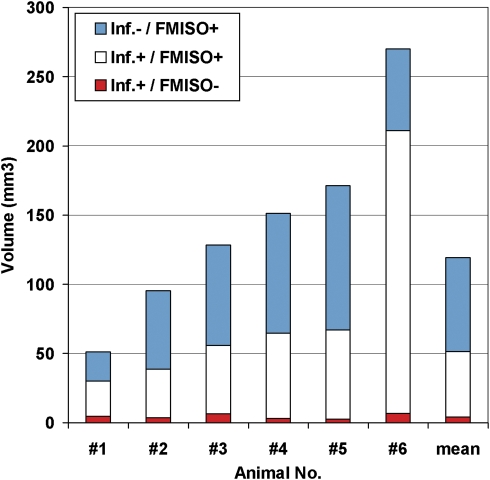
Figure 2.
Volumes of FMISO retention and infarction after 2-hour MCAo with 24-hour survival. Quantification of individual animal tissue volumes from Figure 1 is shown. After 2-hour temporary MCAo, the mean proportion of the [3H]FMISO-retaining area that did not progress to infarction in the ensuing 24 hours was 48% (range 23% to 62%). [3H]FMISO, hydrogen-3 fluoromisonidazole; MCAo, middle cerebral artery occlusion.
Microscopic inspection of the location of macroscopically classified infarcted areas not showing FMISO retention was undertaken (red areas, Figure 1). Contrary to what would be expected if these regions were the necrotic infarct core, they were predominantly peripherally located relative to the infarct, and were most commonly at external or periventricular edges of the histologic sections. Light microscope examination of a sample of these regions from each experimental animal revealed that in almost all, cellular morphology appeared normal or near normal, and that the original assignment to the infarcted region from low-power digitised images appeared to have been made erroneously based only on H&E pallor. The pallor in these regions appeared to be artefactual, related to their location at the edge of the section.
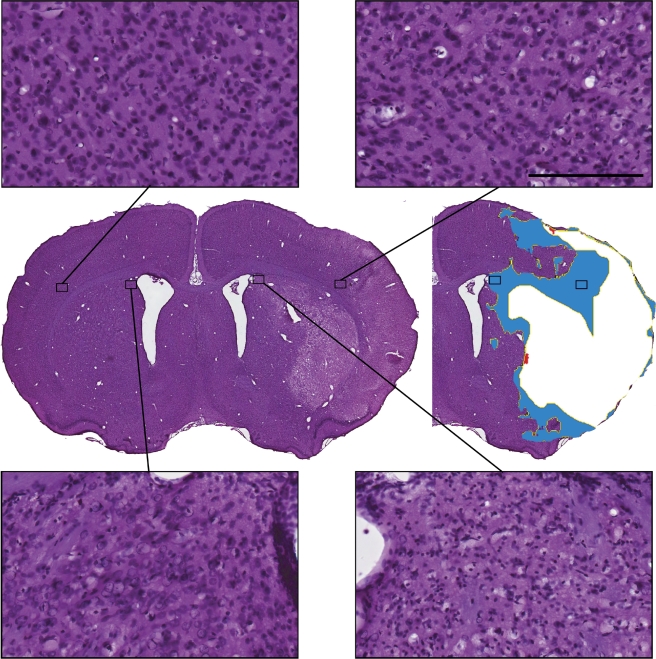
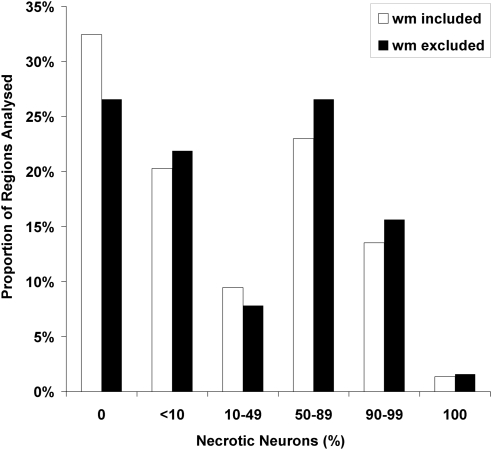
Examination of noninfarcted, [3H]FMISO-retention regions under high-power light microscopy revealed a mixed picture (Figures 3 and 4). In some regions, histology appeared normal or near normal. However, particularly in some regions adjacent to areas of H&E pallor, there was widespread cellular loss. Semiquantitative analysis of cellular morphology in the areas of noninfarcted [3H]FMISO retention was performed on 75 high-power fields from 6 animals (range=7 to 19 regions per animal, dependant on the number of histologic sections with any noninfarcted FMISO-retention region). In all, 11 fields contained predominantly white matter. In the analysis excluding these fields, 27% of regions sampled showed no neuronal necrosis and a further 22% had <10% neuronal loss; however, 45% of sampled high-power fields showed ⩾50% necrotic neurons (Figure 4).
