Abstract
Recombinant human growth hormone is used for the treatment of growth failure in children and metabolic dysfunction in adults with growth hormone deficiency. However, conventional growth hormone therapy requires daily subcutaneous injections that may affect treatment adherence, and subsequently efficacy outcomes. To enhance potential treatment adherence, improved ease of use of growth hormone delivery devices and long-acting growth hormone formulations are now being developed. Flexpro®, approved by the US Food and Drug Administration in March 2010, is the most recent pen device developed by Novo Nordisk A/S to deliver Norditropin®. It is a multidose, premixed, preloaded, disposable pen device that requires relatively less force to inject and does not require refrigeration after initial use. Dose adjustments can be optimized by small dose increments of the pen delivery device at 0.025 mg, 0.05 mg and 0.1 mg. In addition, for patients with needle anxiety, NovoFine® needles, some of the shortest and thinnest available, and Autocover®, which hides the needle during injections, can be used with the Flexpro pen device. This article reviews the Norditropin Flexpro pen device in the context of other growth hormone delivery devices, sustained-release growth hormone formulations in development, and future prospects.
Keywords: growth hormone, administration, adherence, treatment, pen, device
Introduction
Early preparations of growth hormone were extracted from human pituitary glands, but their use for treating growth hormone deficiency was discontinued in 1985 following the reports of four cases of Creutzfeld-Jacob disease in patients who had received growth hormone.1 Later in the same year, the first recombinant form of human growth hormone became available, and was produced using genetically engineered bacterial cells (Escherichia coli),2 but in 1987, a mammalian-cell derived recombinant growth hormone preparation (produced by murine C127 cells) was introduced.3 With the availability of recombinant growth hormone, researchers have been able to explore the use of growth hormone for other conditions associated with growth retardation and metabolic abnormalities. Growth hormone has since been approved in the US for the treatment of chronic renal insufficiency in children in 1997, for short stature related to Turner’s syndrome in children in 1998, for short children born small for gestational age without catch-up growth by the age of two years in 2001, for Noonan syndrome in children in 2007,4,5 and for the treatment of growth hormone deficiency in adults in 1997.6
To achieve optimal therapeutic results with growth hormone, continuous long-term adherence is essential. However, at present, all existing recombinant growth hormone products are administered daily as a subcutaneous injection, and this can lead to issues with adherence,7 avoidance of therapy,8 and early termination of growth hormone treatment.9 Therefore, it is important that devices used for recombinant growth hormone administration are convenient, practical, user-friendly, and acceptable to patients. The device should be easy to learn and easy to use for patients, particularly in children and in elderly patients with impaired vision and manual dexterity.
To increase choice and satisfy patient needs, several pen devices for administering recombinant growth hormone have been developed. In this review, developments in the administration of growth hormone treatment for children and adults are discussed, with a particular focus on the most recently available pen device developed by Novo Nordisk A/S (Bagsvaerd, Denmark), Flexpro®, that delivers Norditropin®, and its place in the context of other growth hormone delivery devices, sustained-release growth hormone formulations in development, and future prospects.
Adherence to therapy
Poor adherence to growth hormone treatment is associated with reduced efficacy outcomes and increased health care costs.10 Nonadherence is most often seen in teenagers, with up to 23% of teenagers missing at least two injections per week.11 However, there is no direct and reliable method of assessing adherence to treatment. Current data are derived from user questionnaires which rely on recall and goodwill. In a survey of 158 adult patients, 326 adolescents, and 398 parents of children currently receiving or previously treated with growth hormone, the key factors associated with nonadherence were misperceptions about the consequences of missed doses, discomfort with injections, dissatisfaction with treatment results, and inadequate contact with health care providers.12 Patients not offered a free choice of pen device at the start of treatment have also been shown to be less likely to adhere to growth hormone treatment.11
Given these results, endocrinology units, particularly in larger centers, in recent years have moved towards offering patients starting growth hormone treatment a free choice of a greater range of injection devices and products. This process involves an endocrine nurse specialist handling, with issues such as dialing up doses, assembling, growth hormone reconstitution, considering facilities provided by manufacturer, injecting (both patient and parent), showing additional material, and cost consideration.13 However, discomfort with injections and ease of use remains an ongoing problem that affects treatment adherence in both children and adults alike.
Current administration devices
Considerable technological progress in the methods of administering growth hormone in recent years has led to the availability of a range of delivery devices (see Table 1). There are five broad categories of devices, ie, syringe with needle, injection pen, autoinjector pen, needle-free injector, and electronic injector. The overall convenience and ease of use of devices within each class also depend on the preparation of growth hormone, and presently, there are two types of recombinant growth hormone on the market, ie, Norditropin® (Novo Nordisk) and Nutropin AQ® (Ipsen, Paris, France; Genentech, CA) that are available as prefilled liquid formulations.
