Abstract
There is some evidence that in animal models of acute ischaemic stroke, combinations of neuroprotective agents might be more efficacious than the same agents administered alone. Hence, we developed pragmatic, empirical criteria based on therapeutic target, cost, availability, efficacy, administration, and safety to select drugs for testing in combination in animal models of acute stroke. Magnesium sulphate, melatonin, and minocycline were chosen from a library of neuroprotective agents, and were tested in a more ‘realistic' model favoured by the STAIR (Stroke Therapy Academic Industry Roundtable). Outcome was assessed with infarct volume, neurologic score, and two newly developed scales measuring general health and physiologic homeostasis. Owing to the failure to achieve neuroprotection in aged, hypertensive animals with drug delivery at 3 hours, the bar was lowered in successive experiments to determine whether neuroprotection could be achieved under conditions more conducive to recovery. Testing in younger animals showed more favourable homeostasis and general health scores than did testing in older animals, but infarct volume and neurologic scores did not differ with age, and treatment efficacy was again not shown. Testing with shorter occlusions resulted in smaller infarct volumes; nevertheless, treatment efficacy was still not observed. It was concluded that this combination, in these stroke models, was not effective.
Keywords: animal experiments, cerebral ischaemia, combination neuroprotection, combination therapy, meta-analysis, multimodal therapy
Introduction
No individual neuroprotective agent has yet progressed beyond the experimental phase to become a fully fledged, acute stroke treatment. Combination therapy may circumvent some limitations of single-agent therapy by boosting efficacy, by limiting side effects, or by extending the time window for treatment (Cheng et al, 2004; Danton, 2004; Adams et al, 2003; Rogalewski et al, 2006; Fisher, 2003). Nevertheless, combining therapies increases the complexity of experimental design (Saver and Kalafut, 2001); hence, a framework is required for drug selection and prioritising experiments.
In this study, a pragmatic approach was adopted; we sought to eliminate combinations with limited clinical prospects by selecting only those with reasonable evidence of efficacy from animal experiments. The selected combination was then subjected to a preclinical testing process comparable with clinical trials in terms of comorbidities, time windows, and bias avoidance. As the validity of extrapolating experimental results from young, healthy animals to aged humans with comorbidities has been questioned (STAIR, 1999; Wiebers et al, 1990), we used aged, hypertensive rats. We also sought to initiate treatment at delays consistent with clinical time frames, and to control bias through blinding and randomisation (STAIR, 1999).
The first aim was to develop and apply a process for selecting treatments from a library of candidate therapies. The second aim was to undertake individual meta-analyses of past performance of selected therapies in animal stroke. The third aim was to test the selected therapies in combination under experimental conditions commensurate with clinical trials in terms of comorbidities, time windows, and bias avoidance, with regard to what could realistically be achieved in a laboratory setting.
Materials and methods
Part 1: Drug Selection Process
The process involved: (1) determination of selection criteria; (2) identification of candidate drugs; (3) collection of drug data; and (4) drug ranking. The final selection of drugs was made by a panel of clinical staff and scientists (see the ‘Acknowledgements' section).
Selection Criteria
Drugs selection was based on: (1) target mechanism; (2) efficacy in stroke models; (3) cost and availability; (4) stability; (5) delivery method; and (6) safety.
Targets chosen were excitotoxicity, oxidative stress, and inflammation because these were well-recognised pathophysiolgical processes in the cascade of ischaemic damage (Hass, 1983; Heiss and Graf, 1994; Peruche and Krieglstein, 1993). Furthermore, they were among the best studied and the most effective targets (O'Collins et al, 2006). By targeting three mechanisms, we anticipated that overall efficacy could be increased. With more than three targets, we believed that any benefit could be outweighed by negative interactions and complexities in trial design.
As there is currently little incentive for the industry to develop drugs that are out of patent but nevertheless may benefit patients, the criteria also included cost and availability. Lower costs may increase access to therapies; lower-income countries bear the brunt of cardiovascular disease (WHO, 2009); yet there is no parity in access to medical capital.
We hoped to develop a therapy that could be administered in the field (ambulance), prehospital. Consequently, it was imperative that the agents be safe, stable, and easily administered. Drugs available as intravenous preparations were preferred because dysphagia (difficulty swallowing) is a frequent sequela of ischaemic stroke.
Drug Identification
Candidates were identified by: (1) a PubMed search of ‘neuroprotection' (all years) or of ‘cerebral ischaemia/ischemia' (1960 to 1980) and (2) drugs cross-referenced from other articles. In this manner, 1,026 therapies were identified and have been reported in a previously published systematic review (O'Collins et al, 2006).
Data Collection
Information on drug efficacy was obtained through PubMed, using the search criteria ‘<drug name>‘ and ‘cerebral isch(a)emia' or ‘stroke' or ‘neuroprotection'. This search identified 8,516 experimental results published from 1965 to 2004. The scope of this search covered controlled tests of drug efficacy in both animal and tissue culture models of neuroprotection (O'Collins et al, 2006). Information pertaining to safety, Food and Drug Administration (FDA) approval, administration, and putative primary mechanism of action was obtained from MIMS (2004), the Merck Manual (Beers, 2003), PubMed, and the FDA.
Drug Ranking
Candidates were scored for their performance in experimental models (O'Collins et al, 2006) Target mechanism, FDA approval status, and the availability of an intravenous preparation were noted. Evaluation of safety, stability, availability, and cost and target mechanism were qualitative, rather than quantitative.
Part 2: Meta-Analyses of Selected Therapies
Random-effects meta-analyses were undertaken for the three selected agents (DerSimonian and Laird, 1986; Macleod et al, 2004). Data were extracted from published results from controlled focal ischaemia experiments using infarct size as an outcome. Forest plots were used to display the mean overall effect sizes, together with effect sizes for subgroups relating to characteristics of the stroke model and treatment. Study quality was measured on a 10-point scale, with higher scores representing superior quality (Macleod et al, 2004). The range of testing was scored on a scale of 0 to 10 points, with 10 representing broader testing (O'Collins et al, 2006).
Part 3: Experimental Testing of the Drug Combination
Animals
Spontaneous hypertensive rats (Animal Research Centre, Perth, WA, Australia) were used because of their similarity to human hypertension and their smaller infarct variability (Okamoto and Aoki, 1963). Experiments 1 to 2 used aged rats (M±s.d.=100±2 weeks), whereas young rats were used in experiments 3 (M±s.d.=12.8±0.2 weeks) and 4 (M±s.d.=13.4±0.7 weeks). Animals were housed in pairs under standard laboratory conditions (12 hours light–dark) with unlimited access to food and water. The Austin Health Animal Ethics Committee approved the project, and care was taken to minimise animal suffering.
Stroke Model
Rats were anaesthetised with isoflurane (5% induction, 1.5% to 2.0% maintenance) in 50:50 air/oxygen. Atropine (120 μg per rat intraperitoneal) was administered to inhibit mucus secretions. Rectal temperature was monitored and maintained at 37°C using a controlled heat-mat system. Heart rate and blood oxygen saturation were monitored using pulse oximeter probes at the right hind limb. Ipsilateral cortical cerebral blood flow (CBF) velocity (4 mm lateral and 1 mm caudal to the bregma) was monitored over the thinned skull using laser Doppler flowmetry (Moor Instruments, Axminster, UK). The femoral vein was cannulated before stroke induction.
