Abstract
Cerebrovascular endothelial cells (CECs) are crucial components of the blood–brain barrier. Our previous study showed that oxidized low-density lipoprotein (oxLDL) induces apoptosis of CECs. This study was designed to further evaluate the effects of resveratrol on oxLDL-induced CEC insults and its possible molecular mechanisms. Resveratrol decreased the oxidation of LDL into oxLDL. Additionally, the oxLDL-caused oxidative stress and cell damage were attenuated by resveratrol. Exposure of CECs to oxLDL induced cell shrinkage, DNA fragmentation, and cell apoptosis, but resveratrol defended against such injuries. Application of Lox-1 small interference (si)RNA into CECs reduced the translation of this membrane receptor, and simultaneously increased resveratrol protection from oxLDL-induced cell apoptosis. By comparison, overexpression of Lox-1 attenuated resveratrol protection. Resveratrol inhibited oxLDL-induced Lox-1 mRNA and protein expressions. Both resveratrol and Lox-1 siRNA decreased oxLDL-enhanced translocation of proapoptotic Bcl-2-associated X protein (Bax) from the cytoplasm to mitochondria. Sequentially, oxLDL-induced alterations in the mitochondrial membrane potential, cytochrome c release, and activities of caspases-9, -3, and -6 were decreased by resveratrol. Pretreatment with Z-VEID-FMK (benzyloxycarbonyl-Leu-Glu-His-Asp-fluoromethyl ketone) synergistically promoted resveratrol's protection against DNA fragmentation and cell apoptosis. Therefore, this study shows that resveratrol can protect CECs from oxLDL-induced apoptotic insults via downregulating Lox-1-mediated activation of the Bax-mitochondria–cytochrome c–caspase protease pathway.
Keywords: antiapoptotic events, cerebrovascular endothelial cells, Lox-1, oxidized low-density lipoprotein, resveratrol
Introduction
Resveratrol (3,4′,5-trihydroxystilbene), a polyhydroxyphenolic antioxidant, is synthesized by various plant species including grapes (Pervaiz, 2003). Resveratrol has gained considerable attention because of its potential cancer chemopreventive and anticancer properties (Baur and Sinclair, 2006). In addition, resveratrol has the potential to control atherosclerosis, heart disease, arthritis, and autoimmune disorders (Yoshida et al, 2007). These numerous biological activities may explain resveratrol's antiinflammatory, anticarcinogenic, and anticancer characteristics (Pervaiz, 2003). During the processes of acute and chronic inflammation, there is a wide variety of oxidative mediators that participate in the physiological and pathophysiological regulation of tissue/cell functions (El Bekay et al, 2003). Reactive oxygen species (ROS), including superoxide, peroxynitrite, hydroxyl anions, and hydrogen peroxide, are representative factors. The ROS can regulate cardiovascular diseases such as hypertension, as well as hyperlipidemia and diabetes (El Bekay et al, 2003). Thus, reducing levels of ROS and their toxic effects may promote the prevention and treatment of cardiovascular diseases. Resveratrol is valuable in the chemoprevention of the development of heart disease (Lin and Tsai, 1999).
Plasma low-density lipoprotein (LDL) is readily transformed into oxidized (ox)LDL at the moieties of apolipoprotein B or lipids, which are either catalyzed by divalent cations, such as Cu2+ or Fe2+, or promoted by incubation with endothelial cells, smooth muscle cells, and monocytes (Matsuura et al, 2008). A body of evidence shows that oxLDL is one of the earliest events in atherosclerosis via causing a process of binding to macrophage scavenger receptors to form lipid-laden foam cells (Sneck et al, 2005; Matsuura et al, 2008). In addition, high levels of oxLDL can induce pathophysiological conditions in various tissues and cells. In ventricular myocytes, oxLDL is reported to induce cell damage and cause irregular electrical activity (Zorn-Pauly et al, 2005). Zettler et al (2004) showed that oxLDL inhibits nuclear translocation of cell cycle proteins and retards the growth of proliferating vascular smooth muscle cells. The oxLDL was also implicated as having neurotoxicity because of its effects on decreasing neuronal viability due to stimulation of Ca2+-dependent activations of extracellular signal-regulated kinases 1/2 and c-Jun N-terminal kinase (Schroeter et al, 2001). Lectin-like oxLDL receptor-1 (Lox-1) is a scavenger receptor that primarily binds to and regulates oxLDL (Mehta and Li, 2002). Oxidative stress is a crucial factor that mediates oxLDL-caused atherothrombotic events, including endothelial cell lining defects, necrotic core formation, and plaque rupture or erosion (Chen et al, 2007).
The blood–brain barrier (BBB), an important barricade situated in cerebral capillaries, maintains the homeostasis of the cerebral microenvironment (Abbott, 2002; Engelhardt, 2003). Cerebrovascular endothelial cells (CECs) are vital components of the BBB; they form complex tight junctions to force most molecular traffic to take a transcellular route across the barrier (Rubin, 1992; Abbott, 2002). However, a variety of intrinsic and extrinsic factors or disease conditions may affect the physiology and pathophysiology of CECs. A previous study reported that glutamate at excitotoxic levels decreases the integrity of the human cerebral endothelial barrier, causing the BBB to break down (Sharp et al, 2003). Lipopolysaccharide, an endotoxin, can induce injuries to bovine cerebral endothelial cell layers (de Vries et al, 1996). In ischemia, CECs are involved in the processes that alter the BBB functions and induce brain damage (Betz, 1996). Our previous study further showed that oxLDL can induce CEC apoptosis via a mitochondrion-dependent mechanism (Chen et al, 2007). Data from those previous studies reveal that dysfunction of CECs can influence the BBB's permeability and lead to brain damage (Abbott, 2002). Thus, determining how to protect CECs from insults is a critical issue in preventing and treating cerebrovascular diseases.
The oxLDL can pass through the BBB and exists in brain tissues where it participates in neurodegeneration associated with oxidative stress-related cerebral diseases (Kim et al, 2006) oxLDL can also be an effector for death regulation (Kashiwakura et al, 2004; Chen et al, 2007). Apoptosis is an energy-dependent type of programmed cell death (Goyal, 2001). There are many intrinsic and extrinsic factors involved in the regulation of cell apoptosis (Goyal, 2001; Chen et al, 2002). Bax, a proapoptotic protein, functions as an essential gateway, which mediates mitochondrion-dependent apoptosis (Pagliari et al, 2005; Lee et al, 2009). Bax translocated from the cytoplasm to mitochondria can insert itself into the outer mitochondrial membrane, permeabilizing the membrane and triggering the release of apoptotic factors such as cytochrome c and ROS (Pagliari et al, 2005). Cytochrome c can trigger cascade activation of caspases-9, -3, and -6, which leads to the cleavage of key cellular proteins, for example lamin and nuclear mitotic apparatus proteins, which consequently damages genomic DNA (Rao et al, 1996). Resveratrol can ameliorate atherosclerosis, heart disease, arthritis, and autoimmune disorders because of its chemopreventive characteristics (Yoshida et al, 2007). Thus, this study was aimed to evaluate the effects of resveratrol on oxLDL-induced insults to CECs and its possible molecular mechanisms.
Materials and methods
Isolation and Culture of Mouse Cerebrovascular Endothelial Cells
Mouse CECs were prepared from cerebral microvessels according to a previously described method (Xu et al, 1998). This investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996), and all procedures were preapproved by the Institutional Animal Care and Use Committee of Taipei Medical University, Taipei, Taiwan. Briefly, after the cerebral cortex was homogenized and filtered, the filtrate was digested with collagenase B and then centrifuged in a 40% Percoll solution. The second band containing microvessels was collected and plated onto collagen-coated dishes. Mouse CECs were seeded in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, -glutamine, penicillin (100 IU/mL), and streptomycin (100 μg/mL) in 75 cm2 flasks at 37°C in a humidified atmosphere of 5% CO2. To verify whether the isolated brain cells were CECs in our preparation, immunocytochemical analyses of vimentin and factor VIII were performed following the standard protocol provided by the VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA, USA) as described previously (Ho et al, 2009). Cells were grown to confluence before drug treatment. Only the first 10 passages of mouse CECs were used in this study.