Table 1.
How Norditropin® Flexpro® compares with other devices for injecting recombinant growth hormone
| Device type | Device | Recombinant GH manufacturer | Device features | Reconstitution | Storage |
|---|---|---|---|---|---|
| Syringe and needle | Miniquick® | Genotropin® (Pfizer) | Single-use, disposable syringe; available in dose 0.2–2.0 mg in 0.2 mg steps | Syringes are prefilled with lyophilized r-GH and diluents in a two-chamber cartridge | Can be kept nonrefrigerated for 3 months |
| Injector pen | Genotropin® pen | Genotrin® (Pfizer) | Multiuse pen in two sizes; needle guard; dose dialed before each injection; digital dose-display window | Lyophilized r-GH is mixed internally through the use of a two-chamber cartridge | Cartridge is stored in the pen; the pen is refrigerated |
| HumatroPen™ | Humatrope® (Lilly) | Multiuse pen; dose dialed; digital display of dose selected | Lyophilized r-GH is reconstituted before use using a prefilled syringe of diluents | The cartridge is stored in the device between in injections; the device is refrigerated | |
| NordiPen® | Norditropin® (Novo Nordisk) | Multiuse pen; dose dialed before each injection; can be used with an optional guard to hide the needle and autoinserter mechanism (NordiPen Mate®) | Prefilled cartridge contains premixed liquid r-GH | Can be stored at room temperature for 3 weeks | |
| NordiFlex® | Norditropin® (Novo Nordisk) | Disposable pen in 5, 10 and 15 mg sizes; dose dialed before each injection; can be used with a retractable needle cover (NovoFine® Autocover® needle); no longer available in the US after May 31, 2011 | Prefilled, multidose cartridge contains premixed liquid r-GH | Can be stored at room temperature for 3 weeks | |
| Flexpro® | Norditropin® (Novo Nordisk) | Disposable pen in 5, 10, and 15 mg sizes and shorter by 11 mm than the Nordiflex®; dose dialed before each injection; smallest dose increment 0.025 mg; a click confirms dose delivery; can be used with a retractable needle cover (NovoFine® Autocover® needle) | Prefilled, multidose cartridge contains premixed liquid r-GH | Can be stored at room temperature for 3 weeks | |
| Nutropin AQ Pen® | Nutropin AQ® (Ipsen/Genentech) | Multiuse pen; dose dialed before each injection or dose recall function used; digital dose display; optional needle shield | Premixed, cartridge prefilled with liquid r-GH | Cartridge, stored within device; the device is refrigerated | |
| Autoinjector pen | one.click™ | Saizen® (Merck Serono International SA) | Multiuse autoinjector pen; dose dialed; adjustable injection depth; hidden needle | Lyophilized GH is reconstituted using click.easy device | Cartridge stored within the device; the device is refrigerated |
| Needle-free injector | cool.click™ | Saizen® (Merck Serono International SA) | Needle-free device; dose dialed before each use | Lyophilized r-hGH is reconstituted using click.easy device; cartridge is inserted at each use | The cartridge is stored outside the device and is refrigerated |
| Zomajet® 2 vision* | Zomacton® (Ferring) | Needle-free device; dose dialed before each use | Lyophilized r-GH is reconstituted before use | The vial is kept outside the device and is refrigerated | |
| Electronic injector | easypod™ | Saizen® (Merck Serono) | Electronic autoinjector; dose programmed; adjustable injection depth; hidden needle | Lyophilized r-GH is reconstituted using click.easy device | Cartridge stored within the device; the device is refrigerated |
Note:
Not available in the US.
Abbreviations: GH, growth hormone; r-GH, recombinant growth hormone.
Syringes and needles that do not require refrigeration offer a simpler disposable method of administering growth hormone. The prime example is the Miniquick® (Pfizer Inc, New York, NY) that offers patients increased convenience and portability over conventional needles and syringes. By containing a two-chamber cartridge prefilled with growth hormone powder and diluents, the user only needs to turn the plunger to reconstitute the drug. If not reconstituted, the Miniquick® syringe can be stored for up to three months without refrigeration.
Injector pens incorporate a dial so that the dose can be set before the injection is administered. Handling is easier than for a needle and syringe, but the user still needs a reasonable amount of manual dexterity to insert the needle, reach the button to deliver the growth hormone, and remove the needle from the pen after use.14 Some devices have a hidden needle for those with needle anxiety, but this does not necessarily improve safety. Needle-stick injury is more common with injection pens than with conventional syringes, and most injuries are related to disassembly.15 To improve safety, needle shields that automatically cover the needle after injection have been designed.