Stroke was induced by inserting a thread into the external carotid artery and guiding it through the internal carotid artery to the middle cerebral artery origin (Koizumi et al, 1986; Takano et al, 1997; Spratt et al, 2006). Threads were tipped with silicon cylinders (diameter: 0.35 mm; length: 5 mm (experiments 1 to 3), 2 mm (experiment 4)). To permit reperfusion, threads were withdrawn at 2 hours (experiments 2 to 3) or at 90 minutes (experiment 4) after occlusion. At 24 hours, animals were killed (Lethobarb: 15 mg/100 g rat intraperitoneal) and perfused with saline and then 4% paraformaldehyde (Sigma-Aldrich, Castle Hill, NSW, Australia) in 0.1 mol/L phosphate-buffered saline over 15 minutes at 18 mL/min. Originally, the killing was planned for 1 week; owing to high mortality in aged rats, this was discontinued.
Outcome Measures
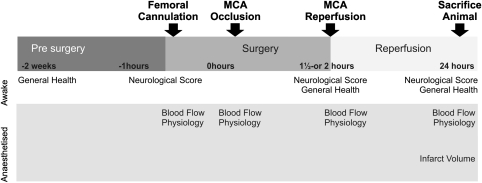
The primary outcome measure was infarct volume at 24 hours, and the secondary outcomes were 24 hours neurologic score, general health, and homeostasis (Figure 1). The infarct was visualised with haematoxylin and eosin, and automatically segmented using a threshold derived from relative optical density of the images using MCID (Imaging Research, St. Catherines, Ontario, Canada). Neurologic score was measured on a scale adapted from Petullo measuring lateral push, torso twisting, forelimb flexion (1 point each), and mobility (2 points) (Petullo et al, 1999), with 0 indicating no deficit, 5 maximal deficits, and 6 denoting death.
Figure 1.
Experimental timeline. MCA, middle cerebral artery.
In response to ethical concerns about the well-being of aged animals, a scale was devised to measure general health (Table 1). Animals were scored against criterion indicative of disease or distress (0=no symptom, 1=moderate/unilateral deficit, 2=severe/bilateral deficit) and a total was obtained. A maximum of 30 was given if all symptoms were present or if the animal died before assessment.
Table 1. General health assessment.
| General health | Not present | Mild deficita | Severe deficitb |
|---|---|---|---|
| Restless or lethargic | 0 | 1 | 2 |
| Posture hunched | 0 | 1 | 2 |
| Shaking (e.g., postanaesthetic) | 0 | 1 | 2 |
| Breathing: laboured, rasping, shallow | 0 | — | 2 |
| Rough hair coat (piloerection) | 0 | 1 | 2 |
| Subcutaneous tumours | 0 | 1 | 2 |
| Pressure sores | 0 | 1 | 2 |
| Cataracts | 0 | 1 | 2 |
| Discharge from the Harderian gland | 0 | 1 | 2 |
| Discharge from nose | 0 | 1 | 2 |
| Bleeding (e.g., wound sites) | 0 | 1 | 2 |
| Noises of distress when in cage | 0 | 1 | 2 |
| Distressed when touched | 0 | 1 | 2 |
| Self-mutilation, alopecia | 0 | 1 | 2 |
| Diarrhoea | 0 | 1 | 2 |
| Total | 30 |
Moderate or unilateral deficit.
Severe or bilateral deficit.
A scale was devised to measure the extent to which stroke disrupts homeostasis (Table 2). The rationale for the scale was that perturbations from the physiologic norm were related to stroke outcome (Sena et al, 2007b). Furthermore, in the discontinued pilot study in which aged animals were kept out to 1 week, a higher mortality was observed together with greater difficulties in maintaining physiology within normal limits. The score was calculated for rats at 24 hours after occlusion using physiologic parameters routinely monitored in animals under anaesthesia: the scale ranged from 0 (healthy) to 4 (disrupted homeostasis). The test was exploratory and further validation is required.
Table 2. Homeostasis scale.
| Criteria | Description | Score |
|---|---|---|
| Blood oxygen saturation | <90% | 1 |
| Breathing | <20 or >60 breaths per minute | 1 |
| Heart rate | <200 or >450 beats per minute | 1 |
| Temperature | <36.0°C or >38.5°C | 1 |
| Total | 4 |
The homeostasis scale measured at 24 hours after occlusion was used as a secondary end point. Steps were taken to control temperature during surgery, but at 24 hours, the temperature may not have stabilised as it was measured just after induction of anaesthesia for killing. Respiration was not regulated by mechanical ventilation.
Animals were randomised using a dynamic, permuted block system to balance treatment allocation. Outcomes were undertaken blind to treatment allocation.
Drug Delivery
Drugs were prepared according to established protocols and tested at the maximally effective dose reported in the literature, which also fell within the safe limits for humans assuming an identical dose–weight relationship. Magnesium sulphate heptahydrate (BDH Chemicals, Kilsyth, VIC, Australia) was delivered in 0.9% saline at 0.75 mmol/kg (Westermaier et al, 2003). Melatonin (99.5%, Sigma-Aldrich) was delivered in ethanol (<5%) and saline at 8 mg/kg (Kilic et al, 1999; Kilic, 2004). Minocycline was delivered in saline at 9 mg/kg (Xu et al, 2004). The combination was prepared from the same stock solutions as the single drug preparations. All preparations were diluted to identical volumes with saline and then filtered. Saline was used as the control condition. Light exposure was minimised during preparation, storage, and delivery. Drugs were prepared before each experiment and then stored at −20°C until required (up to several weeks later). No loss in activity was expected (Barry and Badal, 1978; Cavallo and Hassan, 1995).
Therapy was administered through an intravenous femoral line starting immediately after occlusion (experiments 1 and 4) or 3 hours after occlusion (experiments 2 and 3). Each rat received 0.5 mL/100 g, with 1/3 delivered over 30 minutes, and 2/3 delivered over the remaining time to 24 hours. As minocycline is bright yellow, femoral lines were primed with saline and the infusion equipment was concealed to ensure blinded induction of surgery. Consequently, a delay of 10 minutes ensued before drugs entered circulation.
Statistics
Sample sizes were calculated (Stata, version 10, Statacorp, College Station, TX, USA) using an independent group design (P=0.05, power=80%). For a 25% effect and s.d. of 19%, 10 animals are required per group. Analyses of variance were undertaken to determine group differences in continuous variables (two tailed, P=0.05, Stata version 10). Regression (SPSS, version 15, St Leonards, NSW, Australia) was used to determine whether the combination influenced infarct volume after accounting for CBF changes. Secondary end points were analysed using the Hodges–Lehmann estimates of shift parameters in Stata version 10 (Wang, 1999). This method yields a point estimate (θ) and 95% confidence intervals (95% CIs) for the shift parameter.
Inclusion Criteria
Animals were included if: (1) the presurgery neurologic score was <1; (2) experiments followed protocol; and (3) they showed signs of occlusion, e.g., neurologic symptoms at reperfusion (where applicable) and reduction in CBF velocity. Animals were killed where evidence indicated subarachnoid haemorrhage or pain after surgery. Exclusions were made before analysis of results.
Results
Part 1: Drug Selection Process
Of 1,026 candidates (O'Collins et al, 2006), 250 treatments approved by the FDA for use in humans (not necessarily for stroke) were selected for further consideration. The panel further reduced this to 45 interventions after considering the potential adverse effects on blood pressure and sedation. After eliminating drugs lacking intravenous preparations, consideration was given to efficacy, safety, and complementary mechanisms.
