Abstract
The need for bone tissue engineering has increased as the world population ages. The objectives of this study were to (1) develop a novel human umbilical cord mesenchymal stem cell (hUCMSC)-encapsulating, fiber-reinforced injectable calcium phosphate cement (CPCF) scaffold, and (2) investigate the effects of osteogenic media delivery, preosteodifferentiation, and bone morphogenetic protein-2 (BMP-2) delivery on hUCMSC osteodifferentiation inside CPCF for the first time. CPCF was developed using calcium phosphate powders, chitosan, and absorbable fibers. Four types of hUCMSC-encapsulating constructs were fabricated: control media in alginate hydrogel microbeads in CPCF; osteogenic media in microbeads; preosteodifferentiation; and recombinant human BMP-2 (rhBMP-2) in microbeads. The hUCMSCs inside CPCF maintained good viability, successfully differentiated into the osteogenic lineage, and synthesized bone minerals. The preosteodifferentiation method yielded high gene expressions of alkaline phosphatase, osteocalcin, collagen, and osterix, as well as alkaline phosphatase protein synthesis. The mineralization for the preosteodifferentiation constructs exceeded those of the rhBMP-2 group at 1–7 days, and was slightly lower than the rhBMP-2 group at 21 days. Mineralization of the rhBMP-2 group was 12-fold that of the control constructs at 21 days. In conclusion, although the BMP-2 delivery promoted osteodifferentiation, the preosteodifferentiation method and the ostegenic media method with hUCMSCs in CPCF were also promising for bone regeneration. hUCMSCs may be an effective alternative to the gold-standard bone marrow MSCs, which require an invasive procedure to harvest. The novel injectable stem cell–CPCF construct may be useful in minimally invasive and other orthopedic surgeries.
Introduction
The need for bone repair arises from skeletal diseases, congenital malformations, trauma, and cancer surgery. Nearly 7 million people suffer bone fractures each year in the United States, and musculoskeletal conditions cost $215 billion annually.1–5 Stem cells offer immense potential for tissue engineering.6–9 Although human bone marrow mesenchymal stem cells (hBMSCs) are useful,4,8,9 they require an invasive procedure to harvest, and have lower self-renewal potential with aging. Human umbilical cord MSCs (hUCMSCs) had multipotent stem cell characteristics and could differentiate into adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells.10–16 Umbilical cords can provide an inexpensive cell source, without the invasive procedure of hBMSCs. Studies showed that hUCMSCs behaved as primitive MSCs, exhibited a high plasticity and developmental flexibility,12 and caused no immunorejection in transplantation in a preliminary animal study.17
Scaffolds serve as a matrix for cell function while maintaining the volume and supporting the external stresses. hUCMSCs were cultured with polystyrene,13 polymer scaffolds,16 and calcium phosphates.18,19 Calcium phosphate scaffolds are bioactive, mimic the bone minerals, and can bond to bone,20–23 in contrast to bioinert implants that can form undesirable fibrous capsules. However, for preformed bioceramic scaffolds to fit into a bone cavity, the surgeon needs to machine the graft or carve the surgical site, leading to increases in bone loss, trauma, and surgical time.5 Preformed scaffolds have other drawbacks, including the difficulty in seeding cells deep into the scaffold, and inability for injection in minimally invasive surgeries.4 In contrast, calcium phosphate cements (CPCs) can set in situ to form a bioactive scaffold that bonds to bone.24–27 The first CPC was approved by the Food and Drug Administration in 1996 for craniofacial repairs.24,28,29 For cell delivery, alginate hydrogel beads were used to encapsulate cells in CPC to protect the cells during the CPC mixing and setting reactions.30–32 Once the CPC had set, the beads could then dissolve and release the cells throughout the CPC scaffold, while concomitantly creating macroporosity.
Recently, an injectable, hUCMSC-encapsulating CPC scaffold was developed with mechanical strength matching that of cancellous bone.33 hUCMSCs were encapsulated into alginate microbeads. The microbead-CPC paste was readily injectable through a 10-gauge needle.33 The hUCMSCs after the injection had a viability similar to that without injection.33 The injectable, hUCMSC-encapsulating CPC is promising for a wide range of load-bearing orthopedic applications. To enhance cell function, an important approach is to deliver growth factors such as bone morphogenetic protein-2 (BMP-2) with the stem cells. Previous studies showed that BMP-2 enhanced osteogenic differentiation and bone formation.5,34–37 A second approach is to deliver the osteogenic media, instead of BMP-2. Osteogenic media with supplements, including dexamethasone, β-glycerophosphate, and ascorbic acid, guided stem cells to differentiate down the osteogenic lineage.13,16,38,39 For delivery with cells in CPC, the osteogenic media could be used to replace the saline in forming the alginate microbeads. A third approach is to culture the hUCMSCs to undergo preosteogenic differentiation in vitro first, and then incorporate the hUCMSC-microbeads into the CPC paste. These three approaches have not been investigated and compared for hUCMSC encapsulation and delivery via CPC.
