Abstract
Poststroke hyperglycaemia (PSH) is common, has an unclear pathophysiology, and is associated with poor outcomes. Animal studies report conflicting findings. We systematically reviewed the effects of hyperglycaemia on infarct volume in middle cerebral artery occlusion (MCAO) models, generating weighted mean differences between groups using random effects models summarised as effect size (normalised to control group infarct volume as 100%) and 95% confidence interval. Of 72 relevant papers, 23 reported infarct volume. Studies involved 664 animals and 35 distinct comparisons. Hyperglycaemia was induced by either streptozotocin (STZ, 17 comparisons, n=303) or dextrose (18 comparisons, n=356). Hyperglycaemic animals had infarcts that were 94% larger, but STZ was associated with significantly greater increase in infarct volumes than dextrose infusion (140% larger versus 48% larger). In seven studies, insulin did not significantly reduce infarct size and results were heterogeneous. Although hyperglycaemia exacerbates infarct volume in MCAO models, studies are heterogeneous, and do not address the common clinical problem of PSH because they have used either the STZ model of type I diabetes or extremely high glucose loads. Insulin had a nonsignificant and significantly heterogeneous effect. Further studies with relevant models may inform clinical trial design.
Keywords: blood glucose, cerebrovascular disease, focal cerebral ischaemia, hyperglycaemia, insulin, stroke
Introduction
Poststroke hyperglycaemia (PSH) is common, with up to 68% of patients with acute stroke being documented as having a blood glucose concentration >6.0 mmol/L at some point within 48 hours of stroke symptom onset (Fuentes et al, 2009; Matz et al, 2006; Scott et al, 1999; Yong and Kaste, 2008). Hyperglycaemia in acute stroke is associated with a poor outcome (Baird et al, 2003; Bruno et al, 1999; Candelise et al, 1985; Capes et al, 2001; Fuentes et al, 2009; Weir et al, 1997; Yong and Kaste, 2008), particularly in patients who have not been diagnosed with diabetes. Clinical intervention for PSH has a limited evidence base. The only large clinical trial of acute intervention to date, namely the GIST-UK (UK Glucose-Insulin Stroke Trial), found no significant benefit from insulin therapy started within 24 hours of stroke onset, but had a number of significant flaws that render interpretation difficult (Gray et al, 2007). Clinical guidelines generally advise that blood glucose be monitored, and offer varied recommendations on thresholds for management or optimal treatment regimes. In addition to GIST-UK, a number of studies in other clinical scenarios have raised concerns about the safety of insulin treatment in acutely hyperglycaemic patients, primarily through a high incidence of hypoglycaemia (Finfer and Heritier, 2009; Griesdale et al, 2009). Therefore, it is important to consider whether there is adequate evidence to support intervention in all patient groups before embarking on further large clinical trials. Important considerations include uncertainty about the importance of hyperglycaemia at different times after stroke onset, the balance of risk and benefit in intervention with insulin treatment, whether this differs according to the level of blood glucose, and whether the underlying insulin resistance states (including undiagnosed diabetes and more commonly impaired glucose tolerance) and ‘stress' hyperglycaemia are equally important.
Understanding the pathophysiology of PSH in animal focal ischaemia models may inform clinical study design. However, studies have reported divergent results, e.g., some studies have reported that hyperglycaemia increases infarct growth, whereas others have suggested that high blood glucose may be beneficial (de Courten-Myers et al, 1988; Ginsberg et al, 1987; Kraft et al, 1990; Nedergaard et al, 1987; Prado et al, 1988; Venables et al, 1985; Zasslow et al, 1989). Few clinical studies have addressed pathophysiological processes associated with hyperglycaemia. The possible mechanistic explanations for the adverse effect of hyperglycaemia that have been advanced include higher intracranial bleeding risk after thrombolytic treatment and exacerbation of infarct growth resulting from reduced penumbral survival associated with lactic acidosis (Baird et al, 2003; Parsons et al, 2002; Wahlgren et al, 2008).
We undertook a systematic review of the literature and meta-analysis primarily of studies of hyperglycaemia in animal models of focal ischaemia induced by middle cerebral occlusion (MCAO) and secondarily of the effect of insulin in these models. We aimed at clarifying the effect of hyperglycaemia on infarct volume in models of focal cerebral ischaemia and at elucidating the effect of insulin in such models. We hypothesised that clinically relevant concentrations of blood glucose would not have been widely used in animal studies and that the evidence for insulin affecting infarct volume would be inconsistent.
Materials and methods
Systematic Review
Studies of hyperglycaemia in animal models of MCAO were identified from Ovid Medline (1950 to March 2009) and Embase (1980 to March 2009). The search strategy is specified in Supplementary Appendix 1.
Titles were screened for relevance, and abstracts for all potentially relevant papers were read by one investigator. We also performed hand searches of abstracts of scientific meetings including the 2008 World Stroke Conference, the 1981 to 2009 International Stroke Conferences, the 2005 to 2009 Brain Meetings, the 1992 to 2009 European Stroke Conference, and the Marburg Conferences from 1994 to 1998, and then screened reference lists of identified publications.
Inclusion Criteria
We included models of focal ischaemia induced by MCAO in which data were presented on infarct volume, defined either histologically or on brain imaging. The subset of papers that included data on the use of insulin, while meeting other inclusion criteria, was identified for additional analysis. We excluded models of global or forebrain ischaemia and studies, the data of which did not include volumetric data.
Data Extraction
We extracted data from the included papers on species, strain, gender, and weight of animals, model and timing of ischaemia, presence or absence of reperfusion, number of animals and experimental groups, experimental interventions, method of induction and timing of hyperglycaemia, level of hyperglycaemia, insulin use: timing of outcomes, as well as method of measuring infarct and mean final infarct size and s.d.
Studies that clearly reported the method of inducing hyperglycaemia and volume of final infarction with s.d. were included in further analyses.
If published data were incomplete, we contacted authors to obtain further information (Gisselsson et al, 1999; Kamada et al, 2007; Li et al, 1998c; Martin et al, 2006; Quast et al, 1995; Rizk et al, 2006; Zhang et al, 2003).