Magnesium sulphate was chosen to target excitotoxicity, melatonin was included on the strength of its antioxidative properties, and minocycline was selected on the basis of its antiinflammatory effects. No drug was deemed a perfect fit, with concerns regarding the efficacy of magnesium, potential sedation from melatonin, and mixed results from nonstroke neuroprotection models with minocycline.
Part 2: Meta-Analyses of Selected Therapies
Trial Characteristics, Quality, and Range of Testing
Meta-analyses of previously published experiments were undertaken for magnesium, melatonin, and minocycline. Study characteristics are given in Table 3.
Table 3. Characteristics of the experiments included in the meta-analysis.
| Factor | Magnesium | Melatonin | Minocycline |
|---|---|---|---|
| Sample size | |||
| Total number of experimental comparisons | 26 Experiments | 42 Experiments | 34 Experiments |
| Total number of animals in the treatment group | 245 Animals | 366 Animals | 425 Animals |
| Total number of animals in the control group | 270 Animals | 392 Animals | 412 Animals |
| Mean sample size in the treatment group | 9 Animals | 9 Animals | 13 Animals |
| Mean sample size in the control group | 10 Animals | 9 Animals | 12 Animals |
| Mean sample size in the control group adjusted for the number of treatment groups | 5 Animals | 5 Animals | 7 Animals |
| Species: strain (number of experiments) | |||
| Mice | 1 | 6 | 15 |
| Gerbil | 2 | 0 | 0 |
| Rat: Sprague Dawley | 14 | 27 | 14 |
| Rat: Wistar | 8 | 9 | 4 |
| Rat: Fisher 344 | 1 | 0 | 0 |
| Rat: SHR | 0 | 0 | 1 |
| Sex (number of experiments) | |||
| Male | 24 | 35 | 32 |
| Female | 0 | 2 | 0 |
| Both | 0 | 0 | 0 |
| Not stated/not known | 2 | 5 | 2 |
| Risk factors (number of experiments) | |||
| Diabetes | 0 | 0 | 0 |
| Hypertension | 0 | 0 | 1 |
| Agea | 2 | 0 | 0 |
| Median time of drug delivery (after stroke) | −15 minutes | −1 minute | 60 minutes |
| Experimental quality (number of experiments) | |||
| Randomisation | 21 | 11 | 8 |
| Blinded surgery | 7 | 8 | 2 |
| Blinded assessment of outcome | 12 | 8 | 8 |
| Peer-reviewed publication | 25 | 42 | 34 |
| Temperature controlled | 24 | 39 | 28 |
| Animals: aged or comorbidities | 1 | 0 | 1 |
| Conflict of interest statement | 0 | 0 | 3 |
| Funding statement | 11 | 32 | 26 |
| Ethics approval | 22 | 33 | 28 |
| Sample-size calculation | 0 | 0 | 0 |
| Average quality (s.d., range) | 4.7 points (1.5, 0–7) | 4.1 points (1.0, 2–6) | 4.1 points (1.4, 2–8) |
SHR, spontaneous hypertensive rat.
Weight, not age was generally reported for animals studies. If age was not reported, it was assumed that young adult animals were used in the experiment. ‘Young' is defined as <6 months of age.
The study quality ranged from 0 to 8 points, with medians of 4.1 to 4.7 (Table 3). The use of animals with comorbidities was low, and no study undertook sample-size calculations. Randomisation was common place only in the magnesium experiments, and reported levels of blinding during surgery and outcome assessment were low.
Drugs scored 8 to 9 on the range of testing checklist (Table 4). Only melatonin was also tested in female animals. Only magnesium was tested in aged rats (Davis et al, 1997). Only minocycline was tested in hypertensive animals (Murata et al, 2008).
Table 4. Range of testing across focal ischaemia experiments.
| Factors | Description | Magnesium | Melatonin | Minocycline |
|---|---|---|---|---|
| Laboratory | Tested in 2+ independent laboratories | ✓ | ✓ | ✓ |
| Species | Tested in 2+ species | ✓ | ✓ | ✓ |
| Comorbidities | Tested in old or diseased animals | ✓ | × | ✓ |
| Sex | Tested in males and females | × | ✓ | × |
| Reperfusion | Tested in temporary and permanent occlusions | ✓ | ✓ | ✓ |
| Time window | Administered at least 1 hour after occlusion | ✓ | ✓ | ✓ |
| Dose response | Administered at 2+ doses | ✓ | ✓ | ✓ |
| Delivery | Feasible mode of delivery | ✓ | ✓ | ✓ |
| End point | Both behavioural and histologic outcomes | ✓ | ✓ | ✓ |
| Long term | Outcome measured at 4+ weeks | × | × | ✓ |
| Total | 8 | 8 | 9 |
Overall Reported Efficacy
Magnesium
The effect of magnesium on infarct size ranged from 61% reduction in infarct size to 44% expansion compared with the control condition (6 of 26 treated groups had larger infarcts than did their controls). Overall, magnesium had a treatment effect of 25.9% (M=25.9%, s.d.=2.2%, 95% CI=23.6% to 28.1%, N=26). The effect was lower for chloride salt (M=8.9%, s.e.=4.8%, 95% CI=−0.6% to 18.2%, N=5) than for sulphate salt (M=30.7%, s.e.=2.6%, 95% CI=−25.7% to 35.7%, N=5), consistent with the hypothesis that magnesium chloride may give rise to hyperglycaemia (Muir and Lees, 1998).
Melatonin
Individual outcomes for melatonin experiments ranged from a 10% to 76% reduction in infarct size in the treated group compared with control. When combined in the meta-analysis, this yielded an overall effect of 40% (M=40.0%, s.d.=2.0%, 95% CI=38.0% to 42.1%).
Minocycline
The experimental efficacy of minocycline ranged from −35% to 69% (2 studies in 33 yielded negative effects), with an overall effect size of 30.6% (M=30.6%, s.d.=1.7%, 95% CI=28.9% to 32.3%).
No evidence of publication bias was detected for the three drugs.
Influence of Experimental Characteristics
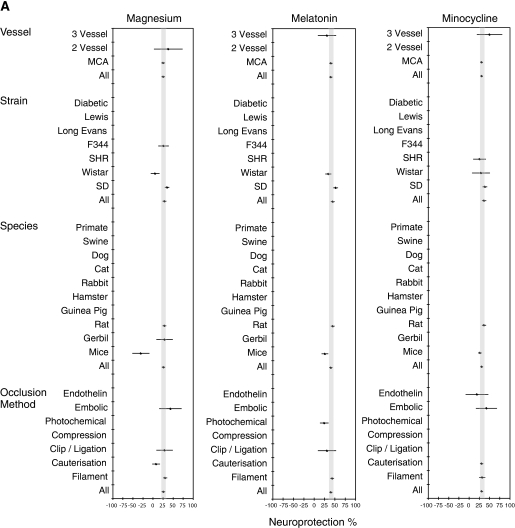
Subgroup analyses were undertaken to investigate efficacy under different experimental conditions. Findings displayed in forest plots (Figure 2A) reveal that drugs were tested under a narrow range of conditions. A limited range of strains and species were used, with experiments conducted only in rats, gerbils, or mice. For all drugs, efficacy was higher in Sprague Dawley than in Wistar rats and outcomes were better in rats than in mice. When tested in mice, magnesium was detrimental but that single study used magnesium chloride. All drugs performed better in models in which reperfusion was possible (e.g., filament models, endothelin models, embolic models) compared with permanent occlusion models (e.g., cauterisation, models, clip, or ligation models).
Figure 2.