Therefore, the objectives of this study were to investigate the osteogenic differentiation and mineralization of hUCMSCs encapsulated in the injectable CPC scaffold, and compare the efficacy of osteogenic media delivery, preosteodifferentiation, and BMP-2 delivery. It was hypothesized that (1) the encapsulated hUCMSCs will undergo osteogenic differentiation in CPC under all three treatments; (2) mineralization via encapsulated hUCMSCs in all constructs will increase with time; (3) the hUCMSCs via the osteogenic media method and the preosteodifferentiation method will match the bone marker gene expressions and mineralization via the BMP-2 method.
Materials and Methods
Fabrication of CPC composite scaffold
Tetracalcium phosphate (TTCP), Ca4(PO4)2O, was synthesized from a solid-state reaction between dicalcium phosphate anhydrous (DCPA, CaHPO4) and CaCO3. TTCP was ground to particles of 1–80 μm (median = 17 μm). DCPA was ground to particles of 0.4–3.0 μm (median = 1.0 μm). TTCP and DCPA were mixed at 1:3 molar ratio to form the CPC powder. A biopolymer, chitosan, was used to make the CPC fast-setting.40 Chitosan and its derivatives are natural biopolymers that are biodegradable and osteoconductive.41 Chitosan lactate (Vanson) was mixed with water at a chitosan/(chitosan + water) mass fraction of 15%.42 An absorbable suture fiber (Vicryl, polyglactin 910; Ethicon) was used due to its relatively high strength.43 The fiber was cut to a length of 3 mm so that the CPC-fiber paste was injectable, following a previous study.33 The materials were sterilized in an ethylene oxide sterilizer (Andersen).32
Encapsulating hUCMSCs in alginate hydrogel beads
The harvest of hUCMSCs was approved by the University of Kansas. The pregnancy was at least 37 weeks, the birth weight of the baby was at least 6 pounds, and the cells were harvested within 24 h after the delivery. As described previously,10,14 the cords obtained from an obstetrician were incubated in hyaluronidase (MP Biomedical) and collagenase type I (Sigma) for 30 min at 37°C. Then, the vascular tissue was removed, and the cords were minced and plated in a modified Dulbecco's modified Eagle's medium for 1 week. The cord remnants were removed and the attached cells were harvested. In a preliminary study, to ensure a homogeneous population of stem cells, passage-4 cells were characterized by flow cytometry to analyze specific surface antigens of MSC lineage, including CD29, CD49e, CD73, CD90, and CD105. The results confirmed that the hUCMSCs had a purity of above 95%. For example, the preliminary study found that 98% of the cells were positive to CD29. hUCMSCs isolated by the methods used herein have been demonstrated to go down the osteogenic and chondrogenic lineages when stimulated with the appropriate supplements.14,16
The use of hUCMSCs was approved by the University of Maryland. Cells were cultured in a low-glucose Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen) (control media).19 At 80%–90% confluence, hUCMSCs were detached by trypsin and passaged. Passage-4 hUCMSCs were used for the experiments. The osteogenic media contained the control media plus 100 nM dexamethasone, 10 mM β-glycerophosphate, 0.05 mM ascorbic acid, and 10 nM 1α,25-dihydroxyvitamin (Sigma).16,32
Alginate is a natural polysaccharide extracted from seaweed, is noncytotoxic, and can form a crosslinked gel under mild conditions.9 A 1.2% (mass) sodium alginate solution was prepared by dissolving alginate (molecular weight = 75,000–220,000 g/mol; ProNova) in saline (155 mM NaCl).30,31 hUCMSCs were encapsulated at 1 million cells/mL of alginate solution.32 The alginate–cell solution was loaded into a syringe that was placed into a syringe pump and connected to a bead-generating device (Var J1; Nisco), as described in a recent study.33 This produced microbeads that gelled in a calcium chloride solution for 5 min. These cell-encapsulating microbeads had a median diameter of 207 μm.33
CPC constructs to deliver hUCMSCs
The previous study only used saline to prepare the hUCMSC-encapsulating alginate microbeads.33 In the present study, four different types of hUCMSC-encapsulating, microbead-CPC constructs were fabricated.