In contrast, autoinjectors are spring-driven systems that automatically inject the drug from a cartridge or prefilled syringe, and have dials that allow the dose to be preset. One autoinjector recently developed for growth hormone cartridges is the one.click™ (Merck Serono International SA) system, which requires only a single click of the button to both insert the needle and inject the drug. Other autoinjectors have been developed for use across multiple injectable drugs by fitting around standard insulin syringes.
Two needle-free injector systems for administering growth hormone are also currently available on the market, ie, cool. click™ (Merck Serono International SA, Geneva, Switzerland) and Zomajet® (Ferring, Saint-Prex, Switzerland [not available in the US]). These devices expel growth hormone solution at high pressure through a small nozzle that forces the drug through the skin and disperses the drug subcutaneously.16 Although the needle-free devices avoid using needles, they are not totally painless due to the high pressure on drug administration. However, they do offer an alternative to administration by needle injection and eliminate the risk of needle-stick injury.
An electronic injector is a fully automated, programmable device, and easypod™ (Merck Serono International SA) is the only electronic, fully automated, injection device for recombinant growth hormone delivery. This device is of similar size to a cell/mobile phone and through a menu system displayed on the screen, the user is able to view their dose history and personalize their injection settings. The dose is electronically preset and dose adjustments are also set at this stage. The screen displays information regarding the dosing history and the need to change the cartridge. The “home” screen displays the date and time of the last dose injected, the number of doses remaining in the cartridge, and a reminder to the user not to reinject if an injection was given within the previous 24 hours, or if the user has not injected within 24 hours of the last injection. Health care personnel can download data from the easypod to a computer, and records of injection dates (differentiated into completed injections, missed injections, and partial injections) can then be accessed.
Supply, stability, storage, and administration
The potency of growth hormone products is expressed as IU/mg,17 and all commercially available growth hormone products have a potency of approximately 3.0 IU/mg. Growth hormone preparations are available as lyophilized powder in vials for reconstitution (Humatrope®, Nutropin®, Serostim®, Saizen®, Zorbtive®, Tev-Tropin®, and Omnitrope®), in two-chamber cartridges requiring reconstitution (Genotropin® and Saizen®), as a liquid in prefilled cartridges (Norditropin®, Humatrope®, Nutropin AQ®, Saizen® and Omnitrope®), or as prefilled pens (Norditropin® Flexpro®, Norditropin® Nordi-Flex®, and Nutropin AQ® Nuspin™). All growth hormone products should be stored at 36–46°F (2–8°C) after reconstitution. However, an alternative in-use storage option is permissible for Norditropin® 5 mg/1.5 mL or 10 mg/1.5 mL of up to three weeks at not more than 77°F (25°C). Storage times can range from 14–28 days. Growth hormone diluted and preservative-free diluents must be used within 24 hours, with the exception of Serostim® 5 mg and 6 mg vials, which should be administered immediately after mixing.18–27
Growth hormone administration for growth hormone-deficient adults and children originally involved intramuscular injections given during the daytime two to three times weekly.28,29 However, several studies have shown an improvement in growth rate when growth hormone-deficient children were switched over to daily subcutaneous injections administered in the evening without changing the total weekly dose.30,31 The improved growth response may be attributed to an increased frequency of injections, use of the subcutaneous route of administration resulting in prolonged intervals with sustained elevations in plasma growth hormone levels, and administration of growth hormone in the evening which more closely approximates the physiological endogenous secretory pattern of growth hormone.
Development of sustained-release growth hormone formulations
Recombinant growth hormone therapy in humans requires daily injections because the half-life of growth hormone after subcutaneous administration is 5.3 hours32 and is rapidly cleared by the liver and kidney. Thus, a controlled and sustained delivery system for growth hormone is potentially attractive in providing enhanced therapeutic efficacy and patient convenience. To achieve sustained growth hormone delivery, various formulations, including fusion of stabilizing peptide, crystal formulation, PEGylation, and loading to a microsphere or hydrogel, have been proposed.33–39 However, the most investigated formulation so far is an encapsulation method into the biodegradable poly(lactic acid-co-glycolic acid, PLGA) microsphere system,40 which has shown a sustained growth hormone release profile for one month.36 Pharmacokinetic and pharmacodynamic data have been published on two such preparations, ie, Nutropin Depot® and hGH-Bioshepe®. Nutropin Depot® was the first launched sustained growth hormone delivery system that used the PLGA microsphere system but was later withdrawn from the market due to the high cost of manufacturing,34 while hGH-Bioshepe® has a superior release profile. However, outcomes data from multicenter trials in both children and adults for Nutropin Depot® have shown that catch-up growth was observed in children, although to a lesser degree than historic comparative data obtained with the use of daily system injections, and the effects on metabolic derangements in growth hormone-deficient patients appeared similar to those observed with daily injections.