Results from meta-analyses of historical data from animal stroke experiments testing magnesium, melatonin or minocycline. (A) Meta-analysis of stroke model characteristics on treatment efficacy. (B) Meta-analysis of drug delivery characteristics on treatment efficacy. Results from the meta-analyses of previously published experiments testing the efficacy of the drugs individually are displayed graphically in forest plots. On the vertical axes of the plots are given the characteristics for which subgroup analyses were performed. The horizontal axes depict the level of efficacy in the animal models of stroke, from −100% (detrimental) through 0 (no effect) to 100% (complete protection of the infarct). The grey bars depict the 95% confidence intervals bounding the mean effect size for each treatment. Where no experimental data were identified for a particular experimental condition, no point estimate of efficacy is shown. For the purpose of comparison with standard drug testing methods (O'Collins et al, 2006), categories are included even where no testing has been undertaken. ICV, intracerebroventricular; MCA, middle cerebral artery; SHR, spontaneous hypertensive rat.
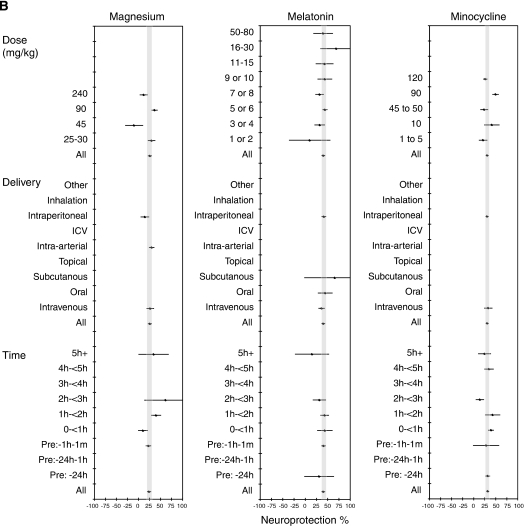
Influence of Treatment Characteristics
Subgroup analyses probed the effect of dose, delivery, and time of administration on drug efficacy (Figure 2B). Magnesium was effective at 25 to 30 mg/kg and at 90 mg/kg, with reduced efficacy at 240 mg/kg and a negative effect at 45 mg/kg. This suggested a complex dose–response relationship (Meloni et al, 2006) or confounding by other factors. However, there was some discrepancy with how the dose was reported, and most studies did not specify whether the anhydrous form was used.
Melatonin was effective at all doses tested, with the 95% CIs above 0 for a dose of 3 to 4 mg/kg or higher, with the maximum effect at 16 to 30 mg/kg. For minocycline, doses up to 10 mg/kg were administered intravenously and above this level intraperitoneally. Therefore, these findings were consistent with two separate dose–response curves—one for each mode of delivery—with 10 mg/kg intravenous and 90 mg/kg intraperitoneal being the most effective.
The maximum efficacy for magnesium was observed when delivered at 2 to 3 hours after occlusion. Melatonin showed a decline in efficacy when administered beyond 1 hour. The greatest response for minocycline was at 4 to 5 hours, with 2 to 3 hours showing the least response.
Magnesium was typically administered with saline, but in four cases, had been delivered with either water (N=1) or dextrose in saline or water (N=3). For melatonin, Tween vehicle resulted in the greatest efficacy (M=59.5%, 95% CI=37.0% to 82.0%, N=7) and ethanol or polyethylene glycol the lowest (M=35.2%, 95% CI=29.5% to 41.0%, N=16), with saline and dimethyl sulfoxide having intermediate results. Tween has been shown to increase the absorption of lipid soluble drugs (Anello and Levy, 1969); hence, it may have increased the bioavailability of melatonin. Minocycline was sometimes administered with water and sometimes with saline as a vehicle, with results supporting saline as the preferred option (saline: M=31.5%, 95% CI=1.8% to 28.0%, N=28; water: M=17.4%, 95% CI=2.8% to 32.1%, N=4).
Magnesium had little effect on infarct size in adult rats, and increased infarct size in aged rats; however, these experiments were undertaken with magnesium chloride not with magnesium sulphate (Davis et al, 1997). One minocycline study was conducted in hypertensive animals with outcome 5% worse than overall estimates of efficacy (Murata et al, 2008); however, drug delivery was at 4 hours after stroke.
Summary of Meta-Analysis Data
Overall, the drugs met the efficacy criteria but not unreservedly: each drug reduced the infarct size in stroke models, but the findings are limited by the narrow time windows and by scant testing in animals with comorbidities, such as hypertension. This is a common limitation across the field of preclinical testing.
Part 3: Experimental Testing of the Drug Combination
The first experiment was designed to test for a pharmacokinetic interaction between magnesium and minocycline in aged, hypertensive animals subjected to cerebral ischaemia. Three experiments then tested the efficacy of the combination (magnesium, melatonin, and minocycline) in successively less damaging models. It was hypothesised that animals receiving the combination therapy would have reduced infarct volumes and superior secondary outcomes than would those in the control condition. Post hoc comparisons were also made between different experiments to explore effects of age (experiments 2 versus 3) and stroke severity (experiments 3 versus 4).
Experiment 1: Test for Drug Interaction in Aged Rats
Owing to a potential effect of the uptake of magnesium on minocycline, an experiment was undertaken to test for the effect of magnesium on the bioavailability of minocycline. Plasma levels of magnesium and minocycline (Colovic and Caccia, 2003) were determined from blood collected through a cardiac puncture at the time of killing, i.e., 24 hours after ischaemia onset and drug delivery. There was no significant difference (t=−0.97, P>0.05, N=14) between plasma levels of minocycline (microgram/millilitre plasma) in the group receiving only minocycline (M=1.39, s.d.=0.8, N=7), compared with animals receiving a combination of magnesium, melatonin, and minocycline (M=1.02, s.d.=0.6, N=7). Brain concentrations were not measured, but these were expected to be linearly related to plasma levels for minocycline (Colovic and Caccia, 2003).
Experiment 2: Efficacy in Aged Rats With Large Strokes
The efficacy of the combination (magnesium, melatonin, and minocycline) was tested against a melatonin group and a saline control, with delivery starting 3 hours after occlusion. Owing to cost, only melatonin was tested individually: melatonin was chosen as the single-drug control because we had undertaken a meta-analysis of melatonin (Macleod et al, 2005), and at the time, its efficacy was superior to magnesium and minocycline.
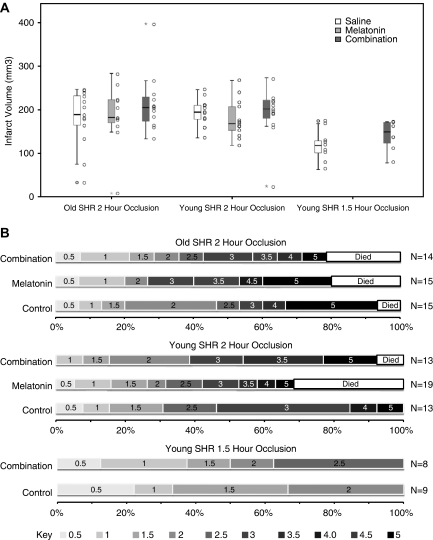
Combination treatment failed to reduce infarct volume in aged rats subjected to a 2-hour occlusion (F(2, 36)=0.425, P>0.05, partial eta square=0.051). No shift in the distribution of scores was observed between control and combination groups for either the neurologic score (θ=0, 95% CI=−1.5 to 1.5) or for the homeostasis score (θ=0, 95% CI=−1 to 0). The infarct results are presented graphically in Figure 3. Summary statistics for primary and secondary end points for experiments 2 to 4 are given in Table 5.
