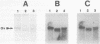Abstract
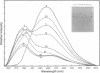
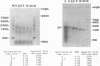
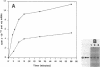
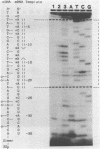
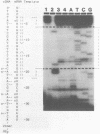
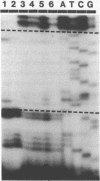
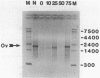
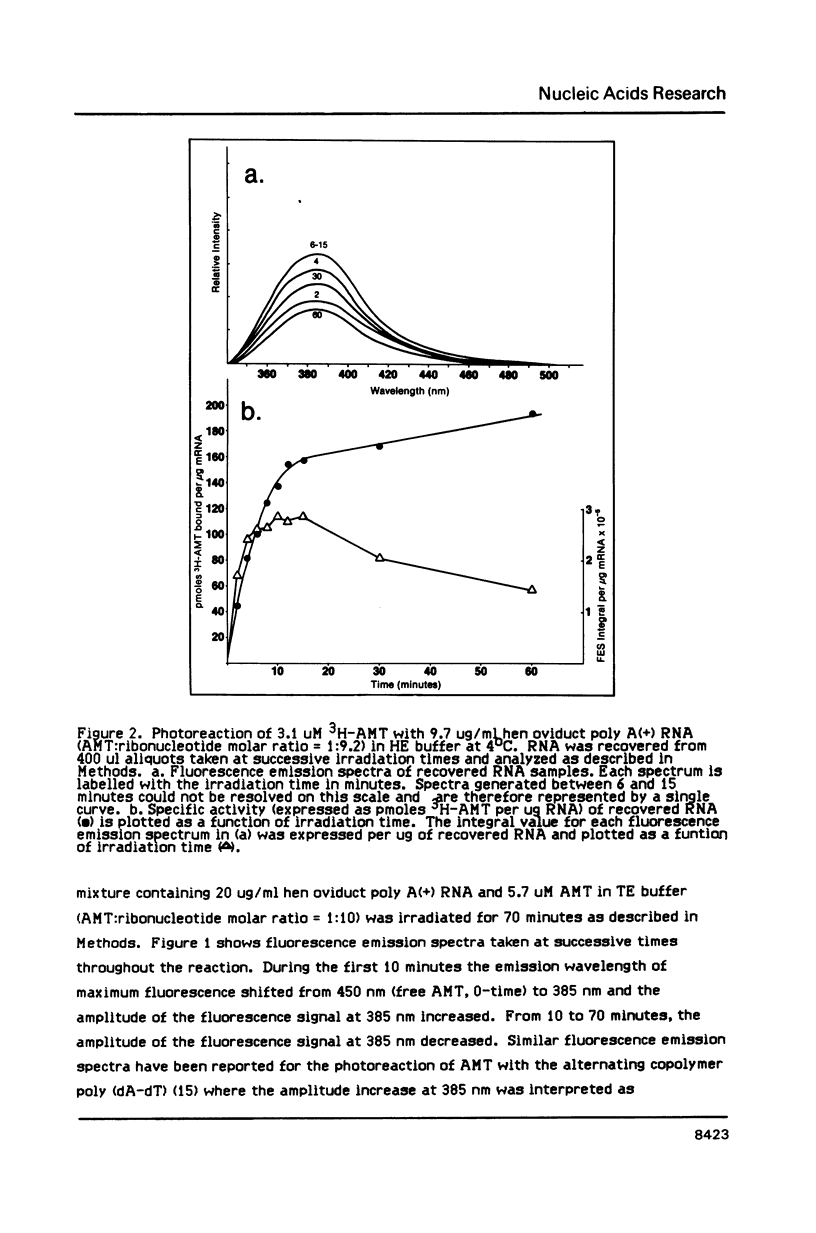
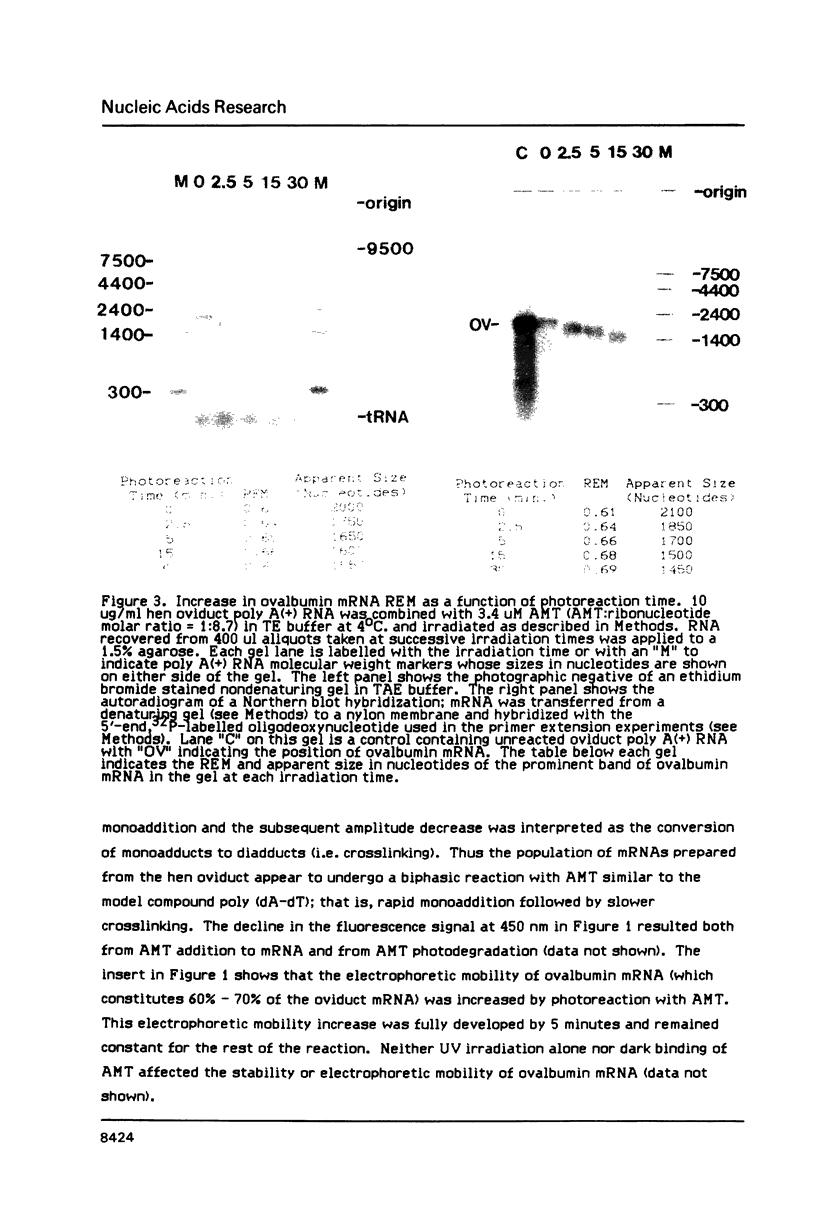
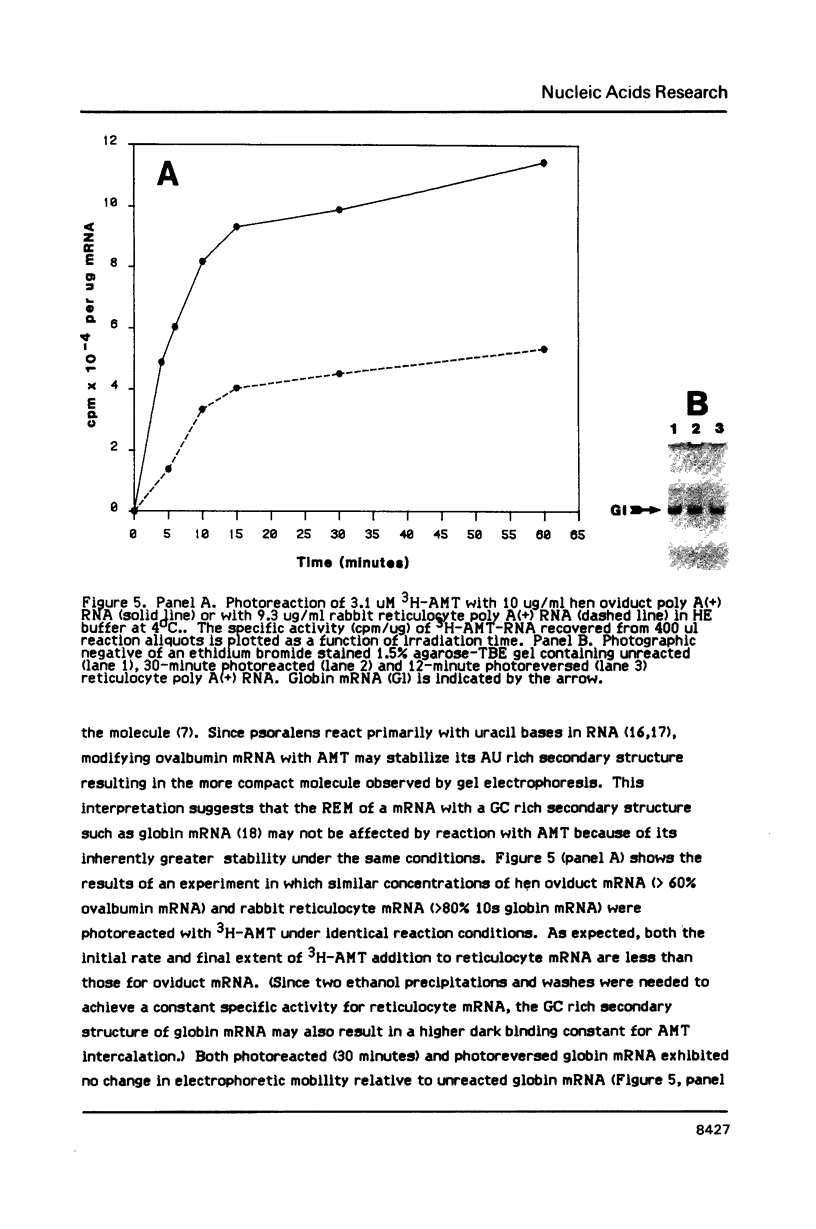
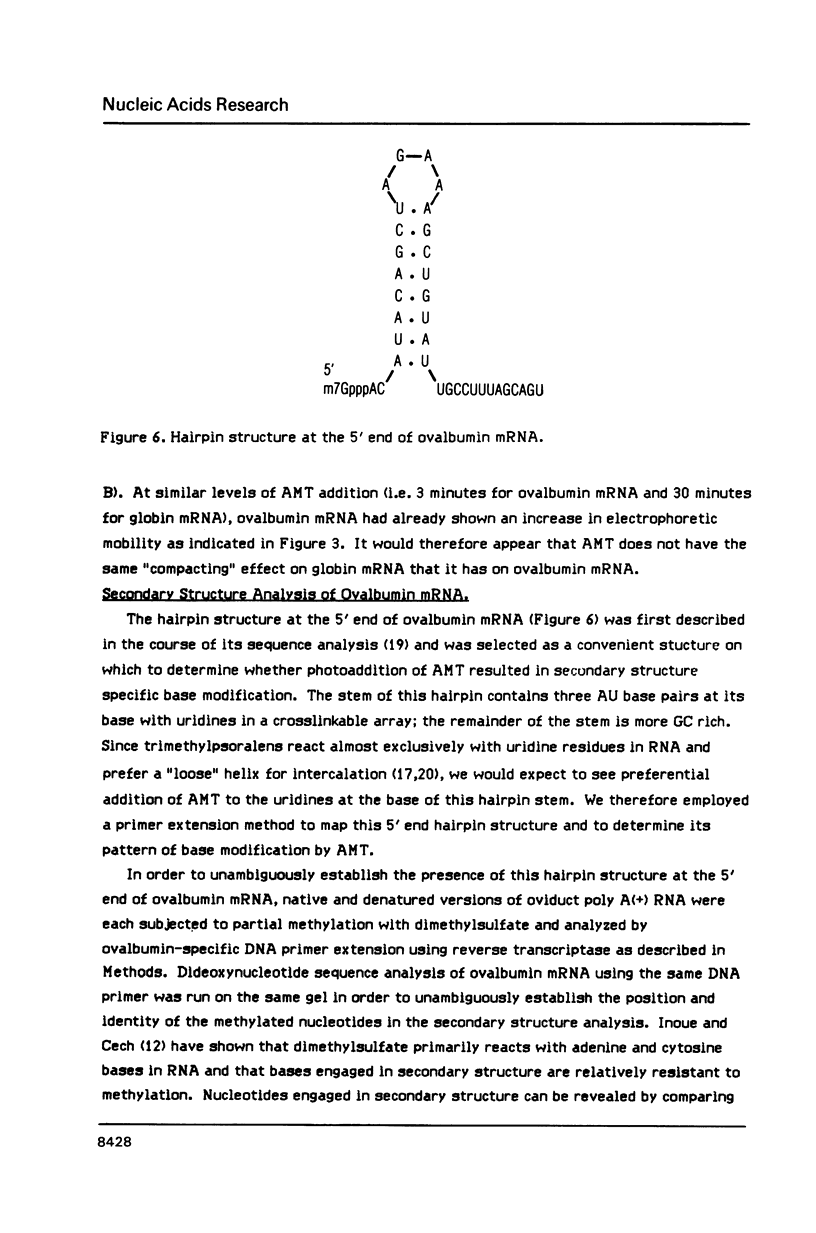
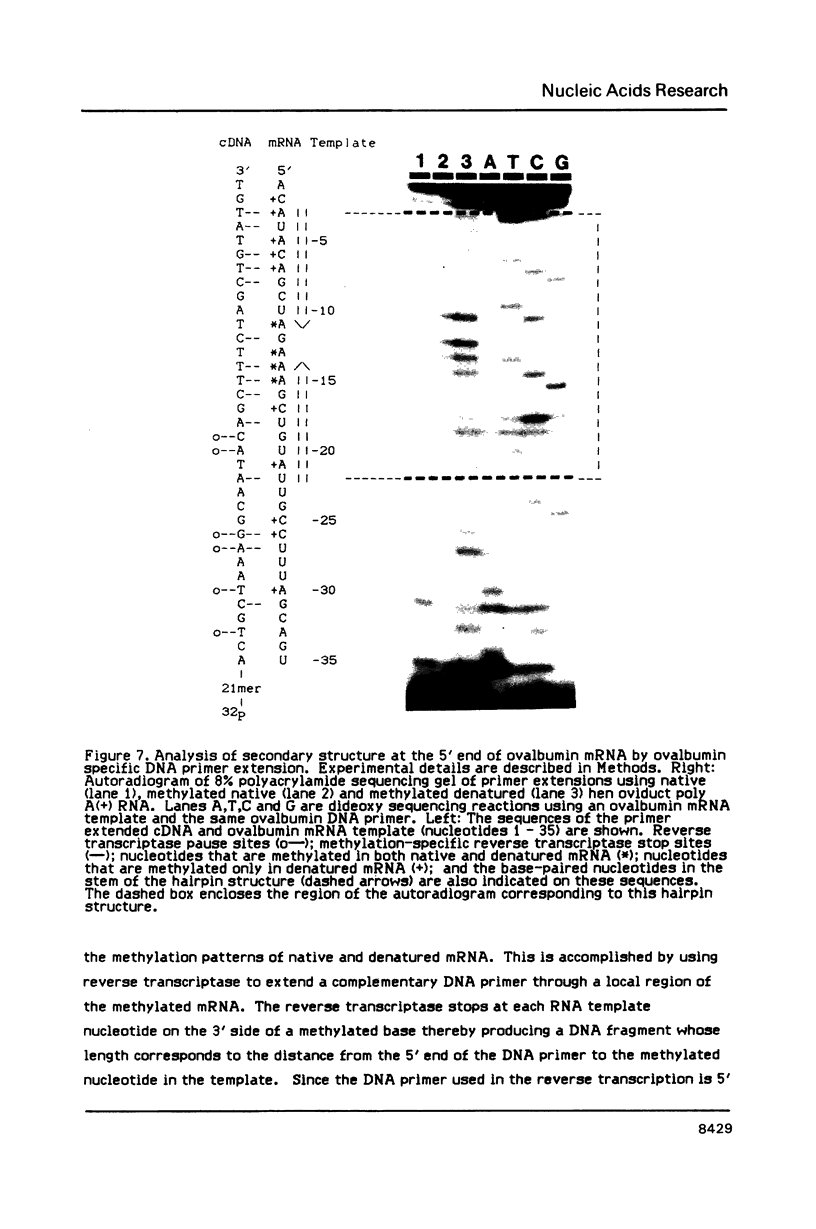
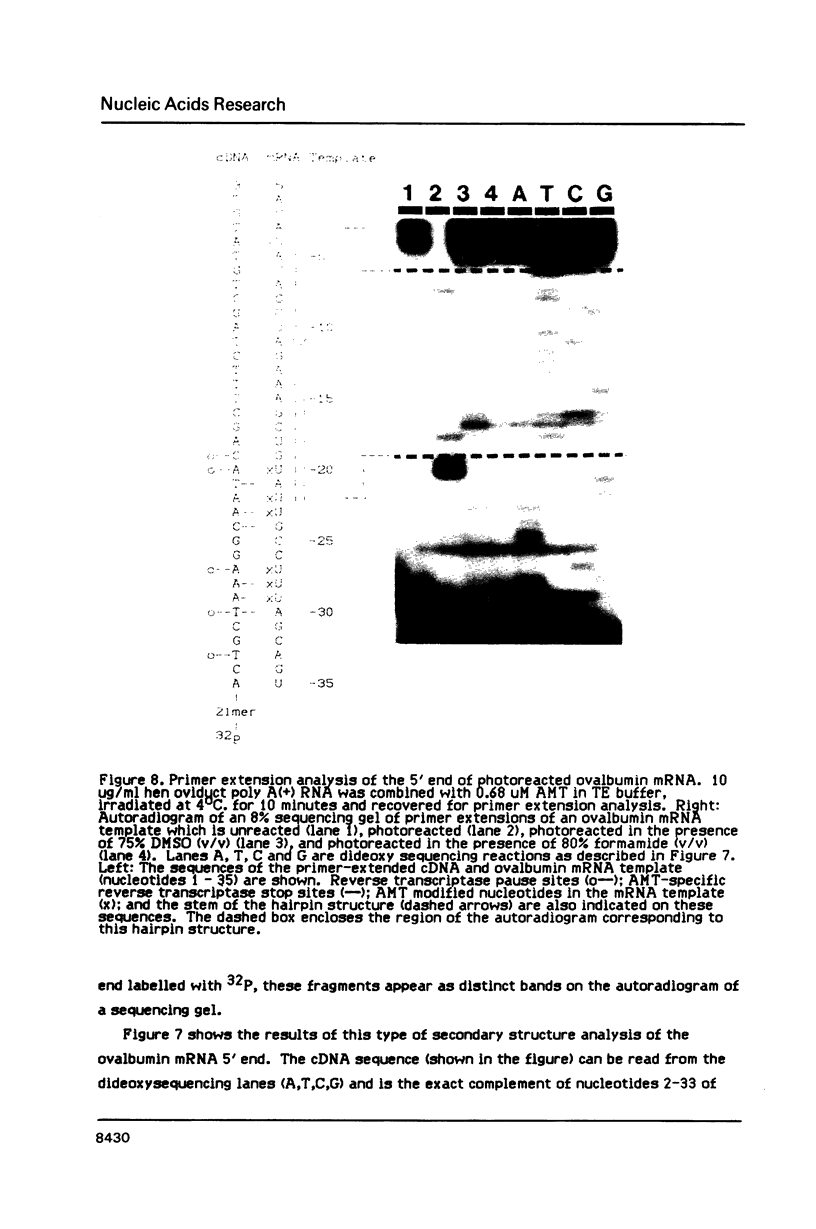
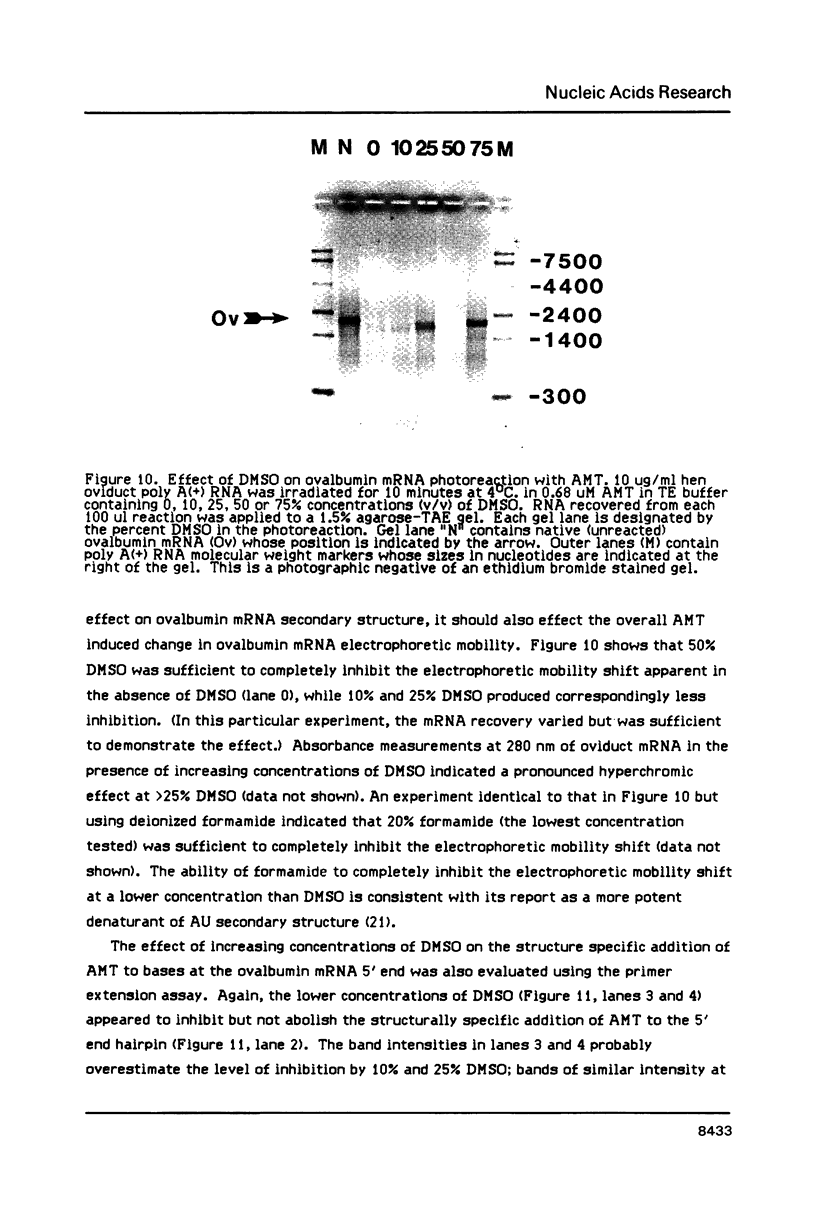
We have described the reaction of 4'-aminomethyl-4,5',8-trimethylpsoralen (AMT) with hen oviduct mRNA and have investigated the specific effects of AMT photoaddition on ovalbumin mRNA which constitutes 60-70% of oviduct mRNA. The photoreaction of AMT with hen oviduct mRNA appeared to