Preparation of Low-Density Lipoprotein and Oxidized Low-Density Lipoprotein
The LDL and oxLDL were prepared according to a previously described method (Li et al, 2003). The protocol of this study was approved by the Institutional Review Board of Taipei Medical University-Municipal Wan-Fang Hospital (approval no.: 96039), and all participants provided written informed consent. Briefly, LDL was purified from human whole blood following a process of centrifugation, reactions with sodium bromide and N,N,N′,N′-ethylenediaminetetraacetic acid (EDTA), and dialysis against phosphate-buffered saline (PBS; 0.14 mol/L NaCl, 2.6 mmol/L KCl, 8 mmol/L Na2HPO4, and 1.5 mmol/L KH2PO4). The oxLDL was prepared by reacting purified LDL with copper sulfate at 37°C for 24 hours. To remove the copper ions, the oxLDL product (1 mL) was extensively dialyzed against PBS (3 L) for 3 hours. The amounts of malondialdehyde were measured to evaluate levels of oxLDL according to the method of Liu et al (1996).
Quantification of Cellular Reactive Oxygen Species
Levels of cellular ROS were quantified according to a previously described method (Chang et al, 2010). Briefly, 5 × 105 mouse CECs were cultured in 12-well tissue culture plates overnight, and then cotreated with drugs and 2′,7′-dichlorofluorescin diacetate, an ROS-sensitive dye. After drug treatment, mouse CECs were harvested and suspended in × 1 PBS buffer. Relative fluorescence intensities of cells were quantified using a flow cytometer (FACS Calibur, Becton Dickinson, San Jose, CA, USA).
Cytotoxicity Assay
A viability assay was performed using a trypan blue exclusion method described previously (Ho et al, 2009). Briefly, mouse CECs (2 × 105 cells) were cultured in 24-well tissue culture plates (Corning-Costar, Cambridge, MA, USA). After drug administration, cells were trypsinized with 0.1% trypsin-EDTA (Gibco-BRL, Grand Island, NY, USA). Following centrifugation and washing, mouse CECs were suspended in PBS and stained with trypan blue dye (Sigma, St Louis, MO, USA). Fractions of dead cells with a blue signal were visualized and counted using a reverse-phase microscope (Nikon, Tokyo, Japan).
DNA Ladder Analysis
Genomic DNA from control and drug-treated mouse CECs were analyzed by a classical DNA electrophoresis method to determine DNA ladders (Chen et al, 2002). After drug treatment, CECs were harvested and lysed in 0.05 mL lysis buffer (5% sarcosyl, 10 mmol/L Tris–Cl, 10 mmol/L EDTA, and 20 units of proteinase K) at 50°C overnight. The lysates were treated with 10 μg DNase-free RNase for 1 hour, and then extracted with 0.2 mL phenol–chloroform (1:1) solution several times. The water layer was electrophoretically separated in a 1.2% agarose gel containing 0.1 μg/mL ethidium bromide. The DNA bands were visualized and photographed under UV-light exposure.
Quantification of DNA Fragmentation
DNA fragmentation in mouse CECs was quantified following a previously described method (Wu et al, 2007). The 5-bromo-2-deoxyuridine-labeled histone-associated DNA fragments in the cytoplasm of cell lysates were detected according to the instructions of the cellular DNA fragmentation enzyme-linked immunosorbent assay kit (Boehringer Mannheim, Indianapolis, IN, USA). Briefly, mouse CECs (2 × 105) were subcultured in 24-well tissue culture plates and labeled with 5-bromo-2-deoxyuridine overnight. Cells were harvested and suspended in the culture medium. A measure of 100 μL of cell suspension was added to each well of 96-well tissue culture plates. The CECs were cocultured with oxLDL for another 8 hours at 37°C in a humidified atmosphere of 5% CO2. Amounts of 5-bromo-2-deoxyuridine-labeled DNA in the cytoplasm were quantified using an Anthos 2010 microplate photometer (Anthos Labtec Instruments, Lagerhausstrasse, Wals/Salzburg, Austria) at a wavelength of 450 nm.
Analysis of Apoptotic Cells
Apoptotic cells were determined according to the method of Tai et al (2007). After drug treatment, mouse CECs were harvested and fixed in cold 80% ethanol. Following a process of centrifugation and washing, fixed cells were stained with propidium iodide and analyzed using a FACScan flow cytometer (Becton Dickinson) on the basis of a 560-nm dichromic mirror and a 600-nm bandpass filter.
Lox-1 Knockdown
Translation of Lox-1 messenger (m)RNA in mouse CECs was knocked down using an RNA interference (RNAi) method following a small interfering (si)RNA transfection protocol provided by Santa Cruz Biotechnology (Santa Cruz, CA, USA) (Wu et al, 2009). Lox-1 siRNA was purchased from Santa Cruz Biotechnology, and is a pool of three target-specific 20–25-nt siRNAs designed to knockdown Lox-1's expression. Briefly, after culturing mouse CECs in antibiotic-free Dulbecco's modified Eagle's medium at 37°C in a humidified atmosphere of 5% CO2 for 24 hours, the siRNA duplex solution was added to mouse endothelial cells. After being transfected for 24 hours, the medium was replaced with normal Dulbecco's modified Eagle's medium, and mouse CECs were treated with resveratrol, oxLDL, or a combination of resveratrol and oxLDL.
Expression of Lox-1 Full-Length Complementary (c)DNA
pCMV6-XL5-Lox-1 plasmids, which were constructed with full-length human Lox-1 cDNA, were purchased from Origene Technologies (Rockville, MD, USA). pCMV6-XL5-Lox-1 plasmids were transfected into mouse CECs using a FuGene 6 transfection reagent (Roche Diagnostics, Mannheim, Germany) as previously described (Cherng et al, 2008). Empty vectors were transfected as the controls. After transfection for 48 hours, CECs were exposed to resveratrol and oxLDL. Then, cells were harvested for an apoptotic analysis.
Immunoblot Analyses for Lox-1, Bax, Cytochrome c, and β-Actin
Protein analyses were performed according to a previously described method (Chen et al, 2005). After drug treatment, cell lysates were prepared in ice-cold radioimmunoprecipitation assay buffer (25 mmol/L Tris–HCl (pH 7.2), 0.1% sodium dodecylsulfate, 1% Triton X-100, 1% sodium deoxycholate, 0.15 mol/L NaCl, and 1 mmol/L EDTA). Protein concentrations were quantified using a bicinchonic acid protein assay kit (Pierce, Rockford, IL, USA). Proteins (50 μg/well) were subjected to sodium dodecylsulfate–polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. After blocking, Lox-1 was immunodetected using a goat monoclonal antibody against mouse Lox-1 (Santa Cruz Biotechnology). Cytochrome c was immunodetected using a mouse monoclonal antibody against the human cytochrome c protein (Transduction Laboratories, Lexington, KY, USA). Cellular β-actin protein was immunodetected using a mouse monoclonal antibody against mouse β-actin (Sigma) as the internal standard. These protein bands were quantified with the aid of the UVIDOCMW vers. 99.03 digital imaging system (Uvtec, Cambridge, UK).