Figure 3.
Effect of drug combination on primary end points. (A) Infarct volume: box plots depict infarct volumes for each treatment group in experiment 2 old rats with 2-hours occlusion, in experiment 3 young rats with 2-hour occlusions, and in experiment 4 young rats with 1.5-hour occlusions. Columns of dots to the right of each box plot represent values from each individual animal in the treatment group. Refer to Table 5 for the exact values of M, s.e., and N for each group. (B) Neurologic score: horizontal bars represent the distribution of neurologic scores at 24 hours after occlusion. Each individual bar represents the distribution of outcomes for a single treatment group within each experiment. The greater the width of the bar for any one colour, the greater the proportion of animals who had that score. Better outcomes (lower scores) are represented by lighter greys. Worse outcomes (higher scores) are represented by darker greys. Death is represented by white bars.
Table 5. Primary and secondary end points.
| Outcome | Experiment 2 | Experiment 3 | Experiment 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Old rats | Young rats | Young rats | |||||||||
|
2-hour occlusion |
2-hour occlusion |
1.5-hour occlusion |
|||||||||
| Treatment group | C | Mel | Com | All | C | Mel | Com | All | C | Com | All |
| Infarct volume (mm3) | |||||||||||
| Mean | 181 | 187 | 215 | 194 | 194 | 178 | 192 | 188 | 118 | 142 | 130 |
| s.e. | 18 | 22 | 20 | 11 | 9 | 12 | 17 | 7 | 14 | 13 | 9 |
| N | 13 | 11 | 12 | 36 | 13 | 13 | 12 | 38 | 9 | 8 | 17 |
| Neurologic score (0–6, 0=normal) | |||||||||||
| Mean | 3.1a | 3.6a | 3.2 | 3.3 | 2.6a | 2.9a | 3.2a | 2.9 | 1.4a | 1.7a | 1.5 |
| s.e. | .5 | 0.5 | 0.5 | 0.4 | 0.3 | 0.4 | 0.4 | 0.2 | 0.2 | 0.3 | 0.2 |
| N | 15 | 15 | 14 | 44 | 13 | 19 | 13 | 45 | 9 | 8 | 17 |
| General health (0–30, 0=normal) | |||||||||||
| Mean | 8.3 | 5.0 | 6.0 | 6.6b | 5.8a | 5.1 | 4.1a | 5.0b | 6.0a | 2.3a | 4.3 |
| s.e. | 3.5 | 0 | 1.0 | 1.0 | 2.1 | 2.2 | 0.1 | 1.0 | 3.0 | 0.7 | 1.6 |
| N | 3 | 2 | 3 | 8 | 13 | 13 | 13 | 39 | 9 | 8 | 17 |
| Physiologic homeostasis (0–4, 0=normal) | |||||||||||
| Mean | 1.0 | 1.1 | 0.8 | 1.0b | 0.2 | 0.6 | 0.3 | 0.4b | 0.7 | 0.5 | 0.6 |
| s.e. | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 |
| N | 13 | 10 | 9 | 32 | 9 | 10 | 6 | 25 | 6 | 6 | 12 |
| Mortality | |||||||||||
| N (Died) | 1 | 3 | 3 | 7 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| N (Total) | 15 | 15 | 14 | 44 | 13 | 13 | 13 | 39 | 9 | 8 | 17 |
All, all treatment groups in the experiment; C, control group; Com, combination treatment group (magnesium, melatonin, and minocycline); M, mean; Mel, melatonin group; N, number of animals.
Shifts were seen between the different treatment groups within experiments, but the confidence intervals were wide and also consistent with no effect. The general health scale was only developed during experiment 2; consequently, there are only a few animals scored along this scale. It was designed in response to the problems associated with the use of aged animals. Complete physiologic records were not available for all animals at 24 hours; consequently, homeostasis scores are not available for all animals.
Evidence of shift in distribution using shift analysis in combined groups in experiments 2 and 3 (aged versus young animals).
Experiment 3: Efficacy in Young Rats With Large Strokes
The third experiment sought to replicate the second experiment, but using younger rats. Each animal received the control condition (saline), the single drug (melatonin), or the drug combination at 3 hours after occlusion, with animals killed at 24 hours after occlusion. Treatment failed to elicit a difference in infarct volume between groups (F(2, 38)=0.435, P⩾0.05, partial eta square=0.024). Combination treatment increased (worsened) the point estimate of the neurologic score by 0.5 compared with control (θ=0.5, 95% CI=−1 to 2) and to melatonin (θ=0.5, 95% CI=−1 to 1.5), but the CIs were wide. General health declined by 1 point in the combination group compared with control (θ=−1, 95% CI=−3 to 2), but the CIs were wide. There was no shift in the homeostasis score between control and combination groups (θ=0, 95% CI=−1 to 1) nor between single drug and combination groups (θ=0, 95% CI=−1 to 0).
Experiment 4: Test for Efficacy in Young Rats With Small Strokes
Owing to concerns that the bar was still too high, efficacy was tested again in young rats, but this time using a 90-minute occlusion with a shorter filament (2 mm length) and with treatment starting immediately after occlusion. It was anticipated that by reducing stroke severity and by delivering the drugs early that neuroprotection might be shown. Melatonin was not tested alone because the combination had not shown any effect in earlier experiments, let alone an additive effect over the single treatment.
Average infarct volumes were substantially lower under these conditions. However, again no difference in volume was found between the saline control and combination groups (F(1, 17)=1.94, P=0.184, partial eta square=0.115). Combination treatment was linked to a small nonsignificant increase in neurologic score (θ=0.5, 95% CI=−0.5 to 1). There was a shift towards the combination group having a better general health outcome, but the CIs were wide (θ=−1, 95% CI=−3 to 1). There was no difference in homeostasis between treatment groups (θ=0, 95% CI=−1 to 0).
Regression analysis was used to determine whether the drug combination significantly affected infarct volume after CBF velocity changes after occlusion were taken into account. Both within individual experiments and across all experiments, the combination was not found to be a significant predictor of outcome, nor was CBF velocity decrease itself related to infarct volume.
Effect of Age on Outcome
Before stroke, there was no difference in the neurologic score in aged versus young rats (θ=0, 95% CI=0 to 0) nor homeostasis (θ=0, 95% CI=0 to 1), but the general health was 1 point worse in aged animals (θ=−1, 95% CI=−4 to −1). At reperfusion, the neurologic score did not differ between old and young rats (θ=0, 95% CI=−0.5 to 0.5). At 24 hours, the neurologic score was worse by half a point in aged rats (θ=−0.5, 95% CI=−1 to 0.5), and homeostasis had declined in aged animals compared with young animals (θ=−1, 95% CI=−1 to 0), but the CIs were also consistent with no change. The general health in aged animals deteriorated by a large 3-point shift after stroke compared with young animals (θ=−3, 95% CI=−5 to −1), but the numbers analysed in the aged group were small. There was no difference in infarct volume between aged animals (194 mm3) and young animals (188 mm3) at 24 hours (F(1, 73)=0.2, P=0.6). Mortality was higher in aged rats (7/44) versus young rats (1/39) (Table 5).
