Abstract
Although aging-induced changes in urinary bladder neurotransmission have been studied in some detail, information regarding alterations in detrusor muscle is scanty and addresses only partial aspects of the myogenic response of detrusor. Rodent bladder aging shows several features similar to those reported in humans. The aim of this study was to characterize in aged mouse the alterations of detrusor muscle contraction and the putative underlying changes in Ca2+ signals. We studied in vitro the myogenic contraction induced by agonists in detrusor strips from adult (3 months old) or aged (23–25 months old) mice. In addition, we determined the agonist-induced [Ca2+]i signals by epifluorescence microscopy in fura-2 loaded isolated detrusor cells. Aging impaired the contractile response of bladder strips to cholinergic stimulation with bethanechol and to chemical depolarization with KCl-containing solutions. On the contrary, the response to purinergic stimulation (ATP) was enhanced. Aging also diminished the transient Ca2+ signal evoked by bethanechol and the Ca2+ influx induced by KCl in bladder strips. Treatments aimed to release calcium from intracellular stores (caffeine and a low level of ionomycin in Ca2+-free medium) showed that aging reduces the size of agonist-releasable stores. Similar to contraction, the mobilization of Ca2+ by ATP was increased in aged cells. Therefore, the differential effects of aging on detrusor contraction are associated to alterations of [Ca2+]i signals: the cholinergic inhibition is due to inhibition of voltage-operated Ca2+ influx and reduction of the size of intracellular Ca2+ stores, while the age-induced ATP response is accompanied by an enhanced Ca2+ mobilization.
Keywords: Mouse, Detrusor, Acetylcholine, ATP, Aging, Ca2+ signals, Ca2+ stores
Introduction
It is well known that disturbances of micturition cycle are common in the elderly population (Diokno et al. 1992; Milsom et al. 2001). These alterations are considered the result of modifications of both the neural and the myogenic components of the detrusor contraction. In aged human bladder both cholinergic transmission (Yoshida et al. 2001, 2004) and M3 muscarinic receptors (Mansfield et al. 2005) are reduced, but purinergic neurotransmission is enhanced (Yoshida et al. 2001, 2004). This pattern is also present in aged rat bladder, which shows a loss of cholinergic innervation (Pagala et al. 2001), accompanied by a non contractile up-regulation of muscarinic receptors (Schneider et al. 2005) and an increase of the contractile effect of purinergic (Saito et al. 1993; Lieu et al. 1997; Kageyama et al. 2000), α-adrenergic (Suzuki et al. 1999; Lluel et al. 2003), and serotoninergic (Saito et al. 1993; Lieu et al. 1997) neurotransmission. While these changes can contribute to bladder overactivity, this is also due to a decrease in the relaxing effect of β-adrenergic receptors (Nishimoto et al. 1995) and a reduced expression of mucosal NO synthase III (Lluel et al. 2003).
Part of the alterations of aged urinary bladder occurs downstream of smooth muscle receptors, such as changes of Gi proteins (Derweesh et al. 2000) and impairment of mitochondrial enzymatic activity and ATP production (Lin et al. 2000). The main contributor to detrusor contraction is cytosolic Ca2+ concentration ([Ca2+]i). This signalling system is directly determined by transport through plasma membrane (Ca2+ influx channels and active Ca2+ extrusion) and by uptake and release from intracellular Ca2+ stores. Aging increases the dependence of detrusor contraction on extracellular Ca2+ influx (Yu et al. 1996), reduces Ca2+ extrusion mechanisms (Gomez-Pinilla et al. 2007b), and alters Ca2+-dependent and independent contraction pathways (Gomez-Pinilla et al. 2008). However, there is no information regarding the involvement of these disturbances in the contraction changes observed in aged detrusor.
Given that most of the studies in the field are focused on the nervous plexus, we decided to study in mouse detrusor the effects of aging in the contraction and the Ca2+ signals induced by the main physiological stimulus. Our data indicate that aging differentially alters the contractile response to ATP and cholinergic stimulation, underlaid by parallel changes in Ca2+ signals.
Materials and methods
Animals and tissue preparation
Male mice were divided in two groups according to age: young-adult (2–3 months old) and aged (23–25 months old). After deep halothane anaesthesia and cervical dislocation, urinary bladder was removed and placed in cold Krebs–Henseleit solution (K–HS; for composition see “Solutions and drugs”), cleaned of fatty tissue, opened longitudinally, washed with K-HS solution and the urothelium was carefully dissected away. All the experiments were carried out according to the guidelines of Animal Care and Use and approved by the Animal Ethics Committee of the University of Extremadura.