Meta-Analysis
Data on infarct volume were recorded. To allow for different species in studies, the effect size was normalised to the mean of the control group (assumed 100%) before analysis in Review Manager 5.0.2 (Cochrane Collaboration; http://www.cochrane.org/) and StatsDirect, version 2.7.3 (StatsDirect Ltd, Cheshire, UK) by means of a DerSimonian–Laird random effects model that expresses the difference between groups as a weighted mean difference for effect size and 95% confidence interval (95% CI). The significance of difference between groups was assessed by partitioning heterogeneity and using the χ2 distribution with n−1 degrees of freedom (d.f.), where n equals the number of groups.
Several stratified analyses were planned. We looked for a differential effect caused by either streptozotocin (STZ) or dextrose. The insulin studies were stratified by the control group (normoglycaemic or hyperglycaemic). We also compared permanent and transient MCAO models. To allow for multiple comparisons, we adjusted the significance level to P<0.02 using the Bonferroni method.
Study Quality
We assessed the quality of the individual papers analysed in this study using a modified version of the CAMARADES (Collaborative Approach to Meta-Analysis and Review of Animal Data in Experimental Stroke) score (Macleod et al, 2004; Sena et al, 2007), omitting a score for neuroprotective properties of anaesthetic agents used, and therefore giving a maximum score of 10 points.
Assessment of Bias
Funnel plots comparing s.e.m. treatment effect with effect size for each study were obtained and analysed by Egger's method to identify possible publication bias (Sterne and Egger, 2001).
Results
Identification of Papers
The initial search produced 1,482 titles that were screened to identify 178 abstracts that were read in detail. From these abstracts, 57 papers were initially identified for data extraction. A further 15 papers were identified by hand searches. This is detailed in the flow chart shown in Figure 1.
Figure 1.
Flow chart of systematic review process.
A total of 22 papers reported data in a format suitable for meta-analysis (Araki et al, 1992; Berger and Hakim, 1989; Bomont and MacKenzie, 1995; Combs et al, 1990; de Courten-Myers et al, 1989, 1994; Duverger and MacKenzie, 1988; Ginsberg et al, 1987; Huang et al, 1996; Kraft et al, 1990; Li et al, 2004; Liu et al, 2007; Nedergaard et al, 1986; Nedergaard, 1987; Nedergaard and Diemer, 1987; Quast et al, 1997; Slivka, 1991; Wei et al, 1997, 2003; Wei and Quast, 1998; Zasslow et al, 1989). One further paper was included when an author kindly contacted us with additional data for analysis (Martin et al, 2006). Six papers had insufficient data to include in the meta-analysis (see Table 1). The remaining papers did not report infarct size, but included measurements of cerebral blood flow (Kawai et al, 1997; Nakai et al, 1988; Zhao et al, 1997), brain biochemistry analysis (Marsh et al, 1986), blood–brain barrier function (Ennis and Keep, 2007), genetic analysis, evaluation of tissue energy states (Chew et al, 1991; Folbergrova et al, 1992; Nedergaard et al, 1987), behavioural tests (Rejdak et al, 2001), and immunohistochemical analysis.
Table 1. Summary of excluded studies.
| Study | Hyperglycaemic agent | No. animals with normal glucose | Size of infarct in normal glucose | No. animals with hyperglycaemia | Size of infarct in hyperglycaemic animals | Reason for exclusion |
|---|---|---|---|---|---|---|
| Kamada et al (2007) | STZ | 6 | 100 (value estimated from graph) | 6 | 300 (value estimated from graph) | Incomplete data on infarct size |
| Rizk et al (2006) | STZ | 6 | 3.02±2.4 | 6 | ‘∼10 × greater' | No report of infarct size |
| Gisselsson et al (1999) | DEX | Unclear | 115 (value estimated from graph) | Unclear | 125 (value estimated from graph) | Incomplete data on animal numbers and infarct size |
| Li et al (1998b) | DEX | None | None | Unclear | 50 | Incomplete data on animal numbers and infarct size |
| Quast et al (1995) | STZ | 7 | 60 (value estimated from graph) | 7 | 400 (value estimated from graph) | Incomplete data on infarct size |
| Zhang et al (2003) | STZ | 6 | 100 (value estimated from graph) | 6 | 500 (value estimated from graph) | Incomplete data on infarct size |
DEX, dextrose; STZ, streptozotocin.
From the 23 papers included, a total of 36 different comparisons of infarct size between hyperglycaemic and normoglycaemic controls after MCAO were described (see Table 2). These experiments used a total of 664 animals. In two cases, two comparisons used the same control group. This is noted below (Table 2). Hyperglycaemia was induced with dextrose infusion or injection in seven cat and one rabbit experiments. In rats, STZ was used in 18 comparisons, whereas dextrose infusion was used in 10. One study had useable data on infarct size but was excluded from analysis because it used a photosensitising model to induce local infarction instead of MCAO (Ginsberg et al, 1987).
Table 2. Characteristics of studies and comparisons included in analysis.
