Abstract
Our laboratory recently reported a new source of smooth muscle cells (SMCs) derived from hair follicle (HF) mesenchymal stem cells. HF-SMCs demonstrated high proliferation and clonogenic potential as well as contractile function. In this study, we aimed at engineering the vascular media using HF-SMCs and a natural biomaterial, namely small intestinal submucosa (SIS). Engineering functional vascular constructs required application of mechanical force, resulting in actin reorganization and cellular alignment. In turn, cell alignment was necessary for development of receptor- and nonreceptor-mediated contractility as soon as 24 h after cell seeding. Within 2 weeks in culture, the cells migrated into SIS and secreted collagen and elastin, the two major extracellular matrix components of the vessel wall. At 2 weeks, vascular reactivity increased significantly up to three- to fivefold and mechanical properties were similar to those of native ovine arteries. Taken together, our data demonstrate that the combination of HF-SMCs with SIS resulted in mechanically strong, biologically functional vascular media with potential for arterial implantation.
Introduction
Venous grafts have been the golden standard for bypass and vascular replacement surgery, but they are limited by donor availability and repeated surgical procedures.1 Although tissue-engineered vascular grafts provide a promising alternative, the two major challenges facing the field remain the use of bioactive materials with appropriate mechanical properties and the source of cells. Polymeric scaffolds have been used with varying degrees of success,2 and natural biopolymers such as collagen and fibrin support enhanced cellular functions but lack adequate mechanical strength for arterial implantation.3–6 In this regard, native decellularized tissues may provide biological signals inherent to each tissue as well as mechanical support with the potential for arterial implantation.
Previous studies employed decellularized blood vessels that were seeded with endothelial cells and myofibroblasts before implantation to improve patency and vasoreactivity.7,8 More recently, decellularized human umbilical arteries were shown to preserve their extracellular matrix (ECM) composition and to support endothelialization and in vivo function for up to 8 weeks as interpositional grafts into the rat abdominal aorta.9 Similarly, decellularized porcine carotid arteries were shown to support rat smooth muscle cell (SMC) attachment and proliferation, which was enhanced by mechanical preconditioning using pulsatile flow.10
Another decellularized natural biomaterial, namely small intestinal submucosa (SIS), has been approved by the U.S. Food and Drug Administration and has been used in various surgical applications. It possesses wall compliance and mechanical strength that closely resemble those of native vessels.11,12 In vivo implantation studies showed that SIS exhibited good endothelialization, significant tissue remodeling, and better patency rates than synthetic grafts.13–16 However, the majority of studies conducted to date have largely focused on endothelialization of SIS, but engineering of functional vascular media has not been adequately explored. The present study explored this issue.
Regardless of the biomaterial used, the source of cells remains a major challenge in vascular tissue engineering, particularly because of the limited replicative capacity of somatic cells from older donors, the population mostly in need for vascular grafts.17,18 In this regard, stem cells from adult tissues may provide alternative sources of proliferative vascular cells for vascular engineering. Recently, highly proliferative and contractile SMCs were derived from bone marrow mesenchymal stem cells (BM-MSCs) using the alpha-smooth muscle actin (αSMA) promoter and flow cytometry sorting. Notably, vascular grafts were engineered with these cells and implanted as interpositional grafts into ovine jugular veins, where they remained patent for several weeks and exhibited high potential for matrix remodeling including significant elastin fiber deposition.5
Although bone marrow and adipose tissues have been extensively studied as sources of MSCs, recent studies indicated that the hair follicle (HF) may be an easily accessible, alternative source of multipotent adult stem cells for tissue regeneration. In addition to epidermal stem cells,19 dermal papilla or dermal sheath cells from the rat HF displayed similar biomarker profile and differentiation potential as BM-MSCs.20,21 More recently, human HF cells were also found to have multilineage differentiation potential toward adipogenic, osteogenic, chondrogenic, and myogenic lineages.22 Using the αSMA promoter and flow cytometry sorting, our group obtained functional SMCs from both ovine and human HF cells (HF-SMCs) that exhibited high proliferation potential and vascular contractility in response to receptor- and nonreceptor-mediated vasoactive agonists.22,23
In this study, we extended our previous findings to engineer vascular grafts using ovine HF-SMCs and the natural decellularized matrix, SIS. We found that application of uniaxial strain was necessary to prevent matrix deformation and achieve cellular alignment with concomitant development of SMC contractility. After 2 weeks in culture, HF-SMCs migrated into SIS and secreted key ECM molecules of the vascular wall, such as collagen and elastin. The HF-SMC–based vascular constructs (HF-SMC/SIS) exhibited constriction and relaxation in response to various vascular agonists as well as considerable mechanical strength with the potential to withstand arterial implantation.
Materials and Methods
Isolation and culture of HF-SMCs
HF-SMCs were isolated and characterized as described previously.23 Briefly, skin tissue was harvested from a newborn lamb under aseptic conditions. The skin tissue was cut into small pieces and subsequently digested with 1 mg/mL of collagenase type I (Invitrogen Life Technologies, Carlsbad, CA) at 37°C for 4 h. Single HFs were removed from the tissue using forceps, filtered through a 40-μm cell strainer (BD Biosciences, San Jose, CA), and washed extensively with phosphate-buffered saline (PBS). Then, HFs were placed each in a well of a 96-well plate (BD Biosciences) and cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Eugene, OR) supplemented with 10% fetal bovine serum (FBS; Invitrogen) to allow for cell migration onto the tissue culture plastic.