Contraction recording of mouse urinary bladder smooth muscle strips
The bladder smooth muscle sheet was cut into three strips along the longitudinally axis (~3 × 10 mm). A 4-0 silk thread was tied to each edge of a strip, and was used then to secure the strip to the hooks in both the isometric transducer and in the bottom of a 10 ml vertical organ bath chamber (four chamber organ bath, Cibertec, Spain), filled with K–HS maintained at 37°C and gassed with 95% O2–5% CO2. Isometric contractions were measured using force displacement transducers interfaced with a Macintosh computer using MacLab hardware and dedicated software (ADInstruments; Colorado Spring, CO, USA). The strips were placed under an initial resting tension of 1 g, and allowed to equilibrate for 60 min, with solution changes every 20 min. At the end of each experiment, the strips were dried and weighed to normalize detrusor contractile responses (mN/mg).
The detrusor contractile response was assessed performing concentration–response curves for bethanechol (BE), ATP (agonists for muscarinic and purinergic smooth muscle receptors respectively), and depolarizing K-HS solutions containing high levels of KCl (20, 40, and 60 mM). To obtain the desired concentrations in the bath, increasing amounts of BE or ATP were added in a cumulative way (no washouts) from stocks concentrated 100–1,000 times the bath concentration. Subsequent concentrations were added when tonic tension phase of the contraction became evident. For KCl, a noncumulative curve was performed, allowing the strips to return to baseline (about 60 min) before addition of the next KCl solution to avoid Ca2+ channel desensitization. Strips from the same animal were not exposed to multiple tests with the same agonist.
Cell isolation and [Ca2+]i determination
Approximately 20 mg of detrusor was cut into small pieces and incubated for 27 min at 37°C in enzyme solution (ES, for composition see “Solutions and drugs”) supplemented with 1 mg/ml BSA, 1 mg/ml papain, and 1 mg/ml dithioerythritol (DTT). Next, the tissue was transferred to fresh ES containing 1 mg/ml BSA, 1 mg/ml collagenase, and 100 μM CaCl2 and incubated for 5 min at 37°C. The tissue was then washed three times using cold ES, and the single smooth muscle cells were isolated by several passages of the tissue pieces through the tip of a fire-polished glass Pasteur pipette. The cell suspension was kept in ES at 4°C until use, generally within 6 h.
Isolated cells were loaded with 4 μM fura 2-AM at room temperature for 15 min. An aliquot of cell suspension was placed in an experimental chamber made with a glass poly-D-lysine treated coverslip (0.17 mm thick) filled with Na+-HEPES solution (for composition see “Solutions and drugs”) and mounted on the stage of an inverted microscope (Eclipse TE2000-S; Nikon). After cell sedimentation, a gravity-fed system was used to perfuse the chamber with Na+-HEPES solution in the absence or presence of the experimental agents. Cells were illuminated at 340 and 380 nm at 0.3 cycles/s using a monochromator (Optoscan, Cairn Research), and the emitted fluorescence was captured with a cooled digital charge-coupled device camera (ORCAII-ERG; Hamamatsu Photonics) and recorded using dedicated software (Metafluor, Universal Imaging). Fluorescence ratio (F340/F380) was calculated pixel by pixel and used to indicate the changes in [Ca2+]i. A calibration of the ratio for [Ca2+]i was not performed in view of the many uncertainties related to the binding properties of fura 2 with Ca2+ inside of smooth muscle cells. Experiments were performed at room temperature (22°C).
The size of the agonist releasable pools was assessed applying a low level of ionomycin (50 nM) in Ca2+-free solution, a treatment which releases intracellular stores bypassing channels and receptors (Camello-Almaraz et al. 2008, 2009). In other series of experiments, the size of intracellular stores was estimated activating ryanodine receptors (present at the sarcoplasmic reticulum) with the cell permeant drug caffeine. The [Ca2+]i signal induced by both treatments allow indirect assessment of the size of intracellular stores.