| Comparison | Hyperglycaemic agent | Permanent or reversible MCAO | Species | Strain | Timing of induction of hyperglycaemia in relation to MCAO | Experimental glucose level (mmol/L) | Control glucose level (mmol/L) |
|---|---|---|---|---|---|---|---|
| Araki et al (1992) | Dextrose | Reversible (1 hour) | Cat | After | >27 | <8.9 | |
| Berger and Hakim (1989) | Dextrose | Permanent | Rat | Sprague | Before | 19.2 | 8.2 |
| Bomont and MacKenzie (1995), 1st comparison a | Dextrose | Permanent | Rat | Fischer | Before | 30.8 | <11.1 |
| Bomont and MacKenzie (1995), 2nd comparison b | Streptozotocin | Permanent | Rat | Fischer | Unclear | 30.1 | <11.1 |
| Combs et al (1990) | Dextrose | Permanent | Cat | Before | 25.5 | 12 | |
| de Courten-Myers et al (1989), 1st comparison a | Dextrose | Reversible (4 hours) | Cat | Before | 22 | 6 | |
| de Courten-Myers et al (1989), 2nd comparison b | Dextrose | Permanent | Cat | Before | 22 | 6 | |
| de Courten-Myers et al (1994), 1st comparison a | Dextrose | Permanent | Cat | Before | 12.9 to 24.7 | <9.2 | |
| de Courten-Myers et al (1988) | Dextrose | Permanent | Cat | Before | 20 | 6.5 | |
| de Courten-Myers et al (1994), 2nd comparison b | Dextrose | Reversible (8 hours) | Cat | Before | 18.2 to 22.5 | <9.4 | |
| Duverger and MacKenzie (1988), 1st comparison a | Streptozotocin | Permanent | Rat | Fischer | 72 hours | 25.3 | 8.3 |
| Duverger and MacKenzie (1988), 2nd comparison b | Streptozotocin | Permanent | Rat | Wistar | 72 hours | 30.6 | 8.8 |
| Huang et al (1996), 1st comparison a | Streptozotocin | Permanent | Rat | Sprague | 48 hours | 25 | 8.1 |
| Huang et al (1996), 2nd comparison b | Streptozotocin | Reversible (1 hour) | Rat | Sprague | 48 hours | 25.8 | 8 |
| Kraft et al (1990) | Dextrose | Permanent | Rabbit | Before | >22.8 | <9.2 | |
| Li et al (2004) | Streptozotocin | Permanent | Rat | Fischer | 5 to 6 weeks | 24.3 | 6.1 |
| Liu et al (2007), 1st comparison a | Dextrose | Permanent | Rat | Sprague | Unclear | 22.4 | 4.8 |
| Liu et al (2007), 2nd comparison b | Dextrose | Reversible (1 hour) | Rat | Sprague | Unclear | 22.7 | 4.8 |
| Nedergaard (1987), 1st comparison a | Streptozotocin | Reversible (10 minutes) | Rat | Wistar | 48 hours | >20 | <9.2 |
| Nedergaard (1987), 2nd comparison b | Streptozotocin | Reversible (15 minutes) | Rat | Wistar | 48 hours | >20 | <9.5 |
| Nedergaard (1987), 3rd comparison c | Streptozotocin | Reversible (5 minutes) | Rat | Wistar | 48 hours | >20 | <9.5 |
| Nedergaard and Diemer (1987), 1st comparison a | Streptozotocin (2 days) | Permanent | Rat | Wistar | 48 hours | 25 | 7.3 |
| Nedergaard and Diemer (1987), 2nd comparison b | Streptozotocin (4 months) | Permanent | Rat | Wistar | 4 months | 28 | 7.3 |
| Nedergaard and Diemer (1987), 3rd comparison c | Dextrose | Permanent | Rat | Wistar | After | 32 | 7.3 |
| Quast et al (1997), 1st comparison a | Streptozotocin | Permanent | Rat | Sprague | 48 hours | 26.5 | 9 |
| Quast et al (1997), 2nd comparison b | Streptozotocin | Reversible (2 hours) | Rat | Sprague | 48 hours | 26.5 | 9 |
| Slivka (1991), 1st comparison a | Dextrose | Permanent | Rat | SHR | Before | 22.2 | 7 |
| Slivka (1991), 2nd comparison b | Streptozotocin | Permanent | Rat | SHR | 48 hours | 26.4 | 8.8 |
| Wei et al (1997) | Streptozotocin | Reversible (2 hours) | Rat | Sprague | 48 hours | 20.9 | 6.1 |
| Wei and Quast (1998) | Streptozotocin | Reversible (2 hours) | Rat | Sprague | 48 hours | 25.6 | 7.2 |
| Wei et al (2003) | Dextrose | Reversible (90 minutes) | Rat | Sprague | Before | 19.6 | 4.5 |
| Zasslow et al (1989) | Dextrose | Permanent | Cat | Sprague | Before | 31.2 | 11.6 |
| Martin et al (2006) | Dextrose | Reversible (1 hour) | Rat | Sprague | Before | 18.4 | 8.6 |
| Kittaka (1996), 1st comparison a | Streptozotocin | Reversible (1 hour) | Rat | Sprague | 7 days | 15.5 | 4.3 |
| Kittaka (1996), 2nd comparison b | Dextrose | Reversible (1 hour) | Rat | Sprague | Before | 14.8 | 4.3 |
MCAO, middle cerebral artery occlusion; SHR, spontaneously hypertensive rat.
Streptozotocin was administered 48 hours before the experiment in 8 papers. In other studies, STZ was administered at earlier times (72 hours earlier in 2 studies, 4 days earlier in 1 study, 7 days earlier in 1 study, 5 to 6 weeks earlier in 3 studies, and 4 months earlier in 1 study; it must be noted that these numbers do not add up to 18 because some studies included more than 1 comparison group).
Dextrose infusions were started between 15 and 120 minutes (median time 30 minutes) before MCAO in comparisons in which hyperglycaemia was induced before occlusion. Dextrose infusion concentration varied from 10% to 50%. In comparisons in which hyperglycaemia was induced after MCAO, this was performed with an injection of 50% dextrose 5 to 20 minutes after ictus.
In nine papers, the frequency of monitoring of blood glucose was unclear. Glucose concentration was reported at the time of arterial occlusion and at an unstated time after occlusion in the remaining papers. Peak blood glucose values ranged from 18.4 to 31.2 mmol/L in hyperglycaemic groups and from 4.6 to 11.1 mmol/L in control groups. In the hyperglycaemic groups, the mean blood glucose was 23.9 mmol/L (95% CI: 22.4 to 25.3). In the control groups, the mean glucose was 8 mmol/L (95% CI: 7.3 to 8.7).
Study Quality
Study quality was generally low based on the modified CAMARADES score (median 3/10, range 1 to 6 points). All papers were published in peer-reviewed journals. Blood pressure monitoring was documented in 20 of 24 papers, whereas temperature monitoring was documented in 22 of 24. Random allocation of animals to experimental groups was documented in only 6 of 24 papers, whereas blinded assessment of outcomes was only detailed in 7 of 24. No papers documented the blinded induction of ischaemia, sample size calculations, or a clear conflict of interest statement. The complete quality score table is included as Supplementary Appendix 2.
Assessment of Bias
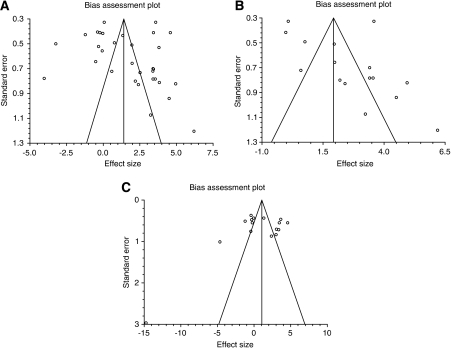
Funnel plots (Figure 2, Graphs A to C) suggested possible publication bias, with paucity of small, negative studies (particularly using the STZ model), but formal statistical analysis was not significant (Egger's bias test 2.88 (95% CI: −1.53 to 7.29, P=0.1925)) (Sterne and Egger, 2001).