Figure 3.
Cellular outcomes in the FMISO-retaining, noninfarcted tissue (FMISO-defined ischaemic penumbra) after 2-hour MCA occlusion, 22-hour reperfusion: representative morphology within FMISO-retaining regions. A diversity of cellular outcomes is illustrated (contralateral homotypic regions illustrated for comparison). Scale bar=100 μm (HP images). FMISO, fluoromisonidazole; MCA, middle cerebral artery.
Figure 4.
Cellular outcomes in the FMISO-defined ischaemic penumbra—quantification of morphologic outcome ( × 40 objective lens). A total of 6 animals were assessed, yielding 75 regions for analysis. Twenty-two hours after 2-hour ischaemia, the morphologic outcome of most [3H]FMISO-bound tissues showed at least some selective neuronal loss. [3H]FMISO, hydrogen-3 fluoromisonidazole.
Large volumes of [3H]FMISO retention were seen after [3H]FMISO administration at 4, 5, or 6 hours after pMCAo (292±34 mm2). Retention appeared strongest in the infarct core in all animals (Figure 5). A tissue plane at the approximate midpoint of the infarction was chosen for illustrative purposes. The most intense [3H]FMISO retention was seen within the striatum (infarct core) in all animals, irrespective of whether they had large (striatum+cortical) or relatively small (predominantly striatal) infarcts. Although there was a ring-like area of very intense retention in some animals, the slightly less intense central region still exhibited retention levels well above the threshold.
Figure 5.
FMISO retention in the infarct core. The autoradiograph and corresponding histology from a single tissue plane of each of the four animals with [3H]FMISO administered 6 hours after the onset of permanent MCA occlusion are shown. The histologic image has the outline of the FMISO retention superimposed, merely for illustrative purposes, because the threshold used was derived for 2-hour MCA occlusion and may overestimate the penumbra in permanent MCA occlusion (Spratt et al, 2009). Avid striatal retention is seen in all animals, corresponding to the regions of greatest H&E pallor. Arrows represent small areas of artefact caused by overlabelled fiducial marks, used to align histologic and radiologic images. [3H]FMISO, hydrogen-3 fluoromisonidazole; H&E, haematoxylin and eosin; MCA, middle cerebral artery.
Discussion
This study has expanded on findings obtained from human studies that FMISO is retained in ischaemic tissues at risk of infarction after stroke. The distribution of the noninfarcted FMISO-retaining tissue followed a penumbral pattern closely resembling that seen in humans. Selective neuronal injury was quite widespread in these regions, indicating submaximal injury, and suggesting that the injured regions are threatened penumbra, and not merely unthreatened oligaemic areas. However, for the first time, we have also shown consistent FMISO retention within areas that progress to infarction after >6-hour ischaemia. Although this tissue is still alive when exposed to the tracer, it has passed the point at which salvage is credible. This indicates that FMISO does not accurately distinguish the infarct from the penumbra at time points that would likely be relevant to clinical decision making.
Distribution of the FMISO-defined penumbra (Figure 1) was seen to mirror that in human [18F]FMISO PET studies (Markus et al, 2003). The largest volumes were seen in the periphery of the ischaemic MCA territory, lateral and superior to areas of infarction in a ‘penumbral' distribution. It also fits the pattern of infarct expansion seen in studies of the evolution of rat MCA stroke with progressive increases in the duration of vessel occlusion (Garcia et al, 1995; Kaplan et al, 1991; Memezawa et al, 1992). In contrast, the location of small volumes of infarcted, non-[3H]FMISO-retention tissues was peripheral, rather than centrally within the infarct as seen in human PET studies. Microscopic examination of these regions suggested that they were largely regions erroneously marked as infarcted on low-power ( × 1) images of H&E-stained tissue slices, using the technique of manual outlining of area of infarction as is widely used in the literature. A more accurate infarct definition could have been achieved by mapping the infarct under the microscope; however, this was not done to avoid the possibility of unblinding and bias. It also highlights the slight inaccuracy inherent to the very commonly used technique of mapping the area of pallor on low-power histologic images. The observation of [3H]FMISO retention within white-matter tracts confirms results from human studies, suggesting that in contrast to many other putative penumbral imaging techniques, FMISO may be equally adept at detecting hypoxia within the white matter as in the grey matter (Falcao et al, 2004). In the white matter, conventional H&E may significantly underestimate the degree of injury (Dewar et al, 1999; Dietrich et al, 1998; Irving et al, 2001).