Solutions and drugs
The K–HS contained (in mM): 113 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, and 11.5 D-glucose. This solution had a final pH of 7.35 after equilibration with 95% O2–5% CO2. To prepare KCl-rich solutions, the increased amount of KCl was substituted by NaCl. The ES used to disperse cells contained (in mM): 10 HEPES, 55 NaCl, 5.6 KCl, 80 sodium glutamate, 2 MgCl2, and 10 D-glucose, with pH adjusted to 7.3 with NaOH. The Na+–HEPES solution contained (in mM): 10 HEPES, 140 NaCl, 4.7 KCl, 2 CaCl2, 2 MgCl2, and 10 D-glucose, with pH adjusted to 7.3 with NaOH. The Ca2+-free Na+–HEPES solution included EGTA (1 mM) instead of CaCl2. Drug concentrations are expressed as final bath concentrations of active species. Drugs and chemicals were obtained from the following sources: ATP, bethanechol, caffeine, and ionomycin from Sigma Chemical (St. Louis, MO); fura 2-AM from Molecular Probes (Molecular Probes Europe, Leiden, Netherlands); collagenase from Fluka (Madrid, Spain), and papain from Worthington Biochemical (Lakewood, NJ). Stock solution of ionomycin was prepared in DMSO. The final concentration of DMSO was ≤ 0.1% vol/vol. This concentration of DMSO did not interfere with fura 2 fluorescence.
Quantification and statistics
Results are expressed as means ± SEM of n cells or bladder strips. [Ca2+]i is expressed as ΔF340/F380 and contractile responses in millinewtons (mN)/mg of tissue. Differences between concentration–response curves were tested using adequate analysis of variance for repeated measures (ANOVA), followed by Bonferroni post test. Differences between two groups were assessed by Student's test.
Results
Effects of aging on detrusor contractile response
To evaluate the effect of aging on the contractile response of detrusor to the main types of stimulus in this tissue, i.e., muscarinic and purinergic receptors and depolarization, we performed bethanechol (BE), ATP or KCl concentration–response curves. Bethanechol (10 nM–1 mM) produced a concentration-dependent contraction with a maximal effect at 10-4 M (Fig. 1a). Analysis of variance (ANOVA) showed that age produced a statistically significant (P < 0.001) decrease, especially at the four higher concentrations (P < 0.01 or better), although no shift was observed in the dose–response curve, indicating that potency was not influenced by aging. In the case of ATP, the maximal effect in detrusor from young individuals was more than ten times smaller than for BE, although the curve did not reach the maximal effect at the range of concentrations assayed (1 μM–1 mM, Fig. 1b). Contrary to the response to BE, the effect of aging on purinergic response was an increase (P < 0.05, ANOVA), which was statistically significant at 1 mM ATP (P < 0.01, Fig. 1b).
Fig. 1.
Aging affects the myogenic response of murine urinary bladder. Mouse detrusor strips were exposed to increasing concentrations of bethanechol (BE) (a), ATP (b), and KCl (c). The curves for BE and ATP were constructed in a cumulative way while for KCl a non-cumulative approach was performed. Contractile responses are expressed as mean ± SEM. n = 6–12 strips from five to six animals (**P < 0.01, ***P < 0.001 aged vs adult)
Contraction due to voltage-operated calcium channels was studied by chemical depolarization with K-enriched physiological solution. Figure 1c shows the increasing effect of depolarizing solutions, reaching a maximum contraction close to the maximal effect for BE. As it can be observed, in aged strips the depolarization-evoked contraction was reduced compared to adult tissue (P < 0.01, ANOVA), reaching statistical significance at 60 mM KCl (P < 0.001, Fig. 1c).
Effects of aging on calcium mobilization
To test if the age-induced changes in bladder contractile response are due to changes in Ca2+ mobilization, we challenged fura-2 loaded isolated detrusor cells with the same chemical stimulus used in the functional studies. Both BE and ATP induced a biphasic Ca2+ signal, with an initial peak increase, due to release from internal stores, followed by a sustained phase of low amplitude (Fig. 2). In the case of KCl, the [Ca2+]i response displayed a sustained plateau level preceded by a slow increase. Aged cells showed an impairment of calcium mobilization in response to BE (adult 1.088 ± 0.099 ΔF340/F380, aged 0.315 ± 0.020 ΔF340/F380, n = 30 and 18 cells, respectively, P < 0.001, Fig. 2a) and to KCl application (adult 0.304 ± 0.040 ΔF340/F380, aged 0.094 ± 0.004 ΔF340/F380, n = 20 and 14 cells, respectively, P < 0.001, Fig. 2c). This finding is in keeping with the effect of aging in cholinergic- and depolarization-induced contraction (Fig. 1).