Effect of Stroke Severity on Outcome
There was a significant difference in infarct volume between young animals with a 2-hour occlusion (188 mm3) and a 90-minute occlusion (130 mm3) (F(1, 54)=21.7, P<0.001, partial eta square=0.29). Mortality did not differ between longer (1/39) and shorter (0/17) occlusions, nor did homeostasis at 24 hours (θ=0, 95% CI=0 to 1). However, at 24 hours, the neurologic score was worse in animals with long occlusions (θ=−1.0, 95% CI=−2 to −0.5), and general health was also poorer in this group, although the CIs were wide (θ=−1, 95% CI=−2 to 1).
Discussion
A pragmatic approach was adopted in this study. To improve prospects at the clinical trial, we sought to test a combination of drugs in a clinically relevant animal model so that poor candidates could be eliminated early. We mined the existing literature for inexpensive, safe, and readily available agents which—when combined—might increase efficacy. We chose interventions from the pool of 1,026 candidates tested in previous animal studies. Three drugs were selected because they targeted complementary mechanisms in the ischaemic cascade, and because they were inexpensive, purportedly effective in animal models, safe, stable, easily administered, and readily available.
Testing was initiated under conditions that sought to meet the gold standard at the time experimentation was started—the STAIR (1999) recommendations. When this failed, the drugs were tried in a less severe stroke model to see whether neuroprotection might be ‘reinstated' to levels typically found in the literature. However, even with smaller lesions and prompt drug delivery, neuroprotection was still not shown. Three reasons for the failure are considered, one relating to the drugs, the second to the animal model, and the third to the experimental method.
Explanation 1: Failure Attributable to Lack of Drug Efficacy
A parsimonious explanation for the results is simply that the combination of magnesium, melatonin, and minocycline does not work. Drugs were selected after a broad review of neuroprotective agents: The drugs looked good on paper, but with hindsight, there were several indications that they might fail to deliver:
1. Relative Frame of Reference: In an earlier review, it was found that the average neuroprotective efficacy across hundreds of interventions was ∼25% (O'Collins et al, 2006). However, the dearth of clinically effective neuroprotectants suggests that some of this overall effect may be attributed to experimental ‘noise' or publication bias. To remove contamination of noise from the literature, a relative frame of reference may be preferable. The drugs here were only marginally superior to the average neuroprotective efficacy of 25%: magnesium (+1%); melatonin (+15%), and minocycline (+6%). Furthermore, when ranked against other interventions on the basis of performance in combination, these three agents were average performers (manuscript in preparation).
2. Inconsistent Results: Not all published results consistently supported the use of magnesium and minocycline. Almost one-quarter of magnesium studies (6 of 26) showed a worsening of infarct size. Furthermore, magnesium was not effective when combined with mexiletine (Lee et al, 1999). The use of minocycline also has its detractors, in part owing to instances of failure in nonstroke animal models. The issue of inconsistency is not an easy one; however, as drugs with narrow therapeutic windows may elicit toxic responses at high doses but still be effective at their optimal dose or in different diseases.
3. Dose Response: Hackam and Redelmeier (2006) reported that animal studies incorporating dose–response gradients were more likely to translate successfully to humans. In this study, both melatonin and minocycline show signs of an increased response to higher doses when the method of administration is taken into account, but they were only tested over a narrow range of doses for each mode of delivery. No clear dose–response relationship was observed in the magnesium meta-analysis, compatible either with a lack of effect or with a complex dose–response relationship (Meloni et al, 2006)). Meta-regression would be preferable to stratified meta-analysis when assessing dose–response relationships (e.g., Minnerup et al, 2008), and comprehensive individual dose–response studies would be better still. In vivo neuroprotection dose–response studies rarely test more than three doses, and perhaps this is an area deserving greater focus. However, for combination trials, dose–response studies may prove difficult logistically.
4. Time Response: It is believed that treatments are generally more effective if administered early after ischaemic injury, and that the relevant targets may change as time after stroke progresses. Findings from the meta-analysis were not entirely consistent with the idea that each drug targets a consecutive point in the ischaemic cascade. Magnesium was least effective immediately after stroke, contrary to what would be expected if its main target is acute excitotoxicity. Minocycline is believed to counter the inflammatory processes that evolve over longer periods than the initial excitotoxic surge; however, the minocycline meta-analysis did not show a clear change in therapeutic response with time. In reality, the ischaemic cascade may not easily be subdivided into discrete temporal blocks relating to the damage caused by excitotoxicity, oxidation, or inflammation, and each of the drugs may have broader actions on the ischaemic cascade than generally emphasised. Nevertheless, a temporal response curve consistent with the pathophysiology of stroke and with known time courses for the drug target may help drug selection. Testing of the temporal response should occur at relevant time frames; only minocycline had a median time to delivery that occurred after the onset of ischaemia in the previously published data (Table 3).
5. Synergistic Interactions: The potential of these drugs to interact synergistically and increase the effectiveness of other agents was only average when rated against other drugs (O'Collins, 2010 personal communication—it must be noted that this information was not available at the time of drug selection). Lower synergism might result from these agents being tested with other highly effective agents already operating near the ceiling for neuroprotection; nevertheless, synergism may be a useful indicator of therapeutic potential.
6. Experimental Quality: Bias can influence experimental results, and this may have a negative impact on translation into positive clinical trial outcomes (Macleod et al, 2008; Sena et al, 2007a; Philip et al, 2009). No previous studies using magnesium, melatonin, or minocycline reported having undertaken the full gamut of randomisation, blinded surgery, blinded outcome, and sample-size calculations. Randomisation and blinding of surgery and of outcome assessment was undertaken in 27% of magnesium experiments, in 17% of melatonin experiments, and in 6% of minocycline experiments.
7. Range of Testing: The range of testing was high across the published experiments, but the test criteria may have been too readily satisfied and some criteria may be more important than others (e.g., comorbidities). Only magnesium sulphate has previously been tested in aged animals, and only minocycline has been tested in a hypertensive model.
In summary, the results of this study suggest that this combination does not work, and a more detailed reading of the results of the meta-analyses might have anticipated a negative outcome. However, this does not preclude the possibility that other combinations containing these agents might not be effective.
Explanation 2: Failure Attributable to the Stroke Model
The second broad explanation for the results is that when the stroke is large or attempts are made to model comorbidities such as hypertension, then neuroprotective potential is lost. At first, it was believed that age might have a large bearing on outcome and that by shifting to younger animals, neuroprotection would be observed. However, even in younger animals, neuroprotection was not shown. Next, testing was undertaken in a model with smaller infarcts as the ‘malignant' infarct induced in the 2-hour occlusion model may be too severe to permit a therapeutic effect (animals experienced damage to most of their middle cerebral artery territory, with the damage sometimes extending into the lateral hypothalamus). Consequently, a thread with a smaller tip was used to induce inclusion for a shorter duration with the idea that it would be less likely to block the superior hypophyseal artery where it branches off the internal carotid artery (proximal to the middle cerebral artery). However, even when a smaller stroke was obtained, no protection was observed. Rectal temperature was similar across all groups and experiments, suggesting that damage to the lateral hypothalamus did not aggravate injury through disruptions to temperature regulation (or if it did, it was obscured by other factors). All animals used were of the spontaneous hypertensive rat strain; hence, it may be that hypertension was an important moderator of outcome.
Explanation 3: Bias and Imprecision in the Experiment
The drug combination may have the potential to work, but not as used in this study owing to the preparation, dose, or delivery of the drugs, or owing to a pharmacokinetic interaction affecting the concentration of drugs. We adopted a pragmatic approach by using the maximally effective doses also considered to be safe in humans, and this may have swamped any potential efficacy. The alternate of using minimally effective doses may better reveal potential synergism.