Figure 2.
Bias assessment plot for effect of hyperglycaemia on infarct size. Graph A is a bias assessment plot for all hyperglycaemic studies. Graph B is a bias assessment plot for studies using streptozotocin. Graph C is a bias assessment plot for studies using dextrose.
Measurement of Effect Size
Infarct size was quantified by tissue staining (TTC (2,3,5-triphenyltetrazolium chloride) in 9 studies, cresyl violet in 4 studies, and haematoxylin and eosin in 11 studies) or by magnetic resonance imaging (MRI). Two histology studies used both cresyl violet and haematoxylin and eosin staining. One study reported only MRI measurement, and three others undertook both MRI and histology. In studies in which both techniques were used, we included MRI data as reported by the original authors. Infarct volume was measured at times ranging from 3 hours after arterial occlusion up to 2 weeks.
Streptozotocin Model and Dextrose Infusion Models
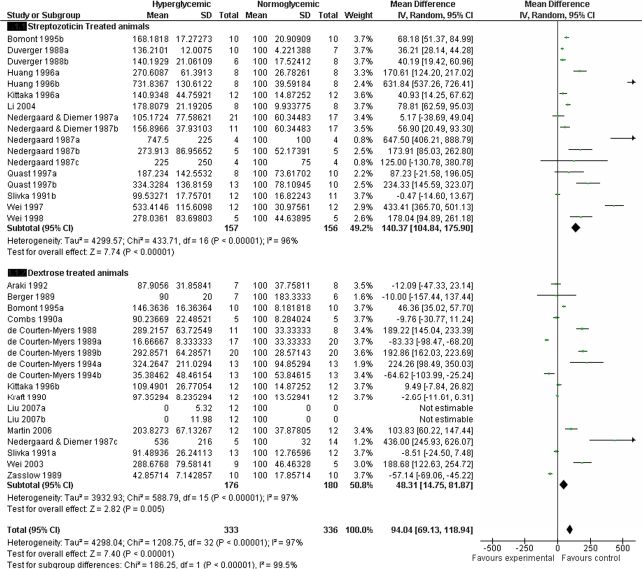
Hyperglycaemic animals had significantly larger infarcts (effect size 94, 95% CI: 69.1 to 118.9, P<0.00001), but STZ was associated with greater exacerbation of infarct volume compared with dextrose alone (effect size 140.3, 95% CI: 104.8 to 175.9, P<0.00001 versus 48.3, 95% CI: 14.8 to 81.9, P=0.005) (Figure 3). There was significant statistical heterogeneity between studies with χ2=1,208.7 with d.f.=32 (P<0.00001). There was also statistical difference between subgroups (χ2=186.3, d.f.=1, P<0.00001).
Figure 3.
Meta-analysis of effect of hyperglycaemia on infarct size. d.f., degrees of freedom; 95% CI, 95% confidence interval.
Permanent Versus Transient Middle Cerebral Artery Occlusion
The STZ model was associated with larger infarcts in both transient and permanent MCAO. In 9 comparisons involving 190 STZ-treated animals with permanent MCAO, the effect estimate for infarct size was 55.2 (95% CI: 31.2 to 79.1, P<0.0001). There was statistically significant heterogeneity between studies (χ2=99.3, d.f.=8, P<0.00001). In 8 comparisons involving 115 STZ-treated animals with transient MCAO, the effect estimate for infarct size was 319.1 (95% CI: 191 to 447, P<0.000001). There was statistically significant heterogeneity between studies (χ2=116.6, d.f.=7, P<0.00001).
In 10 comparisons involving 227 dextrose-treated animals with permanent MCAO, the effect size was less than for STZ at 43.1 (95% CI: −0.05 to 86.2, P=0.05) with statistically significant interstudy heterogeneity (χ2=509, d.f.=9, P<0.00001), and in 6 comparisons involving 140 animals with dextrose-induced hyperglycaemia and transient MCAO, there was no significant effect on infarct size (effect size 19.2, 95% CI: −42.9 to 81.2, P=0.54). There was statistically significant heterogeneity between studies (χ2=149.8, d.f.=5, P<0.00001).
Effects of Insulin Treatment
In comparing insulin treatment with either hyperglycaemic or normoglycaemic control groups, infarct volume was smaller with insulin, but not significantly so.
Insulin did not significantly reduce infarct size (10 comparisons, n=194, effect size −13.4, 95% CI: −41 to −5, P=0.01), and results were heterogeneous in a statistically significant manner (χ2=54.8, d.f.=7, P<0.00001) (see Table 3 and Figure 4).
Table 3. Details of insulin studies.