Fig. 2.
a and b Original recordings showing representative [Ca2+]i transients in response to stimulation of isolated detrusor cells with 100 μM bethanechol (BE) or ATP. c shows the peak values (average ± SEM) in response to BE, ATP and KCl (60 mM) in adult and aged cells. n = 14–33 cells from five to six animals (**P < 0.01, ***P < 0.001 aged vs adult)
In the case of the [Ca2+]i transient evoked by ATP, it was increased in aged cells (adult 0.608 ± 0.085 ΔF340/F380, aged 0.900 ± 0.047 ΔF340/F380, n = 33 and 28 cells, respectively, P < 0.01, Fig. 2b), similar to the enhancement of contraction observed in the functional studies (Fig. 1b). Therefore, for each stimulus, the effect of aging in Ca2+ signals paralleled the effects on contraction.
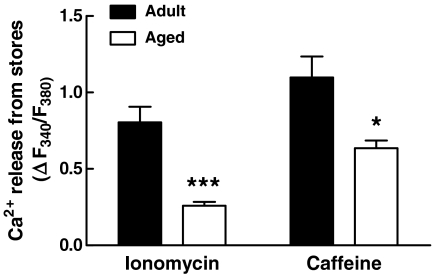
The transient [Ca2+]i peak induced in detrusor by cholinergic stimulation is mainly due to release from intracellular pools (Wu et al. 1999). To assess whether the size of the stores is diminished in aged cells, we used two stimuli known to mobilize the stored Ca2+ with little or negligible contribution from extracellular calcium (ionophore- or ryanodine receptor-mediated Ca2+ depletion). Figure 3 shows the average results from both series of experiments. The [Ca2+]i transient induced by ionomycin (used as index of intracellular Ca2+ release; see “Materials and methods”) was smaller in aged than in adult cells (adult 0.804 ± 0.103 ΔF340/F380, aged 0.260 ± 0.025 ΔF340/F380, n = 30 and 18 cells, respectively, P < 0.001, Fig. 3). When the size of intracellular pools was assessed with ryanodine, we obtained a similar age-dependent decrease: the Ca2+ released in response to caffeine was impaired in aged cells (adult 1.098 ± 0.138 ΔF340/F380, aged 0.636 ± 0.050 ΔF340/F380, n = 41 and 28 cells, respectively, P < 0.05), confirming the reduced availability of intracellular stores.
Fig. 3.
Histogram showing the release from internal stores (estimated as the peak response after treatment) induced by application of a low level of ionomycin (50 nM) in Ca2+-free solution or by application of caffeine (1 mM). n = 18–41 cells from six animals (*P < 0.05, **P < 0.001, aged vs adult)
Discussion
The results of the present study show that aging impairs detrusor contraction in response to cholinergic and depolarizing stimulus, but enhances the purinergic responses. The contractile response changes are paralleled by changes in Ca2+ signals, and are associated to a reduction of intracellular Ca2+ stores and to inhibition of extracellular Ca2+ entry.
It is known that urinary bladder disturbances, as a consequence of alterations in the micturition cycle, increase with age (Diokno et al. 1992; Milsom et al. 2001; Gomez-Pinilla et al. 2007a), but little is known about the underlying changes in bladder smooth muscle. The main contracting signals for detrusor muscle are ACh and ATP released from cholinergic and purinergic terminals (Ford et al. 2006). The instability observed in aged bladder is considered to be due to changes in neurotransmission, especially to the increase of the purinergic component (Ford et al. 2006) and the decrease of the relaxing components (Nishimoto et al. 1995; Lluel et al. 2003). Our finding of enhanced purinergic responses and depressed cholinergic responses is in keeping with previous reports showing loss of cholinergic and increase of purinergic neurotransmission in rat and human bladder (Saito et al. 1993; Lieu et al. 1997; Kageyama et al. 2000; Yoshida et al. 2001, 2004; Pagala et al. 2001), although in female guinea pig a loss of the purinergic response has been reported (Gomez-Pinilla et al. 2007c, 2008). Our data also show that, irrespective of the functional status of autonomic nerves, aging alters the contraction at the level of smooth muscle cells, given that in our experimental conditions the responses are myogenic.