Passage 4 cells were transduced with retrovirus encoding for enhanced green fluorescence protein (EGFP) under the αSMA promoter and EGFP+ cells were isolated using fluorescence-activated cell sorting as described previously.23 These cells exhibited high proliferation and clonogenic potential, expressed SMCs markers, and showed significant contractility in response to various vasoagonists. HF-SMCs were cultured in DMEM supplemented with 10% FBS.
Cell seeding and vascular construct preparation
HF-SMCs were seeded on hydrated SIS matrix (Cook Biotech, West Lafayette, IN) at a density of 106 cells/cm2 onto the nonbasement membrane surface. The size of SIS was 2 × 3 cm, but cell seeding was restricted to an area of 1 × 1 cm using a customized polydimethylsiloxane mold (Dow Corning, Midland, MI). After the cells adhered on SIS for 4 h, the polydimethylsiloxane mold was removed and uniaxial strain was applied by clamps placed at opposite ends of the SIS for 24 h or 2 weeks as indicated. Strain was applied in the direction corresponding to the circumferential trajectory of the original circularized SIS. Medium (DMEM 10% FBS) was changed every 3 days throughout the experiment. To block actin polymerization, cytochalasin D (EMD Biosciences, La Jolla, CA) was added overnight at 10 μg/mL.
Scanning electron microscopy and quantification of cell alignment
Tissue constructs were fixed at room temperature following a two-step protocol. First, they were fixed in a solution of 2% glutaraldehyde in DMEM for 30 min, followed by a 1.5-h treatment in a solution containing 2% glutaraldehyde in 0.1 M sodium cacodylate (pH 7.2) with 0.1 M sucrose. After fixation, the samples were dehydrated in a sequence of ethanol solutions (15%, 35%, 50%, 75%, 95%, and 100% ethanol) for 5 min each and incubated in hexamethyldisilazane (MP Biomedicals, Solon, OH) for 15 min. After air drying at room temperature, the samples were immediately coated with a 20-nm layer of evaporated carbon and images were acquired with a field emission scanning electron microscope (S4000; Hitachi, Tokyo, Japan).
To quantify cell alignment, the number of aligned cells was determined in three randomly chosen scanning electron microscopy (SEM) images (300× magnification) and normalized to the total cell number (n = 122). A cell was considered aligned when the angle of a cell's long axis deviated by ±10 degrees or less from the direction of strain. Nonattached cells were excluded from the analysis.
Vascular reactivity and mechanical properties of HF-SMC/SIS constructs
At 24 h or 2 weeks, the tissues were released from the clamps and connected to a force transducer in a Radnoti tissue bath system (ADI Instruments, Colorado Springs, CO). The tissue bath contained standard Krebs-Ringer solution that was kept at 37°C and continuously bubbled with 94% O2 and 6% CO2 to obtain a pH of 7.4, PCO2 of 38 mmHg, and PO2 >500 mmHg. The Krebs-Ringer solution consists of (in mM) NaCl 118, potassium chloride (KCl) 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25.5, and glucose 5.6. The vessels were equilibrated for 30–60 min before a passive tension of 1.0 g was applied (basal tone). Over the next 60 min, the constructs were rinsed three times and the tissue tension was readjusted to 1.0 g at a stable stretch length as described previously.6
After equilibration, the following pharmacological agents were added in succession to elucidate contractile function: thromboxane A2 mimetic U46619 (1 μM; Sigma, St. Louis, MO), endothelin-1 (ET-1, 20 nM; Sigma), and KCl (118 mM). Each vasoagonist was added for 15 min or until the tension was stable and the tissues were washed extensively before addition of the next compound. For vascular relaxation, the tissues were first constricted using U46619 before the NO donor, S-nitroso-N-acetylpenicillamine (SNAP, 1 μM; Sigma), was added without a wash. Similarly, the Rho kinase inhibitor, Y27632 (10 μM; Sigma), was added after ET-1 constriction. Isometric contraction was recorded by a PowerLab data acquisition unit and analyzed with Chart5 software (ADI Instruments). The SNAP response was calculated as % relaxation of U46619 constriction and Y27632 was calculated as % relaxation of ET-1 constriction.
For measuring mechanical properties, HF-SMC/SIS constructs were mounted on the force transducer and stretched at a rate of 4 mm/min continuously until failure. The ultimate tensile strength (UTS) was recorded using a PowerLab data acquisition system (ADI Instruments) and the displacement of each tissue during the test was video recorded using a Nikon D90 camera equipped with a Nikon 55-mm close-up lens (Micro-NIKKOR-P; Nikon, Inc., Melville, NY). The initial tissue length, l0, corresponded to the length under a passive tension of 1.0 g and was measured using an electronic digital caliper; tissue thickness, δ, was measured using a high-precision LED digital micrometer (LS7600; Keyence, Osaka, Japan). The UTS was calculated as the ratio of the break force to the cross-sectional area (l0 × δ) of each construct and expressed in MPa. The physiological elastic modulus was calculated as the slope of the stress–strain curve within the physiological strain range (1%–10%). The same procedures were followed for native carotid arteries that were collected from three adult sheep (4–5 years old). The arteries were cut into 5-mm-wide rings that were used for measuring reactivity and mechanical properties as described earlier.
We also determined the strain experienced by native arteries under physiologic pressure. To this end, ovine carotid arteries were pressurized using the LumeGen Bioreactor (Tissue Growth Technologies, Minnetonka, MN) and vessel diameter was measured using a LED digital micrometer.