Type 1 is referred to as “Control,” in which the microbeads were made by dissolving alginate in saline.33 The CPC power, chitosan liquid, and fibers were mixed at a powder to liquid mass ratio of 2:1 to form a paste,33 which was then mixed with the hUCMSC-encapsulating microbeads. The microbead volume/entire specimen volume was 50%.33 The fiber volume fraction in CPC was 20% because a previous study showed that CPC-microbead with 20% of fibers was readily injected through a 10-gauge needle, whereas at 25% fibers the paste was difficult to inject.33 The microbead and fiber volume fractions were kept the same for all four types of constructs. The CPC with chitosan and fibers is referred to as “CPCF.” Each hUCMSC-CPCF construct was set in a well of 12-well plates at 37°C for 30 min. The construct volume was ∼127 mm3. Then, 2 mL of the control media was added to each well to submerge the construct. The Type 1 constructs received no osteogenic media or BMP-2.
Type 2 is referred to as “Osteogenic media in microbeads.” It used the osteogenic media, instead of saline, to make the alginate solution. The osteogenic media–alginate–hUCMSC solution was used to make the microbeads. The purpose for the hUCMSCs in the microbeads filled with the osteogenic media was to guide the cells for osteogenic differentiation. If this method would be feasible in this in vitro study, a future goal would be to deliver hUCMSCs in CPC filled with the osteogenic media for bone regeneration in an animal model. The hUCMSC-laiden microbeads were mixed with CPCF and cultured in the control media without osteogenic supplements, as described for Type 1. The osteogenic differentiation of hUCMSCs for Type 2 would be caused only by the osteogenic media used to fabricate the microbeads.
Type 3 was designated as “pre-osteodifferentiation.” hUCMSCs were encapsulated in microbeads, as for Type 1. The microbeads were first cultured in the osteogenic media for 7 days, and then incorporated into CPCF. The constructs were then cultured in the control media without osteogenic supplements. The purpose was to investigate if the preosteodifferentiation treatment was sufficient for the hUCMSCs to continue to differentiate and synthesize bone minerals while being encapsulated in CPCF.
Type 4 is referred to as “BMP-2 in microbeads.” Recombinant human BMP-2 (rhBMP-2) (PeproTech), expressed in Escherichia coli and with a molecular mass of 26 kDa, was used. rhBMP-2 was added to the alginate–saline solution and the microbeads were allowed to gel in the calcium chloride bath for 5 min. Once removed from the calcium chloride bath, the microbeads were mixed with the CPCF paste, and the cell culture experiment was started within 1 h from the alginate gelation. The rhBMP-2 concentration was 5 μg for each construct in each well, with the construct volume being ∼127 mm3 (9 mm diameter and 2 mm thickness). This rhBMP-2 concentration is within the range of those used in previous studies, for example, 5 μg in an implant with volume = 75 mm3,44 and 5 μg in an implant volume of 135 mm3.45 The constructs were cultured in the control media without osteogenic supplements, so that the rhBMP-2 in the microbeads would be responsible for the osteogenic differentiation of the hUCMSCs, as determined in the experiments below.
Viability of encapsulated hUCMSCs
Each construct described above was cultured in the control media without osteogenic supplements. The medium was changed every 2 days. After 1, 7, and 14 days, the CPCF constructs were carefully broken and the cell-encapsulating microbeads were harvested, following previous studies.32,33 Cells were live/dead stained (Invitrogen) and viewed by epifluorescence microscopy (TE2000S; Nikon). Three randomly chosen fields of view were photographed for each sample. Five samples (n = 5) yielded 15 photos for each type of construct at each time point. The percentage of live cells was P = NLive/(NLive + NDead), where NLive = number of live cells, and NDead = number of dead cells.46 In addition, the live cell density, D, was calculated: D = NLive/A, where A is the area of the view field in which NLive was measured.