Real-time Polymerase Chain Reaction Assay
mRNA from mouse CECs exposed to resveratrol, oxLDL, or a combination of resveratrol and oxLDL were prepared for real-time polymerase chain reaction (PCR) analyses of Lox-1 and β-actin mRNA. The oligonucleotides for the PCR analyses of Lox-1 and β-actin mRNA were designed and synthesized by Clontech Laboratories (Palo Alto, CA, USA). The oligonucleotide sequences for these mRNA analyses were 5′-TCTTAGCATGAATTTGGAAAT-3′ and 5′-CCCAGCTAAAGGGCCCATGG-3′ for Lox-1, and 5′-GTGGGCCGCTCTAGGCACCAA-3′ and 5′-CTCTTTGATGTCACGCACGATTTC-3′ for β-actin (Chen et al, 2009). A real-time PCR analysis was performed using iQSYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad).
Confocal Microscopic Analysis of Bax Translocation
Bax protein in mouse CECs was recognized by a specific antibody, and visualized using confocal microscopy following a previously described method (Tai et al, 2007). Briefly, after oxLDL administration, mouse CECs were fixed with a fixing reagent (acetone:methanol, 1:1) at −20°C for 10 minutes. After rehydrating with 1 × PBS at room temperature for 15 minutes, the cells were incubated with 0.2% Triton X-100 at room temperature for 15 minutes. The mouse monoclonal antibody used in this study was generated against human Bax-α (Santa Cruz Biotechnology). This antibody can detect Bax protein in whole cells, including the cytoplasm and mitochondria. Bax was immunodetected at 4°C overnight. After washing, cells were sequentially reacted with second antibodies and biotin-SP-conjugated AffiniPure goat anti-rabbit immunoglobulin G (IgG; Jackson ImmunoResearch, West Grove, PA, USA) at room temperature for 1 hour. After washing, the third antibody with Cy3-conjugated streptavidin (Jackson ImmunoResearch) was added to mouse CECs and reacted at room temperature for 30 minutes. Mitochondria of mouse CECs were stained with 3,3′-dihexyloxacarbocyanine (DiOC6) (Molecular Probes, Eugene, OR, USA), a cell-permeant, positively charged, green fluorescent, and lipophilic dye, at 37°C for 30 minutes. A confocal laser scanning microscope (Model FV500, Olympus, Tokyo, Japan) was used for sample observation. Illumination for the existence of Bax protein was demonstrated by the appearance of ‘hot spots' in both the cytoplasm and membranes. Images were acquired and quantified using FLUOVIEW software (Olympus). The increased densities of hot spots were analyzed by automated recordings within the same region in a cell.
Quantification of the Mitochondrial Membrane Potential
The membrane potential of mitochondria in mouse CECs was determined according to a previously described method (Chang et al, 2006). Briefly, mouse CECs (5 × 105 cells) were seeded in 12-well tissue culture plates overnight. After drug administration, cells were harvested and incubated with DiOC6, at 37°C for 30 minutes in a humidified atmosphere of 5% CO2. After washing and centrifugation, the cell pellets were suspended in 1 × PBS buffer. The fluorescent intensities of mouse CECs were analyzed by flow cytometry (Becton Dickinson).
Fluorogenic Substrate Assay for Caspase Activities
Activities of caspases-3, -6, and -9 in mouse CECs were determined using fluorometric assay kits (R&D Systems, Minneapolis, MN, USA) as previously described (Lee et al, 2009). Briefly, after oxLDL administration, CECs were lysed using a buffer containing 1% Nonidet P-40, 200 mmol/L NaCl, 20 mmol/L Tris/HCl (pH 7.4), 10 μg/mL leupeptin, 0.27 U/mL aprotinin, and 100 μm phenylmethylsulfonyl fluoride. Cell extracts (25 μg total protein) were incubated with 50 μmol/L of specific fluorogenic peptide substrates in 200 μL of a cell-free system buffer comprising 10 mmol/L Hepes (pH 7.4), 220 mmol/L mannitol, 68 mmol/L sucrose, 2 mmol/L NaCl, 2.5 mmol/L KH2PO4, 0.5 mmol/L EGTA, 2 mmol/L MgCl2, 5 mmol/L pyruvate, 0.1 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L dithiothreitol. The peptide substrates for the assays of caspase-3, -6, and -9 activities were DEVD (Asp-Glu-Val-Asp), VEID (Val-Glu-Ile-Asp), and LEHD (Leu-Glu-His-Asp), respectively. These peptides were conjugated to 7-amino-4-trifluoromethyl coumarin for fluorescence detection. Intensities of the fluorescent products were measured using an LS 55 spectrometer of PerkinElmer Instruments (Shelton, CT, USA). In the inhibition study, mouse CECs were pretreated with 50 μmol/L of Z-VEID-FMK (benzyloxycarbonyl-Leu-Glu-His-Asp-fluoromethyl ketone), an inhibitor of caspase-6, for 1 hour, and then exposed to oxLDL for another 12 hours.
Statistical Analysis
The statistical significance of differences between the control and drug-treated groups was evaluated using Student's t-test, and differences were considered statistically significant at P values of <0.05. Differences between resveratrol- and oxLDL-treated mouse CECs were considered significant when the P value of Duncan's multiple-range test was <0.05. Statistical analysis between groups over time or concentrations was performed by a two-way analysis of variance.
Results
Toxicity of Resveratrol to Mouse Cerebrovascular Endothelial Cells
Treatment of mouse CECs with 0. 5, 1, 5, and 10 μmol/L resveratrol for 24, 48, and 72 hours did not affect cell viability (data not shown). Meanwhile, after exposure for 72 hours, resveratrol at 25 μmol/L caused a significant 30% decrease in cell viability. When the treated concentration reached 50 μmol/L, administration of resveratrol for 48 and 72 hours, respectively, decreased the viability of mouse CECs by 44% and 59% (data not shown).
Resveratrol Suppresses Low-Density Lipoprotein Oxidation and Reactive Oxygen Species Production
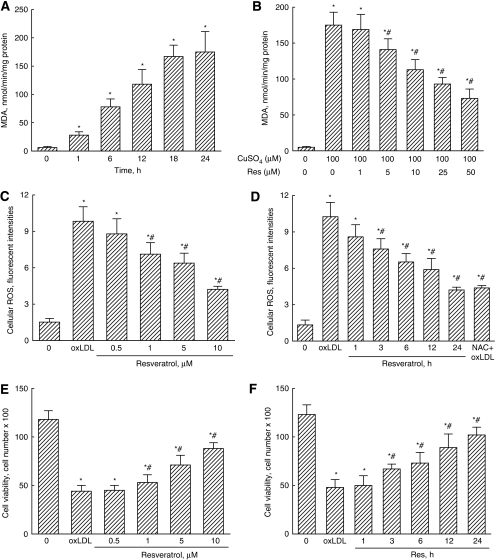
When LDL was reacted with copper sulfate for 1, 6, 12, 18, and 24 hours, amounts of malondialdehyde significantly increased by 5-, 13-, 20-, 28-, and 29-fold, respectively (Figure 1A). Resveratrol at 1 μmol/L did not affect the oxidation of LDL by copper sulfate (Figure 1B). Meanwhile, when the concentrations reached 5, 10, 25, and 50 μmol/L, resveratrol decreased copper sulfate-caused oxidation of LDL by 19%, 35%, 47%, and 58%, respectively. Exposure of mouse CECs to oxLDL significantly increased cellular ROS by sevenfold (Figure 1C). Meanwhile, the oxLDL-caused enhancements of cellular ROS significantly decreased by 28%, 35%, and 57% following exposure to 1, 5, and 10 μmol/L resveratrol for 24 hours, respectively. Treatment of CECs with 10 μmol/L resveratrol for 3, 6, 12, and 24 hours caused significant 24%, 35%, 41%, and 60% decreases in oxLDL-induced cellular ROS, respectively (Figure 1D). Pretreatment of CECs with N-acetylcystein, an antioxidant, caused a significant 58% decrease in oxLDL-induced cellular ROS (Figure 1D).