Avid striatal uptake of [3H]FMISO was seen in 9 of 9 animals with [3H]FMISO administered 4 to 6 hours after the onset of pMCAo. The consistency of this finding across the spectrum of infarct severities lends weight to the findings. In particular, even in the 2 animals administered [3H]FMISO 6 hours after the onset of permanent ischaemia and whose resultant 24-hour infarct volumes were >200 mm3, avid striatal uptake was observed (animals no. 1 and no. 3, Figure 5). In these animals, this region would be expected to have been profoundly hypoxic soon after the onset of MCAo and progress to irreversible injury very rapidly. In models of rat MCAo, the bulk of the evidence indicates that by 6 hours MCAo, the fate of the tissue is already decided and that subsequent reopening of the MCA leaves the animal with the same infarct volume as measured after permanent occlusion, regardless of whether the assessment is made at 1 or 7 days (Buchan et al, 1992; Kaplan et al, 1991; Memezawa et al, 1992). There was a discrepancy between avid central [3H]FMISO uptake and areas of reduced uptake seen in previous [18F]FMISO studies using autoradiography in rat (Saita et al, 2004), PET in stroke patients (Markus et al, 2003, 2004; Read et al, 2000), and in experimental stroke in rats (Takasawa et al, 2007). In all instances, fundamental differences in design from this study provide the likely explanation for the discrepancy. In the rat [18F]FMISO autoradiographic study, a 2-hour MCAo was used, and central pallor was seen only in postreperfusion cohorts (6 and 24 hours after the onset of 2-hour MCAo). In retrospect, we believe the presence of any retention in these cohorts to have been caused by experimental artefact resulting from the use of poly--lysine-coated occluding threads. These may have caused endothelial injury at the time of reperfusion owing to tissue adherence, and thereby result in ongoing ischaemia (Spratt et al, 2006b). In both human and rat PET studies showing central pallor, [18F]FMISO was administered much later after the onset of ischaemia—at a median of 16.5 hours in the human study (Markus et al, 2004), and 48 hours after MCAo in the rat study, with the aim in the latter of showing the lack of uptake in a completely necrotic core (Takasawa et al, 2007). In addition, owing to the larger volume of the human brain, diffusion limitation (slower and less complete diffusion of FMISO into the very poorly perfused core regions) could have contributed to the central pallor seen in some of the patients in the human studies. In support of the presence of FMISO retention in the infarct core, a previous human [18F]FMISO PET+magnetic resonance imaging study has shown considerable overlap between the area of FMISO retention and diffusion-weighted imaging lesion 18 hours after stroke onset (Takasawa et al, 2008). We believe that the 6-hour time point used in this study is relevant for differentiation of the penumbra and infarct core, and that this should settle the controversy regarding the possibility of retention occurring in the infarct core, comprehensively reviewed by Takasawa et al (2008).
We have not elucidated the mechanism for retention of FMISO in the irreversibly injured tissue. Initially, it may be considered improbable, given that retention of nitroimidazoles such as FMISO is an active process requiring sequential reduction reactions. We hypothesise that the reducing enzymes required for these processes are not degraded for some time after the cell itself reaches the point of irreversible injury.