Quantitative real-time reverse transcription polymerase chain reaction measurement of osteogenic differentiation
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR, 7900HT; Applied Biosystems) was used. Each type of construct was cultured for 1, 7, and 14 days. The total cellular RNA of the cells was extracted with TRIzol reagent (Invitrogen) and reverse-transcribed into cDNA using a High-Capacity cDNA Archive kit. TaqMan gene expression assay kits, including two predesigned specific primers and probes, were used to measure the transcription levels of the proposed genes on human alkaline phosphatase (ALP, Hs00758162_m1), osteocalcin (OC, Hs00609452_g1), collagen type I (Hs00164004), osterix (Hs00541729), and glyceraldehyde 3-phosphate dehydrogenase (Hs99999905). Relative expression for each target gene was evaluated using the 2−ΔΔCt method.47 Ct values of target genes were normalized by the Ct of the TaqMan human housekeeping gene glyceraldehyde 3-phosphate dehydrogenase to obtain the ΔCt values. The Ct of hUCMSCs cultured on tissue culture polystyrene in the control media for 1 day served as the calibrator.32,48
Colorimetric assay of ALP activity
To measure the hUCMSC synthesis of the ALP protein, the cell-encapsulating microbeads from CPCF were dissolved by 55 mM sodium citrate tribasic solution (Sigma). A colorimetric p-nitrophenyl phosphate (pNPP) assay (Stanbio) was used to measure the ALP activity. A microplate reader (M5 SpectraMax; Molecular Devices) was used and the ALP was normalized by the DNA content.23,35 DNA was quantified using the Quant-iT PicoGreen Kit (Invitrogen) following standard protocols.32,46
Staining of mineral synthesis by the encapsulated hUCMSCs
Mineralization via the hUCMSCs was investigated at 1, 7, 14, and 21 days, since previous in vitro studies found a significant increase in calcium content from 12 to 21 days.49 Xylenol orange, a fluorescent probe that chelates to calcium and stains the mineral red, was used. Xylenol orange is not harmful to cells; hence, staining can be performed on live cells.49 Xylenol orange (Sigma) was dissolved in water to make a 5 mM solution. Minerals synthesized by hUCMSCs in the microbeads harvested from the constructs were stained and examined using phase-contrast and fluorescence images. Three photos per specimen, with n = 5, yielded 15 photos for each type of construct at each time point. Following a previous study,32 the mineral area percentage was calculated as AMineral/ATotal, where AMineral is the area of mineralization (red fluorescence), and ATotal is the total area of the field of view of the image.
Statistical analysis
One-way and two-way analyses of variance were performed to detect significant (α = 0.05) effects of the variables. Tukey's multiple comparison procedures were used to group and rank the measured values, and Dunn's multiple comparison tests were used on data with non-normal distribution or unequal variance, both at a family confidence coefficient of 0.95.
Results
Viability of encapsulated hUCMSCs
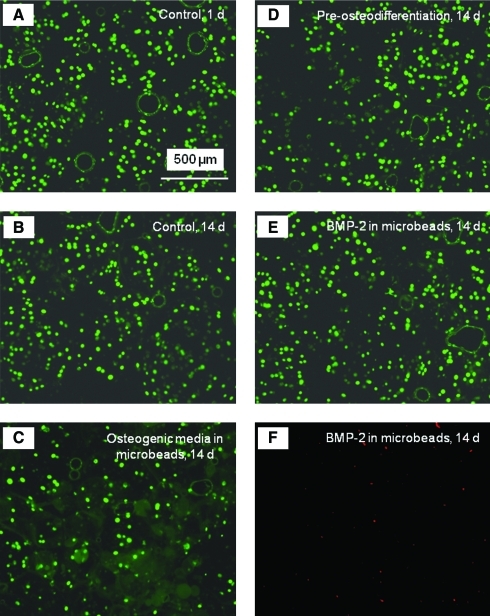
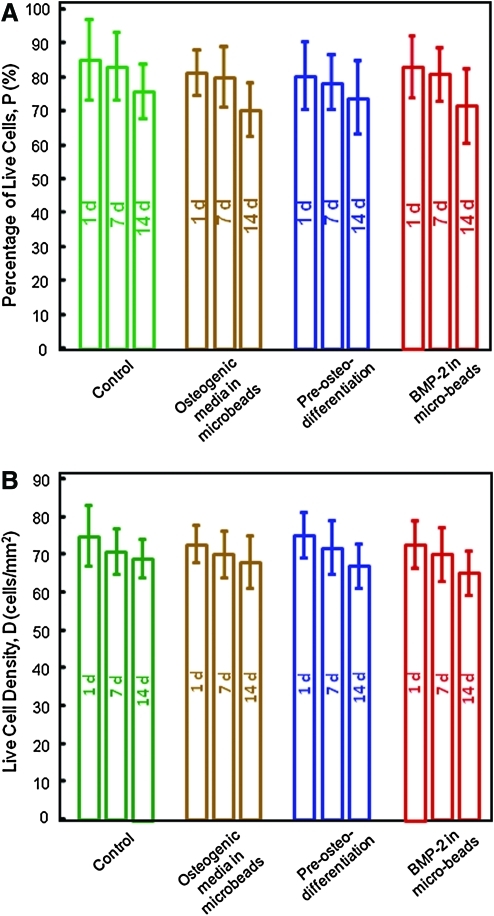
The live/dead staining photos of the encapsulated hUCMSCs are shown in Figure 1. Live cells were stained green and were numerous for all constructs. Dead cells were stained red and were relatively few in all constructs, indicating a good viability for the encapsulated hUCMSCs. Visual examination indicated that the live cell densities were similar for the different types of constructs. Figure 2 shows the quantitative cell viability results. The percentages of live cells for all four types of hUCMSC-encapsulating constructs at different time periods were not significantly different from each other (p > 0.1). This indicates that there was no significant decrease in the number of live cells, and no significant increase in the number of dead cells over time. The live cell density was also similar among the different groups (p > 0.1).