occur in two phases - a rapid monoaddition followed by a slower conversion of monoadducts to diadducts (i.e. crosslinking). Both nondenaturing and denaturing gel electrophoresis revealed a photoreaction time dependent increase in ovalbumin mRNA electrophoretic mobility indicating the formation of a progressively more compact molecular structure. Identical analysis of photoreacted rabbit globin mRNA revealed no change in electrophoretic mobility suggesting that AMT was stabilizing the AU rich secondary structure of ovalbumin mRNA but having no similar effect on the relatively GC rich secondary structure of globin mRNA. Ovalbumin-specific DNA primer extension was used to demonstrate the selective and secondary structure specific photoaddition of AMT to uracil bases at the 5' end of ovalbumin mRNA.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachellerie J. P., Hearst J. E. Specificity of the photoreaction of 4'-(hydroxymethyl)-4,5',8-trimethylpsoralen with ribonucleic acid. Identification of reactive sites in Escherichia coli phenylalanine-accepting transfer ribonucleic acid. Biochemistry. 1982 Mar 16;21(6):1357–1363. doi: 10.1021/bi00535a039. [DOI] [PubMed] [Google Scholar]
- Bachellerie J. P., Thompson J. F., Wegnez M. R., Hearst J. E. Identification of the modified nucleotides produced by covalent photoaddition of hydroxymethyltrimethylpsoralen to RNA. Nucleic Acids Res. 1981 May 11;9(9):2207–2222. doi: 10.1093/nar/9.9.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser T. H., Bruce B. J. Chicken ovalbumin is synthesized and secreted by Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5936–5940. doi: 10.1073/pnas.75.12.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Wheeler E., Lockard R. E., Kumar A. Mapping of psoralen cross-linked nucleotides in RNA. Nucleic Acids Res. 1984 Apr 11;12(7):3405–3423. doi: 10.1093/nar/12.