Abstract
Genetic study can provide insight into the biologic mechanisms underlying inter-individual differences in susceptibility to (or resistance to) organisms’ aging. Recent advances in molecular genetics and genetic epidemiology provide the necessary tools to perform a study of the genetic sources of biological aging. However, to be successful, the genetic study of a complex condition requires a heritable phenotype to be developed and validated. Genome-wide association studies offer an unbiased approach to identify new candidate genes for human diseases. It is hypothesized that convergent results from multiple aging-related traits will point out the genes responsible for the general aging of the organism. This perspective focuses on the musculoskeletal aging as an example of an approach to identify a downstream common pathway that summarizes aging processes. Since the musculoskeletal traits are linked to the state of many vital functions, disability, and ultimately survival rates, we postulate that there is significance in studying musculoskeletal aging. Construction of an integrated phenotype of aging can be achieved based on shared genetics among multiple musculoskeletal biomarkers. Valid biomarkers from other systems of the organism should be similarly explored. The new composite aging score needs to be validated by determining whether it predicts all-cause mortality, incidences of major chronic diseases, and disability late in life. Comprehensive databases on biomarkers of musculoskeletal aging in multiple large cohort studies, along with information on various health outcomes, are needed to validate the proposed measure of biological aging.
Keywords: Biological aging, Human, Phenomics, Genome-wide association studies, Musculoskeletal system
There are multiple definitions of the aging process. Aging may be perceived as the random, systemic loss of molecular fidelity that, after reproductive maturity, accumulates to levels that eventually exceed tissue repair, turnover, or maintenance capacity (Hayflick 2004). The underlying molecular mechanisms of aging remain a subject of debates (de Magalhaes et al. 2009): tissue deterioration might not be programmed, being just a function of increase in entropy (Hayflick 2004). No genes are necessary to drive a stochastic process; however, there are genes that act to prevent an organism from destruction and disorganization. It may be due to the absence of specific disease-causing alleles or due to the presence of favorable alleles (Halaschek-Wiener et al. 2009). These genes may inhibit entropy, regulate inflammation, maintain DNA repair (such as telomere maintenance factors), or provide antioxidant functions (e.g., antagonists of reactive oxygen species). As healthy cells adapt to degeneration, differential expression of genes with age may indicate a transcriptional response to aging rather than a deleterious mechanism of aging per se (de Magalhaes et al. 2009). It might be postulated that there exist alleles that confer a pleiotropic effect on structure and function during aging (Lunetta et al. 2007). These alleles should regulate the ability of an organism to withstand challenging endogenous and exogenous influences.
In addition, environmental factors influence the organism’s ability to withstand the increase in entropy with aging: for example, caloric restriction and smoking can exert opposite effects on the rate of aging (Colman et al. 2009; Fraser and Shavlik 2001). Both protective alleles and a benevolent environment contribute to excess physiological capacity, which in turn indirectly determines an individual’s healthy life span and longevity (Martin et al. 2007). The well-recognized increase in variability with aging reflects the precarious balance between the stochastic destruction, environmental influences, and correcting effect of genes responsible for repair.
In this review, we will focus on the genetic factors providing protection against aging-related degeneration in a major system of the body—musculoskeletal. The process of bone and muscle involution is a general phenomenon and a typical manifestation of tissue atrophy with age (Plato et al. 1994). Studying the musculoskeletal aging provides a “local” model from which “global” inference for aging can be driven since the musculoskeletal biomarkers are linked to the state of many vital functions, age-related conditions of different bodily systems (Zhang et al. 1997; Samelson et al. 2004; Browner et al. 1993), cardiovascular disease (Hak et al. 2000; Kiel et al. 2001), cancer, mortality, disability, and ultimately survival rates (Kiel et al. 2001; Johansson et al. 1998). We therefore postulate that by studying musculoskeletal aging—as a proof of principle—we can come closer to the understanding of an organism’s aging.
Current progress and problems of genetic studies of aging and longevity
In spite of aging being a risk factor for many diseases, a phenotype of aging to date is still tabula rasa. Yet, the choice of a phenotype is critical for the study of a complex genetic process, such as aging (Melzer et al. 2007). Furthermore, proposed treatments to delay or alleviate aging require that validated outcomes exist, which can be measurable earlier rather than later in the life (thus, longevity per se is impractical). To date, however, most of the twin and family studies focused on broad survival measures, primarily on age at death or survival to some arbitrary advanced age (Nicholas et al. 1994). Thus, it has been demonstrated that longevity has moderate heritability 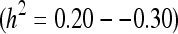 (McGue et al. 1993; Herskind et al. 1996; Gillespie et al. 1998). There are several challenges in using longevity as a phenotype (reviewed in Karasik et al. 2005 and below). A better strategy would be to investigate a broader outcome such as “successful” or “healthy” aging (Mulsant et al. 1994; Seeman et al. 2004). However, there is no consensus definition for the latter categories, especially for a genetic study. Similarly, at present, there is no consensus about how to measure aging starting in midlife despite a plethora of publications on the biomarkers and risk factors of aging (Newman et al. 2008). Yet, researchers (Nilsson et al. 2003; Crabtree et al. 2002; Vaillant and Mukamal 2001) have argued that studies of aging genetics should be initiated earlier in life, when there are life expectations permissive of longitudinal studies as well as information on environmental exposures traceable to the outcomes.
(McGue et al. 1993; Herskind et al. 1996; Gillespie et al. 1998). There are several challenges in using longevity as a phenotype (reviewed in Karasik et al. 2005 and below). A better strategy would be to investigate a broader outcome such as “successful” or “healthy” aging (Mulsant et al. 1994; Seeman et al. 2004). However, there is no consensus definition for the latter categories, especially for a genetic study. Similarly, at present, there is no consensus about how to measure aging starting in midlife despite a plethora of publications on the biomarkers and risk factors of aging (Newman et al. 2008). Yet, researchers (Nilsson et al. 2003; Crabtree et al. 2002; Vaillant and Mukamal 2001) have argued that studies of aging genetics should be initiated earlier in life, when there are life expectations permissive of longitudinal studies as well as information on environmental exposures traceable to the outcomes.
Biomarkers of aging
Despite growing interest surrounding the topic, data on valid predictors of active life expectancy and successful aging are relatively scarce (Jackson et al. 2003; Anstey et al. 1996; Nakamura and Miyao 2003; Seeman et al. 2004). With aging, a discernable decline occurs in most bodily systems, including metabolic, cognitive, reproductive, and endocrine. The aging phenotype may be best represented by the construction of an index derived from several biological parameters of an organism (biomarkers), encompassing functional parameters that predictably change with age (Dean and Morgan 1988; Johnson 2006) and are closely related to the maintenance of life, and are recognized as risk factors for age-related degeneration and diseases (Borkan and Norris 1986). The ability to predict life span is a traditional but imperfect criterion used to validate these biomarkers of aging; a more important requirement for a good biomarker is its ability to discriminate between adverse aging-related events, such as frailty (Mitnitski et al. 2002), immobility (Simonsick et al. 2001), and propensity to fall (Lord et al. 1994).
There are additional considerations when choosing biomarkers to characterize aging. First, biomarkers measured at a given age are merely snapshots of important regulatory systems (Seeman et al. 2004); there is no information on system dynamics if each biomarker is measured only once. Having longitudinal measures should be important to approximate trajectories of change in each system. Yet, cross-sectional and longitudinally measured biomarkers of aging correlate to some extent (Nakamura and Miyao 2007). The best approach seems to be investigating both cross-sectional measures at a given point of ontogenesis as well as dynamic trajectories of changes. Secondly, how specific a biomarker should be and which bodily system best represents general aging is questionable since the organism functions as a whole and not as a sum of parts. In this review, we argue a case for the major system of the body—musculoskeletal—to serve as an example of how the aging phenotype can be approximated based on genetic analysis of validated heritable biomarkers.