Previous data have shown that the volume of FMISO retention after 2-hour MCAo closely matches infarction volume after permanent vessel occlusion, implying no significant retention of FMISO in the tissue not at risk of infarction (the oligaemic zone) (Spratt et al, 2009). The current findings expand on this by showing that much of the FMISO-retaining noninfarcted tissue shows evidence of significant cellular injury. This was true even though tissue preparation was performed at 24 hours (to permit sufficient autoradiographic signal), days before selective injury is maximal (Du et al, 1996; Garcia et al, 1997). There is evidence that such selective cellular injury resulting from temporary MCAo does not progress to pannecrosis, even after 7 days, although the uncorrected infarct volume may increase because of the effects of oedema, which peak at ∼72 hours (Garcia et al, 1997; Persson et al, 1989). The finding of significant cellular injury even on the outer margin of the FMISO-retaining tissue indicates that hypoxia of sufficient duration and severity to result in FMISO retention above threshold will normally result in at least a degree of injury of vulnerable cells within such tissues. Similar selective cellular injury has previously been shown in computed tomography-negative regions in humans (Lassen et al, 1983) and in areas with transient, or even permanent, diffusion-weighted imaging renormalisation on magnetic resonance imaging in a rat stroke model (Li et al, 2000). Data obtained from human combined [15O] PET and perfusion-diffusion magnetic resonance imaging studies have shown increased oxygen extraction (another indicator of tissue hypoxia) in such areas of diffusion-perfusion mismatch (magnetic resonance imaging-defined penumbra) at 6 hours after stroke (Shimosegawa et al, 2005). Peri-infarct selective cellular loss has been shown to exhibit features of apoptosis by both light- and electron-microscopy criteria (Li et al, 1995). These and our study indicate that there may be a significant degree of neuronal injury in the ‘salvaged' penumbra. This supports previous data showing that macroscopic tissue integrity does not always equate with preservation of neurons, and may even compromise neurologic recovery—as reviewed by Baron (2005).
There were some limitations to this study. Haematoxylin and eosin staining was used as the classical light microscopy method to identify ischaemic cell death, but does not provide definitive evidence that dead cells were all neuronal. We did not attempt to show an association between selective cellular loss and functional outcome, given the current sensitivity of rat behavioural test batteries after stroke and the variability inherent in animal stroke models, showing that such an association would require large animal numbers. We cannot show that all noninfarcted FMISO-retaining regions were destined to death in the absence of reperfusion at 2 hours. Similarly, we are unable to prove that in pMCAo models, none of the central, avidly FMISO-retaining regions were potentially salvageable at >4 to 6 hours—this relies on our own and published experience of this model suggesting that 100% tissue salvage is not possible at such a late time point, and might be said to be a ‘common-sense' test. Similarly, we cannot rule out a degree of hypoxaemia during the period of FMISO retention, because it was administered to awake animals 4 to 6 hours after MCAo, and we were unable to monitor oxygen saturations in free-moving animals. However, no increased FMISO retention was seen outside the ischaemic MCA territory in any animal (e.g., contralateral watershed territory) to suggest that this may have occurred.
In conclusion, these data from experimental stroke in Sprague–Dawley rats support the concept that FMISO is retained in the potentially salvageable ischaemic penumbra soon after stroke. Detailed histologic analysis indicates that there may be significant neuronal injury in salvaged hypoxic tissues, despite preservation of tissue integrity. There was little FMISO retention in the unthreatened tissue with only mildly reduced perfusion (oligaemia). However, there was avid FMISO retention in the infarct core even >6 hours after the onset of MCAo. This indicates that this tracer does not reliably distinguish between the core and the penumbra at clinically relevant time points.
Acknowledgments
Dr Spratt was supported for this work by a NHMRC Postgraduate Scholarship and by a Hunter Medical Research Institute/Greater Building Society Stroke Research Fellowship.
The authors declare no conflict of interest.
Footnotes
The work was funded by a Cardiovascular Lipid Research Grant (Pfizer).