A growth hormone-loaded hyaluronate microparticle (LB03002), which was developed and launched as a once-a-week injection formulation in Korea by LG Life Sciences in 2007, is the only commercially available sustained-release formulation of growth hormone to date. In a Phase III study (ClinicalTrials.gov identifier: NCT 00596037), treatment with LB03002 for 26 or 52 weeks in adults with growth hormone deficiency demonstrated a sustained reduction of fat mass and other body composition parameters, with a good safety profile and acceptable tolerability.41 More recently, Park et al40 has shown that that the development of a combined system of a polyelectrolyte complex and injectable, biodegradable and thermosensitive poly (organophosphazene) hydrogel also appears to show much promise as an effective delivery system for sustained-release growth hormone.
Norditropin® Flexpro®
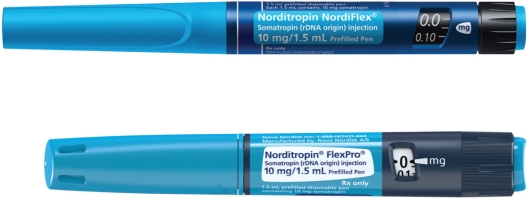
With the exception of the Norditropin® Nordiflex® 30 mg pen, Novo Nordisk A/S announced in January 2011 that it would discontinue the manufacture of Norditropin® Nordiflex® 5 mg, 10 mg, and 15 mg pens after May 31, 2011 in the US, and all patients on these pens would need to be transitioned eventually over to Flexpro®. Norditropin® FlexPro® (Figure 1), approved by the US Food and Drug Administration in March 2010, is a multidose, disposable pen device developed by Novo Nordisk A/S for the administration of recombinant growth hormone. Compared with Norditropin® Nordiflex® (Novo Nordisk A/S), the Norditropin® FlexPro® is 11 mm shorter (Figure 2), and therefore fits more comfortably in small hands, and requires a four-fold lower force for injection. It has an easy-to-push dose button that does not extend out, thus reducing the length the thumb must reach to inject. Norditropin® FlexPro® is a premixed, preloaded pen that requires no reconstitution and no loading of cartridges. The pen device is prefilled with Norditropin® (a liquid formulation of growth hormone) and is available in three doses, ie, 5 mg/1.5 mL, 10 mg/1.5 mL, and 15 mg/1.5 mL (Figure 1). The pen device must be refrigerated prior to first use, but after initial use, the pen devices can then either be stored outside of the refrigerator (at up to 77°F) for use within three weeks, or in the refrigerator (between 36°F and 46°F) for use within four weeks. The pen device is based on a spring mechanism that allows medication to be delivered by continuous depression of the dose button with negligible force. A different clicking sound alerts the patient to the dialing direction and an audible end-of-dose click confirms dose delivery. In addition, to improve ease of use in patients requiring large doses of growth hormone, the device is suitable for injecting doses up to 8 mg (15 mg Norditropin® FlexPro®). Small dose increments of 0.025 mg, 0.05 mg, and 0.1 mg provides the opportunity for finer dose adjustments, resulting in less product wastage and consequently saves cost.42 The Norditropin® Flexpro® pen device can also be used with a NovoFine® needle, which is the shortest, sharpest and thinnest commercially available needle to reduce injection pain, with the 6 mm needles reported to reduce the rate of intramuscular deposition of drug significantly compared with the 12 mm needles.43 For patients with needle anxiety, there is the option of using the NovoFine® Autocover®, which hides the needle by a shield before, during, and after injections, retracts during each injection, and then covers the needle again. The NovoFine®Autocover® can also reduce the risk of needle-stick injury.
Figure 1.
Norditropin® Flexpro® pen. The yellow pen is a 5 mg/1.5 mL pen with 0.025 mg increments, the blue pen is a 10 mg/1.5 mL pen with 0.05 mg increments, and the green pen is a 15 mg/1.5 mL pen with 0.1 mg increments. Photo courtesy of Novo Nordisk A/S.
Figure 2.
Norditropin® Flexpro® and Norditropin Nordiflex® pen. The Norditropin Flexpro pen is 11 mm shorter than the Norditropin Nordiflex pen. Photo courtesy of Novo Nordisk A/S.