The musculoskeletal system as a “barometer” of aging
There are multiple arguments for focusing on the musculoskeletal status as a proxy for aging and longevity. Musculoskeletal health is vital for animal survival: the skeleton, first and foremost, greatly facilitates mobility; therefore, both skeleton (bones, joints, ligaments) and neuromuscular components of the musculoskeletal system are crucial in locomotion, by which mammals seek food and shelter (Rubin and Rubin 2008). Therefore, evolutionary factors worked to prevent this system from early decay (Karasik 2008). Furthermore, the aging process is characterized by changes in hormonal networks, which have strong effects on musculoskeletal aging: effects of menopause, andropause, and somatopause (the latter is manifesting as sarcopenia) are apparent in the musculoskeletal system.
The musculoskeletal system involves several main components, which can be measured by imaging, physiological assays, and blood biochemistry; most of these components are age-dependent and clinically significant and therefore can be considered biomarkers. Age-related musculoskeletal phenotypes are characterized by substantial heritability, as reported by multiple groups. Table 1 illustrates biomarkers that are (a) heritable, (b) predict declines in musculoskeletal and related systems, and (c) have been shown to be highly correlated with age-related loss of function and, ultimately, survival.
Table 1.
Heritable musculoskeletal biomarkers
| Biomarker | Aging-related changes | Predicts disease/condition: | Reference | h2 in Caucasiansa | Reference |
|---|---|---|---|---|---|
| Bone mineral density | Decrease (mostly in women), ∼1%/year | Fractures, CVD, dementia, mortality | Tan et al. (2005), Van Der Klift et al. (2002), Bauer et al. (2002) | 0.45–0.75 (∆ 0.37–0.49) | Hui et al. (2006) |
| Joint/cartilage | Increased Prevalence and severity of joint space narrowing and osteophytes increases | Osteoarthritis, walking disability; mortality | Munger et al. (1999), Haara et al. (2003), Haara et al. (2004) | 0.37 (knee OA) | MacGregor et al. (2009), Zhai et al. (2007) |
| Height | Decrease: 0.42% in men and 0.55% women, over 2 years (Cesari et al., 2009) | Osteoporosis | Soranzo et al. (2009) | >0.90 | Karasik et al. (2007) |
| Muscle mass | Muscle degeneration/loss | Muscle loss (sarcopenia) | Tan (2005) | 0.60–0.80 | Huygens et al., (2004), Seeman et al. (1996), Hsu et al. (2005) |
| Grip strength | Mean annual grip strength loss 0.59 kg in men and 0.31 kg in women (Frederiksen et al. 2002); 2.4% per year (Forrest et al. 2007) | Sarcopenia; mortality and disability; overall functioning | Melzer et al. (2007), Frederiksen et al. (2002), Rantanen et al. (2003) | 0.48–0.65 (∆ 0.32) | Frederiksen et al. (2002), Arden and Spector (1997), Tiainen et al. (2004), Silventoinen et al. (2008), Katzmarzyk et al. (2001) |
| Osteocalcin | Age-related decline | Osteoporosis; metabolic syndrome | (Kanazawa et al. 2009), (Vanderschueren et al. 1990) | 0.62 in a Mexican American population | Mitchell et al. (2000) |
ah2: heritability of biomarkers, both cross-sectional and of their change with age (∆), if reported
Bone mass Adult bone metabolism represents a delicate balance between old tissue removal (resorption) and new bone formation. With advanced age, the placement and quality of new bone are altered due to intrinsic and extrinsic causes, therefore affecting its mineral content and spatial organization. In the majority of older adults, bone mineral density (BMD) predictably decreases with age, with osteoporotic fractures as an end product (Cauley et al. 2009). Osteoporosis traits have been associated with risk for multiple diseases, including coronary disease in women (Samelson et al. 2004), and with risk for breast, colon and prostate cancer (Zhang et al. 1997, 2002), memory impairment (Zhang et al. 2001) and dementia in women (Tan et al. 2005) and a higher risk of mortality independent of age and comorbidities (Van Der Klift et al. 2002; Bauer et al. 2002). Most recently, Cauley et al. (2009) suggested that maintenance of BMD may represent a clinical phenotype of successful aging. It should be noted that many measurements of bone mass and morphology are technically reliable and relatively stable; hence, measurement of bone mass phenotypes is an ideal biomarker of aging.
Aging of joints Osteoarthritis (OA or degenerative arthritis) is an aging-related joint disease associated with cartilage loss in a joint. Among the signs of OA, there are such radiographic features as the presence and severity of joint space narrowing (cartilage defect) and osteophytes (reactive bony proliferation). Kalichman et al. (2006) found a statistically significant association between radiographic hand OA and ischemic heart disease and gastrointestinal diseases. OA is also associated with a higher risk of mortality independent of age and comorbidities (Munger et al. 1999; Haara et al. 2003, 2004).
Heritability of knee OA was 37% in some studies (MacGregor et al. 2009; Zhai et al. 2007); in others, however, familial aggregation of osteoarthritis was most striking for hand and hip, but remarkably absent for the knee (Riyazi et al. 2005). There was a substantial genetic influence also on the progression of knee OA, mostly due to osteophytes and joint space narrowing (Zhai et al. 2007).
Height loss This occurs as a result of loss of hydration of the intervertebral disks with the ensuing disk degeneration, core muscle weakness leading to kyphosis, and vertebral fractures due to bone loss. Since height loss is related to aging changes in the bones, muscles, and joints, it may be a great proxy to many aging-related musculoskeletal processes. Height loss is largely explained by the change in spine length (Chinappen-Horsley et al. 2008). Therefore, unsurprisingly, recent genome-wide association studies (GWAS) for adult height identified, among other bone-related genes, GDF5, a cartilage-derived morphogenetic protein (Soranzo et al. 2009; Sanna et al. 2008; Weedon et al. 2008). Earlier, it was suggested that ESR1 polymorphisms influence the age-related decrease in stature (Ioannidis et al. 2004).
Muscle mass and strength Aging of muscle manifests in the decreases in muscle strength and muscle mass that accompany aging, mainly as a result of the alterations in muscle morphology. Sarcopenia (muscle wasting or “shrinking”) is a common condition which is associated with functional impairment and disability. Reduction in muscle mass and hand grip strength is about ∼25–30% between ages 30 and 70 (Tan 2005). Fat-free (lean) mass measured by dual energy X-ray absorptiometry has been shown to be associated with mobility disability (Visser et al. 1998, 2000, 2002) and overall better functioning (Visser et al. 2002; Broadwin et al. 2001; Huygens et al. 2004).
Notably, mass of muscle is a proxy of its strength and quality, yet is not synonymous with muscle strength, although they are often used interchangeably (see Tiainen et al. 2008). Hand grip strength is a measurement of choice, known to be associated with muscular functioning in other muscle groups and with activities of daily living (Melzer et al. 2007; Frederiksen et al. 2002; Rantanen et al. 2003). Previous research has shown that hand grip strength is a significant predictor of health status, postoperative recovery, recovery from injury, protein loss, cause-specific and total mortality (Metter et al. 2002), and disability (Frederiksen et al. 2002; Rantanen et al. 2003). Muscle mass and cross-sectional area are highly heritable, ranging from 60% to 90% (Huygens et al. 2004; Seeman et al. 1996; Hsu et al. 2005). Muscle strength is also influenced by genetic factors, with h2 estimates ranging from 30% to 65% (De Mars et al. 2008).