Histology and immunohistochemistry
Histology and immunostaining were performed as described previously23 by using the following antibodies in PBS containing 2% goat serum (Sigma): mouse monoclonal anti-human αSMA (1:50 dilution, overnight at 4°C; SeroTec, Raleigh, NC); mouse anti-human calponin (1:100 dilution, overnight at 4°C; DakoCytomation, Carpinteria, CA); mouse anti-human myosin heavy chain (1:100 dilution; overnight at 4°C; Biomedical Technologies, Stoughton, MA); anti-human collagen I (Clone I-8H5; 1:50 dilution; overnight at 4°C; EMD Biosciences); anti-human collagen III (Clone III-53; 1:50 dilution; overnight at 4°C; EMD Biosciences); antiproliferating cell nuclear antigen (anti-PCNA; 1:200 dilution, 4°C overnight; Biolegend, San Diego, CA); polyclonal anti-human tropoelastin (1:50, overnight at 4°C; Abcam, Cambridge, MA); anti-human elastin (Clone BA-4; 1:50, overnight at 4°C; Sigma). For secondary antibody, we used Alexa Fluor564-conjugated (red) secondary goat anti-mouse (1:100 dilution, 1 h at room temperature; Invitrogen) or Alexa Fluor488-conjugated (green) secondary goat anti-IgG (1:200 dilution, 1 h at room temperature; Invitrogen). Staining with secondary antibody only served as negative control. To visualize actin filaments, tissue sections were fixed with 4% paraformaldehyde in PBS for 2 h and stained with Alexa Fluor® 594 phalloidin (1:20 dilution, 15 min at room temperature; Invitrogen). Cell nuclei were stained with Hoechst (EMD Biosciences).
Statistical analysis
Data were expressed as means ± standard deviation. Unpaired Student's t-test was performed using Microsoft excel software and statistical significance was defined as p < 0.05.
Results
HF-derived SMCs as a cell source for vascular constructs
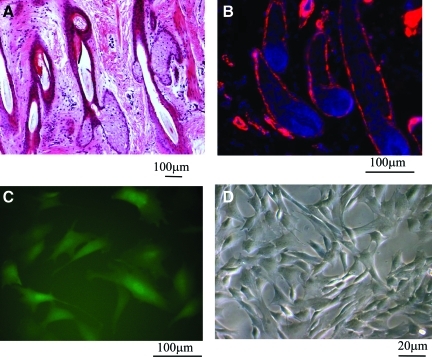
HF is a dynamic miniorgan with the ability to undergo multiple cycles of regeneration and degeneration throughout life. The anatomic location of HF in the skin dermis (Fig. 1A, hematoxylin and eosin [H&E] staining) makes it an attractive target, that is, as a cell source for tissue engineering and regenerative medicine. Interestingly, immunostaining of tissue sections from ovine skin showed that cells along the outer root sheath of HFs expressed αSMA (Fig. 1B, red), a marker of SMCs, suggesting that HFs may be an easily accessible source of contractile SMCs for vascular tissue engineering.
FIG. 1.
Isolation of hair follicle (HF) stem cells. (A) Hematoxylin and eosin (H&E) staining of neonatal ovine dermis containing multiple HFs. (B) Immunostaining of ovine HFs for alpha-smooth muscle actin (αSMA) shows positive cells (red) along the outer root sheath. (C) HF cells were transduced with retrovirus encoding for enhanced green fluorescence protein (EGFP) under the αSMA promoter and EGFP+ cells were isolated using fluorescence-activated cell sorting. These cells were termed HF-smooth muscle cells (SMCs). (D) Brightfield image of HF-SMCs. Color images available online at www.liebertonline.com/tea.
To isolate SMCs from HF cells, we generated a retroviral vector encoding for EGFP under the αSMA promoter. HF cells were transduced with recombinant retrovirus and EGFP+ cells were sorted by fluorescence-activated cell sorting (Fig. 1C, D). As we reported previously, the sorted cells showed high proliferation and clonogenic potential, expressed markers associated with mature SMC phenotype, and exhibited contractile function similar to vascular (V)-SMCs from neonatal ovine umbilical veins.23 For these reasons, they were termed HF-derived smooth muscle progenitor cells (HF-SMCs). In the following, we examined the potential of HF-SMCs as a cell source for engineering the vascular media using a natural biomaterial derived from the porcine SIS.
Application of uniaxial strain resulted in alignment of SIS fibers and HF-SMCs
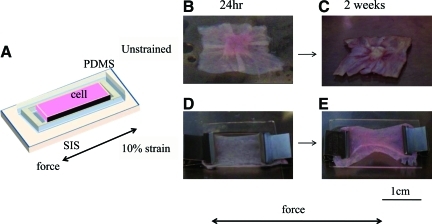
Within 24 h after seeding on SIS (Fig. 2A), highly contractile HF-SMCs pulled the matrix and generated wrinkles extending from the perimeter to the center of SIS (Fig. 2B). Two weeks postseeding, the SIS matrix was folded and cells contracted into a multilayered cluster in the middle of the material (Fig. 2C). This result prompted us to hypothesize that application of an external force might prevent SIS folding and generate functional constructs. To this end, shortly after cell seeding (3–4 h), SIS was subjected to 10% uniaxial strain using clamps at the two opposite sides of the scaffold.
FIG. 2.
Effect of uniaxial strain on gross morphology of HF-SMC/SIS tissue constructs. (A) HF-SMCs were seeded at 106 cells/cm2 onto SIS that was placed inside a customized polydimethylsiloxane (PDMS) frame to ensure uniform surface coverage without cell loss. (B, C) Unstrained tissue constructs at (B) 24 h or (C) 2 weeks after seeding. (D, E) Tissue constructs that were cultured under 10% uniaxial strain (arrows indicate direction of strain) for (D) 24 h or (E) 2 weeks after seeding. SIS, small intestinal submucosa. Color images available online at www.liebertonline.com/tea.