Figure 1.
Suppressive effects of resveratrol (Res) on low-density lipoprotein (LDL) oxidation, cellular reactive oxygen species (ROS) production, and cell death. The LDL was reacted with copper sulfate (CuSO4), and the amounts of malondialdehyde (MDA) were measured to evaluate the levels of oxidized (ox)LDL (A). Effects of Res on LDL oxidation to oxLDL were analyzed (B). Mouse cerebrovascular endothelial cells (CECs) were exposed to 100 μg/mL oxLDL and a combination of oxLDL and 0.5, 1, 5, and 10 μmol/L Res for 24 hours (C), or to 100 μg/mL oxLDL and a combination of oxLDL and 10 μmol/L Res for 1, 3, 6, 12, and 24 hours (D). N-acetylcystein (NAC) was pretreated to the cells as the positive control (D). Levels of cellular ROS were quantified using flow cytometry. Cell viability was assayed using a trypan blue exclusion method (E, F). Each value represents the mean±s.e.m. for n=6. Symbols * and #, respectively, indicate that the values significantly differ from control and oxLDL-treated groups, P<0.05. C, control.
Treatment of mouse CECs with 200 μg/mL oxLDL for 24 hours decreased cell viability by 73% (Figure 1E). However, resveratrol at 1, 5, and 10 μmol/L significantly lowered the oxLDL-caused decrease in viability of mouse CECs by 17%, 38%, and 53%, respectively (Figure 1E). After exposure to 10 μmol/L resveratrol for 3, 6, 12, and 24 hours, the oxLDL-caused reduction in cell viability was, respectively, ameliorated by 28%, 34%, 46%, and 55% (Figure 1F).
Resveratrol was pretreated in mouse CECs for 1 hour, and the medium was then replaced with a new medium containing oxLDL. Such a treatment did not affect oxLDL-induced cell death (Table 1). However, posttreatment of mouse CECs with resveratrol significantly attenuated oxLDL-caused decrease in cell viability by 25%.
Table 1. Effects of pretreatment and posttreatment with resveratrol (Res) on protection against oxLDL-induced death of mouse CECs.
| Treatments | Cell viability (OD values at 550 nm) |
|---|---|
| Control | 1.46±0.08 |
| oxLDL | 0.70±0.07* |
| Pretreatment with Res | 1.38±0.04 |
| Pretreatment with Res+oxLDL | 0.85±0.09* |
| Posttreatment with Res | 1.41±0.08 |
| Posttreatment with Res+oxLDL | 1.07±0.08*,# |
CECs, cerebrovascular endothelial cells; oxLDL, oxidized low-density lipoprotein.
Mouse CECs were pretreated with Res for 1 hour, and then the medium was replaced with a new medium containing oxLDL, or posttreated with Res after exposure to oxLDL for 1 hour. Cell viability was analyzed using a colorimetric method. Each value represents mean±s.e.m. for n=6. Symbols * and # respectively indicate that the values significantly differ from control and oxLDL-treated groups, P<0.05.
Protection Against Oxidized Low-Density Lipoprotein-Induced Insults to Mouse Cerebrovascular Endothelial Cells by Resveratrol Occurs via an Antiapoptotic Mechanism
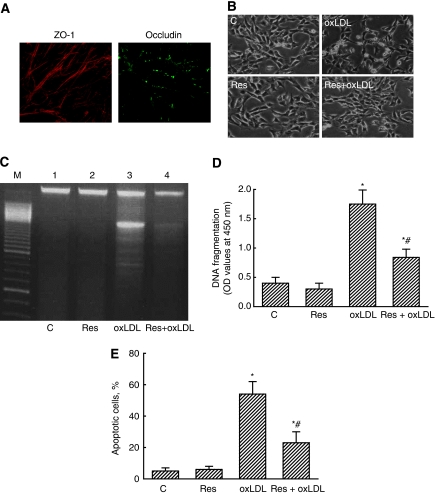
Image analyses using confocal microscopy revealed that ZO-1 and occludin, two tight junction proteins, could be expressed in mouse CECs (Figure 2A). Exposure of mouse CECs to 200 μg/mL oxLDL for 24 hours decreased the cell number and caused cell shrinkage (Figure 2B). Resveratrol at 10 μmol/L did not affect CEC morphologies. However, treatment of mouse CECs with resveratrol decreased oxLDL-caused changes in cell morphologies (Figure 2B). An electrophoretic analysis of genomic DNA showed that treatment with resveratrol alone did not lead to DNA ladder formation (Figure 2C, lane 2). However, exposure to oxLDL induced DNA ladders, but resveratrol lowered such DNA damage (lanes 3 and 4). An enzyme-linked immunosorbent assay further showed that resveratrol at 10 μmol/L did not influence DNA fragmentation (Figure 2D). The oxLDL caused a 3.2-fold increase in DNA fragmentation. Treatment with resveratrol significantly lowered oxLDL-induced DNA fragmentation by 57%. Analysis of the cell cycle revealed that exposure to resveratrol did not cause cell apoptosis (Figure 2E). The oxLDL increased the fraction of mouse CECs, which underwent to apoptosis by 61%. Treatment with resveratrol significantly ameliorated 57% of oxLDL-induced cell apoptosis (Figure 2E).
Figure 2.
Protection by resveratrol (Res) against oxidized low-density lipoprotein (oxLDL)-induced apoptotic insults. Expression of tight junction proteins, ZO-1 and occludin, was analyzed using confocal microscopy (A). Mouse cerebrovascular endothelial cells (CECs) were exposed to 100 μg/mL oxLDL, 10 μmol/L Res, and a combination of Res and oxLDL. Cell morphologies were observed and photographed by a reverse-phase microscope (B). DNA fragmentation was determined following analyses of DNA ladders (C) and an enzyme-linked immunosorbent assay (ELISA) (D). Cell apoptosis was determined using flow cytometry (E). Each value represents the mean±s.e.m. for n=6. The symbols * and #, respectively, indicate that values significantly differ from the control and oxLDL-treated groups, P<0.05. C, control.
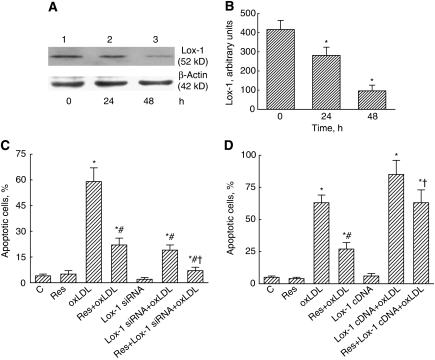
The Lox-1 Receptor Participates in Resveratrol-Involved Cell Protection
Application of Lox-1 siRNA into mouse CECs for 24 and 48 hours decreased the levels of Lox-1 receptor (Figure 3A, lanes 2 and 3). These protein bands were quantified and analyzed (Figure 3B). Exposure to Lox-1 siRNA for 24 and 48 hours caused significant 32% and 79% decreases in the levels of Lox-1. Exposure of mouse CECs to resveratrol or Lox-1 siRNA alone did not induce cell apoptosis (Figure 3C). The oxLDL significantly induced cell apoptosis by 59%. Treatment with resveratrol and Lox-1 siRNA, respectively, caused significant 58% and 65% decreases in oxLDL-induced CEC apoptosis. Cotreatment with resveratrol and Lox-1 siRNA synergistically reduced oxLDL-caused cell apoptosis by 88% (Figure 3C). By comparison, overexpression of Lox-1 alone in mouse CECs did not affect cell apoptosis but completely attenuated resveratrol-involved protection against oxLDL-induced apoptotic insults (Figure 3D).