Biochemical and endocrine serum markers
There are several biochemical traits related to musculoskeletal aging:
Osteocalcin Osteocalcin (OC, or gamma-carboxyglutamic acid protein) comprises about 15% of the non-collagenous protein component of the extracellular bone matrix and is the most abundant non-collagenous protein of the human body (Hauschka et al. 1989). The majority of osteocalcin is synthesized by osteoblasts and becomes incorporated into the bone matrix by binding to hydroxyapatite (Hauschka 1986); as such, OC serum concentrations reflect osteoblastic activity and the rate of bone formation. There is accumulating evidence that OC is also involved in glucose metabolism and insulin secretion (Oz et al. 2006; Pedrazzoni et al. 1989). Serum OC has been shown to be negatively correlated with (a) the degree of glucose intolerance (Bouillon et al. 1995), (b) with fasting glucose in patients with diabetes and with homeostasis model assessment of insulin resistance (HOMA-IR; Kanazawa et al. 2009), and (c) with systolic blood pressure in hypertensive postmenopausal women. Since there is sufficient evidence for a link between serum OC levels with metabolic syndrome and cardiovascular risk factors, OC should be considered not only a bone turnover marker but also an index of general health. The estimated heritability of serum OC level was 62% in the Mexican American population (Mitchell et al. 2000).
Insulin-like growth factor I (IGF1) Growth hormone (GH) and its related factors are active during development and repair in order to generate bone morphological adaptation in a fully coordinated manner with the skeletal muscle (Lovejoy et al. 2002). Concentrations of GH decline progressively with increasing age in men and women (so-called somatopause; Redman and Ravussin 2009). Growth hormone activates IGF1 gene transcription in vivo (Goldspink 2004). IGF1 in turn plays a prominent role in musculoskeletal aging. Elevated levels of IGF1 prompt the differentiation of osteocytes from osteoblasts and thus promote bone formation (Hirukawa et al. 2005); IGF1 also restores satellite cell proliferative potential in immobilized old skeletal muscle. In concert with sex steroids, GH and IGF1 interact to instigate growth during puberty and maintenance of body composition (Redman and Ravussin 2009). Many reports (Langlois et al. 1998; Karasik et al. 2002) have documented a decrement in IGF1 plasma levels with age in humans.
Vitamin D Vitamin D (Vit D) is an important nutritional variable related to aging by virtue of its pleiotropic actions. A growing number of conditions have been linked to vitamin D insufficiency, including type 1 and 2 diabetes (Hypponen et al. 2001; Zipitis and Akobeng 2008), cardiovascular disease (Wang et al. 2008), and cancers of the breast, colon, and prostate (Martinez et al. 1996; Garland et al. 1989; John et al. 1999). A recent meta-analysis of randomized controlled trials suggested that vitamin D supplementation led to significant reductions in mortality (Autier and Gandini 2007). Vit D is crucial for reaching optimal peak bone mass and preserving bone strength throughout life (Klibanski et al. 2001). Therefore, the Vit D receptor (VDR) gene has long been targeted as one of the genetic determinants influencing bone status. There are other genes that have Vit D responsive elements, such as those coding for osteocalcin and osteopontin. In addition, Vit D deficiency causes an increase of serum parathyroid hormone, leading to an increase in bone resorption.
Recent studies have highlighted the importance of Vit D also for muscle. Bischoff et al. (Bischoff et al. 2001; Bischoff-Ferrari et al. 2004) pointed out that the expression of the VDR in human skeletal muscle tissue decreases with age, and several studies have demonstrated that serum 25(OH) Vit D is related to physical performance (including walking test, chair stand and tandem stand; Wicherts et al. 2007) and its rate of decline, body sway, and lower body strength (Pfeifer et al. 2000). In a recent meta-analysis, Vit D status was significantly associated with falls (Bischoff-Ferrari et al. 2009). Notably, 25-OH Vit D is heritable (h2 = 53%; Livshits et al. 2003; Wang et al. 2010).
Sex hormones Special relations exist between the phenotypes of musculoskeletal aging and the sex hormones, such as levels of androgen (testosterone) in men and endogenous estrogen in women, as is evident in the sexual dimorphism in bones, joints dimensions, muscle size, and strength (Frontera et al. 2000). Estrogen is essential in growth plate closure and therefore is important in defining the adult body size. Furthermore, menopause in women leads to bone loss (Karasik and Kiel 2010); women also experience a sudden drop in muscle mass following menopause (Rolland et al. 2008). Not surprisingly, increase in longevity was found in older users of postmenopausal estrogen therapy in the Leisure World Cohort Study (Ross et al. 1990).
Testosterone is an anabolic steroid which induces skeletal muscle hypertrophy by multiple mechanisms, including modulating the commitment of pluripotent mesenchymal cells into the myogenic lineage and inhibiting adipogenesis through an androgen receptor (AR)-mediated pathway (Herbst and Bhasin 2004). The anabolic skeletal actions of androgens may result from direct activation of the AR or may alternatively depend on the stimulation of the estrogen receptors following aromatization of androgens into estrogens that occurs in peripheral tissues (the enzyme, aromatase, catalyzes this conversion). Testosterone stimulates muscle protein synthesis, increases lean body mass, and decreases fat mass in men in a dose-dependent fashion (Ferrando et al. 2003; Bhasin et al. 2006). Notably, older men’s muscles are as responsive to the anabolic effects of testosterone as young men’s (Herbst and Bhasin 2004). In men aged 40–70 years from the Massachusetts Male Aging Study, total testosterone was weakly related to mortality (Frisoli et al. 2005).
There is also a notable relationship between the sex hormones and longevity, which can serve as an example of antagonistic pleiotropy. Thus, higher levels of testosterone in males lead to increased reproductive fitness early in life while causing decreased fitness later in life due to a higher risk of prostate cancer (Summers and Crespi 2008). Both testosterone (Kuijper et al. 2007; Harris et al. 1998) and estrogen (Martini et al. 2001) are heritable; thus, there is a need for researches to step in and link heritability of reproductive hormones to that of the longevity.
Heritability of biomarkers of aging
Table 1 presents a list of the above musculoskeletal traits that have been extensively studied by several cohort studies, along with their change with age. It also summarizes their relationships with diseases and conditions of aging. There is sufficient evidence to consider the above traits as biomarkers that reflect inter-individual differences in aging-related changes in the skeleton and muscle and other important bodily systems. From the table, it also follows that the h2 of “change” in biomarkers with age is lower than the cross-sectional “snapshot” of each trait. This is an important observation which can be attributed to the prominent effect of environmental and lifestyle rather than genetic factors on the rate of musculoskeletal aging (rather than on cross-sectional, one-time measures). It also calls for methods other than analyzing the “change” phenotypes to capitalize on longitudinal biomarkers. On the other hand, while high h2 is important for predicting power for a genetic study, it does not, however, indicate how many and how strong the individual genetic factors are since the effects of all genes are pooled together (Vaessen et al. 2001).
GWAS approach for aging genes discovery
The genetic component of each heritable biomarker is complex; it encompasses contributions from multiple genetic sources not necessarily related to aging. Some of the genes are active mostly in development and others involved in homeostasis. Functional pathways regulating complex diseases are usually multigenic (Ho et al. 2008; Sievänen 2005; Wolf et al. 2006) since biological processes are known to be highly integrated.
To date, the GWAS approach is considered to be productive in uncovering multiple genes responsible for complex diseases. The hypothesis-free genome-wide strategy is sometimes referred to as forward genetics. An alternative strategy (reverse genetics) is to pursue previously recognized candidate genes via association studies. The candidate gene approach needs a robust prior hypothesis and has proven viable when there is a strong biological basis for the inclusion of a gene as a plausible candidate. The downside of such an approach is that it often suggests physiological pathways that appear much less profound when examined at the systems level.