The criteria used to decide the amount of uniaxial strain applied to the tissues were twofold. First, we measured the change in diameter experienced by native arteries under physiologic pressure and found that ovine arteries expanded by 9.7% ± 2.1% (n = 3; animals 4–5 years old) when they were pressurized from 0 to 100 mmHg. Second, we conducted a pilot study in which the tissues were cultured under 0%, 10%, or 20% strain for 2 weeks. We found that unstrained tissue constructs displayed multiple cell layers on the SIS surface, with no infiltration into the matrix and no cellular alignment (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/tea). Their nuclei had a pyknotic appearance (Supplementary Fig. S1B) and they were devoid of PCNA (Supplementary Fig. S1G, H). On the other end, histological analysis (Supplementary Fig. S1E, F) and nuclear staining (Supplementary Fig. S1L) of tissue constructs grown under 20% strain showed that very few—if any—cells remained on the scaffold and there were no PCNA-expressing cells (Supplementary Fig. S1K), suggesting that at 20% strain the stiffness of the substrate might not be supportive of cell attachment and growth. This result is in agreement with a recent study, which reported that it was difficult to apply more that 15% strain because of stiffness of the SIS matrix.24 (Note that in strained samples, SIS appeared thinner and with decreased porosity possibly due to fiber elongation.) In contrast, under 10% strain, HF-SMCs formed multiple cell layers and some migrated into the matrix (Supplementary Fig. S1C, D). Notably, the majority of cells (91.1% ± 4.2%; n = 213) were actively proliferating as indicated by PCNA expression (Supplementary Fig. S1I, J). Based on these results, 10% strain was employed throughout the rest of this study.
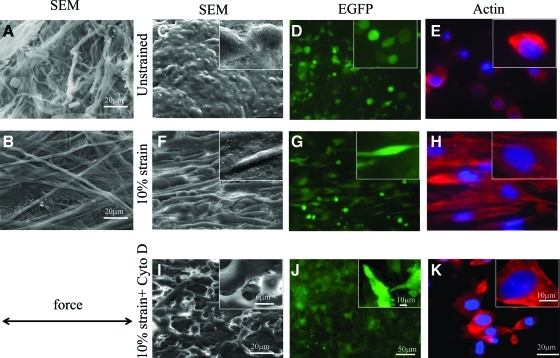
Application of 10% strain prevented wrinkling of the SIS scaffold at 24 h (Fig. 2D) or 14 days (Fig. 2E) after seeding. SEM showed that application of 10% strain induced the entangled SIS fibers to stretch (Fig. 3A, B). Cell morphology and orientation were assessed at 24 h after seeding by SEM and fluorescence microscopy for EGFP and actin phalloidin. After application of 10% strain, the majority of HF-SMCs (97% ± 0.6%, n = 122 cells) elongated and aligned in the direction of force (Fig. 3F, G). In addition, actin–phalloidin stain (red) showed that actin was organized in filaments, which also aligned in the direction of force (Fig. 3H). Treatment with cytochalasin D (10 μg/mL; overnight) disrupted actin filaments and prevented cell alignment (Fig. 3I–K), indicating that cell orientation on the extended SIS matrix was an active process requiring actin polymerization.
FIG. 3.
Application of uniaxial strain induced cell alignment in the direction of the force. SIS fibers (no cells) were visualized by scanning electron microscopy (SEM) before (A) or after (B) application of strain. HF-SMCs were seeded on SIS and cultured for 24 h under the following conditions: (C–E) unstrained; (F–H) 10% uniaxial strain; or (I–K) 10% strain in the presence of cytochalasin D. Cells were visualized by SEM (C, F, I), fluorescence microscopy for EGFP (D, G, J), or actin phalloidin (E, H, K). Arrow indicates the direction of force. Magnification: (A, B, C, F, I) 1000×, insets are 5000× in SEM; (D, G, J) 10×, insets are 60×; (E, H, K) 60× magnification, insets are 100×. Color images available online at www.liebertonline.com/tea.
HF-SMCs migrated into SIS and secreted collagen and elastin
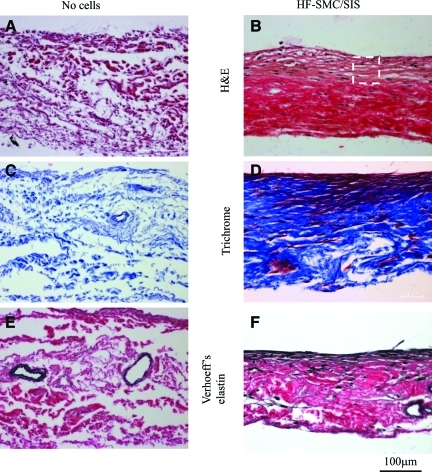
After 2 weeks in culture under 10% strain, HF-SMCs migrated into the SIS with the majority of cells remaining near the surface as evidenced by H&E staining (Fig. 4A, B). As expected, Trichrome staining showed collagen throughout the SIS matrix but also within and around the cells (Fig. 4C, D). Most notably, Verhoeff's staining revealed significant amounts of elastin in areas with high cell density (Fig. 4E, F), suggesting that this may be newly synthesized rather than preexisting elastin in the SIS matrix. Indeed, staining of cell-free SIS showed elastin only in what appeared to be lumens of preexisting vascular conduits (Fig. 4E).
FIG. 4.
HF-SMCs migrated into SIS and secreted extracellular matrix. (A, B) H&E staining; (C, D) trichrome staining; (E, F) Verhoeff's elastin staining. (A, C, E) SIS matrix alone (no cells); (B, D, F) HF-SMC/SIS constructs after 2 weeks of culture under 10% uniaxial strain. The box encloses part of the area with high cell density. Color images available online at www.liebertonline.com/tea.