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey D. R., Turner D. H. Solvent effects on the stability of A7U7p. Biochemistry. 1985 Apr 9;24(8):2086–2094. doi: 10.1021/bi00329a042. [DOI] [PubMed] [Google Scholar]
- Holder J. W., Lingrel J. B. Determination of secondary structure in rabbit globin messenger RNA by thermal denaturation. Biochemistry. 1975 Sep 23;14(19):4209–4215. doi: 10.1021/bi00690a009. [DOI] [PubMed] [Google Scholar]
- Inoue T., Cech T. R. Secondary structure of the circular form of the Tetrahymena rRNA intervening sequence: a technique for RNA structure analysis using chemical probes and reverse transcriptase. Proc Natl Acad Sci U S A. 1985 Feb;82(3):648–652. doi: 10.1073/pnas.82.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Hearst J. E. Characterization of the photoreaction between DNA and aminomethyl-trimethylpsoralen using absorption and fluorescence spectroscopy. Photochem Photobiol. 1981 Jun;33(6):785–791. doi: 10.1111/j.1751-1097.1981.tb05493.x. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Hearst J. E. Low-level psoralen--deoxyribonucleic acid cross-links induced by single laser pulses. Biochemistry. 1981 Feb 17;20(4):739–745. doi: 10.1021/bi00507a012. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Kung A. H., Moore C. B., Hearst J. E. Kinetics of formation of deoxyribonucleic acid cross-links by 4'-(aminomethyl)-4,5',8-trimethylpsoralen. Biochemistry. 1981 Feb 17;20(4):735–738. doi: 10.1021/bi00507a011. [DOI] [PubMed] [Google Scholar]
- Kim C. H., Sarma R. H. Spatial configuration of mRNA 5'-terminus. Nature. 1977 Nov 17;270(5634):223–227. doi: 10.1038/270223a0. [DOI] [PubMed] [Google Scholar]