Although there is accumulating knowledge of the genetic pathways responsible for the longevity-related traits, based on studies using aging animal models (Finch and Ruvkun 2001) and works on people surviving to advanced ages, such as centenarians (Sebastiani et al. 2009; Perls et al. 2002), this information is still far from being complete. Thus, a genome-wide search offers advantages over candidate gene association studies because it provides a more extensive coverage of the genome and the opportunity for truly novel gene discoveries—which is entirely “agnostic” and unconstrained by existing knowledge. In fact, many candidate genes, even if validated by the replication of several studies, were not confirmed by GWAS results (Richards et al. 2009). Recent reports from GWAS demonstrate the potential of this approach to identify novel gene associations for common complex conditions. Thus, GWAS of type II diabetes reported robust novel associations with regards to biology (beta cell), location (non-coding, intergenic), and potential shared biologic pathways with coronary disease (Helgadottir et al. 2007; Saxena et al. 2007; Scott et al. 2007; McPherson et al. 2007). GWAS for BMD similarly provided evidence for novel pathways important for bone metabolism (Rivadeneira et al. 2009). However, attention should be shifted to the many other variants in the human genome, such as rare polymorphisms (not only SNPs), copy number variants, and combinations of thereof (Cluett and Melzer 2009). Studies of rarer variants require much more powerful designs. Both new discovery by GWAS and replication of association findings, especially for complex quantitative phenotypes, require large sample sizes; therefore, multiple consortia, such as GIANT (Lindgren et al. 2009), GEFOS (Richards et al. 2009; Rivadeneira et al. 2009), CHARGE (Psaty et al. 2009), SunLight (Wang et al. 2010), and others, were formed.
The first foray into the GWAS of aging, using longevity and osteoporosis-related traits, was performed in the Framingham Heart Study (FHS) as a part of the FHS SHARe Project (Lunetta et al. 2007; Kiel et al. 2007). Notably, in those first works, the authors made an inquiry into the pleiotropy of the top SNP associations. Thus, a SNP near SOX5 gene was found to be associated with both walking speed and biologic age measured by a bone score and another intronic SNP in FOXO1a associated with age at death and biologic age score (Lunetta et al. 2007). However, in general, results of the associations for phenotypes such as age at death and morbidity-free survival at age 65 were far from genome-wide significant (the latter being usually defined as p values <5 × 10−8).
That first GWAS was underpowered and used a not-too-dense coverage of the genome, by the Affymetrix 100K SNP GeneChip. It was therefore expected that the second phase of the FHS SHARe Project (550K SNP array) would provide more robust results due to the much larger sample (approximately three times) of men and women with more refined phenotypes for longevity. Also, very importantly, this sample became a part of the large consortium for meta-analysis and replication of GWAS, the CHARGE (Psaty et al. 2009). However, the results for the longevity phenotype, defined as survival to age 90 and older, in the CHARGE consortium cohorts (longevity “cases”, n = 1,836, and population comparison “controls”, n = 1,955) did not reach the genome-wide significance threshold (Newman et al. 2010), similarly to Lunetta et al. (2007) GWAS. Genome-wide association studies with extreme long-living individuals to date did provide only two longevity-associated genes (Sebastiani et al. 2009). The question remains, however, how generalizable are these results and whether they replicate beyond the centenarian cohorts.
As was argued previously (Karasik et al. 2005), the longevity phenotype has several limitations for a quantitative genetic study: (a) only a small subset of a general population may be assigned a phenotype (Halaschek-Wiener et al. 2009); (b) it is difficult to obtain a sufficiently large sample of parents and offspring who both possess extreme longevity; (d) lack of a control sample (such as blood samples from the average-lifespan individuals, born at the same time as centenarians) poses a problem for case–control comparison of allelic frequencies; and (e) most importantly, ultimate longevity may not be the phenotype of clinical interest. Quality of life in older age and/or duration of disease- and disability-free aging would be preferable to be brought to the center of attention. Ultimately, interventions should be aimed at compressing morbidity and preventing functional decline along with increasing a healthy life span rather than just increasing life expectancy (Goggins et al. 2005).
One step to extend the utility of the abundant genome data is to have the phenome much better defined (Varki and Altheide 2005). We propose to outline human biological aging using the musculoskeletal aging phenotypes as an example and a starting point, with further addition of other relevant biomarkers. There are benefits in considering pleiotropy for the genetic study of complex phenotypes, including utility of genetic relationships as a means toward a better job of defining a phenotype.
Pleiotropic relationships between genes and aging-related traits and advances of phenomics
Pleiotropy
Functional pathways regulating complex processes are usually characterized by redundancy, being regulated by multiple pleiotropic genes, seemingly to avoid perturbation triggered by the environment (Ho et al. 2008; Sievänen 2005; Wolf et al. 2006). Biological systems are governed by groups of genes that perform a discrete function separable from the rest of the system, called “modules.” A precise definition of what constitutes a module is elusive; a definition of modules as groups of genes co-expressed under specific conditions seems to be most often used.
Theoretically, there may be co-expressed genes that are responsible for the phenotypic correlations among aging traits. It remains unclear to what extent the musculoskeletal biomarkers of aging each have a distinct genetic predisposition and how much of a genetic background is shared among them. We have shown correlations both among osteoporosis-related traits (Karasik et al. 2004, 2010) and between bone and muscle traits (Karasik et al. 2009).
Examples of biological candidate genes with pleiotropic functions, which are involved in aging in general and in musculoskeletal aging in particular, are numerous: (a) in addition to the IGF-1 and vitamin D genes, estrogen metabolism pathway genes, including estrogen receptors and aromatase (CYP19), are associated with fat-free mass (Walsh et al. 2005) and BMD (Shearman et al. 2004), prostate and breast cancer (Gallicchio et al. 2006), and cardiovascular disease risk (Shearman et al. 2003). (b) The klotho gene, which has been originally described as associated with premature aging-like disorders (Kuro-o et al. 1997), was recently found to be a fundamental regulator of calcium and phosphorus homeostasis, as well as to interfere with the intracellular signaling of insulin and IGF-1 (Kuro-o et al. 1997). Therefore, klotho belongs to the group of key factors regulating mineral and vitamin D metabolism. (c) ENPP1, known to be the gene for insulin resistance/type 2 diabetes, hyperglycemia, and obesity (Meyre et al. 2005), was recently shown by us and others to be associated with osteoporosis-related traits (Cheung et al. 2010). (d) Activation of the nuclear protein deacetylase Sirt1 (an antioxidant protein involved in aging) has recently been shown to decrease adipocyte development from pre-adipocytes, therefore promoting differentiation of mesenchymal stem cells into osteoblasts via inhibition of PPARγ (Backesjo et al. 2009). (e) Loss of the carboxyl terminus of Hsp70-interacting protein (CHIP or STUB1), a ubiquitin ligase co-chaperone, is associated with reduced longevity and accelerated aging, including atrophy of skeletal muscle, low BMD, and severe kyphosis in mice (Min et al. 2008). (f) Recently, in a meta-analysis of GWAS for BMD, gene ARHGAP1 (at the 11p12) was found to be genome-wide significantly associated with BMD loci. ARHGAP1 codes for a Rho GTPase-activating protein 1, also known as Cdc42 GTPase-activating protein. It was shown that CDC42GAP-null mice also develop a senescence phenotype (Wang et al. 2007).
The list of candidate genes for musculoskeletal aging with potentially pleiotropic actions is thus constantly growing.
Phenomics
Amid many debates about the role of GWAS in understanding the etiology of common diseases, it seems that at present, the true value of GWAS is in the biological discovery rather than in the medical applications. In parallel, there is a need to capitalize on the wealth of pleiotropic relationships between multiple phenotypes analyzed to date using GWAS.
The phenomic approach orients genetic investigation around common biologic pathways leading to disease (Varki and Altheide 2005). There are two main premises of the phenomic approach: first, since relations between phenotypes often reflect a biologic and functional interaction at the gene level, several investigators (Bilder et al. 2009) have argued for using phenotypic relations to discern the underlying biology (as exemplified by pleiotropic findings for several pathologies; Helgadottir et al. 2007; Saxena et al. 2007; Scott et al. 2007; McPherson et al. 2007). It was argued that a comprehensive collection of systematically obtained phenotypic information (phenome) will aid in the identification of disease genes (Freimer and Sabatti 2003).