| Study | Target blood glucose with insulin treatment | Type of insulin | Timing of insulin | Baseline status | Species | Strain | Blood glucose levels (mmol) | Type of MCAO | Control |
|---|---|---|---|---|---|---|---|---|---|
| Bomont and MacKenzie (1995), 3rd comparison c | Normal | Bovine insulin (Sigma) | Infusion started 2 days earlier | Diabetic streptozotocin | Rat | Fischer | 9.8 | Permanent | |
| Combs et al (1990), 2nd comparison b | Normal | Regular human insulin (Squibb Novo Inc., Princeton, NJ, USA) Somatostatin was also received | Before and after | Glucose infusion | Cat | 24.9 initially, 31.4 at 4 hours | Permanent | ||
| de Courten-Myers et al (1994), 3rd comparison c | Hypoglycaemic | Regular human insulin | Bolus before then infusion | Normoglycaemic | Cat | Between 2.9 and 7.6 | Permanent | Dextrose infusion | |
| de Courten-Myers et al (1994), 4th comparison d | Hypoglycaemic | Regular human insulin | Bolus before then infusion | Normoglycaemic | Cat | Between 3.3 and 3.7 | Reversible | Dextrose Infusion | |
| Zhu (2004), 1st comparison a | Normal | Bovine crystalline zinc insulin (CZI) and longer-acting zinc insulin suspension | 60 minutes before | Normoglycaemic | Rat | Wistar | 5 before ischaemia, 2.5 at 3 hours after ischaemia | Reversible | Normoglycaemic |
| Zhu (2004), 2nd comparison b | Normal | Bovine CZI and longer-acting zinc insulin suspension | 20 minutes after ischaemia | Normoglycaemic | Rat | Wistar | 11.2 before ischaemia, 2.2 at 3 hours | Reversible | Normoglycaemic |
| Hamilton (1995), 1st comparison a | Normal | Porcine/bovine CZI and longer-acting zinc insulin suspension (Lente) | 50 to 70 minutes before | Slightly hyperglycaemic with glucose | Rat | Sprague | Between 3.4 and 3.6 | Reversible | Normoglycaemic |
| Hamilton (1995), 2nd comparison b | Hypoglycaemic | Porcine/bovine CZI and longer-acting zinc insulin suspension (Lente) | 50 to 70 minutes before | Normoglycaemic | Rat | Sprague | Between 7 and 10.1 | Reversible | Normoglycaemic |
| Izumi (1992) | Normal | Actrapid 5 (Novo, France) fast, short acting | Immediately after MCAO | Normoglycaemic | Rat | Fischer | 4.7 | Permanent | Normoglycaemic |
| Nedergaard and Diemer (1987), 4th comparison d | Hypoglycaemic | Crystalline porcine insulin (Leo) | 2 hours before | Hypoglycaemic | Rat | Wistar | 2 | Permanent | Normoglycaemic |
MCAO, middle cerebral artery occlusion.
Figure 4.
Meta-analysis of effect of insulin on infarct growth. d.f., degrees of freedom; pMCAO, permanent middle cerebral artery occlusion; rMCAO, reversible middle cerebral artery occlusion; 95% CI, 95% confidence interval.
Discussion
Although a large body of clinical observational evidence indicates that hyperglycaemia in the acute phase of stroke is associated with poorer outcomes (Capes et al, 2001), typically defined by death or dependence 90 days after the event, few clinical studies have addressed the mechanism by which this may occur (McCormick et al, 2010; Parsons et al, 2002). Many mechanistic proposals are based on inferences drawn from animal models of focal ischaemia. In particular, the rationale for acute intervention to decrease blood glucose levels (usually by administration of insulin) is based on an assumption that the adverse effect of hyperglycaemia predominantly relates to exacerbation of acute infarct evolution. However, insulin treatment carries significant risks, particularly hypoglycaemia (Finfer and Heritier, 2009; Griesdale et al, 2009), and the clinical evidence supporting an acute effect of hyperglycaemia is mainly observational and open to alternative interpretations. Data relating hyperglycaemia to early infarct volume can alternatively be explained by ‘stress' hyperglycaemia resulting from more severe infarcts, for example. Recent observations that suggest that hyperglycaemia carries a higher risk of symptomatic intracerebral haemorrhage in patients treated with intravenous alteplase may be confounded by a similar effect (Bruno et al, 2002; Demchuk et al, 1999). An association of glucose concentration with lactate concentration in the infarct core and with infarct growth in a small observational study using MRI (Parsons et al, 2002) is mechanistically plausible, but without an intervention arm, could not discount the possibility that the relationship with blood glucose is not causal. In addition, the assumption that lactate itself is toxic in the ischaemic brain is not necessarily correct, with other evidence suggesting that lactate is instead produced as an alternative metabolic substrate and is beneficial (Berthet et al, 2009; Parsons et al, 2002). Only one small clinical trial has attempted to address these mechanistic hypotheses using advanced imaging, and has found that insulin treatment reduced blood glucose and lactate concentration in the brain, but had no effect on infarct growth (McCormick et al, 2010).
Our systematic review yields findings that suggest that existing animal model data have limited relevance to the clinical situation, and that further studies may therefore be required to inform clinical study design.
First, although hyperglycaemia at the time of focal ischaemia onset increases infarct size, this is predominantly attributed to its large effect in the STZ rat model, which simulates type I diabetes mellitus (Like and Rossini, 1976). Hyperglycaemia induced by dextrose infusion has a much smaller effect on infarct size, although still significant. Significant heterogeneity of effect size was present among both STZ and dextrose models. Although the animal data may be informative with regard to a biologic effect of hyperglycaemia on acute infarct volume, the clinical inferences that can be drawn are very limited. Dextrose infusion models typically administered hypertonic solution (20% to 50% dextrose) to yield blood glucose exceeding 20 mmol/L, a level considerably greater than is typically encountered in clinical practice (fewer than 2% of subjects in one study), and hence the clinical relevance of findings in this model system is unclear. Although only a minority of stroke patients have established diabetes mellitus (overwhelmingly type II diabetes), a high proportion of patients with acute hyperglycaemia after stroke are found to have unrecognised insulin resistance when followed (impaired glucose tolerance, metabolic syndrome, or undiagnosed type II diabetes) (Gray et al, 2004; McCormick and Muir, 2006). No study of infarct volume used animals that model the insulin-resistant phenotype typical of patient populations.
The underlying mechanism for the difference in final infarct size between dextrose models and STZ models is uncertain. In models of global ischaemia, the anatomic distribution and severity of brain damage in the STZ model is similar to that seen in animals acutely infused with dextrose (Li et al, 1998a). It has been suggested that STZ may increase the rate of apoptosis in models of focal cerebral ischaemia (Britton et al, 2003). The diabetic state induced by STZ may damage the microvasculature of the rat brain or impair the compensatory mechanisms that would normally protect from ischaemia. The difference in effect size raises a central question of whether the adverse effect arises from high glucose or from lack of insulin.
The effect of insulin was nonsignificant, although the effect size estimate is consistent with reduction in infarct volume. However, as insulin may reduce infarct volume when compared with normoglycaemic control groups, it is possible that insulin does not act by reducing blood glucose in these model systems, and we cannot infer that reducing blood glucose (as opposed to administering insulin) represents an effective intervention. As the results of these studies are heterogeneous, a degree of caution is necessary when interpreting them.
To allow for comparisons involving different species and models, we used weighted mean differences in infarct volume normalised to the mean volume of the control group (Banwell et al, 2009; Wheble et al, 2008). Given the small group sizes, and the possible exclusion of animals that either died or exhibited no evidence of infarction from group mean infarct volume measurements—commented on in only a handful of studies—the magnitude of effect size can be regarded only as an approximation.