- Kuebbing D., Liarakos C. D. Nucleotide sequence at the 5' end of ovalbumin messenger RNA from chicken. Nucleic Acids Res. 1978 Jul;5(7):2253–2266. doi: 10.1093/nar/5.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Liarakos C. D., Gupta R. C., Randerath K., O'Malley B. W. Ribosome binding site analysis of ovalbumin messenger ribonucleic acid. Biochemistry. 1979 Dec 25;18(26):5798–5808. doi: 10.1021/bi00593a009. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Bachellerie J. P., Hall K., Hearst J. E. Dependence of 4'-(hydroxymethyl)-4,5',8-trimethylpsoralen photoaddition on the conformation of ribonucleic acid. Biochemistry. 1982 Mar 16;21(6):1363–1368. doi: 10.1021/bi00535a040. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Hearst J. E. Structure of E. coli 16S RNA elucidated by psoralen crosslinking. Cell. 1983 Apr;32(4):1355–1365. doi: 10.1016/0092-8674(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Turner S., Noller H. F. Identification of sites of 4'-(hydroxymethyl)-4,5',8-trimethylpsoralen cross-linking in Escherichia coli 23S ribosomal ribonucleic acid. Biochemistry. 1983 Aug 16;22(17):4159–4164. doi: 10.1021/bi00286a026. [DOI] [PubMed] [Google Scholar]
- Van N. T., Holder J. W., Woo S. L., Means A. R., O'Malley B. W. Secondary structure of ovalbumin messenger RNA. Biochemistry. 1976 May 18;15(10):2054–2062. doi: 10.1021/bi00655a005. [DOI] [PubMed] [Google Scholar]
- Wollenzien P. L., Cantor C. R. Marking the polarity of RNA molecules for electron microscopy by covalent attachment of psoralen-DNA restriction fragments. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3940–3944. doi: 10.1073/pnas.79.13.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. Sequencing psoralen photochemically reactive sites in Escherichia coli 16 S rRNA. Anal Biochem. 1982 Jan 1;119(1):86–89. doi: 10.1016/0003-2697(82)90669-8. [DOI] [PubMed] [Google Scholar]