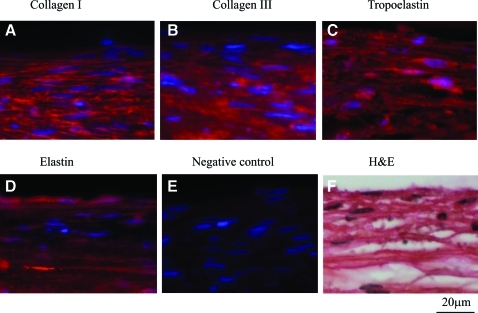
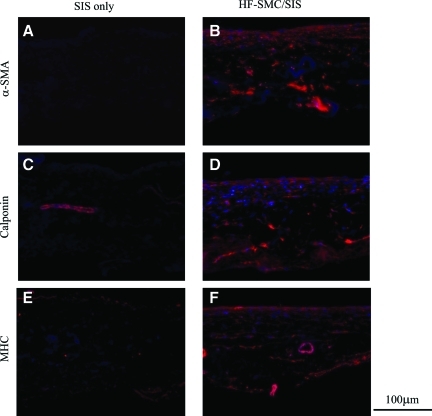
These results were verified by immunostaining for collagen types I and III and tropoelastin, which revealed the presence of all three proteins in the cytoplasm of HF-SMCs (Fig. 5A–C). Deposition of elastin was verified by an additional antibody recognizing mature elastin (Fig. 5D). Tissue sections stained only with secondary antibody served as negative controls (Fig. 5E), and gross morphology was evaluated by H&E staining (Fig. 5F). Once again, in cell-free SIS, elastin staining was absent except in the lumen of preexisting blood vessels. Finally, HF-SMCs infiltrating SIS expressed early (αSMA), intermediate (calponin), and late myosin heavy chain (MHC) smooth muscle markers, indicating a contractile SMC phenotype (Fig. 6).
FIG. 5.
HF-SMCs migrated into SIS and secreted extracellular matrix. HF-SMCs were seeded on SIS and cultured for 2 weeks under 10% uniaxial strain. Immunostaining for (A) collagen type I, (B) collagen type III, (C) tropoelastin, and (D) elastin; (E) staining with secondary antibody only (no primary antibody) served as negative control. Cell nuclei were stained with Hoechst. (F) H&E staining. All panels correspond to the high cell density area enclosed by the box in Figure 4B. Color images available online at www.liebertonline.com/tea.
FIG. 6.
HF-SMCs expressed SMC-specific markers. HF-SMCs were seeded on SIS and cultured for 2 weeks under 10% uniaxial strain. Immunostaining for (A, B) αSMA, (C, D) calponin, and (E, F) MHC. SIS matrix without cells (A, C, E) or in the presence of HF-SMCs (B, D, F). All the images were taken at a similar exposure time of 20–25 ms. MHC, myosin heavy chain. Color images available online at www.liebertonline.com/tea.
HF-SMC/SIS constructs developed significant vascular reactivity
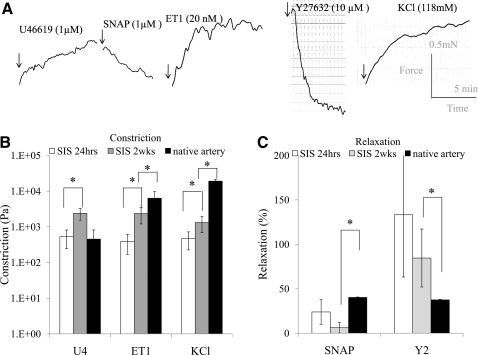
Next we examined the effect of uniaxial strain on the HF-SMCs' contractile function by measuring the isometric tension generated in response to receptor- and nonreceptor-mediated vasoactive agonists (Fig. 7). Receptor-mediated contraction was examined using a thromboxane A2 mimetic (U46619, 1 μM) and ET-1 (20 nM), a ligand of G-protein coupled receptors (ET-A). Nonreceptor-mediated contraction was measured in response to KCl (118 mM). Figure 7A shows characteristic force signatures of HF-SMC/SIS constructs cultured under 10% strain for 24 h as a function of time after addition of the indicated agonist.
FIG. 7.
HF-SMC/SIS constructs exhibited vascular constriction and relaxation. (A) Representative graphs of isometric constriction or relaxation of 10% strained HF-SMC/SIS constructs at 24 h after cell seeding. The scale bars for the force (y-axis, 0.5 mN) and time (x-axis, 5 min) are shown on the right. (B) Vascular reactivity (Pa) was measured in response to U46619, endothelin-1 (ET-1), or potassium chloride (KCl) at 24 h and 2 weeks in culture. (C) Relaxation was measured in response to S-nitroso-N-acetylpenicillamine (SNAP) following U46619 constriction or Y27632 following ET-1 constriction. Each experiment was repeated at least three times with n = 7–10 tissue constructs. Native ovine carotid arteries served as positive control for comparison. The symbol (*) denotes statistical significance between the indicated samples (p < 0.05).
Media equivalents demonstrated contraction in response to all three agonists as soon as 24 h after seeding (Fig. 7B). Specifically, HF-SMC/SIS constructs generated 475 Pa of force in response to KCl, which increased to 1327 Pa after 2 weeks in culture or about 7.5% of the force produced by native arteries (17,461 Pa) (n = 7–10; p < 0.01). The response of HF-SMCs to ET-1 increased from 392 Pa at 24 h to about 2374 Pa at 2 weeks, corresponding to 48% of the force produced by native arteries (4932 Pa) (n = 7–10; p < 0.01). Finally, the response to U46619 was similar to that of native arteries at 24 h (n = 7–10; p > 0.05) and increased by 4.5-fold to about 2400 Pa after 2 weeks in culture (n = 7–10; p < 0.05).