Methodological quality of animal experiments is a significant concern because several reviews suggest that studies that do not report items such as blinding of outcomes and randomisation are more prone to bias than are more rigorous studies (Sena et al, 2007). Quality scores have been based on criteria developed for preclinical evaluation of therapeutic interventions (e.g., the Stroke Therapy Academic Industry Roundtable [STAIR] criteria), which have gone through several iterations over time, rather than physiologic studies such as those predominantly reported herein. The median quality score using a slightly modified system used by the CAMARADES group (Macleod et al, 2004) was only 3 (out of a possible 10), and only 2 papers scored 6, which likely reflects a predominance of older papers and different standards of documentation for physiologic studies (e.g., items such as conflict of interest statements are not likely to be perceived as necessary, in contrast to drug treatment studies). The feasibility of blinding in some circumstances—such as STZ-pretreated animals and dextrose infusions in animals monitored by regular blood sampling—is also unclear.
We did not identify significant publication bias by a conventional analysis, but a recent publication has highlighted alternative methods such as ‘trim-and-fill' analysis (Sena et al, 2010) to estimate the effect of publication bias on efficacy outcomes in systematic reviews and meta-analysis of animal models of stroke (Duval and Tweedie, 2000). This method estimates the number of unpublished studies that may exist based on the estimated proportion of unpublished data from the Egger regression. Although this approach suggested that up to one-sixth of the studies were unpublished (with effect sizes potentially altered by one-third), the relevance of publication bias to physiologic studies rather than therapeutic agents is not established clearly. Even with an effect of this magnitude on overall estimates, hyperglycaemia would have a highly significant adverse effect on infarct volume.
In summary, although animal focal ischaemia models indicate exacerbation of infarct volume by acute hyperglycaemia, this effect reflects a particularly detrimental effect in a model of type 1 (insulin deficient) diabetes, with both a smaller effect size and considerable heterogeneity in acute hyperglycaemia induced by dextrose infusion, which may represent a situation analogous to ‘stress hyperglycaemia'. No study has reported the effects of hyperglycaemia in an insulin-resistant model, which is potentially the most clinically relevant scenario. Few studies have investigated the effect of insulin on infarct volume, and as the concentrations of blood glucose induced in the model systems have generally greatly exceeded those relevant to clinical practice, we have no adequate data to support the current clinical guidelines suggesting intervention at concentrations ⩾140 mg/dL (7.7 mmol/L) (Adams et al, 2007).
Acknowledgments
The authors acknowledge the personal communications from Anna Planas and Jane Montgomery when we were trying to obtain additional data for this paper.
Niall JJ MacDougall declares no conflict of interest. Keith W Muir has received grants from the Stroke Association and the Chief Scientists Office (Scotland) in relation to hyperglycaemia and stroke.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the Early Management of Adults with Ischemic Stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Araki N, Greenberg JH, Sladky JT, Uematsu D, Karp A, Reivich M. The effect of hyperglycemia on intracellular calcium in stroke. J Cereb Blood Flow Metab. 1992;12:469–476. doi: 10.1038/jcbfm.1992.64. [DOI] [PubMed] [Google Scholar]
- Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis. 2009;18:269–276. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Berger L, Hakim AM. Nimodipine prevents hyperglycemia-induced cerebral acidosis in middle cerebral artery occluded rats. J Cereb Blood Flow Metab. 1989;9:58–64. doi: 10.1038/jcbfm.1989.8. [DOI] [PubMed] [Google Scholar]
- Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1780–1789. doi: 10.1038/jcbfm.2009.97. [DOI] [PubMed] [Google Scholar]
- Bomont L, MacKenzie ET. Neuroprotection after focal cerebral ischaemia in hyperglycaemic and diabetic rats. Neurosci Lett. 1995;197:53–56. doi: 10.1016/0304-3940(95)11899-8. [DOI] [PubMed] [Google Scholar]
- Britton M, Rafols J, Alousi S, Dunbar JC. The effects of middle cerebral artery occlusion on central nervous system apoptotic events in normal and diabetic rats. Int J Exp Diabesity Res. 2003;4:13–20. doi: 10.1080/15438600303727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno A, Biller J, Adams HP, Jr, Clarke WR, Woolson RF, Williams LS, Hansen MD. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999;52:280–284. doi: 10.1212/wnl.52.2.280. [DOI] [PubMed] [Google Scholar]
- Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, Lyden PD, Broderick JP, Kwiatkowski TG, Fineberg SE. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59:669–674. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- Candelise L, Landi G, Orazio EN, Boccardi E. Prognostic significance of hyperglycemia in acute stroke. Arch Neurol. 1985;42:661–663. doi: 10.1001/archneur.1985.04060070051014. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Chew W, Kucharczyk J, Moseley M, Derugin N, Norman D. Hyperglycemia augments ischemic brain injury: in vivo MR imaging/spectroscopic study with nicardipine in cats with occluded middle cerebral arteries. AJNR Am J Neuroradiol. 1991;12:603–609. [PMC free article] [PubMed] [Google Scholar]