Current clinical studies of Norditropin® Flexpro®
There is so far only one study that has directly compared the efficacy and safety of Norditropin® with other growth hormone products. Pfutzner et al44 compared injection time, ease of use, usability, overall preference, and dose accuracy of the Norditropin® Flexpro® with two other commercially available injection devices, ie, easypod™ (Merck Serono International SA) and Genotropin® (Pfizer Inc) in growth hormone-treated pediatric patients. These investigators found that Norditropin® Flexpro® was associated with shorter injection times, higher dose accuracy, greater intuitiveness, and was the most preferred of the three devices, but was rated lower than the easypod™ and Genotropin® devices on quality and appearance.
In addition, a study by Shine et al45 was conducted to compare the Norditropin® cartridge and its delivery device, Nordipen®, with three other marketed growth hormone formulations and their delivery devices (Humatrope® [via Humatrope® Pen], Nutropin AQ® [via vial and syringe], and Genotropin® [via Genotropin® Pen]). The investigators in this study found higher patient and parent preferences for the Norditropin® cartridge over the other growth hormone products. The Norditropin® cartridge was also preferred by most parents with respect to ease of preparation, ease of measuring doses, and ease of administration.
The usability and acceptability of Norditropin® Flexpro® was recently evaluated by Fuchs et al46 in a group of pediatric patients with growth hormone deficiency. This open-label, uncontrolled study included 70 patients aged 10–18 years who completed a 21-item questionnaire on the acceptability and usability of the device. All patients reported that using the Flexpro® pen device was very easy or quite easy, with 90% of patients reporting that the pen device was better in terms of stability of the device as compared with their currently used device. Overall, 45 patients (64%) indicated a preference for the Flexpro® over the current pen, 14 patients were uncertain, and 11 patients preferred their current device over the Flexpro® pen. There was also a high level of confidence that growth hormone had been injected properly and that the correct dose was delivered.
More recently, in two multinational, open-label, uncontrolled studies, Kappelgaard et al compared patient acceptability47 and usability48 of Norditropin® Flexpro® and Flexpro® PenMate® with Norditropin Nordiflex® and Flexpro® PenMate® for administration of growth hormone in children and adolescents with growth hormone deficiency. These studies demonstrated that the majority of patients preferred the Flexpro®/PenMate® over the Nordiflex®/PenMate® system, with 84% and 96%, respectively, reporting that they were highly confident and found the Flexpro® PenMate® system user-friendly. These results suggest that the improvements made to the Norditropin® pen with Norditropin® Flexpro® are tangible and recognizable to subjects, and may thus contribute to improved treatment adherence and possibly enhance treatment outcomes.
Conclusion
The administration of recombinant growth hormone to children and adults is likely to require injectable delivery for the foreseeable future. Therefore, in order to improve treatment adherence, user-friendly injection pens that do not require reconstitution and refrigeration after initial use, have design features that can reduce pain on injection such as finer needles and hidden-needle options, small dose increments for finer tuning of dose adjustments, and disposable after use are all important factors which the Norditropin® Flexpro® pen device presently offers. In addition, electronic devices with adherence aids such as reminder messages and tracking functions, multiple sensor and precision cartridge detection, preprogrammed dosing, automatic needle attachment/detachment, and automatic injection using a permanently hidden needle, are now being developed that might all help in increasing levels of treatment adherence and improving treatment efficacy. It is noteworthy that none of these technologies are yet able to mimic the physiological secretion of endogenous growth hormone, which is pulsatile with episodic peaks and primarily nocturnal. For these reasons, for the time being, injection devices, such as the Norditropin® Flexpro® pen device that delivers growth hormone with minimal pain, simplicity, convenience, adjustability, and dosing accuracy, are likely to remain the mainstay in the administration of recombinant growth hormone treatment to children and adults with growth hormone deficiency. However, further prospective studies will be needed to assess whether these new growth hormone injection devices translate into improved treatment efficacy outcomes and reduced overall health care costs in the future.
Future perspectives
Apart from the easypod™, there are, as yet, no indications for further development of another electronic device for recombinant growth hormone administration. Instead, there are more developments in disposable injector devices in other therapy areas, and this trend is likely to continue for recombinant growth hormone.49 Indeed, there is an increasing number of early-phase studies in developing recombinant growth hormone technology that will reduce frequency and increase the interval of injections, such as the sustained-release growth hormone formulations.