Figure 3.
Roles of the Lox-1 receptor in resveratrol (Res)-involved cell protection. Mouse cerebrovascular endothelial cells (CECs) were exposed to 10 μmol/L Res, 100 μg/mL oxidized low-density lipoprotein (oxLDL), and a combination of Res and oxLDL. Lox-1 siRNA was applied to mouse CECs, and the amounts of cellular Lox-1 were immunodetected (A, top panel). Levels of β-actin were detected as the internal controls (bottom panel). These protein bands were quantified and analyzed (B). Effects of Lox-1 siRNA on cell apoptosis were determined using flow cytometry (C). Lox-1 full-length cDNA was overexpressed in mouse CECs, and its effects on cell apoptosis were also analyzed by flow cytometry (D). Each value represents the mean±s.e.m. for n=6. The symbols * and #, respectively, indicate that the values significantly differ from control and oxLDL-treated groups, P<0.05. The symbol † means that the value significantly differs from the Res+oxLDL-treated group, P<0.05. C, control.
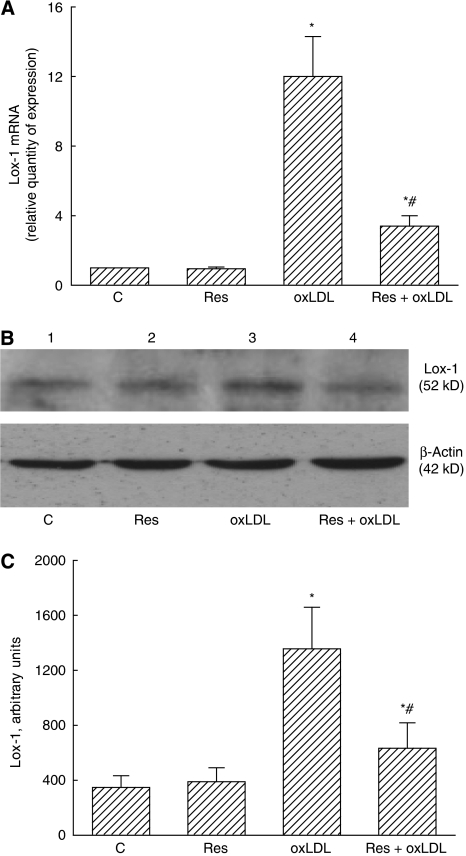
Resveratrol Can Inhibit Oxidized Low-Density Lipoprotein-Induced Lox-1 mRNA and Protein Expressions
Exposure of mouse CECs to 10 μmol/L resveratrol did not affect Lox-1 mRNA production (Figure 4A). After administration of oxLDL, the expression of Lox-1 mRNA was enhanced by 12-fold. Resveratrol significantly decreased oxLDL-induced Lox-1 mRNA production by 72%. An immunoblot analysis revealed that resveratrol did not change Lox-1 protein synthesis (Figure 4B, top panel, lane 2). Exposure of mouse CECs to oxLDL caused an increase in Lox-1 protein synthesis (lane 3). Administration of resveratrol lowered such enhancement (lane 4). β-Actin was immunodetected as an internal control (Figure 4B, bottom panel). These protein bands were quantified and analyzed (Figure 4C). Exposure to oxLDL increased Lox-1 synthesis by 4.2-fold. Meanwhile, oxLDL-induced augmentation significantly decreased by 59%.
Figure 4.
Effects of resveratrol (Res) on oxidized low-density lipoprotein (oxLDL)-induced biosyntheses of Lox-1 mRNA and protein. Mouse cerebrovascular endothelial cells (CECs) were exposed to 10 μmol/L Res, 100 μg/mL oxLDL, and a combination of Res and oxLDL. Productions of Lox-1 and β-actin mRNA were determined by real-time polymerase chain reaction (PCR) (A). Amounts of Lox-1 were immunodetected (B, top panel). Levels of β-actin were determined as the internal control (bottom panel). These protein bands were quantified and analyzed (C). Each value represents the mean±s.e.m. for n=6. The symbols * and #, respectively, indicate that the values significantly differ from the control and oxLDL-treated groups, P<0.05. C, control.
Resveratrol's Protection May Be Involved in Lox-1 Receptor-Mediated Bax Translocation
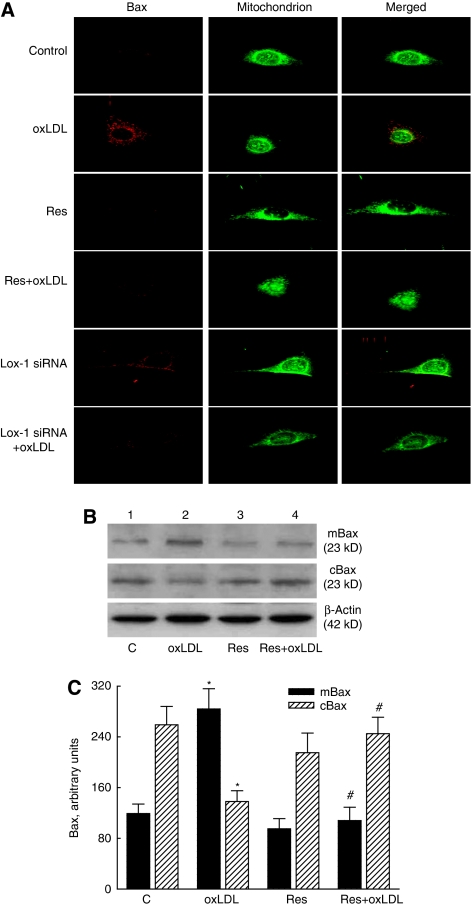
The localization of Bax protein in the cytoplasm is shown in the left panels of Figure 5A, and has red signals. The middle panels with green signals present the distribution of mitochondria in mouse CECs. The merged signals stained with yellow show localization of the Bax protein in CEC mitochondria (right panels). Exposure of mouse CECs to oxLDL enhanced translocation of the Bax protein from the cytoplasm to mitochondria. Cotreatment of resveratrol or Lox-1 siRNA with oxLDL decreased oxLDL-induced Bax translocation to mitochondria of mouse CECs.
Figure 5.
Effects of Lox-1 siRNA and resveratrol (Res) on Bax translocation from the cytoplasm to mitochondria. Mouse cerebrovascular endothelial cells (CECs) were exposed to oxidized low-density lipoprotein (oxLDL) with Res or Lox-1 siRNA (A). The distribution of Bax protein in CECs was immunodetected, and the mitochondria of mouse CECs were stained with DiOC6. The fluorescent images were visualized using a confocal laser scanning microscope. Mouse CECs were exposed to oxLDL, Res, and a combination of Res and oxLDL. Levels of mitochondrial and cytosolic Bax were immunodetected (B, top two panels). β-Actin was detected as the internal control (bottom panel). These protein bands were quantified and analyzed (C). Each value represents the mean±s.e.m. for n=6. The symbols * and #, respectively, indicate that the values significantly differ from the control and oxLDL-treated groups, P<0.05. C, control.
Exposure of mouse CECs to oxLDL increased mitochondrial Bax but decreased cytosolic levels (Figure 5B, top two panels, lane 2). Resveratrol did not affect the levels of mitochondrial or cytosolic Bax, but attenuated oxLDL-caused alteration of this proapoptotic protein in the mitochondria and cytoplasm (lanes 3 and 4). The amounts of β-actin were immunodetected as the internal control (bottom panel). These protein bands were quantified and analyzed (Figure 5C). The oxLDL increased mitochondrial Bax by 2.4-fold but reduced cytosolic Bax by 47%. Treatment with resveratrol completely recovered oxLDL-caused alterations in mitochondrial and cytosolic Bax (Figure 5C).
