Abstract
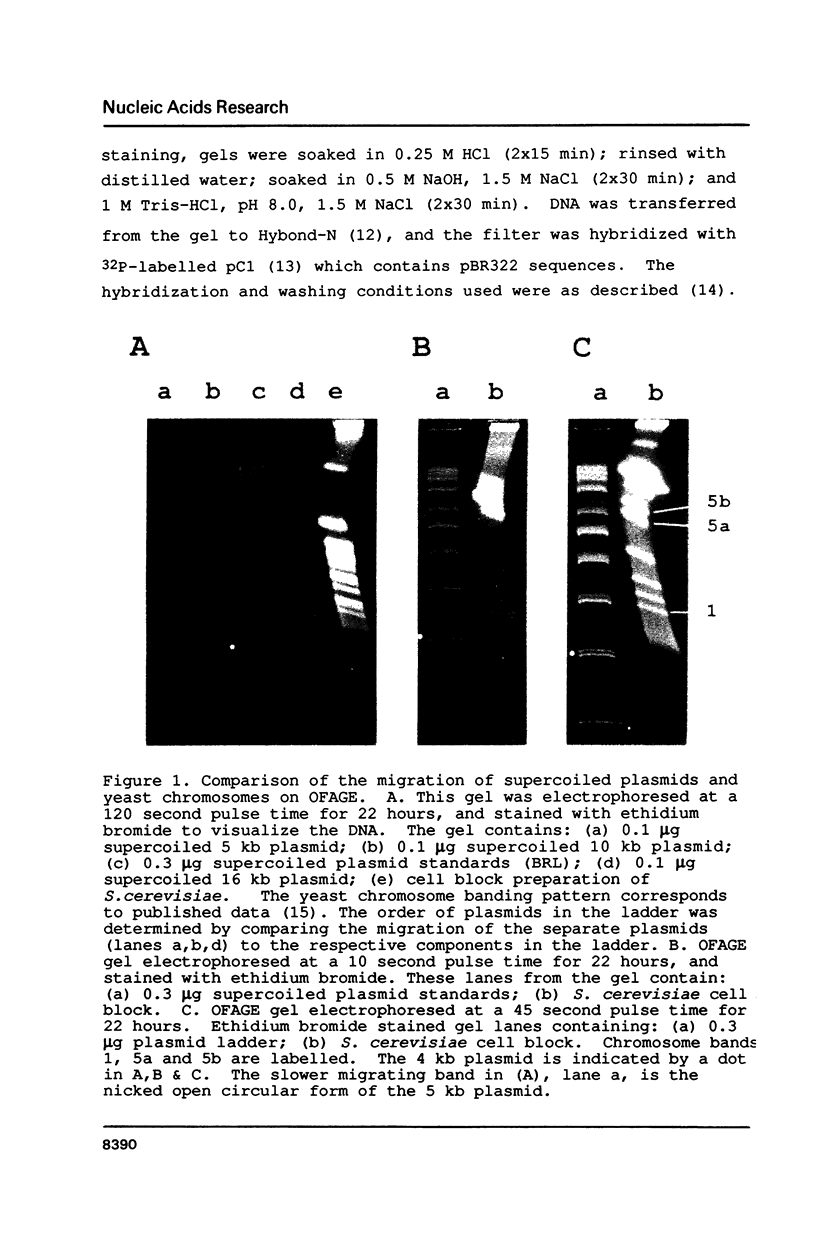
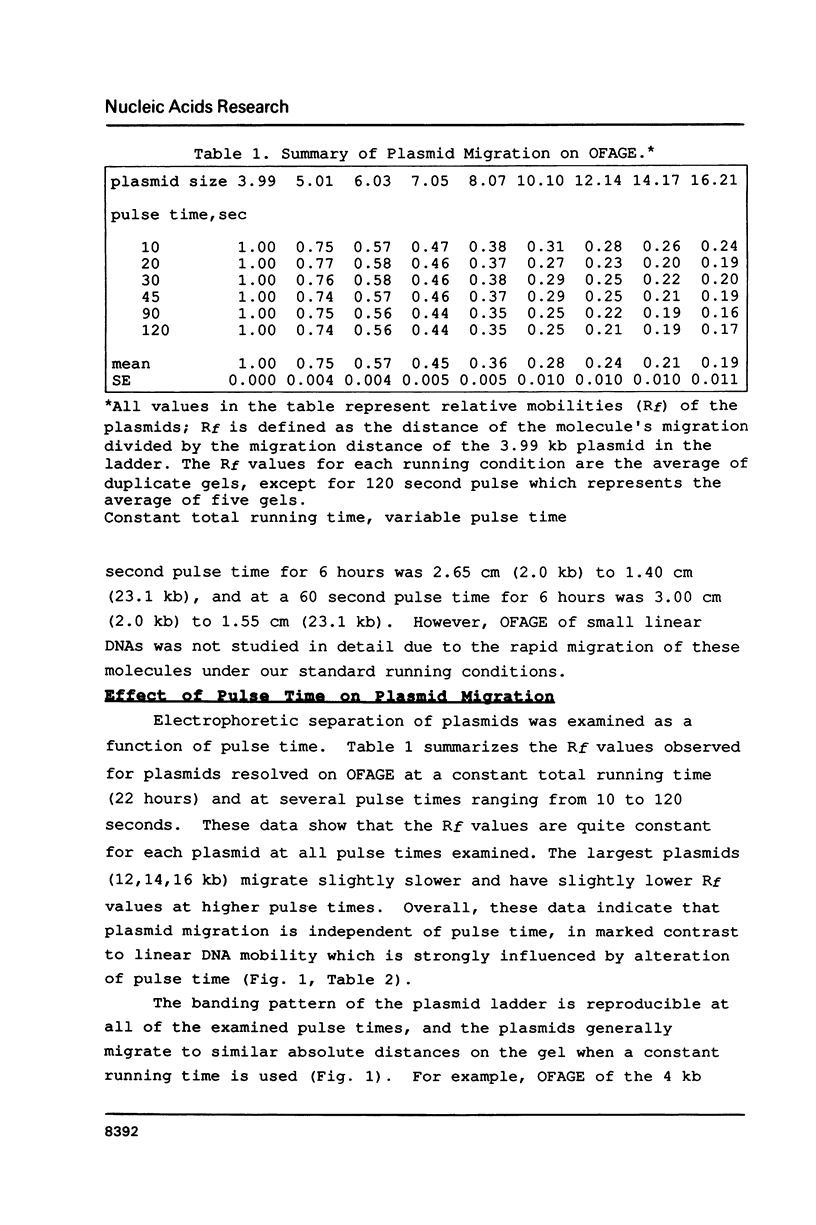
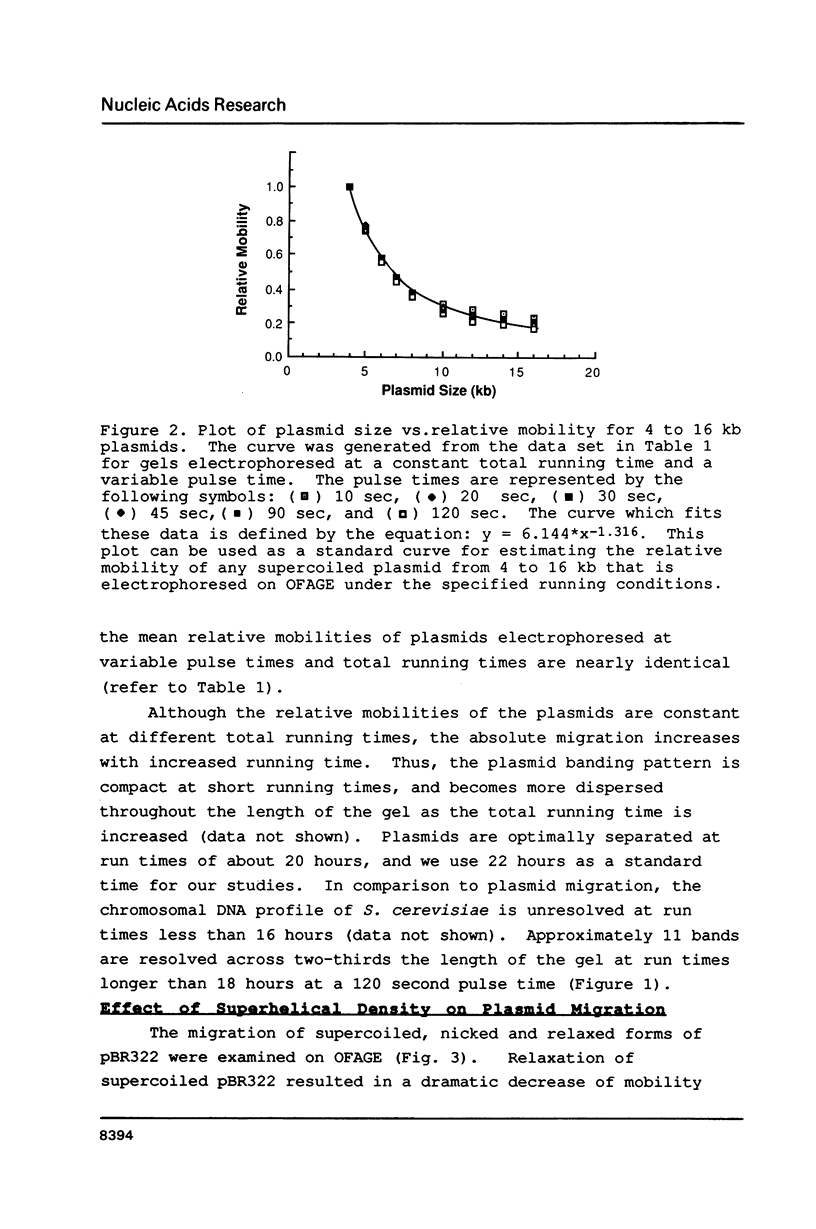
The migration properties of a series of supercoiled plasmids ranging in size from 4 to 16 kilobases (kb) have been analyzed by orthogonal-field-alternation gel electrophoresis (OFAGE). These circular DNAs enter the gel and are well resolved. Unlike linear DNA molecules, the relative mobilities of these plasmids are constant over a wide range of pulse times, from 10 to 120 seconds, as well as over a broad range of total running times, from 6 to 24 hours. Electrophoresis of supercoiled, relaxed, and nicked open circular forms as well as topoisomers of pBR322 shows that the extent of supercoiling has a dramatic effect on plasmid migration on OFAGE. Several practical applications for exploiting the different migration properties of circular and linear DNA molecules on OFAGE are presented.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Bernards A., Kooter J. M., Michels P. A., Moberts R. M., Borst P. Pulsed field gradient electrophoresis of DNA digested in agarose allows the sizing of the large duplication unit of a surface antigen gene in trypanosomes. Gene. 1986;42(3):313–322. doi: 10.1016/0378-1119(86)90235-0. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Grumont R., Washtien W. L., Caput D., Santi D. V. Bifunctional thymidylate synthase-dihydrofolate reductase from Leishmania tropica: sequence homology with the corresponding monofunctional proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5387–5391. doi: 10.1073/pnas.83.15.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Corcoran L. M., Coppel R. L., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F. Size variation in chromosomes from independent cultured isolates of Plasmodium falciparum. Nature. 1985 May 23;315(6017):347–350. doi: 10.1038/315347a0. [DOI] [PubMed] [Google Scholar]
- McPeek F. D., Jr, Coyle-Morris J. F., Gemmill R. M. Separation of large DNA molecules by modified pulsed field gradient gel electrophoresis. Anal Biochem. 1986 Aug 1;156(2):274–285. doi: 10.1016/0003-2697(86)90254-x. [DOI] [PubMed] [Google Scholar]
- Mickel S., Arena V., Jr, Bauer W. Physical properties and gel electrophoresis behavior of R12-derived plasmid DNAs. Nucleic Acids Res. 1977;4(5):1465–1482. doi: 10.1093/nar/4.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae, edition 9. Microbiol Rev. 1985 Sep;49(3):181–213. doi: 10.1128/mr.49.3.181-213.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Shure M., Pulleyblank D. E., Vinograd J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 1977;4(5):1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Matsumoto T., Niwa O., Klco S., Fan J. B., Yanagida M., Cantor C. R. An electrophoretic karyotype for Schizosaccharomyces pombe by pulsed field gel electrophoresis. Nucleic Acids Res. 1987 Jun 11;15(11):4481–4489. doi: 10.1093/nar/15.11.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Cornelissen A. W., Barry J. D., Borst P. Chromosomes of kinetoplastida. EMBO J. 1984 Dec 20;3(13):3109–3115. doi: 10.1002/j.1460-2075.1984.tb02266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washtien W. L., Grumont R., Santi D. V. DNA amplification in antifolate-resistant Leishmania. The thymidylate synthase-dihydrofolate reductase gene and abundant mRNAs. J Biol Chem. 1985 Jul 5;260(13):7809–7812. [PubMed] [Google Scholar]