To address problems associated with injection pain and needle anxiety, new technologies are challenging the problems of using needle-free liquid jet injectors to administer large protein molecules, such as occasional pain, discomfort and local reactions, inconvenience of use compared with injections, and cost. The ViaDerm™ device (Teva Pharmaceutical Industries, Petah Tikva, Israel) uses a radiofrequency signal to generate microchannels in the outer layer of the skin. It is hypothesized that these channels will enable transdermal recombinant growth hormone delivery through a growth hormone patch attached to the skin surface. The safety, tolerability, and pharmacokinetics of this system have been tested in a Phase I trial (ClinicalTrials.gov identifier: NCT 00455260). In addition, an existing needle-free device, ie, the cool.click™, has been shown to deliver subcutaneous recombinant growth hormone exposure that is bioequivalent to exposure following injection by a needle and syringe,50 and is now being further refined for future use.
Recent advances in aerosol technology, by increasing particle size and lowering their density and tendency to agglomerate, have increased efficiency of deep lung delivery and improved systemic absorption compared with conventional micronized particles.51 This has enabled the development of an inhaled formulation of growth hormone, known as somatropin inhalation powder. Somatropin inhalation powder underwent a six-month toxicology study in primates, pharmacokinetic and pharmacodynamic studies in healthy male adult subjects, and a 28-day pharmacokinetic and safety study in adults with mild to moderate asthma. All demonstrated the acute safety and tolerability of somatropin inhalation powder without any untoward effects on pulmonary function tests.52 In a recent study, Walvoord et al53 demonstrated that inhaled growth hormone (somatropin inhalation powder) administered to growth hormone-deficient children for seven days was well tolerated and resulted in dose-dependent increases in serum growth hormone and insulin growth factor-I levels. However, bioavailability was only 50% of what was expected based on previous adult data. Although children preferred this route of therapy, the bioavailability results of this study have somewhat hampered future developments of somatropin inhalation powder.
Finally, sustained-release growth hormone formulations are now also being developed which can offer a lower frequency of injection rates, ranging from once a week to potentially once a month. However, improved sustained-release growth hormone preparations will need further study of their long-term efficacy and safety, but if shown to be effective and reliable in administering recombinant growth hormone, will prove to be highly attractive in terms of patient adherence and convenience.
Footnotes
Disclosure
The authors of this study report no conflicts of interest in this work.
References
- 1.Johnson RT, Gibbs CJ., Jr Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994–2004. doi: 10.1056/NEJM199812313392707. [DOI] [PubMed] [Google Scholar]
- 2.Flodh H. Human growth hormone produced with recombinant DNA technology: Development and production. Acta Paediatr Scand Suppl. 1986;325:1–9. doi: 10.1111/j.1651-2227.1986.tb10356.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeisel HJ, von Petrykowski W, Wais U. Pharmacokinetics and short-term metabolic effects of mammalian cell-derived biosynthetic human growth hormone in man. Horm Res. 1992;37(Suppl 2):5–13. doi: 10.1159/000182369. [DOI] [PubMed] [Google Scholar]
- 4.Dumas H, Panayiotopoulos P, Parker D, Pongpairochana V. Understanding and meeting the needs of those using growth hormone injection devices. BMC Endocr Disord. 2006;6:5. doi: 10.1186/1472-6823-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krysiak R, Gdula-Dymek A, Bednarska-Czerwinska A, Okopien B. Growth hormone therapy in children and adults. Pharmacol Rep. 2007;59:500–516. [PubMed] [Google Scholar]
- 6.Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: A statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 7.Smith SL, Hindmarsh PC, Brook CG. Compliance with growth hormone treatment – are they getting it. Arch Dis Child. 1993;68:91–93. doi: 10.1136/adc.68.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambanini A, Newson RB, Maisey M, Feher MD. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999;46:239–246. doi: 10.1016/s0168-8227(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 9.Coste J, Letrait M, Carel JC, et al. Long-term results of growth hormone treatment in France in children of short stature: Population, register based study. BMJ. 1997;315:708–713. doi: 10.1136/bmj.315.7110.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haverkamp F, Johansson L, Dumas H, et al. Observations of nonadherence to recombinant human growth hormone therapy in clinical practice. Clin Ther. 2008;30:307–316. doi: 10.1016/j.clinthera.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93:147–148. doi: 10.1136/adc.2006.114249. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14:143–154. doi: 10.4158/EP.14.2.143. [DOI] [PubMed] [Google Scholar]