FIG. 1.
Live/dead staining of encapsulated human umbilical cord mesenchymal stem cells (hUCMSCs). (A, B) Live cells encapsulated in the control construct at 1 and 14 days, (C) live cells for the osteogenic media group at 14 days, (D) live cells for the preosteodifferentiation group at 14 days, (E) live cells for the recombinant human bone morphogenetic protein-2 (rhBMP-2) group at 14 days, and (F) dead cells for the rhBMP-2 group at 14 days. Live cells were stained green and were numerous in all constructs. Dead cells were stained red and were relatively few in all constructs. Color images available online at www.liebertonline.com/tea.
FIG. 2.
Viability of the encapsulated hUCMSCs. (A) Percentage of live cells, and (B) live cell density. Each value is the mean of five measurements with the error bar showing one standard deviation (mean ± SD; n = 5). Constructs fabricated with osteogenic media, preosteodifferentiation, and rhBMP-2 incorporation all had good percentages of live cells and cell density, similar to the control construct (p > 0.1). Color images available online at www.liebertonline.com/tea.
Osteogenic differentiation of encapsulated hUCMSCs
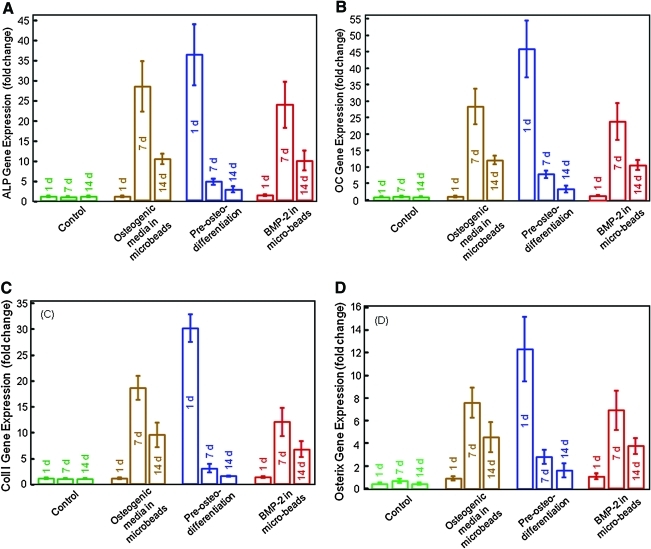
The ALP, OC, collagen I, and osterix gene expressions measured by RT-PCR are plotted in Figure 3. In Figure 3A, the ALP gene expression for the control hUCMSCs (Type 1) was low from 1 to 14 days. For hUCMSCs in osteogenic media in microbeads in CPCF (Type 2), the ALP was low at 1 day, but increased greatly at 7 days, and then decreases at 14 days (p < 0.05). For the preosteodifferentiated hUCMSCs (Type 3), the ALP showed a high peak at 1 day, because these cells were precultured in osteogenic media for 7 days before encapsulation. The rhBMP-2 containing constructs (Type 4) showed a peak in ALP expression similar to that of Type 2.
FIG. 3.
Osteogenic gene expressions measured by real-time reverse transcription polymerase chain reaction. (A) Alkaline phosphatase (ALP), (B) osteocalcin (OC), (C) collagen type I, and (D) osterix gene expressions. Each value is mean ± SD; n = 5. hUCMSCs had high expressions of all four markers. The osteogenic media group and rhBMP-2 group peaked at 7 days. The preosteodifferentition group peaked at 1 day, because the cells were precultured in osteogenic media for 7 days before incorporation into fiber-reinforced injectable calcium phosphate cement (CPCF). The encapsulated hUCMSCs in constructs with osteogenic media, preosteodifferentiation, and rhBMP-2 incorporation all successfully differentiated into the osteogenic lineage. Color images available online at www.liebertonline.com/tea.
In Figure 3B–D, the OC, collagen I, and osterix expressions showed similar trends as ALP. The osteogenic media group and the rhBMP-2 group showed high expressions at 7 days, whereas the preosteodifferentiation group showed a high peak at 1 day. The ALP peak values were not significantly different among the three groups (p > 0.1). The OC, collagen, and osterix peaks were higher for the preosteodifferentiation group than the other groups (p < 0.05).