In addition, the present study demonstrates that voltage-evoked contraction is also depressed in aged muscle bladder cells. Depolarization is a physiological component of regulatory detrusor signals. Actually, Ca2+ influx through voltage-activated calcium is a main component of the cholinergic stimulation of detrusor cells (Wu et al. 2002; Kajioka et al. 2005). Moreover, it has been shown that detrusor operate in part as an electrical syncytium: depolarization waves run along the longitudinal axis of bundles of cells, with poorer propagation between neighbor bundles (Hashitani et al. 2001). Although the impaired response to depolarization reported here could contribute to the diminished pressure of the voiding phase characteristic of aged detrusor (Siroky 2004; Gomez-Pinilla et al. 2007a), further research is needed to be considered.
Cytosolic calcium is the main determinant of contraction. Detrusor Ca2+ signals involve both Ca2+ influx and Ca2+ release from the internal stores. Information regarding how aging influences this Ca2+ signals in urinary bladder is scarce. We have recently shown that in guinea pig detrusor aging disrupts Ca2+ sensitizing mechanisms of the contractile machinery (Gomez-Pinilla et al. 2008), and inhibits the Ca2+ removal mechanisms (Gomez-Pinilla et al. 2007b). The present study shows that both voltage-activated Ca2+ influx and intracellular pools are impaired by aging. This could account for the decreased contraction in response to depolarization and to cholinergic stimulation. A previous report in rat aged detrusor shows that both contraction in response to nerve stimulation and Ca2+ signals display a higher sensitivity to experimental inhibition of Ca2+ influx (Yu et al. 1996), in keeping with our finding.
Contrary to cholinergic or KCl contraction, ATP contraction is enhanced by aging. This finding is likely to be related to differences in Ca2+ signalling mechanisms: while ACh receptors release Ca2+ from intracellular pools through phosphoinositide metabolism followed by subsequent influx (both of them impaired in aged cells), ATP directly induces Ca2+ influx through purinergic receptors (Iacovou et al. 1990; Heppner et al. 2005). Our results showing downsizing of the stores and impairment of Ca2+ influx rule out the possibility that ATP signals operate releasing Ca2+ from internal pools or via co-activation of voltage-operated channels, a process previously reported in smooth muscle (Morales et al. 2004). Note that even if purinergic receptors release Ca2+ from a pool different to that mobilized by ACh or caffeine, the signal would be reduced, as indicated by the results with ionomycin in aged cells (because this treatment releases all the available Ca2+).
A recent report in mouse bladder shows that P2X receptors mediate spontaneous muscle depolarization independently of voltage-activated Ca2+ influx (Young et al. 2008). This activity could be related to the spread of depolarization along bundles of cells, a pattern which is thought to actively accommodate the wall tension to the increasing volume of bladder during the filling phase (Hashitani et al. 2001). If this component is enhanced by an increased effect of ATP, it could explain previous reports of enhanced activity during the filling phase (Gomez-Pinilla et al. 2007a).
The implication for this differential effect of aging on Ca2+ signals is that the alterations are not due to impairment of Ca2+ buffer capacity (Toescu and Verkhratsky 2000) or Ca2+ extrusion (Gomez-Pinilla et al. 2007b), two common findings in aging studies, because it would affect equally to the calcium signals induced by different agonists (enhancing their amplitude and/or duration).
A likely explanation to account for our results is the modification at the receptor level or downstream to it. In fact, cholinergic receptors have been reported to be reduced (Mansfield et al. 2005) or non-functional (Schneider et al. 2005) in aged detrusor, while purinergic receptors are up-regulated by aging in rat (Suadicani et al. 2009). Of course, this mechanism is compatible with other modifications of the purinergic pathway reported in aging and in some pathophysiological conditions: increase of ATP release from parasympathetic fibers in aged human bladder (Yoshida et al. 2004), diminished hydrolysis of ATP (Fry et al. 2002; Harvey et al. 2002) or changes in the purinergic receptors of sensory nerves (see Ford et al. 2006). Our results with isolated cells point that, in addition to these mechanisms, the smooth muscle cells are also a target for age-induced enhancement of the purinergic transmission.
Acknowledgements
This work was supported by the Junta de Extremadura (PRI07A069) and Spanish Ministery of Science and Education (BFU 2007-60563) and Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad Grant RD06/0013/1012.