Resveratrol Sequentially Suppresses Activation of a Mitochondria–Cytochrome c–Caspase Protease Pathway
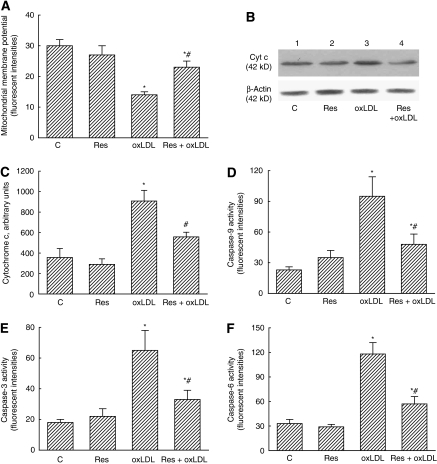
Exposure of mouse CECs to resveratrol did not change the mitochondrial membrane potential (Figure 6A). Meanwhile, administration of oxLDL decreased the membrane potential of CEC mitochondria by 53%. Treatment with resveratrol significantly ameliorated this reduction by 64%. Treatment of mouse CECs with resveratrol did not change cytochrome c release (Figure 6B). The oxLDL enhanced levels of cytosolic cytochrome c by 4.5-fold (Figures 6B and 6C). After administration of resveratrol, the oxLDL-induced augmentation of cytosolic cytochrome c was significantly reduced by 55%.
Figure 6.
Effects of resveratrol (Res) on oxidized low-density lipoprotein (oxLDL)-induced alterations in mitochondrial membrane potential, cytochrome c (Cyt C) release, and activities of caspases-9, -3, and -6. Mouse cerebrovascular endothelial cells (CECs) were exposed to 10 μmol/L Res, 100 μg/mL oxLDL, and a combination of Res and oxLDL. The mitochondrial membrane potential was detected using flow cytometry (A). Amounts of Cyt c were immunodetected and quantified (B, C). Activities of caspases-9, -3, and -6 were, respectively, analyzed by fluorogenic assays using LEHD (Leu-Glu-His-Asp), DEVD (Asp-Glu-Val-Asp), and VEID (Val-Glu-Ile-Asp) as the substrate (D–F). Each value represents the mean±s.e.m. for n=6. The symbols * and #, respectively, indicate that the values significantly differ from the control and oxLDL-treated groups, P<0.05. The symbol † means that the value significantly differs from the Res+oxLDL-treated groups, P<0.05. C, control.
Exposure of mouse CECs to resveratrol did not affect caspase-9 activity (Figure 6D). The oxLDL significantly increased caspase-9 activity by 4.1-fold. After resveratrol administration, the oxLDL-induced enhancement of caspase-9 activity decreased by 51%. The activity of caspase-3 was not affected by resveratrol (Figure 6E). Exposure of mouse CECs to oxLDL caused a 3.6-fold increase in caspase-3 activity. Administration of resveratrol significantly ameliorated oxLDL-caused enhancement of caspase-3 activity by 46%. The oxLDL increased caspase-6 activity by 3.6-fold (Figure 6F). Meanwhile, the oxLDL-induced increase in caspase-6 activation significantly decreased following resveratrol administration.
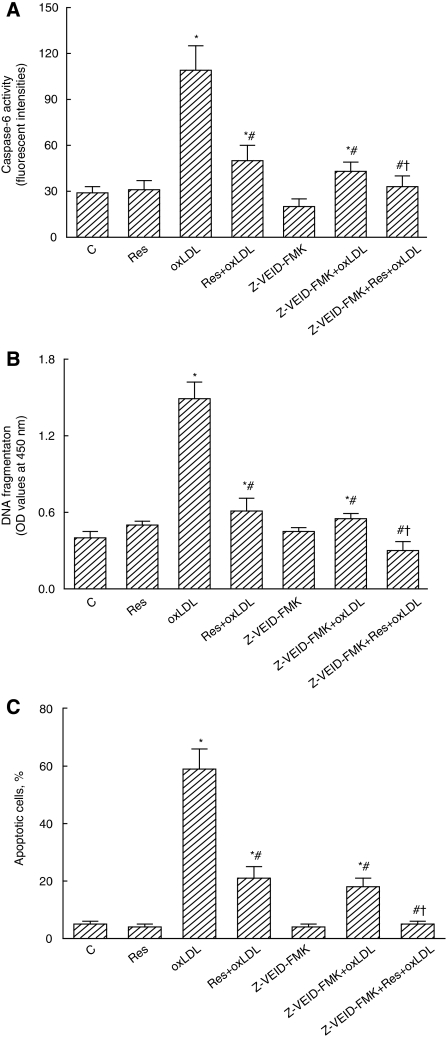
Z-VEID-FMK, an inhibitor of caspase-6, was applied to mouse CECs to further evaluate the roles of this protease in resveratrol-caused protection (Figure 7). Treatment with Z-VEID-FMK significantly decreased oxLDL-induced augmentation of caspase-6 activity by 61% (Figure 7A). Cotreatment with resveratrol and Z-VEID-FMK completely lowered oxLDL-caused enhancement of caspase-6 activity. In parallel with the suppression of caspase-6 activity, administration of Z-VEID-FMK decreased oxLDL-induced DNA fragmentation by 63% (Figure 7B). Treatment with resveratrol and Z-VEID-FMK synergistically and completely alleviated oxLDL-induced DNA damage. The oxLDL-caused CEC apoptosis was significantly ameliorated by 69% following administration of Z-VEID-FMK (Figure 7C). Simultaneous exposure to resveratrol and Z-VEID-FMK completely lowered oxLDL-induced cell apoptosis.
Figure 7.
Effects of resveratrol (Res), oxidized low-density lipoprotein (oxLDL), and Z-VEID-FMK on caspase-6 activity, DNA fragmentation, and cell apoptosis. Mouse cerebrovascular endothelial cells (CECs) were pretreated with 50 μmol/L Z-VEID-FMK, an inhibitor of caspase-6, for 1 hour, and then exposed to 10 μmol/L Res, 100 μg/mL oxLDL, or a combination of Res and oxLDL. Caspase-6 activity was determined by a fluorogenic assay (A). DNA fragmentation was quantified using a 5-bromo-2-deoxyuridine (BrdU)-labeled histone-associated enzyme-linked immunosorbent assay (ELISA) kit (B). Apoptotic cells were quantified using flow cytometry (C). Each value represents the mean±s.e.m. for n=6. The symbols * and #, respectively, indicate that the values significantly differ from control and oxLDL-treated groups, P<0.05. The symbol † means that the value significantly differs from the Res+oxLDL-treated groups, P<0.05. C, control.
Discussion
Resveratrol has multiple biochemical and molecular actions (Pervaiz, 2003; Athar et al, 2009). In animals, resveratrol can protect against oxidative stress-induced insults (Csiszar et al, 2008; Farghali et al, 2009). The present study showed that when exposed to concentrations of ≤10 μmol/L, resveratrol attenuated the oxidation of LDL and the oxLDL-induced cellular ROS and CEC death. Provinciali et al (2005) demonstrated that resveratrol at 0.2 mg/kg body weight in vivo, corresponding to 4 μmol/L, has antitumor activity. Thus, the concentration of resveratrol used in this study may be higher than it can be in vivo applied. The oxLDL is a toxicant present in the blood (Matsuura et al, 2008). Mouse CECs used in this study can express the BBB markers ZO-1 and occludin. A previous study showed that CECs isolated from cerebral microvessels can form tight junction (An and Xue, 2009). Thus, the protective effects of resveratrol on oxLDL-caused insults to CECs afford a considerable means for defending against injury of the BBB.