Although many phenotypic measurements may be collected for each individual, a smaller number of variables (latent factors) may explain most inter-individual differences (Karasik et al. 2004; Freimer and Sabatti 2003). Thus, in a study of approximately one million Swedish young men, the genetic correlations between several muscle strength traits varied from 0.58% to 0.78 (Silventoinen et al. 2008), suggesting that a latent variable might be identified by combining these measures. We and others found many indications of pleiotropy between bone and muscle traits in aging (Karasik et al. 2004; Karasik and Kiel 2008). “Reverse phenotyping” was proposed as an approach whereby genetic marker data are used to define phenotype groupings (to uncover the phenotypic modularity, defined as the separability of the design into units that perform independently, at least to a first approximation; Schulze and McMahon 2004; Harris et al. 2007). This can be done first by utilizing the unique resource of the “Catalog of Published Genome-Wide Association Studies” dataset (Hindorff et al. 2009).
By identifying which measurements contribute most to defining the underlying hidden variables, phenotyping efforts can focus on such “integrating” measures that conceptually resemble phenotypic moduli, which can then be utilized as quantitative traits for gene mapping (Pan et al. 2006; Schulze and McMahon 2004), therefore decreasing the problem of multiple testing. This will generate the “integrator” phenotypes that are probably more proximal to the “endophenotype” of disease.
We have argued (Karasik and Kiel 2008) that there are several incentives for considering pleiotropy for the genetic study of complex phenotypes as a means toward reverse phenotyping. First, the “post-genomic” era provides the opportunity to utilize a technically advanced hypothesis-free selection method to identify the major genetic variants (using GWAS, expression experiments, eSNPs, etc.). Second, the computational and bioinformatic tools (Gajendran et al. 2007), exhaustive databases, and advanced database querying methods support the conduct of multidimensional analyses. Third, analyzing multiple correlated traits can also help control false positives: by looking at multiple affected phenotypes, one is creating a framework for removing potentially spurious phenotype associations due to measurement uncertainty.
Finally, GWAS frequently analyze multiple correlated phenotypes; therefore, in addition to the statistical challenges of increased type 1 error (chance finding), there is uncertainty of what statistical threshold for related traits might be indicative of pleiotropy. The newly proposed and existing analytic approaches, such as multivariate analysis of correlated phenotypes, would allow performing joint analyses of genetically correlated traits. Given that the multivariate analysis is more powerful than univariate (Almasy et al. 1997; Williams et al. 1999; Havill et al. 2006; Liu et al. 2009), there are more chances to unearth the genetic signals that jointly govern the aging-related changes in biomarkers.
Conclusions
In the absence of a consensus phenotype for aging, genetic research is impeded (Melzer et al. 2007). At present, it is difficult to determine whether preventative and therapeutic strategies (such as calorie restriction) have beneficial effects in humans because there are no validated biomarkers that can serve as surrogate markers of aging (Matkovic et al. 1990). To have the “phenome of aging” (Xue et al. 2007) much better defined, we propose using the musculoskeletal aging phenotypes as an example and starting point.
The genetic study of musculoskeletal aging does provide insights into the potential biologic mechanisms underlying inter-individual differences in susceptibility to (or resistance to) an organism’s aging. The GWAS approach is considered to be productive in uncovering multiple genes responsible for aging and the underlying mechanisms leading to variation in musculoskeletal aging and longevity. Several population-based cohorts such as the Framingham Heart Study, Rotterdam Study, AGES-Reykjavik Study (Harris et al. 2007), other members of the Longevity Consortium (http://www.longevityconsortium.org/resources/), and many others are therefore uniquely suited to propel the field, with abundant genome coverage and longitudinal phenotyping done by standardized methods.
Knowledge of genetic interrelationship between the biomarkers of aging may lead to the discovery of a downstream common pathway that summarizes aging processes; the list of biomarkers should be as comprehensive as possible via incorporating other well-known systems involved in aging in addition to the musculoskeletal system. Further development of the pleiotropy-based approaches will be useful for other studies of multiple related phenotypes which employ genome-wide associations to decipher genetics in the absence of disease endophenotypes, which is the case of human aging. With the advent of these approaches, new candidate genes may emerge for further pursuit. In its turn, discovery of the “phenome of aging” may translate into innovative diagnostic and therapeutic interventions to improve the overall health of older men and women.
Acknowledgment
The author would like to express his thanks to the anonymous reviewer for her/his useful suggestions in revising this manuscript.
References
- Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol. 1997;14:953–958. doi: 10.1002/(SICI)1098-2272(1997)14:6<953::AID-GEPI65>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Anstey K, Lord S, Smith G. Measuring human functional age: a review of empirical findings. Exp Aging Res. 1996;22:245–266. doi: 10.1080/03610739608254010. [DOI] [PubMed] [Google Scholar]
- Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res. 1997;12:2076–2081. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- Backesjo CM, Li Y, Lindgren U, Haldosen LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. Cells Tissues Organs. 2009;189:93–97. doi: 10.1159/000151744. [DOI] [PubMed] [Google Scholar]
- Bauer DC, Palermo L, Black D, Cauley JA. Quantitative ultrasound and mortality: a prospective study. Osteoporos Int. 2002;13:606–612. doi: 10.1007/s001980200081. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Sabb FW, Cannon TD, et al. Phenomics: the systematic study of phenotypes on a genome-wide scale. Neuroscience. 2009;164:30–42. doi: 10.1016/j.neuroscience.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff HA, Borchers M, Gudat F, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Borchers M, Gudat F, et al. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkan GA, Norris AH. Assessment of biological age using a profile of physical parameters. J Gerontol. 1986;35:177–184. doi: 10.1093/geronj/35.2.177. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Bex M, Herck E, et al. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995;80:1194–1202. doi: 10.1210/jcem.80.4.7714089. [DOI] [PubMed] [Google Scholar]
- Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49:1641–1645. doi: 10.1046/j.1532-5415.2001.t01-1-49273.x. [DOI] [PubMed] [Google Scholar]
- Browner WS, Pressman AR, Nevitt MC, et al. Association between low bone density and stroke in elderly women: the study of osteoporotic fractures. Stroke. 1993;24:940–946. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Lui LY, Barnes D, et al. Successful skeletal aging: a marker of low fracture risk and longevity. The study of osteoporotic fractures (SOF) J Bone Miner Res. 2009;24:134–143. doi: 10.1359/JBMR.080813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64:377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CL, Livshits G, Zhou Y et al. (2010) Hip geometry variation is associated with bone mineralization pathway gene variants: the framingham study. J Bone Miner Res, still in press; doi:10.1359/jbmr.091102 [DOI] [PMC free article] [PubMed]
- Chinappen-Horsley U, Blake GM, Fogelman I, et al. Quantitative trait loci for bone lengths on chromosome 5 using dual energy X-ray absorptiometry imaging in the Twins UK cohort. PLoS ONE. 2008;3:e1752. doi: 10.1371/journal.pone.0001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluett C, Melzer D. Human genetic variations: beacons on the pathways to successful ageing. Mech Ageing Dev. 2009;130:553–563. doi: 10.1016/j.mad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree NJ, Kroger H, Martin A, et al. Improving risk assessment: hip geometry, bone mineral distribution and bone strength in hip fracture cases and controls. The EPOS Study. European Prospective Osteoporosis Study. Osteoporos Int. 2002;13:48–54. doi: 10.1007/s198-002-8337-y. [DOI] [PubMed] [Google Scholar]
- Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars G, Windelinckx A, Huygens W, et al. Genome-wide linkage scan for maximum and length-dependent knee muscle strength in young men: significant evidence for linkage at chromosome 14q24.3. J Med Genet. 2008;45:275–283. doi: 10.1136/jmg.2007.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean W, Morgan RF. In defense of the concept of biological aging measurement—current status. Arch Gerontol Geriatr. 1988;7:191–210. doi: 10.1016/0167-4943(88)90002-7. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Paddon-Jones D, et al. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88:358–362. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- Finch CE, Ruvkun G. The genetics of aging. Annu Rev Genomics Hum Genet. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- Forrest KY, Zmuda JM, Cauley JA. Patterns and correlates of muscle strength loss in older women. Gerontology. 2007;53:140–147. doi: 10.1159/000097979. [DOI] [PubMed] [Google Scholar]
- Fraser GE, Shavlik DJ. Ten years of life: is it a matter of choice? Arch Intern Med. 2001;161:1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Gaist D, Petersen HC, et al. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol. 2002;23:110–122. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- Freimer N, Sabatti C. The human phenome project. Nat Genet. 2003;34:15–21. doi: 10.1038/ng0503-15. [DOI] [PubMed] [Google Scholar]
- Frisoli A, Jr, Paula AP, Pinheiro M, et al. Hip axis length as an independent risk factor for hip fracture independently of femural bone mineral density in Caucasian elderly Brazilian women. Bone. 2005;37:871–875. doi: 10.1016/j.bone.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Suh D, Krivickas LS, et al. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol. 2000;279:C611–C618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- Gajendran VK, Lin JR, Fyhrie DP. An application of bioinformatics and text mining to the discovery of novel genes related to bone biology. Bone. 2007;40:1378–1388. doi: 10.1016/j.bone.2006.12.067. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Berndt SI, Mcsorley MA, et al. Polymorphisms in estrogen-metabolizing and estrogen receptor genes and the risk of developing breast cancer among a cohort of women with benign breast disease. BMC Cancer. 2006;6:173. doi: 10.1186/1471-2407-6-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- Gillespie LD, Gillespie WJ, Cumming R et al. (1998) Interventions to reduce the incidence of falling in the elderly. Cochrane Database of Systematic Reviews
- Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Age-related muscle loss and progressive dysfunction in mechanosensitive growth factor signaling. Ann NY Acad Sci. 2004;1019:294–298. doi: 10.1196/annals.1297.050. [DOI] [PubMed] [Google Scholar]
- Haara MM, Manninen P, Kroger H, et al. Osteoarthritis of finger joints in Finns aged 30 or over: prevalence, determinants, and association with mortality. Ann Rheum Dis. 2003;62:151–158. doi: 10.1136/ard.62.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haara MM, Heliovaara M, Kroger H, et al. Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. J Bone Joint Surg Am. 2004;86:1452–1457. doi: 10.2106/00004623-200407000-00013. [DOI] [PubMed] [Google Scholar]
- Hak A, Pols H, Hemert A, et al. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- Halaschek-Wiener J, Amirabbasi-Beik M, Monfared N, et al. Genetic variation in healthy oldest-old. PLoS ONE. 2009;4:e6641. doi: 10.1371/journal.pone.0006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Vernon PA, Boomsma DI. The heritability of testosterone: a study of Dutch adolescent twins and their parents. Behav Genet. 1998;28:165–171. doi: 10.1023/a:1021466929053. [DOI] [PubMed] [Google Scholar]
- Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility—Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka PV. Osteocalcin: the vitamin K-dependent Ca2+-binding protein of bone matrix. Haemostasis. 1986;16:258–272. doi: 10.1159/000215298. [DOI] [PubMed] [Google Scholar]
- Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- Havill LM, Rogers J, Cox LA, Mahaney MC. QTL with pleiotropic effects on serum levels of bone-specific alkaline phosphatase and osteocalcin maps to the baboon ortholog of human chromosome 6p23–21.3. J Bone Miner Res. 2006;21:1888–1896. doi: 10.1359/jbmr.060812. [DOI] [PubMed] [Google Scholar]
- Hayflick L. “Anti-aging” is an oxymoron. J Gerontol A Biol Sci Med Sci. 2004;59:B573–B578. doi: 10.1093/gerona/59.6.b573. [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Herskind AM, Mcgue M, Holm NV, et al. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Hindorff LA, Sethupathy P, Junkins HA, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci USA. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirukawa K, Miyazawa K, Maeda H, et al. Effect of tensile force on the expression of IGF-I and IGF-I receptor in the organ-cultured rat cranial suture. Arch Oral Biol. 2005;50:367–372. doi: 10.1016/j.archoralbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Ho AM, Marker PC, Peng H, et al. Dominant negative Bmp5 mutation reveals key role of BMPs in skeletal response to mechanical stimulation. BMC Dev Biol. 2008;8:35. doi: 10.1186/1471-213X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Lenchik L, Nicklas BJ, et al. Heritability of body composition measured by DXA in the diabetes heart study. Obes Res. 2005;13:312–319. doi: 10.1038/oby.2005.42. [DOI] [PubMed] [Google Scholar]
- Hui SL, Koller DL, Foroud TM, et al. Heritability of changes in bone size and bone mass with age in premenopausal white sisters. J Bone Miner Res. 2006;21:1121–1125. doi: 10.1359/jbmr.060412. [DOI] [PubMed] [Google Scholar]
- Huygens W, Thomis MA, Peeters MW, et al. Determinants and upper-limit heritabilities of skeletal muscle mass and strength. Can J Appl Physiol. 2004;29:186–200. doi: 10.1139/h04-014. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Ralston SH, Bennett ST, et al. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA. 2004;292:2105–2114. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Weale MR, Weale RA. Biological age—what is it and can it be measured? Arch Gerontol Geriatr. 2003;36:103–115. doi: 10.1016/s0167-4943(02)00060-2. [DOI] [PubMed] [Google Scholar]
- Johansson C, Black D, Johnell O, et al. Bone mineral density is a predictor of survival. Calcif Tissue Int. 1998;63:190–196. doi: 10.1007/s002239900513. [DOI] [PubMed] [Google Scholar]
- John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971–1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomark Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kalichman L, Malkin I, Livshits G, Kobyliansky E. The association between morbidity and radiographic hand osteoarthritis: a population-based study. Joint Bone Spine. 2006;73:406–410. doi: 10.1016/j.jbspin.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yamamoto M, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:45–49. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- Karasik D. Osteoporosis: an evolutionary perspective. Hum Genet. 2008;124:349–356. doi: 10.1007/s00439-008-0559-8. [DOI] [PubMed] [Google Scholar]
- Karasik D, Kiel D. Genetics of osteoporosis in older age. In: Duque GAK, editor. Senile osteoporosis: advances in pathophysiology and therapeutic approach. London: Springer; 2010. [Google Scholar]
- Karasik D, Kiel DP. Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner Res. 2008;23:788–802. doi: 10.1359/jbmr.080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik D, Rosen CJ, Hannan MT, et al. Insulin-like growth factor binding proteins 4 and 5 and bone mineral density in elderly men and women. Calcif Tissue Int. 2002;71:323–328. doi: 10.1007/s00223-002-1002-0. [DOI] [PubMed] [Google Scholar]