References
- Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Age-related alterations in Ca2+ signals and mitochondrial membrane potential in exocrine cells are prevented by melatonin. J Pineal Res. 2008;45:191–198. doi: 10.1111/j.1600-079X.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- Camello-Almaraz C, Macias B, Gomez-Pinilla PJ, Alcon S, Martin-Cano FE, Baba A, Matsuda T, Camello PJ, Pozo MJ. Developmental changes in Ca2+ homeostasis and contractility in gallbladder smooth muscle. Am J Physiol Cell Physiol. 2009;296:C783–C791. doi: 10.1152/ajpcell.00452.2008. [DOI] [PubMed] [Google Scholar]
- Derweesh IH, Wheeler MA, Weiss RM. Alterations in G-proteins and beta-adrenergic responsive adenylyl cyclase in rat urinary bladder during aging. J Pharmacol Exp Ther. 2000;294:969–974. [PubMed] [Google Scholar]
- Diokno AC, Brown MB, Goldstein N, Herzog AR. Epidemiology of bladder emptying symptoms in elderly men. J Urol. 1992;148:1817–1821. doi: 10.1016/s0022-5347(17)37038-6. [DOI] [PubMed] [Google Scholar]
- Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, Cockayne DA. Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol. 2006;147(Suppl 2):S132–S143. doi: 10.1038/sj.bjp.0706637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CH, Skennerton D, Wood D, Wu C. The cellular basis of contraction in human detrusor smooth muscle from patients with stable and unstable bladders. Urology. 2002;59:3–12. doi: 10.1016/S0090-4295(01)01632-6. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Gomez MF, Hedlund P, Sward K, Hellstrand P, Camello PJ, Pozo MJ, Andersson KE. Effect of melatonin on age associated changes in Guinea pig bladder function. J Urol. 2007;177:1558–1561. doi: 10.1016/j.juro.2006.11.071. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Pozo MJ, Baba A, Matsuda T, Camello PJ. Ca2+ extrusion in aged smooth muscle cells. Biochem Pharmacol. 2007;74:860–869. doi: 10.1016/j.bcp.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Aging impairs neurogenic contraction in guinea pig urinary bladder: role of oxidative stress and melatonin. Am J Physiol Regul Integr Comp Physiol. 2007;293:R793–R803. doi: 10.1152/ajpregu.00034.2007. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Gomez MF, Sward K, Hedlund P, Hellstrand P, Camello PJ, Andersson KE, Pozo MJ. Melatonin restores impaired contractility in aged guinea pig urinary bladder. J Pineal Res. 2008;44:416–425. doi: 10.1111/j.1600-079X.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Harvey RA, Skennerton DE, Newgreen D, Fry CH. The contractile potency of adenosine triphosphate and ecto-adenosine triphosphatase activity in guinea pig detrusor and detrusor from patients with a stable, unstable or obstructed bladder. J Urol. 2002;168:1235–1239. doi: 10.1016/S0022-5347(05)64632-0. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol. 2005;564:201–212. doi: 10.1113/jphysiol.2004.077826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovou JW, Hill SJ, Birmingham AT. Agonist-induced contraction and accumulation of inositol phosphates in the guinea-pig detrusor: evidence that muscarinic and purinergic receptors raise intracellular calcium by different mechanisms. J Urol. 1990;144:775–779. doi: 10.1016/s0022-5347(17)39590-3. [DOI] [PubMed] [Google Scholar]
- Kageyama S, Fujita K, Suzuki K, Shinbo H, Masuda N, Uchida W. Effect of age on the responses of rat bladder detrusor strips to adenosine triphosphate. BJU Int. 2000;85:899–904. doi: 10.1046/j.1464-410x.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- Kajioka S, Nakayama S, Asano H, Brading AF. Involvement of ryanodine receptors in muscarinic receptor-mediated membrane current oscillation in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2005;288:C100–C108. doi: 10.1152/ajpcell.00161.2004. [DOI] [PubMed] [Google Scholar]
- Lieu PK, Sa'adu A, Orugun EO, Malone-Lee JG. The influence of age on isometric and isotonic rat detrusor contractions. J Gerontol A Biol Sci Med Sci. 1997;52:M94–M96. doi: 10.1093/gerona/52a.2.m94. [DOI] [PubMed] [Google Scholar]