At 24 h the presence of the Rho-kinase inhibitor, Y27632, diminished ET-1-mediated constriction, indicating that contractility required an active Rho-kinase pathway (Fig. 7C). HF-SMC/SIS constructs also exhibited physiological vasorelaxation following U46619 constriction in the presence of the nitric oxide donor, SNAP. After 2 weeks in culture, relaxation decreased but was not statistically different.
Finally, either at 24 h or 2 weeks, constriction or relaxation was undetectable in unstrained tissues (n = 6; p < 0.01) or in the presence of the polymerization inhibitor, cytochalasin D (n = 4; p < 0.01), suggesting that actin-mediated cellular alignment was necessary for development of contractility.
HF-SMC/SIS constructs displayed similar mechanical properties as native ovine arteries
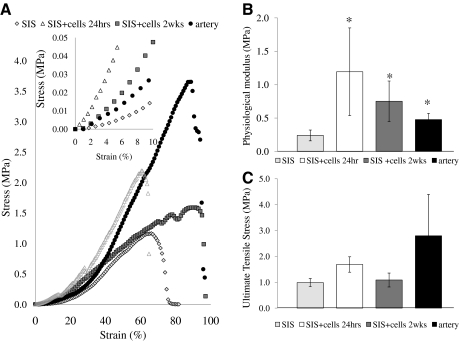
Next, HF-SMC/SIS constructs were mounted on a force transducer and stretched at 4 mm/min continuously until failure to generate the stress–strain curves (Fig. 8A). Similar to native ovine arteries, the stress–strain relationship of HF-SMC/SIS was biphasic, with a low slope at low strains followed by a transition to a higher slope at higher strains. As we are interested in the behavior of these constructs under physiological conditions, the modulus of the stress–strain curve was calculated within the range of strains experienced by native ovine arteries under physiological pressure, that is, in the range of 1%–10% strain (as determined above), and termed “physiological modulus.”
FIG. 8.
HF-SMC/SIS constructs exhibited similar mechanical properties as ovine arteries. HF-SMC/SIS were cultured for 24 h or 2 weeks under 10% uniaxial strain. (A) Representative stress–strain curves (n = 3–5). The inset shows the linear part of the stress–strain curves in the range between 1% and 10% strain. (B) Physiological modulus (MPa) (n = 3–5). (C) Ultimate tensile stress (MPa) (n = 10). SIS without cells served as negative control and native ovine carotid arteries served as positive control for comparison. The symbol (*) denotes statistical significance (p < 0.05) between the indicated samples and the negative control.
Interestingly, the physiological modulus of 10% strained HF-SMC/SIS constructs at 24 h or 2 weeks in culture was similar to that of ovine carotid arteries (HF-SMC/SIS at 24 h: 1.19 ± 0.66 MPa; HF-SMC/SIS at 2 weeks: 0.75 ± 0.3 MPa; ovine arteries: 0.47 ± 0.09 MPa; n = 3–7; p > 0.11) and significantly higher than unstrained cell-free SIS (SIS: 0.24 ± 0.08 MPa; n = 3–7; p < 0.05 compared with ovine arteries or HF-SMC/SIS at 24 h or 2 weeks) (Fig. 8B). In addition, Figure 8C shows that the presence of cells did not alter the UTS of SIS significantly (SIS: 0.98 ± 0.15 MPa; HF-SMC/SIS at 24 h: 1.69 ± 0.30 MPa; HF-SMC/SIS at 2 weeks: 1.09 ± 0.27 MPa; n = 10; p > 0.05). Although the UTS of HF-SMC/SIS was lower than that of native ovine carotid arteries, the difference was not statistically significant (2.8 ± 1.6 MPa; n = 7; p > 0.05 compared with HF-SMC/SIS at 24 h or 2 weeks). Finally, the UTS of HF-SMC/SIS at 24 h or 2 weeks was not significantly higher than the UTS of ovine jugular veins (1.32 ± 0.2 MPa; n = 4; p > 0.05).
Discussion
In our approach to develop an alternative to the standard of vascular grafting, we employed a natural scaffold, SIS, and a novel source of SMCs derived from HFs. We showed that in the appropriate mechanical microenvironment (uniaxial strain), SIS supported HF-SMC alignment and development of vascular contractility as soon as 1 day postseeding. Over a period of 2 weeks, the cells migrated into the matrix and secreted collagen and elastin, the two main components of the vascular wall. These media equivalents also demonstrated mechanical strength and compliance with potential for arterial implantation. Taken together, the present study provides potential solutions to two key challenges facing arterial implantation of bioengineered vascular grafts: scaffold and functional SMCs from a novel and easily accessible stem cell source.
The most important property characterizing SMCs is the ability to generate force in response to vascular agonists. Interestingly, as soon as 24 h after application of mechanical strain, the HF-SMC/SIS constructs exhibited considerable contractility in response to receptor-mediated vasoagonists U46619, ET-1, and nonreceptor-mediated constriction by KCl. This is the first time that vascular reactivity was observed in engineered vascular grafts at such an early time point, suggesting fast development of tissue-like properties. After 2 weeks in culture, the contractile response to all three vasoagonists increased further by three- to fivefold. HF-SMC/SIS constructs preconstricted with ET-1 relaxed in the presence of Y27632, indicating that an active Rho-ROCK pathway was required for vasoconstriction. They also responded to the physiologic regulator NO as they partially relaxed in response to SNAP following preconstruction with U46619. Interestingly, HF-SMC/SIS constructs displayed significantly higher contractility than their HF-SMC/fibrin counterparts.23 Although the reason for this difference is not currently clear, enhanced HF-SMC function may be the result of enhanced cell–SIS interactions under mechanical tension.