- Karasik D, Cupples LA, Hannan MT, Kiel DP. Genome screen for a combined bone phenotype using principal component analysis: the Framingham Study. Bone. 2004;34:547–556. doi: 10.1016/j.bone.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Karasik D, Demissie S, Cupples LA, Kiel DP. Disentangling the genetic determinants of human aging: biological age as an alternative to the use of survival measures. J Gerontol A Biol Sci Med Sci. 2005;60:574–587. doi: 10.1093/gerona/60.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik D, Dupuis J, Cupples LA, et al. Bivariate linkage study of proximal hip geometry and body size indices: the Framingham Study. Calcif Tissue Int. 2007;81:162–173. doi: 10.1007/s00223-007-9052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik D, Zhou Y, Cupples LA, et al. Bivariate genome-wide linkage analysis of femoral bone traits and leg lean mass: Framingham Study. J Bone Miner Res. 2009;24:710–718. doi: 10.1359/JBMR.081222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik D, Hsu Y, Zhou Y et al. (2010) Genome-wide pleiotropy of osteoporosis-related phenotypes: the Framingham Study. J Bone Miner Res, still in press; doi:10.1002/jbmr.38 [DOI] [PMC free article] [PubMed]
- Katzmarzyk PT, Gledhill N, Perusse L, Bouchard C. Familial aggregation of 7-year changes in musculoskeletal fitness. J Gerontol A Biol Sci Med Sci. 2001;56:B497–B502. doi: 10.1093/gerona/56.12.b497. [DOI] [PubMed] [Google Scholar]
- Kiel DP, Kauppila LI, Cupples LA, et al. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- Kiel DP, Demissie S, Dupuis J, et al. Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S14. doi: 10.1186/1471-2350-8-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanski A, Adams-Campbell L, Bassford T, et al. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- Kuijper EA, Lambalk CB, Boomsma DI, et al. Heritability of reproductive hormones in adult male twins. Hum Reprod. 2007;22:2153–2159. doi: 10.1093/humrep/dem145. [DOI] [PubMed] [Google Scholar]
- Kuro-O M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rosen CJ, Visser M, et al. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–4262. doi: 10.1210/jcem.83.12.5308. [DOI] [PubMed] [Google Scholar]
- Lindgren CM, Heid IM, Randall JC, et al. Genome-wide association scan meta-analysis identifies three loci influencing adiposity and fat distribution. PLoS Genet. 2009;5:e1000508. doi: 10.1371/journal.pgen.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, Pei YF, Liu JF, et al. Powerful bivariate genome-wide association analyses suggest the SOX6 gene influencing both obesity and osteoporosis phenotypes in males. PLoS ONE. 2009;4:e6827. doi: 10.1371/journal.pone.0006827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshits G, Yakovenko C, Seibel M. Substantial genetic effects involved in determination of circulating levels of calciotropic hormones in human pedigrees. Biochem Genet. 2003;41:269–289. doi: 10.1023/b:bigi.0000006029.01736.64. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc. 1994;42:1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO, Meindl RS, Ohman JC, et al. The Maka femur and its bearing on the antiquity of human walking: applying contemporary concepts of morphogenesis to the human fossil record. Am J Phys Anthropol. 2002;119:97–133. doi: 10.1002/ajpa.10111. [DOI] [PubMed] [Google Scholar]
- Lunetta KL, D'agostino RB, Sr, Karasik D, et al. Genetic correlates of longevity and selected age-related phenotypes: a genome-wide association study in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S13. doi: 10.1186/1471-2350-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor AJ, Li Q, Spector TD, Williams FM. The genetic influence on radiographic osteoarthritis is site specific at the hand, hip and knee. Rheumatology (Oxford) 2009;48:277–280. doi: 10.1093/rheumatology/ken475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Bergman A, Barzilai N. Genetic determinants of human health span and life span: progress and new opportunities. PLoS Genet. 2007;3:e125. doi: 10.1371/journal.pgen.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ME, Giovannucci EL, Colditz GA, et al. Calcium, vitamin D, and the occurrence of colorectal cancer among women. J Natl Cancer Inst. 1996;88:1375–1382. doi: 10.1093/jnci/88.19.1375. [DOI] [PubMed] [Google Scholar]
- Martini G, Valenti R, Giovani S, et al. Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone. 2001;28:113–117. doi: 10.1016/s8756-3282(00)00408-7. [DOI] [PubMed] [Google Scholar]
- Matkovic V, Fontana D, Tominac C, et al. Factors that influence peak bone mass formation: a study of calcium balance and the inheritance of bone mass in adolescent females. Am J Clin Nutr. 1990;52:878–888. doi: 10.1093/ajcn/52.5.878. [DOI] [PubMed] [Google Scholar]
- Mcgue M, Vaupel JW, Holm N, Harvald B. Longevity is moderately heritable in a sample of Danish twins born 1870–1880. J Gerontol. 1993;48:B237–B244. doi: 10.1093/geronj/48.6.b237. [DOI] [PubMed] [Google Scholar]
- Mcpherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Hurst AJ, Frayling T. Genetic variation and human aging: progress and prospects. J Gerontol A Biol Sci Med Sci. 2007;62:301–307. doi: 10.1093/gerona/62.3.301. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- Meyre D, Bouatia-Naji N, Tounian A, et al. Variants of ENPP1 are associated with childhood and adult obesity and increase the risk of glucose intolerance and type 2 diabetes. Nat Genet. 2005;37:863–867. doi: 10.1038/ng1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JN, Whaley RA, Sharpless NE, et al. CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol Cell Biol. 2008;28:4018–4025. doi: 10.1128/MCB.00296-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BD, Cole SA, Bauer RL, et al. Genes influencing variation in serum osteocalcin concentrations are linked to markers on chromosomes 16q and 20q. J Clin Endocrinol Metab. 2000;85:1362–1366. doi: 10.1210/jcem.85.4.6571. [DOI] [PubMed] [Google Scholar]
- Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulsant BH, Sweet R, Rifai AH, et al. The use of the Hamilton Rate Scale for depression in elderly patients with cognitive impairment and physical illness. Am J Geratr Psychiatry. 1994;2:220–229. doi: 10.1097/00019442-199400230-00006. [DOI] [PubMed] [Google Scholar]
- Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999;69:147–152. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. Further evaluation of the basic nature of the human biological aging process based on a factor analysis of age-related physiological variables. J Gerontol A Biol Sci Med Sci. 2003;58:196–204. doi: 10.1093/gerona/58.3.b196. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. J Gerontol A Biol Sci Med Sci. 2007;62:1096–1105. doi: 10.1093/gerona/62.10.1096. [DOI] [PubMed] [Google Scholar]
- Newman AB, Boudreau RM, Naydeck BL, et al. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. doi: 10.1093/gerona/63.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Walter S, Lunetta KL, et al. A meta-analysis of four genome-wide association studies of survival to age 90 years or older: the Cohorts for Heart and Aging Research in Genome Epidemiology (CHARGE) Consortium. J Gerontol A Biol Sci Med Sci. 2010;65:478–487. doi: 10.1093/gerona/glq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas V, Prewett A, Beltica P, Mohan S, Finkelman R, Baylink DJ, Farley JR. Age-related decreases of insulin-like growth factor-1 and transforming growth factor-b in femoral cortical bone from both men and women: implications for bone loss with aging. J Clin Endocr Metab. 1994;78:1011–1016. doi: 10.1210/jcem.78.5.8175953. [DOI] [PubMed] [Google Scholar]
- Nilsson PM, Engberg M, Nilsson JA, et al. Adverse social factors predict early ageing in middle-aged men and women: the Ebeltoft Health Study, Denmark. Scand J Public Health. 2003;31:255–260. doi: 10.1080/14034940210165019. [DOI] [PubMed] [Google Scholar]
- Oz SG, Guven GS, Kilicarslan A, et al. Evaluation of bone metabolism and bone mass in patients with type-2 diabetes mellitus. J Natl Med Assoc. 2006;98:1598–1604. [PMC free article] [PubMed] [Google Scholar]
- Pan WH, Lynn KS, Chen CH, et al. Using endophenotypes for pathway clusters to map complex disease genes. Genet Epidemiol. 2006;30:143–154. doi: 10.1002/gepi.20136. [DOI] [PubMed] [Google Scholar]
- Pedrazzoni M, Ciotti G, Pioli G, et al. Osteocalcin levels in diabetic subjects. Calcif Tissue Int. 1989;45:331–336. doi: 10.1007/BF02556002. [DOI] [PubMed] [Google Scholar]
- Perls T, Kunkel LM, Puca AA. The genetics of exceptional human longevity. J Mol Neurosci. 2002;19:233–238. doi: 10.1007/s12031-002-0039-x. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, Minne HW, et al. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- Plato C, Fox K, Tobin J. Skeletal changes in human aging. New York: Oxford University Press; 1994. [Google Scholar]