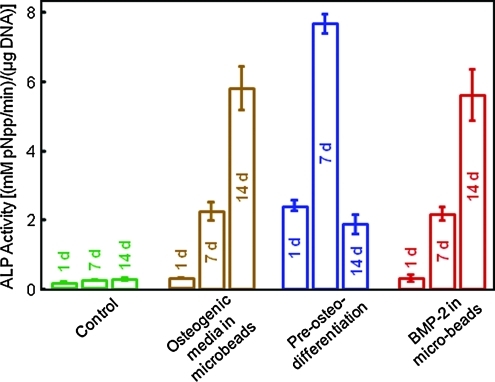
The ALP activity via the pNPP assay is plotted in Figure 4. The control showed low ALP from 1 to 14 days. hUCMSCs of the osteogenic media group had a large increase in ALP at 7 days, then a further increase at 14 days (p < 0.05). For the preosteodifferentiated group, the ALP activity was relatively high at 1 day, further increased at 7 days, then decreased at 14 days. The rhBMP-2 group had an ALP activity that was the highest at 14 days, similar to the osteogenic media group.
FIG. 4.
ALP activity measured by the colorimetric p-nitrophenyl phosphate (pNPP) assay. Each value is mean ± SD; n = 5. The ALP activity was increased by 30–40-fold compared to the control cells, indicating osteogenic differentiation of the encapsulated hUCMSCs in constructs with osteogenic media, preosteodifferentiation, and rhBMP-2 incorporation. Note that after cell encapsulation in alginate microbeads in CPCF, all the constructs were cultured in the control media without the addition of osteogenic supplements. Color images available online at www.liebertonline.com/tea.
Mineral synthesis by the encapsulated hUCMSCs
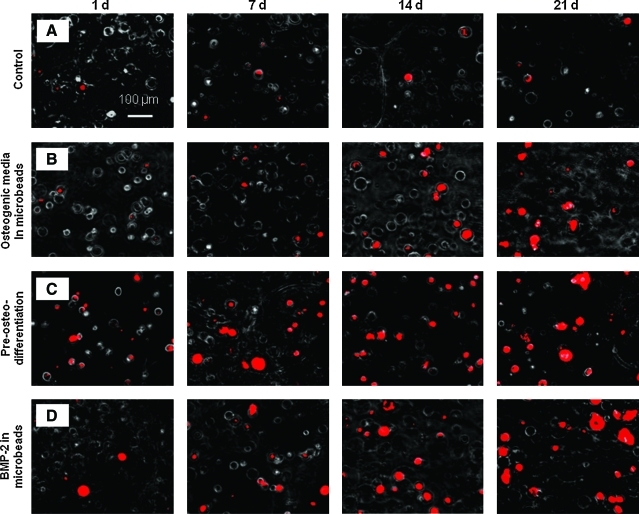
Typical mineral staining photos of the encapsulated hUCMSCs are shown in Figure 5. Minerals emitted red fluorescence when stained with xylenol orange. The control had little mineral staining from 1 to 21 days. For the osteogenic media group, mineral staining was minimal at 1 day, similar to the control. The mineral staining at 1 day was more for the preosteodifferentiation group and the rhBMP-2 group. For the osteogenic media group, the preosteodifferentiation group, and the rhBMP-2 group, mineral staining increased from 1 to 21 days.
FIG. 5.
Xylenol orange staining photos of mineralization via the encapsulated hUCMSCs. (A) The control, (B) the osteogenic media group, (C) the preosteodifferentiation group, and (D) the rhBMP-2 group, each cultured for 1–21 days. There was little mineral staining from 1 to 21 days for the control. For the osteogenic media group, preosteodifferentiation group, and rhBMP-2 group, mineral staining increased significantly from 1 to 21 days. Color images available online at www.liebertonline.com/tea.
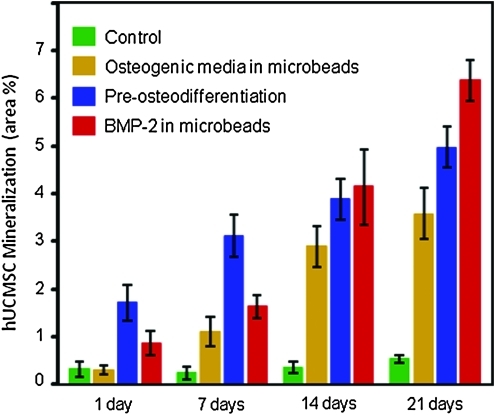
The mineral area percentage is plotted in Figure 6. Except for the control, all other constructs had a significant increase in mineralization area with time. At 1–7 days, the preosteodifferentiation group had the most minerals, followed by the rhBMP-2 group. At 14 days, the rhBMP-2 group matched the preosteodifferentiation group, and at 21-day exceeded the preosteodifferentiation group (p < 0.05). The values were significantly different from each other at 21 days (p < 0.05). The preosteodifferentiation group had more mineral than the osteogenic media group from 1 to 21 days (p < 0.01). At 21 days, the rhBMP-2 group had mineralization that was 12-fold that of the control group, and nearly twice that of the osteogenic media group.