- Lin AT, Hsu TH, Yang C, Chang LS. Effects of aging on mitochondrial enzyme activity of rat urinary bladder. Urol Int. 2000;65:144–147. doi: 10.1159/000064860. [DOI] [PubMed] [Google Scholar]
- Lluel P, Palea S, Ribiere P, Barras M, Teillet L, Corman B. Increased adrenergic contractility and decreased mRNA expression of NOS III in aging rat urinary bladders. Fundam Clin Pharmacol. 2003;17:633–641. doi: 10.1046/j.1472-8206.2003.00187.x. [DOI] [PubMed] [Google Scholar]
- Mansfield KJ, Liu L, Mitchelson FJ, Moore KH, Millard RJ, Burcher E. Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br J Pharmacol. 2005;144:1089–1099. doi: 10.1038/sj.bjp.0706147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- Morales S, Camello PJ, Alcon S, Salido GM, Mawe G, Pozo MJ. Coactivation of capacitative calcium entry and L-type calcium channels in guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1090–G1100. doi: 10.1152/ajpgi.00260.2003. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Latifpour J, Wheeler MA, Yoshida M, Weiss RM. Age-dependent alterations in beta-adrenergic responsiveness of rat detrusor smooth muscle. J Urol. 1995;153:1701–1705. doi: 10.1016/S0022-5347(01)67508-6. [DOI] [PubMed] [Google Scholar]
- Pagala MK, Tetsoti L, Nagpal D, Wise GJ. Aging effects on contractility of longitudinal and circular detrusor and trigone of rat bladder. J Urol. 2001;166:721–727. doi: 10.1016/S0022-5347(05)66050-8. [DOI] [PubMed] [Google Scholar]
- Saito M, Kondo A, Gotoh M, Kato K, Levin RM. Age-related changes in the response of the rat urinary bladder to neurotransmitters. Neurourol Urodyn. 1993;12:191–200. doi: 10.1002/nau.1930120214. [DOI] [PubMed] [Google Scholar]
- Schneider T, Hein P, Michel-Reher MB, Michel MC. Effects of ageing on muscarinic receptor subtypes and function in rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:71–78. doi: 10.1007/s00210-005-1084-0. [DOI] [PubMed] [Google Scholar]
- Siroky MB. The aging bladder. Rev Urol. 2004;6(Suppl 1):S3–S7. [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Urban-Maldonado M, Tar MT, Melman A, Spray DC. Effects of ageing and streptozotocin-induced diabetes on connexin43 and P2 purinoceptor expression in the rat corpora cavernosa and urinary bladder. BJU Int. 2009;103:1686–1693. doi: 10.1111/j.1464-410X.2008.08337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Moriyama N, Kanada A, Okaya Y, Kawabe K, Aisaka K. The role of alpha 1 L-adrenoceptor in rat urinary bladder: comparison between young adult and aged rats. Life Sci. 1999;65:2553–2559. doi: 10.1016/S0024-3205(99)00524-X. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. Parameters of calcium homeostasis in normal neuronal ageing. J Anat. 2000;197(Pt 4):563–569. doi: 10.1046/j.1469-7580.2000.19740563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Bayliss M, Newgreen D, Mundy AR, Fry CH. A comparison of the mode of action of ATP and carbachol on isolated human detrusor smooth muscle. J Urol. 1999;162:1840–1847. doi: 10.1016/S0022-5347(05)68248-1. [DOI] [PubMed] [Google Scholar]
- Wu C, Sui G, Fry CH. The role of the L-type Ca(2+) channel in refilling functional intracellular Ca(2+) stores in guinea-pig detrusor smooth muscle. J Physiol. 2002;538:357–369. doi: 10.1113/jphysiol.2001.013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Homma Y, Inadome A, Yono M, Seshita H, Miyamoto Y, Murakami S, Kawabe K, Ueda S. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol. 2001;36:99–109. doi: 10.1016/S0531-5565(00)00175-3. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Young JS, Meng E, Cunnane TC, Brain KL. Spontaneous purinergic neurotransmission in the mouse urinary bladder. J Physiol. 2008;586:5743–5755. doi: 10.1113/jphysiol.2008.162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HJ, Wein AJ, Levin RM. Age-related differential susceptibility to calcium channel blocker and low calcium medium in rat detrusor muscle: response to field stimulation. Neurourol Urodyn. 1996;15:563–576. doi: 10.1002/(SICI)1520-6777(1996)15:5<563::AID-NAU12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]