- Combs DJ, Dempsey RJ, Kumar S, Donaldson D. Focal cerebral infarction in cats in the presence of hyperglycemia and increased insulin. Metab Brain Dis. 1990;5:169–178. doi: 10.1007/BF00997070. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers G, Myers RE, Schoolfield L. Hyperglycemia enlarges infarct size in cerebrovascular occlusion in cats. Stroke. 1988;19:623–630. doi: 10.1161/01.str.19.5.623. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Wagner KR, Myers RE. Fatal strokes in hyperglycemic cats. Stroke. 1989;20:1707–1715. doi: 10.1161/01.str.20.12.1707. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Wagner KR, Myers RE. Normoglycemia (not hypoglycemia) optimizes outcome from middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1994;14:227–236. doi: 10.1038/jcbfm.1994.29. [DOI] [PubMed] [Google Scholar]
- Demchuk AM, Morgenstern LB, Krieger DW, Linda Chi T, Hu W, Wein TH, Hardy RJ, Grotta JC, Buchan AM. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–39. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. A nonparametric ‘trim and fill' method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8:449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- Ennis SR, Keep RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2007;27:1573–1582. doi: 10.1038/sj.jcbfm.9600454. [DOI] [PubMed] [Google Scholar]
- Finfer S, Heritier S. The NICE-SUGAR (Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) study: statistical analysis plan. Crit Care Resusc. 2009;11:46–57. [PubMed] [Google Scholar]
- Folbergrova J, Memezawa H, Smith ML, Siesjo BK. Focal and perifocal changes in tissue energy state during middle cerebral artery occlusion in normo- and hyperglycemic rats. J Cereb Blood Flow Metab. 1992;12:25–33. doi: 10.1038/jcbfm.1992.4. [DOI] [PubMed] [Google Scholar]
- Fuentes B, Castillo J, San Jose B, Leira R, Serena J, Vivancos J, Davalos A, Nunez AG, Egido J, Diez-Tejedor E. The prognostic value of capillary glucose levels in acute stroke: the GLycemia in Acute Stroke (GLIAS) study. Stroke. 2009;40:562–568. doi: 10.1161/STROKEAHA.108.519926. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Prado R, Dietrich WD, Busto R, Watson BD. Hyperglycemia reduces the extent of cerebral infarction in rats. Stroke. 1987;18:570–574. doi: 10.1161/01.str.18.3.570. [DOI] [PubMed] [Google Scholar]
- Gisselsson L, Smith ML, Siesjo BK. Hyperglycemia and focal brain ischemia. J Cereb Blood Flow Metab. 1999;19:288–297. doi: 10.1097/00004647-199903000-00007. [DOI] [PubMed] [Google Scholar]
- Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- Gray CS, Scott JF, French JM, Alberti KG, O'Connell JE. Prevalence and prediction of unrecognised diabetes mellitus and impaired glucose tolerance following acute stroke. Age Ageing. 2004;33:71–77. doi: 10.1093/ageing/afh026. [DOI] [PubMed] [Google Scholar]
- Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MG, Tranmer BI, Auer RN. Insulin reduction of cerebral infarction due to transient focal ischemia. J Neurosurg. 1995;82:262–268. doi: 10.3171/jns.1995.82.2.0262. [DOI] [PubMed] [Google Scholar]
- Huang NC, Wei J, Quast MJ. A comparison of the early development of ischemic brain damage in normoglycemic and hyperglycemic rats using magnetic resonance imaging. Exp Brain Res. 1996;109:33–42. doi: 10.1007/BF00228624. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Pinard E, Roussel S, Seylaz J. Insulin protects brain tissue against focal ischemia in rats. Neurosci Lett. 1992;144:121–123. doi: 10.1016/0304-3940(92)90730-u. [DOI] [PubMed] [Google Scholar]
- Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N, Keep RF, Betz AL. Effects of hyperglycemia on cerebral blood flow and edema formation after carotid artery occlusion in Fischer 344 rats. Acta Neurochir Suppl. 1997;70:34–36. doi: 10.1007/978-3-7091-6837-0_10. [DOI] [PubMed] [Google Scholar]
- Kittaka M, Wang L, Sun N, Schreiber SS, Seeds NW, Fisher M, Zlokovic BV. Brain capillary tissue plasminogen activator in a diabetes stroke model. Stroke. 1996;27:712–719. doi: 10.1161/01.str.27.4.712. [DOI] [PubMed] [Google Scholar]
- Kraft SA, Larson CP, Jr, Shuer LM, Steinberg GK, Benson GV, Pearl RG. Effect of hyperglycemia on neuronal changes in a rabbit model of focal cerebral ischemia. Stroke. 1990;21:447–450. doi: 10.1161/01.str.21.3.447. [DOI] [PubMed] [Google Scholar]
- Li C, Li PA, He QP, Ouyang YB, Siesjo BK. Effects of streptozotocin-induced hyperglycemia on brain damage following transient ischemia. Neurobiol Dis. 1998a;5:117–128. doi: 10.1006/nbdi.1998.0189. [DOI] [PubMed] [Google Scholar]
- Li PA, Gisselsson L, Keuker J, Vogel J, Smith ML, Kuschinsky W, Siesjo BK. Hyperglycemia-exaggerated ischemic brain damage following 30 minutes of middle cerebral artery occlusion is not due to capillary obstruction. Brain Res. 1998b;804:36–44. doi: 10.1016/s0006-8993(98)00651-9. [DOI] [PubMed] [Google Scholar]
- Li PA, Vogel J, He QP, Smith ML, Kuschinsky W, Siesjo BK. Preischemic hyperglycemia leads to rapidly developing brain damage with no change in capillary patency. Brain Res. 1998c;782:175–183. doi: 10.1016/s0006-8993(97)01150-5. [DOI] [PubMed] [Google Scholar]
- Li ZG, Britton M, Sima AA, Dunbar JC. Diabetes enhances apoptosis induced by cerebral ischemia. Life Sci. 2004;76:249–262. doi: 10.1016/j.lfs.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang Z, Wang X, Song L, Chen H, Bemeur C, Ste-Marie L, Montgomery J. Comparison of two rat models of cerebral ischemia under hyperglycemic conditions. Microsurgery. 2007;27:258–262. doi: 10.1002/micr.20351. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- Marsh WR, Anderson RE, Sundt TM., Jr Effect of hyperglycemia on brain pH levels in areas of focal incomplete cerebral ischemia in monkeys. J Neurosurg. 1986;65:693–696. doi: 10.3171/jns.1986.65.5.0693. [DOI] [PubMed] [Google Scholar]
- Martin A, Rojas S, Chamorro A, Falcon C, Bargallo N, Planas AM. Why does acute hyperglycemia worsen the outcome of transient focal cerebral ischemia? Role of corticosteroids, inflammation, and protein O-glycosylation. Stroke. 2006;37:1288–1295. doi: 10.1161/01.STR.0000217389.55009.f8. [DOI] [PubMed] [Google Scholar]
- Matz K, Keresztes K, Tatschl C, Nowotny M, Dachenhausenm A, Brainin M, Tuomilehto J. Disorders of glucose metabolism in acute stroke patients: an underrecognized problem. Diabetes Care. 2006;29:792–797. doi: 10.2337/diacare.29.04.06.dc05-1818. [DOI] [PubMed] [Google Scholar]