Resveratrol protects CECs from oxLDL-induced damage of CECs via an antiapoptotic mechanism. Exposure of CECs to oxLDL caused fragmentation of genomic DNA, but resveratrol decreases such alterations. DNA fragmentation is one of the typical characteristics of cells undergoing apoptosis (Chen et al, 2002). Data from analysis of the cell cycle further revealed that resveratrol significantly lowered oxLDL-caused increases in the proportion of mouse CECs arrested at the sub-G1 phase. The appearance of a hypodiploid sub-G1 peak indicates that cells are undergoing apoptosis (Schmid et al, 1994; Lee et al, 2009). Treatment of CECs with oxLDL enhanced the levels of cellular ROS, and augmentation of oxidative stress may lead to cell apoptotic insults (Chen et al, 2007). Previous studies showed that oxLDL is an oxidant and can induce lipid peroxidation to produce more-active radicals (Nicholson and Hajjar, 2004; Kim et al, 2006). Thus, the sources of cellular ROS in oxLDL-treated CECs may be derived from oxLDL itself or the other lipid peroxidation products. The mechanisms of resveratrol-involved antioxidation may occur through directly scavenging free radicals or modulating redoxcycling systems (Pervaiz, 2003; Pervaiz and Holme, 2009). Thus, one of the possible mechanisms of resveratrol-caused antiapoptotic protection is due to suppression of oxidative stress. In this study, we provide direct evidence, including DNA fragmentation and apoptotic analysis, to show that resveratrol protects CECs against oxLDL-induced injuries via an antiapoptotic mechanism.
Intrinsic mitochondrion-mediated signal-transducing events participate in resveratrol-caused antiapoptotic protection. This study showed that resveratrol reduced oxLDL-enhanced translocation of Bax proteins from the cytoplasm to mitochondria. Immunoblotting analyses further demonstrated the suppressive effects of resveratrol on oxLDL-caused changes in cytosolic and mitochondrial levels of Bax protein. Bax functions by mediating mitochondrion-dependent apoptosis (Pagliari et al, 2005; Lee et al, 2009). Translocation of Bax to mitochondria can permeabilize the mitochondrial membrane and trigger the release of apoptotic factors such as cytochrome c and ROS (Pagliari et al, 2005). After exposure to oxLDL, the mitochondrial membrane potential decreased, but levels of cellular ROS and cytochrome c were augmented. These alterations indicate an interruption of mitochondrial membranes by oxLDL. However, resveratrol alleviated such injuries. Cytochrome c interacts with cytoplasmic apoptotic protease-activating factor-1 to form apoptosomes and mediates caspase-9 activation (Kagan et al, 2004). The oxLDL-augmented sequential activation of caspases-9, -3, and -6 was lowered following resveratrol treatment. Furthermore, resveratrol had synergistic effects with Z-VEID-FMK, an inhibitor of caspase-6 activity, on protecting CECs from oxLDL-induced DNA fragmentation and cell apoptosis. Consequently, resveratrol protected CECs against oxLDL-induced apoptotic insults through a Bax-mitochondrion–cytochrome c–caspase protease pathway.
The Lox-1 receptor is involved in resveratrol-caused cell protection. This study showed that knocking down the translation of Lox-1 mRNA using RNAi promoted resveratrol-involved defense of CECs against oxLDL-induced apoptotic damage. Contrarily, overexpression of Lox-1 in CECs lowered resveratrol's protection. LOX-1 is a scavenger receptor that primarily binds to and regulates oxLDL (Mehta and Li, 2002). Thus, the Lox-1 receptor contributes to resveratrol's protection against oxLDL-induced apoptotic insults to CECs. This study further showed that oxLDL induced Lox-1 mRNA and protein expression, but resveratrol significantly inhibited such induction. Suppressing the production of the Lox-1 receptor simultaneously ameliorated oxLDL-caused death of CECs. In parallel, when reducing the translation of the Lox-1 receptor by RNAi, oxLDL-induced Bax translocation from the cytoplasm to mitochondria and apoptosis of CECs were attenuated. Therefore, resveratrol can lessen oxLDL-induced apoptosis of CECs through inhibiting Lox-1 expression and receptor-mediated signal-transducing events.
In conclusion, this study shows that resveratrol at low concentrations (≤10 μmol/L) can protect CECs against oxLDL-induced alterations in the levels of cellular ROS, cell viability, DNA fragmentation, and cell apoptosis. Sequentially, the oxLDL-stimulated translocation of Bax, release of cytochrome c, and activation of caspases-9, -3, and -6 were alleviated by resveratrol. Treatment with resveratrol and Z-VEID-FMK synergistically protected CECs from oxLDL-induced apoptosis. Exposure to resveratrol significantly inhibited oxLDL-induced Lox-1 mRNA and protein expressions. Knockdown and overexpression of Lox-1 in CECs had opposite effects on resveratrol-involved protection. Taken together, this study shows that resveratrol can protect CECs from oxLDL-induced apoptotic insults via downregulating Lox-1 receptor-mediated activation of the Bax–mitochondrion–cytochrome c–caspase protease mechanism. There are certain study limitations in the present investigation. In general, the physiology of CECs is a strict monolayer and very tight paracellularly. Meanwhile, this study evaluated the effects of resveratrol and oxLDL on nonconfluent CECs.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants from the Department of Health (DOH098-TD-F-113-098010; DOH99-TD-B-111-003, DOH99-TD-C-111-008), Taipei, Taiwan, ROC.
References
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An P, Xue YX. Effects of preconditioning on tight junction and cell adhesion of cerebral endothelial cells. Brain Res. 2009;1272:81–88. doi: 10.1016/j.brainres.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Betz AL. Alterations in cerebral endothelial cell function in ischemia. Adv Neurol. 1996;71:301–311. [PubMed] [Google Scholar]
- Chang CC, Liao YS, Lin YL, Chen RM. Nitric oxide protects osteoblasts from oxidative stress-induced apoptotic insults via a mitochondria-dependent mechanism. J Orthop Res. 2006;24:1917–1925. doi: 10.1002/jor.20244. [DOI] [PubMed] [Google Scholar]
- Chang HC, Lin KH, Tai YT, Chen JT, Chen RM. Lipoteichoic acid-induced TNF-α and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating toll-like receptor 2-mediated activation of ERK1/2 and NFκB. Shock. 2010;33:485–492. doi: 10.1097/SHK.0b013e3181c3cea5. [DOI] [PubMed] [Google Scholar]
- Chen RM, Chen TL, Lin YL, Chen TG, Tai YT. Ketamine reduces nitric oxide biosynthesis in human umbilical vein endothelial cells through downregulating endothelial nitric oxide synthase expression and intracellular calcium levels. Crit Care Med. 2005;33:1044–1049. doi: 10.1097/01.ccm.0000163246.33366.51. [DOI] [PubMed] [Google Scholar]
- Chen RM, Lin YL, Jean WC, Chen JS, Wang JH, Liu HC. Nitric oxide induces osteoblast apoptosis through the de novol synthesis of Bax protein. J Orthop Res. 2002;20:295–302. doi: 10.1016/S0736-0266(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Chen TG, Chen TL, Chang HC, Tai YT, Cherng YG, Chang YT, Chen RM. Oxidized low-density lipoprotein induces apoptotic insults to mouse cerebral endothelial cells via a Bax-mitochondria-caspase protease pathway. Toxicol Appl Pharmacol. 2007;219:42–53. doi: 10.1016/j.taap.2006.11.031. [DOI] [PubMed] [Google Scholar]