The true effect size may have been smaller than this study was powered to detect. All surgery and infarct analyses were undertaken without knowledge of treatment allocation; however, the behavioural analysis of several animals in experiment 4 was not blinded by staff availability.
An argument exists that neuroprotection could never be established by us in this model because no reduction in infarct volume was seen in single-drug (melatonin) conditions in aged and young rats with large stroke at 3 hours delay. Against this argument is the consideration that the experimental conditions of this study did not precisely replicate those of other publications in which neuroprotection was achieved (in particular, by blinding, randomising, using hypertensive animals, and by delivering at 3-hour time windows). In addition, a marked reduction was seen in infarct volume in the 2-hour occlusion model (experiments 2 and 3) versus the 90-minute occlusion model (experiment 4), suggesting that reduction in infarct damage is possible in this model, if only by reperfusion.
Age, Stroke, and Neuroprotection
No differences in infarct volume and overall neurologic score were noted between aged and young rats, although this may be owing to the focus on acute effects. Evolution of histochemical changes have been found to differ with age (Badan et al, 2003b) and after several weeks, young rats may make total recovery whereas, in aged rats, recovery may only be partial (Badan et al, 2003a) (long-term changes were not monitored in this study because of the high mortality at days 2 to 3 in aged rats). Furthermore, the use of an aggregate score may mask age-related differences because aged rats were much more likely to have a high score owing to inactivity, whereas young rats tended to have a higher score owing to reflexive activity (forelimb flexion, torso twisting). Measurements of mortality, homeostasis, and general health as used in this study might better reflect disability than infarct volume and a scale based heavily dependent on forelimb function.
Validity of the Combination Neuroprotection Approach
Neuroprotection rests on the assumption that there exists some region of the salvageable tissue or penumbra (Marchal et al, 1996). Heiss et al (1999) observed that most tissue damage occurs rapidly after the reduction in blood supply, with only a relatively small amount being attributable to secondary and delayed mechanisms. In this study, we targeted excitotoxicity, inflammation, and oxidative stress, but unlike the role of blood flow in clinical stroke, neuroprotective targets are still in need of solid clinical data. However, the fact that we failed to achieve protection by targeting excitotoxicity, oxidative stress, and inflammation does not invalidate these targets, just the particular combination of agents. Visualisation of molecular targets through advances in imaging, and better characterisation of the multimodal nature of drugs will improve the selection and implementation of therapeutic strategies.
Methods for Drug Selection
The method for selecting drugs applied in this study may need some refinement, with a greater emphasis on proof of efficacy. As noted by the European Ad Hoc Consensus (1998), ‘A treatment that achieves a good functional outcome is the most cost-effective approach' (p. 59). The decision to fulfil other criteria (such as price, safety, availability, stability, etc.) might have been made at the expense of efficacy. Of the 1,026 candidate interventions, many were eliminated because of the absence of regulatory approval (FDA). Only six drugs survived the filtration process—magnesium, melatonin, minocycline, albumin, erythropoietin, and tacrolimus—suggesting that screening was too tight. Of the 1,026 candidates, many might work well together.
As noted above, if meta-analysis is to be a useful tool in drug selection, then the results need to be read in the context of the following factors: (1) efficacy relative to all previously tested drugs; (2) inconsistent results for the particular drug; (3) dose–response relationships; (4) time–response relationships; (5) synergistic interactions; (6) experimental quality; and (7) range of testing.
It is not clear to what extent meta-analysis can guide drug selection when the conditions under which one intends to test differ from those under which meta-analysis studies were conducted. Preclinical data for magnesium, melatonin, and minocycline contained only one study using hypertensive animals; indeed, comorbidities are generally underrepresented in animal experiments compared with their clinical prevalence. A further limitation of meta-analysis as a guide to drug selection is the manner by which it deals with systematic difficulties, such as smaller variance in the spontaneous hypertensive rat strain. New techniques are evolving which may be better equipped to deal with variance and uncertainty (Sutton and Higgins, 2008). In addition, meta-analyses are only as good as the results and the tests purporting to measure the treatment effect.
STAIR Guidelines
This study attempted to follow the STAIR (Stroke Therapy Academic Industry Roundtable) guidelines; however, it may be useful to operationalise the guidelines more strictly than was done here. For example, the range of testing for magnesium, melatonin, and minocycline was 8 to 9/10, suggesting that a simple checklist approach (testing in two species, two laboratories, etc.) is too readily satisfied (O'Collins et al, 2009). Additional considerations might also be useful, e.g., interpretation of experimental findings in a relative frame of reference taking into account the overall body of neuroprotective work, spotlighting inconsistent results, and looking for synergistic interactions with other agents. One of the most important criteria—described by Feuerstein and Chavez (2009) and Feuerstein et al (2008) as a ‘missing step' in STAIR—is to characterise the target.
Conclusions
The experiments failed to support the use of magnesium, melatonin, and minocycline in combination at these doses, at these delays, and using this method of delivery in this model of cerebral ischaemia. These findings do not preclude the possibility that any one of the drugs may still be useful in combination with some other intervention, or in patients, but is on balance unlikely. Caution needs to be exercised when assessing published data for selecting drugs. The challenge remains to raise the bar sufficiently high, so that only useful drugs are taken forward to further testing, but not so high that many potentially useful agents are excluded.