- McCormick M, Hadley DM, McLean J, Macfarlane J, Condon B, Muir KW. Randomised, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol. 2010;67:570–578. doi: 10.1002/ana.21983. [DOI] [PubMed] [Google Scholar]
- McCormick M, Muir KW.2006Prevalence of impaired glucose metabolism and metabolic syndrome in non-diabetic patients with acute post stroke hyperglycaemia Cerebrovasc Dis 21(Suppl 462Abstract [Google Scholar]
- Nakai H, Yamamoto YL, Diksic M, Worsley KJ, Takara E. Triple-tracer autoradiography demonstrates effects of hyperglycemia on cerebral blood flow, pH, and glucose utilization in cerebral ischemia of rats. Stroke. 1988;19:764–772. doi: 10.1161/01.str.19.6.764. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Transient focal ischemia in hyperglycemic rats is associated with increased cerebral infarction. Brain Res. 1987;408:79–85. doi: 10.1016/0006-8993(87)90360-x. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Diemer NH. Focal ischemia of the rat brain, with special reference to the influence of plasma glucose concentration. Acta Neuropathol. 1987;73:131–137. doi: 10.1007/BF00693778. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Gjedde A, Diemer NH. Focal ischemia of the rat brain: autoradiographic determination of cerebral glucose utilization, glucose content, and blood flow. J Cereb Blood Flow Metab. 1986;6:414–424. doi: 10.1038/jcbfm.1986.74. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Gjedde A, Diemer NH. Hyperglycaemia protects against neuronal injury around experimental brain infarcts. Neurol Res. 1987;9:241–244. doi: 10.1080/01616412.1987.11739802. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- Prado R, Ginsberg MD, Dietrich WD, Watson BD, Busto R. Hyperglycemia increases infarct size in collaterally perfused but not end-arterial vascular territories. J Cereb Blood Flow Metab. 1988;8:186–192. doi: 10.1038/jcbfm.1988.48. [DOI] [PubMed] [Google Scholar]
- Quast MJ, Wei J, Huang NC. Nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester decreases ischemic damage in reversible focal cerebral ischemia in hyperglycemic rats. Brain Res. 1995;677:204–212. doi: 10.1016/0006-8993(95)00134-c. [DOI] [PubMed] [Google Scholar]
- Quast MJ, Wei J, Huang NC, Brunder DG, Sell SL, Gonzalez JM, Hillman GR, Kent TA. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab. 1997;17:553–559. doi: 10.1097/00004647-199705000-00009. [DOI] [PubMed] [Google Scholar]
- Rejdak K, Rejdak R, Sieklucka-Dziuba M, Stelmasiak Z, Grieb P. The effects of citicoline and/or MK-801 on survival, neurological and behavioral outcome of mice exposed to transient hyperglycemia and oligemic hypoxia. Eur Neuropsychopharmacol. 2001;11:333–341. doi: 10.1016/s0924-977x(01)00107-9. [DOI] [PubMed] [Google Scholar]
- Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res. 2006;1096:204–212. doi: 10.1016/j.brainres.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke Trial (GIST) Stroke. 1999;30:793–799. doi: 10.1161/01.str.30.4.793. [DOI] [PubMed] [Google Scholar]
- Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke. Trends Neurosci. 2007;30:433–439. doi: 10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka AP. Hypertension and hyperglycemia in experimental stroke. Brain Res. 1991;562:66–70. doi: 10.1016/0006-8993(91)91187-6. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Venables GS, Miller SA, Gibson G, Hardy JA, Strong AJ. The effects of hyperglycaemia on changes during reperfusion following focal cerebral ischaemia in the cat. J Neurol Neurosurg Psychiatry. 1985;48:663–669. doi: 10.1136/jnnp.48.7.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, Erila T, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kohrmann M, Larrue V, Lees KR, Machnig T, Roine RO, Toni D, Vanhooren G. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials. Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST) Stroke. 2008;39:3316–3322. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- Wei J, Cohen DM, Quast MJ. Effects of 2-deoxy-d-glucose on focal cerebral ischemia in hyperglycemic rats. J Cereb Blood Flow Metab. 2003;23:556–564. doi: 10.1097/01.WCB.0000056061.18772.72. [DOI] [PubMed] [Google Scholar]
- Wei J, Huang NC, Quast MJ. Hydroxyl radical formation in hyperglycemic rats during middle cerebral artery occlusion/reperfusion. Free Radic Biol Med. 1997;23:986–995. doi: 10.1016/s0891-5849(97)00127-5. [DOI] [PubMed] [Google Scholar]
- Wei J, Quast MJ. Effect of nitric oxide synthase inhibitor on a hyperglycemic rat model of reversible focal ischemia: detection of excitatory amino acids release and hydroxyl radical formation. Brain Res. 1998;791:146–156. doi: 10.1016/s0006-8993(98)00089-4. [DOI] [PubMed] [Google Scholar]
- Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheble PC, Sena ES, Macleod MR. A systematic review and meta-analysis of the efficacy of piracetam and piracetam-like compounds in experimental stroke. Cerebrovasc Dis. 2008;25:5–11. doi: 10.1159/000111493. [DOI] [PubMed] [Google Scholar]
- Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke. 2008;39:2749–2755. doi: 10.1161/STROKEAHA.108.514307. [DOI] [PubMed] [Google Scholar]
- Zasslow MA, Pearl RG, Shuer LM, Steinberg GK, Lieberson RE, Larson CP., Jr Hyperglycemia decreases acute neuronal ischemic changes after middle cerebral artery occlusion in cats. Stroke. 1989;20:519–523. doi: 10.1161/01.str.20.4.519. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Lu CZ, Ren HM, Xiao BG. Metabolic changes of arachidonic acid after cerebral ischemia-reperfusion in diabetic rats. Exp Neurol. 2003;184:746–752. doi: 10.1016/S0014-4886(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Yang GY, Domino EF. Acute ethanol effects on focal cerebral ischemia in nonfasted rats. Alcohol Clin Exp Res. 1997;21:745–748. [PubMed] [Google Scholar]
- Zhu CZ, Auer RN. Optimal blood glucose levels while using insulin to minimize the size of infarction in focal cerebral ischemia. J Neurosurg. 2004;101:664–668. doi: 10.3171/jns.2004.101.4.0664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.