In the absence of mechanical strain, the cells formed nonfunctional aggregates with diminished contractile response to vasoagonists. In agreement with previous studies,25,26 blocking actin polymerization with cytochalasin D inhibited stress fiber alignment and diminished contractility, suggesting that dynamic actin reorganization was necessary for SMC contractility. Although the mechanism is not clear, cellular alignment may increase the overall force generated by the tissues in the direction of applied strain. Application of uniaxial strain may also upregulate muscle filament proteins such as αSMA, which are necessary for contractility.27,28 It remains to be seen whether application of cyclic stretch or pulsation can further improve the contractile function of HF-SMC/SIS constructs.
Collagen types I and III as well as tropoelastin, and elastin secretion were significantly enhanced in HF-SMC/SIS constructs, indicating SIS remodeling with the major extracellular components of the vessel wall. Although we did not quantify the collagen content in this study, immunostaining clearly showed high expression in the cell cytoplasm as well as around cells, in agreement with previous studies showing higher ECM secretion by SMCs cultured under strain on a collagen matrix.29 Most notably, Verhoff's elastin and immunostaining clearly showed that HF-SMCs synthesized elastin, which was not detectable in SIS except in the lumen of preexisting vascular conduits. This result is in agreement with previous studies showing elastin expression in collagen hydrogels under cyclic distention30 and, more recently, elastin fiber deposition by SMCs that were cultured on SIS and stretched at low frequency.24 Taken together, these results are encouraging as elastin formation is thought to be important in preventing chronic dilation for implanted vascular grafts.31,32 It remains to be seen whether implantation of HF-SMC/SIS grafts results in elastin fiber deposition to a similar extent as shown for vascular grafts with the highly elastogenic BM-SMCs.5
Notably, HF-SMC/SIS constructs displayed mechanical properties with potential for arterial implantation. Specifically, the physiologic modulus of HF-SMC/SIS was similar to that of ovine carotid arteries, a promising result for future implantation studies, as compliance mismatch has been correlated with failure of synthetic vascular grafts.33 Finally, although not significantly affected by the presence of HF-SMCs during the 2-week culture period, UTS was high enough (∼1 MPa) to suggest that the HF-SMC/SIS grafts may be able to withstand arterial implantation. Experiments are under way in our laboratories to explore this possibility.
In summary, our results suggested that vascular grafts based on HF-SMCs and SIS scaffold may be a good candidate for vascular engineering as demonstrated by development of contractile function, ECM remodeling, and comparable mechanical strength to native vessels. An implication of this finding is that HF-SMC/SIS constructs may be engineered into cylindrical, implantable vascular grafts, as previously demonstrated with acellular SIS.34 We are currently optimizing conditions for endothelialization and mechanical preconditioning of circularized HF-SMC/SIS constructs with the ultimate goal to implant them in the arterial circulation of an ovine animal model that was previously developed in our laboratories.5,6
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 HL086582) and the New York State Stem Cell Science (NYSTEM Contract No. C024316) to S.T. Andreadis and D.D. Swartz. The authors thank Evan Schlaich for help with histology and immunostaining.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gaudino M. Cellini C. Pragliola C. Trani C. Burzotta F. Schiavoni G. Nasso G. Possati G. Arterial versus venous bypass grafts in patients with in-stent restenosis. Circulation. 2005;112:I265. doi: 10.1161/CIRCULATIONAHA.104.512905. [DOI] [PubMed] [Google Scholar]
- 2.Gong Z. Niklason L.E. Blood vessels engineered from human cells. Trends Cardiovasc Med. 2006;16:153. doi: 10.1016/j.tcm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Seliktar D. Nerem R.M. Galis Z.S. Mechanical strain-stimulated remodeling of tissue-engineered blood vessel constructs. Tissue Eng. 2003;9:657. doi: 10.1089/107632703768247359. [DOI] [PubMed] [Google Scholar]
- 4.Long J.L. Tranquillo R.T. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 5.Liu J.Y. Swartz D.D. Peng H.F. Gugino S.F. Russell J.A. Andreadis S.T. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Swartz D.D. Russell J.A. Andreadis S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 7.Bader A. Steinhoff G. Strobl K. Schilling T. Brandes G. Mertsching H. Tsikas D. Froelich J. Haverich A. Engineering of human vascular aortic tissue based on a xenogeneic starter matrix. Transplantation. 2000;70:7. [PubMed] [Google Scholar]
- 8.Kaushal S. Amiel G.E. Guleserian K.J. Shapira O.M. Perry T. Sutherland F.W. Rabkin E. Moran A.M. Schoen F.J. Atala A. Soker S. Bischoff J. Mayer J.E., Jr. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo [comment] Nat Med. 2001;7:1035. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui L. Muto A. Chan S.A. Breuer C.K. Niklason L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A. 2009;15:2665. doi: 10.1089/ten.tea.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazdani S.K. Watts B. Machingal M. Jarajapu Y.P. Van Dyke M.E. Christ G.J. Smooth muscle cell seeding of decellularized scaffolds: the importance of bioreactor preconditioning to development of a more native architecture for tissue-engineered blood vessels. Tissue Eng Part A. 2009;15:827. doi: 10.1089/ten.tea.2008.0092. [DOI] [PubMed] [Google Scholar]