- Psaty B, O’Donnell C, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from five cohorts. Circ Cardiovasc Genet. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Volpato S, Ferrucci L, et al. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- Redman LM, Ravussin E. Endocrine alterations in response to calorie restriction in humans. Mol Cell Endocrinol. 2009;299:129–136. doi: 10.1016/j.mce.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Kavvoura FK, Rivadeneira F, et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151:528–537. doi: 10.7326/0003-4819-151-8-200910200-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadeneira F, Styrkarsdottir U, Estrada K, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41:1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riyazi N, Meulenbelt I, Kroon HM, et al. Evidence for familial aggregation of hand, hip, and spine but not knee osteoarthritis in siblings with multiple joint involvement: the GARP Study. Ann Rheum Dis. 2005;64:438–443. doi: 10.1136/ard.2004.024661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland Y, Czerwinski S, Kan Abellan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RK, Bernstein L, Paganini-Hill A, Henderson BE. Effects of cigarette smoking on ‘hormone-related’ diseases in a Southern California retirement community. In: Wald NJ, Baron J, editors. Smoking and hormone-related disorders. New York: Oxford University Press; 1990. pp. 34–54. [Google Scholar]
- Rubin J, Rubin C. Functional adaptation to loading of a single bone is neuronally regulated and involves multiple bones. J Bone Miner Res. 2008;23:1369–1371. doi: 10.1359/jbmr.09901. [DOI] [PubMed] [Google Scholar]
- Samelson EJ, Kiel DP, Broe KE, et al. Metacarpal cortical area and risk of coronary heart disease: the Framingham Study. Am J Epidemiol. 2004;159:589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- Sanna S, Jackson AU, Nagaraja R, et al. Common variants in the GDF5-UQCC region are associated with variation in human height. Nat Genet. 2008;40:198–203. doi: 10.1038/ng.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Mcmahon FJ. Defining the phenotype in human genetic studies: forward genetics and reverse phenotyping. Hum Hered. 2004;58:131–138. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani P, Montano M, Puca A, et al. RNA editing genes associated with extreme old age in humans and with lifespan in C. elegans. PLoS ONE. 2009;4:e8210. doi: 10.1371/journal.pone.0008210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E, Hopper JL, Young NR, et al. Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol. 1996;270:E320–E327. doi: 10.1152/ajpendo.1996.270.2.E320. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Shearman AM, Cupples LA, Demissie S, et al. Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA. 2003;290:2263–2270. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- Shearman AM, Karasik D, Gruenthal KM, et al. Estrogen receptor Beta polymorphisms are associated with bone mass in women and men: the Framingham Study. J Bone Miner Res. 2004;19:773–781. doi: 10.1359/JBMR.0301258. [DOI] [PubMed] [Google Scholar]
- Sievänen H. Hormonal influences on the muscle-bone feedback system: a perspective. J Musculoskelet Neuronal Interact. 2005;5:255–261. [PubMed] [Google Scholar]
- Silventoinen K, Magnusson PK, Tynelius P, et al. Heritability of body size and muscle strength in young adulthood: a study of one million Swedish men. Genet Epidemiol. 2008;32:341–349. doi: 10.1002/gepi.20308. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Kasper JD, Guralnik JM, et al. Severity of upper and lower extremity functional limitation: scale development and validation with self-report and performance-based measures of physical function. WHAS Research Group. Women’s Health and Aging Study. J Gerontol B Psychol Sci Soc Sci. 2001;56:S10–S19. doi: 10.1093/geronb/56.1.s10. [DOI] [PubMed] [Google Scholar]
- Soranzo N, Rivadeneira F, Chinappen-Horsley U, et al. Meta-analysis of genome-wide scans for human adult stature identifies novel loci and associations with measures of skeletal frame size. PLoS Genet. 2009;5:e1000445. doi: 10.1371/journal.pgen.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K, Crespi B. The androgen receptor and prostate cancer: a role for sexual selection and sexual conflict? Med Hypotheses. 2008;70:435–443. doi: 10.1016/j.mehy.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Tan RS. Aging men’s health: a case-based approach. New York: Thieme; 2005. [Google Scholar]
- Tan ZS, Seshadri S, Beiser A, et al. Bone mineral density and the risk of Alzheimer disease. Arch Neurol. 2005;62:107–111. doi: 10.1001/archneur.62.1.107. [DOI] [PubMed] [Google Scholar]
- Tiainen K, Sipila S, Alen M, et al. Heritability of maximal isometric muscle strength in older female twins. J Appl Physiol. 2004;96:173–180. doi: 10.1152/japplphysiol.00200.2003. [DOI] [PubMed] [Google Scholar]
- Tiainen KM, Perola M, Kovanen VM, et al. Genetics of maximal walking speed and skeletal muscle characteristics in older women. Twin Res Hum Genet. 2008;11:321–334. doi: 10.1375/twin.11.3.321. [DOI] [PubMed] [Google Scholar]
- Vaessen N, Heutink P, Janssen JA, et al. A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637–642. doi: 10.2337/diabetes.50.3.637. [DOI] [PubMed] [Google Scholar]
- Vaillant GE, Mukamal K. Successful aging. Am J Psychiatry. 2001;158:839–847. doi: 10.1176/appi.ajp.158.6.839. [DOI] [PubMed] [Google Scholar]
- Klift M, Pols HA, Geleijnse JM, et al. Bone mineral density and mortality in elderly men and women: the Rotterdam Study. Bone. 2002;30:643–648. doi: 10.1016/s8756-3282(02)00670-1. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Gevers G, Raymaekers G, et al. Sex- and age-related changes in bone and serum osteocalcin. Calcif Tissue Int. 1990;46:179–182. doi: 10.1007/BF02555041. [DOI] [PubMed] [Google Scholar]
- Varki A, Altheide TK. Comparing the human and chimpanzee genomes: searching for needles in a haystack. Genome Res. 2005;15:1746–1758. doi: 10.1101/gr.3737405. [DOI] [PubMed] [Google Scholar]
- Visser M, Harris TB, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–M221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- Visser M, Deeg DJ, Lips P, et al. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- Walsh S, Zmuda JM, Cauley JA, et al. Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J Appl Physiol. 2005;98:132–137. doi: 10.1152/japplphysiol.00537.2004. [DOI] [PubMed] [Google Scholar]
- Wang L, Yang L, Debidda M, et al. Cdc42 GTPase-activating protein deficiency promotes genomic instability and premature aging-like phenotypes. Proc Natl Acad Sci USA. 2007;104:1248–1253. doi: 10.1073/pnas.0609149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhang F, Richards JB et al. (2010) Common genetic determinants of vitamin D insufficiency: the SUNLIGHT consortium. Lancet, Published online June 10, 2010 doi:10.1016/S0140-6736(10)60635-6
- Weedon MN, Lango H, Lindgren CM, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicherts IS, Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- Williams JT, Eerdewegh P, Almasy L, Blangero J. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. I. Likelihood formulation and simulation results. Am J Hum Genet. 1999;65:1134–1147. doi: 10.1086/302570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JB, Pomp D, Eisen EJ, et al. The contribution of epistatic pleiotropy to the genetic architecture of covariation among polygenic traits in mice. Evol Dev. 2006;8:468–476. doi: 10.1111/j.1525-142X.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- Xue H, Xian B, Dong D, et al. A modular network model of aging. Mol Syst Biol. 2007;3:147. doi: 10.1038/msb4100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai G, Hart DJ, Kato BS, et al. Genetic influence on the progression of radiographic knee osteoarthritis: a longitudinal twin study. Osteoarthritis Cartilage. 2007;15:222–225. doi: 10.1016/j.joca.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kiel D, Kreger B, et al. Bone mass and the risk of breast cancer among postmenopausal women. N Engl J Med. 1997;336:611–617. doi: 10.1056/NEJM199702273360903. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Seshadri S, Ellison RC, et al. Bone mineral density and verbal memory impairment: Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2001;154:795–802. doi: 10.1093/aje/154.9.795. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kiel DP, Ellison RC, et al. Bone mass and the risk of prostate cancer: the Framingham Study. Am J Med. 2002;113:734–739. doi: 10.1016/s0002-9343(02)01382-7. [DOI] [PubMed] [Google Scholar]
- Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]