- 13.Langham S, Kirk J. National audit of patient choice in pediatric GH therapy. Horm Res Paediatr. 2010 Sep 2; doi: 10.1159/000318784. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Fidotti E. A history of growth hormone injection devices. J Pediatr Endocrinol Metab. 2001;14:497–501. doi: 10.1515/jpem.2001.14.5.497. [DOI] [PubMed] [Google Scholar]
- 15.Pellissier G, Migueres B, Tarantola A, Abiteboul D, Lolom I, Bouvet E. Risk of needlestick injuries by injection pens. J Hosp Infect. 2006;63:60–64. doi: 10.1016/j.jhin.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov. 2006;5:543–548. doi: 10.1038/nrd2076. [DOI] [PubMed] [Google Scholar]
- 17.Bristow AF, Gaines-Das R, Jeffcoate SL, Schulster D. The First International Standard for Somatropin: Report of an international collaborative study. Growth Regul. 1995;5:133–141. [PubMed] [Google Scholar]
- 18.Serostim. Randolph, MA: EMD Serono, Inc; Sep, 2007. Product information. [Google Scholar]
- 19.Tev-Tropin. Sellersville, PA: Gate Pharmaceuticals; Oct, 2007. Product information. [Google Scholar]
- 20.Zorbtive. Rockland, MA: EMD Serono Inc; Jan, 2009. Product information. [Google Scholar]
- 21.Humatrope. Indianapolis, IN: Eli Lilly and Company; Aug, 2009. Product information. [Google Scholar]
- 22.Nutropin AQ. South San Francisco, CA: Genetech, Inc; Jan, 2008. Product information. [Google Scholar]
- 23.Saizen. Rockland, MA: EMD Serono, Inc; Sep, 2007. Product information. [Google Scholar]
- 24.Norditropin. Princeton, NJ: Novo Nordisk Inc; Mar, 2010. Product information. [Google Scholar]
- 25.Genotropin. New York, NY: Pfizer, Inc; Aug, 2009. Product information. [Google Scholar]
- 26.Nutropin AQ. South San Francisco, CA: Genentech, Inc; Jun, 2006. Product information. [Google Scholar]
- 27.Omnitrope. Princeton, NJ: Sandoz, Inc; Jun, 2010. Product information. [Google Scholar]
- 28.Hermanussen M, Geiger-Benoit K, Sippell WG. Catch-up growth following transfer from three times weekly im to daily sc administration of hGH in GH deficient patients, monitored by knemometry. Acta Endocrinol (Copenh) 1985;109:163–168. doi: 10.1530/acta.0.1090163. [DOI] [PubMed] [Google Scholar]
- 29.Johansson JO, Wiren L, Oscarsson J, Bengtsson BA, Johannsson G. Growth hormone (GH) replacement in GH-deficient adults: A crossover trial comparing the effect on metabolic control, well-being and compliance of three injections per week versus daily injections. Growth Horm IGF Res. 2003;13:306–315. doi: 10.1016/s1096-6374(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 30.Albertsson-Wikland K, Westphal O, Westgren U. Daily subcutaneous administration of human growth hormone in growth hormone deficient children. Acta Paediatr Scand. 1986;75:89–97. doi: 10.1111/j.1651-2227.1986.tb10163.x. [DOI] [PubMed] [Google Scholar]
- 31.Kastrup KW, Christiansen JS, Andersen JK, Orskov H. Increased growth rate following transfer to daily sc administration from three weekly im injections of hGH in growth hormone deficient children. Acta Endocrinol (Copenh) 1983;104:148–152. doi: 10.1530/acta.0.1040148. [DOI] [PubMed] [Google Scholar]
- 32.Laursen T, Jorgensen JO, Susgaard S, Moller J, Christiansen JS. Subcutaneous absorption kinetics of two highly concentrated preparations of recombinant human growth hormone. Ann Pharmacother. 1993;27:411–415. doi: 10.1177/106002809302700402. [DOI] [PubMed] [Google Scholar]
- 33.Chung HJ, Lee Y, Park TG. Thermo-sensitive and biodegradable hydrogels based on stereocomplexed Pluronic multi-block copolymers for controlled protein delivery. J Control Release. 2008;127:22–30. doi: 10.1016/j.jconrel.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Govardhan C, Khalaf N, Jung CW, et al. Novel long-acting crystal formulation of human growth hormone. Pharm Res. 2005;22:1461–1470. doi: 10.1007/s11095-005-6021-x. [DOI] [PubMed] [Google Scholar]
- 35.Johnson OL, Cleland JL, Lee HJ, et al. A month-long effect from a single injection of microencapsulated human growth hormone. Nat Med. 1996;2:795–799. doi: 10.1038/nm0796-795. [DOI] [PubMed] [Google Scholar]