References
- Baron JC. How healthy is the acutely reperfused ischemic penumbra. Cerebrovasc Dis. 2005;20 (Suppl 2:25–31. doi: 10.1159/000089354. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Xue D, Slivka A. A new model of temporary focal neocortical ischemia in the rat. Stroke. 1992;23:273–279. doi: 10.1161/01.str.23.2.273. [DOI] [PubMed] [Google Scholar]
- Dewar D, Yam P, McCulloch J. Drug development for stroke: importance of protecting cerebral white matter. Eur J Pharmacol. 1999;375:41–50. doi: 10.1016/s0014-2999(99)00280-0. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Kraydieh S, Prado R, Stagliano NE. White matter alterations following thromboembolic stroke: a beta-amyloid precursor protein immunocytochemical study in rats. Acta Neuropathol. 1998;95:524–531. doi: 10.1007/s004010050833. [DOI] [PubMed] [Google Scholar]
- Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis. J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Falcao AL, Reutens DC, Markus R, Koga M, Read SJ, Tochon-Danguy H, Sachinidis J, Howells DW, Donnan GA. The resistance to ischemia of white and gray matter after stroke. Ann Neurol. 2004;56:695–701. doi: 10.1002/ana.20265. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu K-F, Ye Z-R, Gutierrez JA. Incomplete infarct and delayed neuronal death after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2303–2309. doi: 10.1161/01.str.28.11.2303. [DOI] [PubMed] [Google Scholar]
- Giffard C, Landeau B, Kerrouche N, Young AR, Barre L, Baron JC. Decreased chronic-stage cortical 11C-flumazenil binding after focal ischemia-reperfusion in baboons: a marker of selective neuronal loss. Stroke. 2008;39:991–999. doi: 10.1161/STROKEAHA.107.489419. [DOI] [PubMed] [Google Scholar]
- Guadagno JV, Jones PS, Aigbirhio FI, Wang D, Fryer TD, Day DJ, Antoun N, Nimmo-Smith I, Warburton EA, Baron JC. Selective neuronal loss in rescued penumbra relates to initial hypoperfusion. Brain. 2008;131:2666–2678. doi: 10.1093/brain/awn175. [DOI] [PubMed] [Google Scholar]
- Hughes JL, Beech JS, Jones PS, Wang D, Menon DK, Baron JC. Mapping selective neuronal loss and microglial activation in the salvaged neocortical penumbra in the rat. NeuroImage. 2009;49:19–31. doi: 10.1016/j.neuroimage.2009.08.047. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bentley DL, Parsons AA. Assessment of white matter injury following prolonged focal cerebral ischaemia in the rat. Acta Neuropathol (Berl) 2001;102:627–635. doi: 10.1007/s004010100416. [DOI] [PubMed] [Google Scholar]
- Jones TH, Morawetz RB, Crowell RM, Marcoux FW, FitzGibbon SJ, DeGirolami U, Ojemann RG. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54:773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- Kaplan B, Brint S, Tanabe J, Jacewicz M, Wang XJ, Pulsinelli W. Temporal thresholds for neocortical infarction in rats subjected to reversible focal cerebral ischemia. Stroke. 1991;22:1032–1039. doi: 10.1161/01.str.22.8.1032. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Losen TS, Højgaard K, Skriver E. Incomplete infarction: a CT-negative irreversible ischemic brain lesion. J Cereb Blood Flow Metab. 1983;3:S602–S603. [Google Scholar]
- Li F, Liu K-F, Silva M, Omae T, Sotak CH, Fenstermacher JD, Fisher M. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats. Correlation with histopathology. Stroke. 2000;31:946–953. doi: 10.1161/01.str.31.4.946. [DOI] [PubMed] [Google Scholar]
- Li Y, Sharov VG, Jiang N, Zaloga C, Sabbah HN, Chopp M. Ultrastructural and light microscopic evidence of apoptosis after middle cerebral artery occlusion in the rat. Am J Pathol. 1995;146:1045–1051. [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Wong JY, Krenus D, Jeffreys BJ, Porritt MJ, Howells DW. Tissue fixation prevents contamination of tritium-sensitive storage phosphor imaging plates. Biotechniques. 1999;26:432–434. doi: 10.2144/99263bm13. [DOI] [PubMed] [Google Scholar]
- Marcoux FW, Morawetz RB, Crowell RM, DeGirolami U, Halsey JH., Jr Differential regional vulnerability in transient focal cerebral ischemia. Stroke. 1982;13:339–346. doi: 10.1161/01.str.13.3.339. [DOI] [PubMed] [Google Scholar]