- 11.Roeder R. Wolfe J. Lianakis N. Hinson T. Geddes L.A. Obermiller J. Compliance, elastic modulus, and burst pressure of small-intestine submucosa (SIS), small-diameter vascular grafts. J Biomed Mater Res. 1999;47:65. doi: 10.1002/(sici)1097-4636(199910)47:1<65::aid-jbm9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Raghavan D. Kropp B.P. Lin H.K. Zhang Y. Cowan R. Madihally S.V. Physical characteristics of small intestinal submucosa scaffolds are location-dependent. J Biomed Mater Res A. 2005;73:90. doi: 10.1002/jbm.a.30268. [DOI] [PubMed] [Google Scholar]
- 13.Shell D.H.T. Croce M.A. Cagiannos C. Jernigan T.W. Edwards N. Fabian T.C. Comparison of small-intestinal submucosa and expanded polytetrafluoroethylene as a vascular conduit in the presence of gram-positive contamination. Ann Surg. 2005;241:995. doi: 10.1097/01.sla.0000165186.79097.6c. discussion 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernigan T.W. Croce M.A. Cagiannos C. Shell D.H. Handorf C.R. Fabian T.C. Small intestinal submucosa for vascular reconstruction in the presence of gastrointestinal contamination. Ann Surg. 2004;239:733. doi: 10.1097/01.sla.0000124447.30808.c7. discussion 8–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemcova S. Noel A.A. Jost C.J. Gloviczki P. Miller V.M. Brockbank K.G. Evaluation of a xenogeneic acellular collagen matrix as a small-diameter vascular graft in dogs—preliminary observations. J Invest Surg. 2001;14:321. doi: 10.1080/089419301753435693. [DOI] [PubMed] [Google Scholar]
- 16.Huynh T. Abraham G. Murray J. Brockbank K. Hagen P.O. Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat Biotechnol. 1999;17:1083. doi: 10.1038/15062. [DOI] [PubMed] [Google Scholar]
- 17.Poh M. Boyer M. Solan A. Dahl S.L. Pedrotty D. Banik S.S. McKee J.A. Klinger R.Y. Counter C.M. Niklason L.E. Blood vessels engineered from human cells. Lancet. 2005;365:2122. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 18.McKee J.A. Banik S.S. Boyer M.J. Hamad N.M. Lawson J.H. Niklason L.E. Counter C.M. Human arteries engineered in vitro. EMBO Rep. 2003;4:633. doi: 10.1038/sj.embor.embor847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs E. Horsley V. More than one way to skin. Genes Dev. 2008;22:976. doi: 10.1101/gad.1645908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahoda C.A. Whitehouse J. Reynolds A.J. Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol. 2003;12:849. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoogduijn M.J. Gorjup E. Genever P.G. Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev. 2006;15:49. doi: 10.1089/scd.2006.15.49. [DOI] [PubMed] [Google Scholar]
- 22.Liu J.Y. Peng H.F. Gopinath S. Tian J. Andreadis S.T. Derivation of functional smooth muscle cells from multipotent human hair follicle mesenchymal stem cells. Tissue Eng Part A. 2010;16:2553. doi: 10.1089/ten.tea.2009.0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J.Y. Peng H.F. Andreadis S.T. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res. 2008;79:24. doi: 10.1093/cvr/cvn059. [DOI] [PubMed] [Google Scholar]
- 24.Heise R.L. Ivanova J. Parekh A. Sacks M.S. Generating elastin-rich small intestinal submucosa-based smooth muscle constructs utilizing exogenous growth factors and cyclic mechanical stimulation. Tissue Eng Part A. 2009;15:3951. doi: 10.1089/ten.tea.2009.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeidan A. Nordstrom I. Albinsson S. Malmqvist U. Sward K. Hellstrand P. Stretch-induced contractile differentiation of vascular smooth muscle: sensitivity to actin polymerization inhibitors. Am J Physiol Cell Physiol. 2003;284:C1387. doi: 10.1152/ajpcell.00508.2002. [DOI] [PubMed] [Google Scholar]
- 26.Grenier G. Remy-Zolghadri M. Larouche D. Gauvin R. Baker K. Bergeron F. Dupuis D. Langelier E. Rancourt D. Auger F.A. Germain L. Tissue reorganization in response to mechanical load increases functionality. Tissue Eng. 2005;11:90. doi: 10.1089/ten.2005.11.90. [DOI] [PubMed] [Google Scholar]
- 27.Flavahan N.A. Bailey S.R. Flavahan W.A. Mitra S. Flavahan S. Imaging remodeling of the actin cytoskeleton in vascular smooth muscle cells after mechanosensitive arteriolar constriction. Am J Physiol Heart Circ Physiol. 2005;288:H660. doi: 10.1152/ajpheart.00608.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.R. Gallant C. Leavis P.C. Gunst S.J. Morgan K.G. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol. 2008;295:C768. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim B.S. Nikolovski J. Bonadio J. Mooney D.J. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol. 1999;17:979. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 30.Isenberg B.C. Tranquillo R.T. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng. 2003;31:937. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 31.Opitz F. Schenke-Layland K. Cohnert T.U. Starcher B. Halbhuber K.J. Martin D.P. Stock U.A. Tissue engineering of aortic tissue: dire consequence of suboptimal elastic fiber synthesis in vivo. Cardiovasc Res. 2004;63:719. doi: 10.1016/j.cardiores.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell S.L. Niklason L.E. Requirements for growing tissue-engineered vascular grafts. Cardiovasc Pathol. 2003;12:59. doi: 10.1016/s1054-8807(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 33.Abbott W.M. Megerman J. Hasson J.E. L'Italien G. Warnock D.F. Effect of compliance mismatch on vascular graft patency. J Vasc Surg. 1987;5:376. [PubMed] [Google Scholar]
- 34.Roeder R.A. Lantz G.C. Geddes L.A. Mechanical remodeling of small-intestine submucosa small-diameter vascular grafts—a preliminary report. Biomed Instrum Technol. 2001;35:110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.