- Chen TL, Chang CC, Lin YL, Chen RM. Signal-transducing mechanisms of ketamine-caused inhibition of interleukin-1β gene expression in lipopolysaccharide-stimulated murine macrophage-like Raw 264.7 cells. Toxicol Appl Pharmacol. 2009;240:15–25. doi: 10.1016/j.taap.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Cherng YG, Chang HC, Lin YL, Kuo ML, Chiu WT, Chen RM. Apoptotic insults to human chondrocytes induced by nitric oxide are involved in sequential events, including cytoskeletal remodeling, phosphorylation of mitogen-activated protein kinase kinase kinase-1, and Bax-mitochondria-mediated caspase activation. J Orthop Res. 2008;26:1018–1026. doi: 10.1002/jor.20578. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Blom-Roosemalen MC, de Boer AG, Breimer DD, Kuiper J. Effect of endotoxin on permeability of bovine cerebral endothelial cell layers in vitro. J Pharmacol Exp Ther. 1996;277:1418–1423. [PubMed] [Google Scholar]
- El Bekay R, Alvarez M, Monteseirin J, Alba G, Chacon P, Vega A, Martin-Nieto J, Jimenez J, Pintado E, Bedoya FJ, Sobrino F. Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NF-kappaB. Blood. 2003;102:662–671. doi: 10.1182/blood-2002-09-2785. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- Farghali H, Cerny D, Kamenikova L, Martinek J, Horinek A, Kmonickova E, Zidek Z. Resveratrol attenuates lipopolysaccharide-induced hepatitis in D-galactosamine sensitized rats: role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxide. 2009;21:216–225. doi: 10.1016/j.niox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Goyal L. Cell death inhibition: keeping caspases in check. Cell. 2001;104:805–808. doi: 10.1016/s0092-8674(01)00276-8. [DOI] [PubMed] [Google Scholar]
- Ho WP, Chan WP, Hsieh MS, Chen RM. Runx2-mediated Bcl-2 gene expression contributes to nitric oxide protection against oxidative stress-induced osteoblast apoptosis. J Cell Biochem. 2009;108:1084–1093. doi: 10.1002/jcb.22338. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Rad Biol Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kashiwakura Y, Watanabe M, Kusumi N, Sumiyoshi K, Nasu Y, Yamada H, Sawamura T, Kumon H, Takei K, Daida H. Dynamin-2 regulates oxidized low-density lipoprotein-induced apoptosis of vascular smooth muscle cell. Circulation. 2004;110:3329–3334. doi: 10.1161/01.CIR.0000147828.86593.85. [DOI] [PubMed] [Google Scholar]
- Kim OS, Lee CS, Joe EH, Jou I. Oxidized low density lipoprotein suppresses lipopolysaccharide-induced inflammatory responses in microglia: oxidative stress acts through control of inflammation. Biochem Biophys Res Commun. 2006;342:9–18. doi: 10.1016/j.bbrc.2006.01.107. [DOI] [PubMed] [Google Scholar]
- Lee ST, Wu TT, Yu PY, Chen RM. Apoptotic insults to human HepG2 cells induced by S-(+)-ketamine occurs through activation of a Bax-mitochondria-caspase protease pathway. Br J Anaesth. 2009;102:80–89. doi: 10.1093/bja/aen322. [DOI] [PubMed] [Google Scholar]
- Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–617. doi: 10.1161/01.cir.0000047276.52039.fb. [DOI] [PubMed] [Google Scholar]
- Lin JK, Tsai SH. Chemoprevention of cancer and cardiovascular disease by resveratrol. Proc Natl Sci Council ROC Life Sci. 1999;23:99–106. [PubMed] [Google Scholar]
- Liu SX, Zhou M, Chen Y, Wen WY, Sun MJ. Lipoperoxidative injury to macrophages by oxidatively modified low density lipoprotein may play an important role in foam cell formation. Atherosclerosis. 1996;121:55–61. doi: 10.1016/0021-9150(95)05683-1. [DOI] [PubMed] [Google Scholar]
- Matsuura E, Hughes GR, Khamashta MA. Oxidation of LDL and its clinical implications. Autoimm Rev. 2008;7:558–566. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Li D. Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J Am College Cardiol. 2002;39:1429–1435. doi: 10.1016/s0735-1097(02)01803-x. [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Hajjar DP. CD36, oxidized LDL and PPAR gamma: pathological interactions in macrophages and atherosclerosis. Vasc Pharmacol. 2004;41:139–146. doi: 10.1016/j.vph.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Aantioxid Red Sign. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Rel F, Donnini A, Orlando F, Bartozzi B, di Stasiol G, Smorlesi A. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer. 2005;115:36–45. doi: 10.1002/ijc.20874. [DOI] [PubMed] [Google Scholar]
- Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL. Endothelial cells: adhesion and tight junctions. Curr Opin Cell Biol. 1992;4:830–833. doi: 10.1016/0955-0674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- Schmid I, Uittenbogaart CH, Giorgi JV. Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry. 1994;15:12–20. doi: 10.1002/cyto.990150104. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Spencer JP, Rice-Evans C, Williams RJ. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J. 2001;358:547–557. doi: 10.1042/0264-6021:3580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp CD, Hines I, Houghton J, Warren A, Jackson TH, Jawahar A, Nanda A, Elrod JW, Long A, Chi A, Minagar A, Alexander JS. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am J Physiol. 2003;285:H2592–H2598. doi: 10.1152/ajpheart.00520.2003. [DOI] [PubMed] [Google Scholar]
- Sneck M, Kovanen PT, Oorni K. Decrease in pH strongly enhances binding of native, proteolyzed, lipolyzed, and oxidized low density lipoprotein particles to human aortic proteoglycans. J Biol Chem. 2005;280:37449–37454. doi: 10.1074/jbc.M508565200. [DOI] [PubMed] [Google Scholar]
- Tai YT, Cherng YG, Chang CC, Hwang YP, Chen JT, Chen RM. Pretreatment with low nitric oxide protects osteoblasts from high nitric oxide-induced apoptotic insults through regulation of c-Jun N-terminal kinase/c-Jun-mediated Bcl-2 gene expression and protein translocation. J Orthop Res. 2007;25:625–635. doi: 10.1002/jor.20365. [DOI] [PubMed] [Google Scholar]
- Wu GJ, Chen TG, Chang HC, Chiu WT, Chang CC, Chen RM. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem. 2007;101:1520–1531. doi: 10.1002/jcb.21268. [DOI] [PubMed] [Google Scholar]
- Wu TT, Chen TL, Chen RM. Lipopolysaccharide triggers macrophage activation of inflammatory cytokine expression, chemotaxis, phagocytosis, and oxidative ability via a toll-like receptor 4-dependent pathway: validated by RNA interference. Toxicol Lett. 2009;191:195–202. doi: 10.1016/j.toxlet.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Xu J, Yeh CH, Chen S, He L, Sensi SL, Canzoniero LMT, Choi DW, Hsu CY. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-α/cycloheximide-induced cerebral endothelial cell death. J Biol Chem. 1998;273:16521–16526. doi: 10.1074/jbc.273.26.16521. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Shioi T, Izumi T. Resveratrol ameliorates experimental autoimmune myocarditis. Circ J. 2007;71:397–404. doi: 10.1253/circj.71.397. [DOI] [PubMed] [Google Scholar]
- Zettler ME, Prociuk MA, Austria JA, Zhong G, Pierce GN. Oxidized low-density lipoprotein retards the growth of proliferating cells by inhibiting nuclear translocation of cell cycle proteins. Arterioscler Thromb Vasc Biol. 2004;24:727–732. doi: 10.1161/01.ATV.0000120373.95552.aa. [DOI] [PubMed] [Google Scholar]
- Zorn-Pauly K, Schaffer P, Pelzmann B, Bernhart E, Wei G, Lang P, Ledinski G, Greilberger J, Koidl B, Jurgens G. Oxidized LDL induces ventricular myocyte damage and abnormal electrical activity—role of lipid hydroperoxides. Cardiovasc Res. 2005;66:74–83. doi: 10.1016/j.cardiores.2004.12.009. [DOI] [PubMed] [Google Scholar]