- Markus R, Reutens DC, Kazui S, Read S, Wright P, Chambers BR, Sachinidis JI, Tochon-Danguy HJ, Donnan GA. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke. 2003;34:2646–2652. doi: 10.1161/01.STR.0000094422.74023.FF. [DOI] [PubMed] [Google Scholar]
- Markus R, Reutens DC, Kazui S, Read S, Wright P, Pearce DC, Tochon-Danguy HJ, Sachinidis JI, Donnan GA. Hypoxic tissue in ischaemic stroke: persistence and clinical consequences of spontaneous survival. Brain. 2004;127:1427–1436. doi: 10.1093/brain/awh162. [DOI] [PubMed] [Google Scholar]
- Memezawa H, Smith ML, Siesjo BK. Penumbral tissues salvaged by reperfusion following middle cerebral artery occlusion in rats. Stroke. 1992;23:552–559. doi: 10.1161/01.str.23.4.552. [DOI] [PubMed] [Google Scholar]
- Nakagawara J, Sperling B, Lassen NA. Incomplete brain infarction of reperfused cortex may be quantitated with iomazenil. Stroke. 1997;28:124–132. doi: 10.1161/01.str.28.1.124. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Persson L, Hardemark HG, Bolander HG, Hillered L, Olsson Y. Neurologic and neuropathologic outcome after middle cerebral artery occlusion in rats. Stroke. 1989;20:641–645. doi: 10.1161/01.str.20.5.641. [DOI] [PubMed] [Google Scholar]
- Read SJ, Hirano T, Abbott DF, Sachinidis JI, Tochon-Danguy HJ, Chan JG, Egan GF, Scott AM, Bladin CF, McKay WJ, Donnan GA. Identifying hypoxic tissue after acute ischemic stroke using PET and 18F-fluoromisonidazole. Neurology. 1998;51:1617–1621. doi: 10.1212/wnl.51.6.1617. [DOI] [PubMed] [Google Scholar]
- Read SJ, Hirano T, Abbott DF, Markus R, Sachinidis JI, Tochon-Danguy HJ, Chan JG, Egan GF, Scott AM, Bladin CF, McKay WJ, Donnan GA. The fate of hypoxic tissue on 18F-fluoromisonidazole positron emission tomography after ischemic stroke. Ann Neurol. 2000;48:228–235. [PubMed] [Google Scholar]
- Saita K, Chen M, Spratt NJ, Porritt MJ, Liberatore GT, Read SJ, Levi CR, Donnan GA, Ackermann U, Tochon-Danguy HJ, Sachinidis JI, Howells DW. Imaging the ischemic penumbra with 18F-fluoromisonidazole in a rat model of ischemic stroke. Stroke. 2004;35:975–980. doi: 10.1161/01.STR.0000121647.01941.ba. [DOI] [PubMed] [Google Scholar]
- Saur D, Buchert R, Knab R, Weiller C, Rother J. Iomazenil-single-photon emission computed tomography reveals selective neuronal loss in magnetic resonance-defined mismatch areas. Stroke. 2006;37:2713–2719. doi: 10.1161/01.STR.0000244827.36393.8f. [DOI] [PubMed] [Google Scholar]
- Shimosegawa E, Hatazawa J, Ibaraki M, Toyoshima H, Suzuki A. Metabolic penumbra of acute brain infarction: a correlation with infarct growth. Ann Neurol. 2005;57:495–504. doi: 10.1002/ana.20427. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Ackerman U, Tochon-Danguy HJ, Donnan GA, Howells DW. Characterization of fluoromisonidazole binding in stroke. Stroke. 2006a;37:1862–1867. doi: 10.1161/01.STR.0000226908.93295.9d. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, Hogan L, Howells DW. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006b;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Donnan GA, Howells DW. Characterisation of the timing of binding of the hypoxia tracer FMISO after stroke. Brain Res. 2009;1288:135–142. doi: 10.1016/j.brainres.2009.06.102. [DOI] [PubMed] [Google Scholar]
- Takasawa M, Beech JS, Fryer TD, Hong YT, Hughes JL, Igase K, Jones PS, Smith R, Aigbirhio FI, Menon DK, Clark JC, Baron JC. Imaging of brain hypoxia in permanent and temporary middle cerebral artery occlusion in the rat using 18F-fluoromisonidazole and positron emission tomography: a pilot study. J Cereb Blood Flow Metab. 2007;27:679–689. doi: 10.1038/sj.jcbfm.9600405. [DOI] [PubMed] [Google Scholar]
- Takasawa M, Moustafa RR, Baron J-C. Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke. 2008;39:1629–1637. doi: 10.1161/STROKEAHA.107.485938. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–3074. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- Weiller C, Willmes K, Reiche W, Thron A, Isensee C, Buell U, Ringelstein E. The case of aphasia or neglect after striatocapsular infarction. Brain. 1993;116:1509–1525. doi: 10.1093/brain/116.6.1509. [DOI] [PubMed] [Google Scholar]