- 36.Johnson OL, Jaworowicz W, Cleland JL, et al. The stabilization and encapsulation of human growth hormone into biodegradable microspheres. Pharm Res. 1997;14:730–735. doi: 10.1023/a:1012142204132. [DOI] [PubMed] [Google Scholar]
- 37.Lee EN, Kim YM, Lee HJ, et al. Stabilizing peptide fusion for solving the stability and solubility problems of therapeutic proteins. Pharm Res. 2005;22:1735–1746. doi: 10.1007/s11095-005-6489-4. [DOI] [PubMed] [Google Scholar]
- 38.Lee HJ, Riley G, Johnson O, et al. In vivo characterization of sustained-release formulations of human growth hormone. J Pharmacol Exp Ther. 1997;281:1431–1439. [PubMed] [Google Scholar]
- 39.Tae G, Kornfield JA, Hubbell JA. Sustained release of human growth hormone from in situ forming hydrogels using self-assembly of fluoroalkyl-ended poly(ethylene glycol) Biomaterials. 2005;26:5259–5266. doi: 10.1016/j.biomaterials.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 40.Park MR, Chun C, Ahn SW, Ki MH, Cho CS, Song SC. Sustained delivery of human growth hormone using a polyelectrolyte complex-loaded thermosensitive polyphosphazene hydrogel. J Control Release. 2010;147:359–367. doi: 10.1016/j.jconrel.2010.07.126. [DOI] [PubMed] [Google Scholar]
- 41.Biller BMK, Ji HJ, Lee S, et al. Twelve months efficacy and safety of a novel once-a-week sustained release rhGH (LB03002): A Phase III study in adults with GH deficiency. Presented at the 92nd annual meeting of the Endocrine Society; San Diego, CA. June 19–22, 2010. [Google Scholar]
- 42.Bazalo GR, Joshi AV, Germak J. Comparison of human growth hormone products’ cost in pediatric and adult patients. A budgetary impact model. Manag Care. 2007;16:45–51. [PubMed] [Google Scholar]
- 43.Solvig J, Christansen JS, Lytzen L. Needle length affects insulin deposition in normal and obese diabetes patients. Diabetes Res Clin Pract. 2001;50(Suppl 2):A132. [Google Scholar]
- 44.Pfutzner A, Hartmann K, Winter F, Fuchs GS, Kappelgaard AM, Rohrer TR. Intuitiveness, ease of use, and preference of a prefilled growth hormone injection pen: A noninterventional, randomized, open-label, crossover, comparative usability study of three delivery devices in growth hormone-treated pediatric patients. Clin Ther. 2010;32:1918–1934. doi: 10.1016/j.clinthera.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Shine B, Musial W, Owens L, Deeb L, Luetjen T, Howard C. Patient and parent preference for growth hormone products. Am J Health Syst Pharm. 2003;60:89–90. doi: 10.1093/ajhp/60.1.89. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs GS, Mikkelsen S, Knudsen TK, Kappelgaard AM. Ease of use and acceptability of a new pen device for the administration of growth hormone therapy in pediatric patients: An open-label, uncontrolled usability test. Clin Ther. 2009;31:2906–2914. doi: 10.1016/j.clinthera.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Kappelgaard AM, Mikkelsen S, Bagger C. Fuchs GS Patient acceptability of a new injection device system to administer human growth hormone: Results from a multinational handling and questionnaire survey. Presented at the 92nd annual meeting of the Endocrine Society; San Diego, CA. June 19–22, 2010. [Google Scholar]
- 48.Kappelgaard AM, Mikkelsen S, Bagger C, Knudsen TK, Fuchs GS. Usability testing of a new pen injector and a combination of the new pen injector and a new penmate system to administer human growth hormone in three open-label user surveys. Presented at the 92nd annual meeting of the Endocrine Society; San Diego, CA. June 19–22, 2010. [Google Scholar]
- 49.French D. Market trends for injection devices for pharmaceuticals. Touch briefings. Future Drug Delivery. 2006:20–24. [Google Scholar]
- 50.Brearley C, Priestley A, Leighton-Scott J, Christen M. Pharmacokinetics of recombinant human growth hormone administered by cool.click 2, a new needle-free device, compared with subcutaneous administration using a conventional syringe and needle. BMC Clin Pharmacol. 2007;7:10. doi: 10.1186/1472-6904-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards DA, Hanes J, Caponetti G, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 52.Chipman J, Lucas R, Jackson B, et al. Pharmacokinetics and safety results of a human single dose comparison of somatropin inhalation powder vs subcutaneous injection of somatropin. Presented at the 87th annual meeting of the Endocrine Society; San Diego, CA. June 4–7, 2005. [Google Scholar]
- 53.Walvoord EC, de la Pena A, Park S, et al. Inhaled growth hormone (GH) compared with subcutaneous GH in children with GH deficiency: Pharmacokinetics, pharmacodynamics, and safety. J Clin Endocrinol Metab. 2009;94:2052–2059. doi: 10.1210/jc.2008-1897. [DOI] [PubMed] [Google Scholar]