FIG. 6.
Percentage of the stained mineral area via encapsulated hUCMSCs. Except for the control, all other constructs had a significant increase in mineral area percentage with time. Each value is mean ± SD; n = 5. After the hUCMSCs were encapsulated in alginate microbeads in CPCF, they were cultured in the control media without osteogenic supplements. Hence the mineralization was due to the osteogenic media in the microbeads, the preosteodifferentiation treatment, or the rhBMP-2 in the microbeads, when the hUCMSCs were encapsulated. Color images available online at www.liebertonline.com/tea.
Discussion
The present study investigated the osteogenic differentiation and mineralization of encapsulated hUCMSCs, and compared the osteogenic media in microbeads, preosteodifferentiation, and rhBMP-2 in microbeads in CPCF for the first time. While the use of hUCMSCs for tissue regeneration is still in its early stage, as a future strategy, both autologous hUCMSCs and hUCMSCs from a donor have the potential. For autologous applications, the future could hold great promise for those who have stored their cords at birth. For allogenic uses, tissue typing as is done with organ transplant could likely be performed when hUCMSC banks become more common in the future. In a previous study, CPCF with 50% hUCMSC-encapsulating microbeads and 20% fibers was able to be injected through a 10-gauge needle, without compromising hUCMSC viability.33 Injectable scaffolds for cell delivery are advantageous because they can shorten the surgical time and minimize the damaging effect of large muscle retraction, thereby reduce postoperative pain and scar size. They may also achieve rapid recovery and reduce cost.4,5 Novel injectable hydrogel and polymer carriers developed in previous studies were important for cell delivery4,50; however, they cannot be used in load-bearing repairs. For example, it was concluded that hydrogels do not possess the mechanical strength to be used in load-bearing applications.50 Mechanical properties are important for the regeneration of load-bearing tissues such as bone, to withstand stresses to avoid scaffold fracture, and to maintain the structure to define the shape of the regenerated tissue. The previous injectable polymeric and hydrogel carriers for cell delivery had strengths of 0.1–0.7 MPa.51,52 The injectable hUCMSC-CPC construct containing 50% by volume of alginate hydrogel microbeads and 20% fibers had a flexural strength of (4.0 ± 0.8) MPa. Although it was lower than the (10.6 ± 1.9) MPa for the CPC with 20% fibers without the microbeads, it matched the reported tensile strength of 3.5 MPa for cancellous bone.33 Literature search indicated that this was the first injectable stem cell-encapsulating construct that matched the strength of cancellous bone.33
The present study investigated the hUCMSC delivery via CPCF using three fabrication methods: osteogenic media in the hUCMSC-microbeads; preosteodifferentiation of hUCMSC-microbeads; and rhBMP-2 incorporation in the hUCMSC-microbeads. The microbeads were mixed with CPCF and cultured in the control media, without osteogenic supplements. Therefore, the subsequent osteogenic differentiation and mineralization of the encapsulated hUCMSCs would be related to these three fabrication methods, and not due to further external osteogenic stimulation. The following is the rationale for this design. During in vitro culture, the hUCMSC construct can be immersed in osteogenic media to induce osteogenic differentiation. However, when delivering the hUCMSC construct for bone regeneration, the construct will not be immersed in osteogenic media in vivo all the time. It may not absorb sufficient growth factors from the neighboring tissues, due to the age of the patient or local inflammation. Therefore, it would be important for the cell-encapsulating construct to be able to have osteogenic differentiation and synthesize the bone matrix on its own, even in the absence of an external infusion of osteogenic factors. The present study showed that all three fabrication methods yielded hUCMSC constructs that successfully osteodifferentiated and synthesized bone minerals, while being cultured in the control media without further osteogenic supplements. In contrast, the control constructs had minimal expression of bone markers and made little mineral. These results indicate that all three fabrication methods for the hUCMSC-CPCF constructs may be feasible to promote bone regeneration in vivo, and warrant further animal study.