Acknowledgments
The authors most grateful for the wisdom and assistance of the following people who helped with animal experimentation and/or drug evaluation: Peter Batchelor, Brian Chambers, Helen Dewey, John Fernandez, Amy Jeffries, Lisa Hogan, Laura Horky, Gabrielle Liberatore, Anna Marcon, Romesh Markus, Ian Mosley, Sarah Rewell, Emily Sena, Neil Spratt, Velandai Srikanth, Bart van der Worp, John Williams, Dennis Young, Jorge Zavala. The following people participated in the drug selection process: Geoffrey Donnan, Laura Horky, David Howells, Malcolm Macleod, Gabrielle Liberatore,Victoria O'Collins, Neill Spratt, Velandai Srikanth, Dennis Young, John Williams, and Jorge Zavala. Victoria O'Collins was the recipient of an APA scholarship.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Adams HP, Adams RJ, Brott T, Del Zoppo GJ, Furlan AJ, Goldstein LB, Grubb RL, Higashida R, Kidwell CS, Kwiatkowski TG, Marler JR, Hademenos GJ. Guidelines for the early management of patients with ischemic stroke. A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–1108. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- Anello JA, Levy G. Effect of complex formation on drug absorption. 10 Effect of polysorbate 80 on permeability of biologic membranes. J Pharm Sci. 1969;58:721. doi: 10.1002/jps.2600580616. [DOI] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003a;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Badan I, Platt D, Kessler C, Popa-Wagner A. Temporal dynamics of degenerative and regenerative events associated with cerebral ischemia in aged rats. Gerontology. 2003b;49:356–365. doi: 10.1159/000073763. [DOI] [PubMed] [Google Scholar]
- Barry AL, Badal RE. Stability of minocycline, doxycycline, and tetracycline stored in agar plates and microdilution trays. Curr Microbiol. 1978;1:33–36. [Google Scholar]
- Beers MH.2003The Merck Manual of Medical Information2nd home ed.Whitehouse Station, NJ: Merck Research Laboratories [Google Scholar]
- Cavallo A, Hassan M. Stability of melatonin in aqueous solution. J Pineal Res. 1995;18:90–92. doi: 10.1111/j.1600-079x.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovic M, Caccia S. Liquid chromatographic determination of minocycline in brain-to-plasma distribution studies in the rat. J Chromatogr B. 2003;791:337–343. doi: 10.1016/s1570-0232(03)00247-2. [DOI] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. The search for neuroprotective strategies in stroke. AJNR Am J Neuroradiol. 2004;25:181–194. [PMC free article] [PubMed] [Google Scholar]
- Davis M, Perry RH, Mendelow AD. The effect of non-competitive N-methyl-D-aspartate receptor antagonism on cerebral oedema and cerebral infarct size in the aging ischaemic brain. Acta Neurochir Suppl (Wien) 1997;70:30–33. doi: 10.1007/978-3-7091-6837-0_9. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trial. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- European Ad Hoc Consensus Group European Ad Hoc Consensus Group: Neuroprotection as initial therapy in acute stroke. Third Report of an Ad Hoc Consensus Group Meeting. Cerebrovasc Dis. 1998;8:59–72. doi: 10.1159/000015817. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Chavez J. Translational medicine for stroke drug discovery. The pharmaceutical industry perspective. Stroke. 2009;40:S121–S125. doi: 10.1161/STROKEAHA.108.535104. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Zaleska MM, Krams M, Wang X, Day M, Rutkowski JL, Finklestein SP, Pangalos MN, Poole M, Stiles GL, Ruffolo RR, Walsh FL. Missing steps in the STAIR case: a Translational Medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28:217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- Fisher M. Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003;34:1539–1546. doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. doi: 10.1001/jama.296.14.1731. [DOI] [PubMed] [Google Scholar]
- Hass WK. The cerebral ischemic cascade. Neurol Clin. 1983;1:345–353. [PubMed] [Google Scholar]
- Heiss WD, Graf R. The ischemic penumbra. Curr Opin Neurol. 1994;7:11–19. doi: 10.1097/00019052-199402000-00004. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A, Grond M, Graf R. Which targets are relevant for therapy of acute ischemic stroke. Stroke. 1999;30:1486–1489. doi: 10.1161/01.str.30.7.1486. [DOI] [PubMed] [Google Scholar]
- Kilic A.2004Preparation of melatonin for use in ischemia experimentsPersonal communication
- Kilic E, Ozdemir YG, Bolay H, Kelestimur H, Dalkara T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J Cereb Blood Flow Metab. 1999;19:511–516. doi: 10.1097/00004647-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema, I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area [reference unverified] Jnp J Stroke. 1986;8:1–8. [Google Scholar]
- Lee EJ, Ayoub IA, Harris FB, Hassan M, Ogilvy CS, Maynard KI. Mexiletine and magnesium independently, but not combined, protect against permanent focal cerebral ischemia in Wistar rats. J Neurosci Res. 1999;58:442–448. [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res. 2005;38:35–41. doi: 10.1111/j.1600-079X.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39:2824–2829. doi: 10.1161/STROKEAHA.108.515957. [DOI] [PubMed] [Google Scholar]
- Marchal G, Beaudouin V, Rioux P, de la Sayette V, Le Doze F, Viader F, Derlon JM, Baron JC. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke. 1996;27:599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- Meloni BP, Zhu H, Knuckey NW. Is magnesium neuroprotective following global and focal cerebral ischaemia? A review of published studies. Magnes Res. 2006;19:123–137. [PubMed] [Google Scholar]
- MIMS . Crows Nest, Australia: MIMS; 2004. Monthly Index of Medical Specialities (Australia) [Google Scholar]
- Minnerup J, Heidrich J, Wellmann J, Rogalewski A, Schneider A, Schäbitz WR. Meta-analysis of the efficacy of granulocyte-colony stimulating factor in animal models of focal cerebral ischemia. Stroke. 2008;39:1855–1861. doi: 10.1161/STROKEAHA.107.506816. [DOI] [PubMed] [Google Scholar]
- Muir KW, Lees KR. Dose optimization of intravenous magnesium sulfate after acute stroke. Stroke. 1998;29:918–923. doi: 10.1161/01.str.29.5.918. [DOI] [PubMed] [Google Scholar]
- Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Collins VE, Donnan GA, Macleod MR, Howells DW. Scope of preclinical testing versus quality control within experiments. Stroke. 2009;40:e497. doi: 10.1161/STROKEAHA.109.550335. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Peruche B, Krieglstein J. Mechanisms of drug actions against neuronal damage caused by ischemia—an overview. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:21–70. doi: 10.1016/0278-5846(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Petullo D, Masonic K, Lincoln C, Wibberley L, Teliska M, Yao DL. Model development and behavioral assessment of focal cerebral ischemia in rats. Life Sci. 1999;64:1099–1108. doi: 10.1016/s0024-3205(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Philip M, Benatar M, Fisher M, Savitz SI. Methodological quality of animal studies of neuroprotective agents currently in phase II/III acute ischemic stroke trials. Stroke. 2009;40:577–581. doi: 10.1161/STROKEAHA.108.524330. [DOI] [PubMed] [Google Scholar]
- Rogalewski A, Schneider A, Ringelstein EB, Schabitz WR. Toward a multimodal neuroprotective treatment of stroke. Stroke. 2006;37:1129–1136. doi: 10.1161/01.STR.0000209330.73175.34. [DOI] [PubMed] [Google Scholar]
- Saver JL, Kalafut M. Combination therapies and the theoretical limits of evidence-based medicine. Neuroepidemiology. 2001;20:57–64. doi: 10.1159/000054762. [DOI] [PubMed] [Google Scholar]
- Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke. Trends Neurosci. 2007a;30:433–439. doi: 10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Sena E, Wheble P, Sandercock P, Macleod M. Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke. Stroke. 2007b;38:388–394. doi: 10.1161/01.STR.0000254462.75851.22. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, Hogan L, Howells DW. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- STAIR Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Sutton AJ, Higgins JPT. Recent developments in meta-analysis. Statist Med. 2008;27:625–650. doi: 10.1002/sim.2934. [DOI] [PubMed] [Google Scholar]
- Takano K, Tatlisumak T, Bergmann AG, Gibson DG, III, Fisher M. Reproducibility and reliability of middle cerebral artery occlusion using a silicone-coated suture (Koizumi) in rats. J Neurol Sci. 1997;153:8–11. doi: 10.1016/s0022-510x(97)00184-6. [DOI] [PubMed] [Google Scholar]
- Wang D. Hodges-Lehmann estimates of shift parameters. Stata Technical Bulletin. 1999;9:52–53. [Google Scholar]
- Westermaier T, Hungerhuber E, Zausinger S, Baethmann A, Schmid-Elsaesser R. Neuroprotective efficacy of intra-arterial and intravenous magnesium sulfate in a rat model of transient focal cerebral ischemia. Acta Neurochir (Wien) 2003;145:393–399. doi: 10.1007/s00701-003-0013-6. [DOI] [PubMed] [Google Scholar]
- WHO 2009Cardiovascular diseases WHO; Fact sheet no. 317. Last updated September, 2009. . http://www.who.int/mediacentre/factsheets/fs317/en/index.html [Google Scholar]
- Wiebers DO, Adams HP, Jr, Whisnant JP. Animal models of stroke: are they relevant to human disease. Stroke. 1990;21:1–3. doi: 10.1161/01.str.21.1.1. [DOI] [PubMed] [Google Scholar]
- Xu L, Fagan SC, Waller JL, Edwards D, Borlongan CV, Zheng J, Hill WD, Feuerstein G, Hess DC. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 2004;4:7. doi: 10.1186/1471-2377-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.