The hUCMSCs encapsulated in the CPCF constructs maintained their viability. A previous study showed that without the encapsulation in the hydrogel microbead carrier, cells in direct contact with freshly mixed CPC paste died.31 Although that study used MC3T3-E1 mouse osteoblasts and not human MSCs (hMSCs), it demonstrated the need to use hydrogel encapsulation to protect the cells. The present study used hydrogel microbead encapsulation and achieved a good cell viability, yielding the percentage of live cells of being around 70%–80%, which is consistent with previous studies on cell encapsulation in hydrogels.9,53 Although the encapsulated hUCMSCs were alive, they did not proliferate from 1 to 14 days, as indicated by cell counting in Figure 2B. This is also consistent with previous studies.54,55 The MSCs are anchorage-dependent and need a bioactive surface to attach and spread.54 In previous studies, investigators modified the hydrogels with RGD peptides to promote cell attachment and function.3,4,35,50 However, whether the gel was inert or bioactive, there was evidence that the hMSCs did not proliferate while being encapsulated, although they did attach and spread in bioactive gels. This is likely because that the cells were packed in the gel and were contact-inhibited; hence, the proliferation became arrested.55 The alginate hydrogel of the present study was not modified with RGD peptides because the purpose of using the alginate microbeads was to protect the hUCMSCs from the CPC paste mixing force and setting reaction. Once the CPC has set in a day, it is biocompatible and supports hUCMSC attachment and viability.33 Therefore, it would be desirable for the microbeads in the CPC to dissolve away in a few days and release the cells in CPC, while concomitantly creating macropores in CPC. Since the eventual goal was for the alginate microbeads to degrade in several days, peptide modification of the alginate was not attempted in the present study. The alginate hydrogel microbeads of the present study were not degradable, to facilitate the harvest of the microbeads with hUCMSCs for analysis. Once the method of osteodifferentiation is established through this study, a future study should develop degradable alginate microbead-CPCF construct, and investigate bead degradation and hUCMSC release and function in CPCF.
The methods of guiding the MSCs for osteogenic differentiation include the use of growth factors such as BMP-2 and other agents.5,27,34–37 For example, the combined delivery of angiogenic and osteogenic factors enhanced MSC osteodifferentiation and bone regeneration in animal models.36,37 Other in vitro studies showed that osteogenic media containing dexamethasone, β-glycerophosphate, and ascorbic acid reliably guided MSCs to differentiate into the osteogenic lineage.13,16,38 For example, dexamethasone was shown to be an effective factor leading to the osteodifferentiation of hMSCs.39 Although several meritorious studies examined growth factor delivery via CPCs to enhance bone regeneration,27,44,45,56 they did not investigate stem cell encapsulation and delivery via CPCs.
In the present study, osteogenic media delivery (Type 2), preosteodifferentiation (Type 3), and rhBMP-2 delivery (Type 4) all succeeded in hUCMSC differentiation and mineralization, while being encapsulated in CPCF in control media. For the rhBMP-2 containing constructs, because the hUCMSCs and the rhBMP-2 were encapsulated in the same alginate microbeads, the cells were exposed to the rhBMP-2 in the microbeads. Hence, it would be preferable for the rhBMP-2 to stay in the constructs to have an effect on the cells, rather than being released away from the construct. A preliminary experiment used an enzyme-linked immunosorbent assay and measured the rhBMP-2 release from the CPC-microbead construct. The cumulative percentage of rhBMP-2 release (mass of released rhBMP-2/mass of rhBMP-2 incorporated into the construct) was (2.3 ± 1.0)% at 1 day, (7.5 ± 0.9)% at 10 days, and (8.8 ± 3.0)% at 20 days. Therefore, the majority of rhBMP-2 was retained in the construct where the hUCMSCs were encapsulated. This is consistent with the hUCMSCs in the rhBMP-2 group showing successful osteogenic differentiation. Two other points should be discussed here. First, the osteogenic media group and the rhBMP-2 group showed peaks in ALP, OC, collagen I, and osterix at 7 days, whereas the markers for the preosteodifferentiation group peaked at 1 day. This is likely because for the preosteodifferentiation group, the encapsulated hUCMSCs in the microbeads were precultured in the osteogenic media for 7 days before being incorporated into CPC. The second point is that the ALP activity in Figure 4 peaked at a later time that the ALP gene expression peak in Figure 3A. ALP is an enzyme expressed by MSCs during osteogenesis and is a well-defined marker for their differentiation.23,35 During osteogenic differentiation for the MSCs, the genetic expression of ALP is first upregulated at the early stage of differentiation. This upregulation sets off a cascade of events that then lead to the production of the ALP protein. The ALP gene expression was measured by the RT-PCR method. The latter event, that is, the synthesis of ALP protein, was measured by the pNPP assay. Therefore, the ALP gene expression increased at 7 days, whereas the ALP activity increased at 14 days for the osteogenic media group and the rhBMP-2 group. The ALP activity for the preosteodifferentiation group peaked at 7 days, which plus the 7 days of preculture in the osteogenic media, was also equal to 14 days. This is consistent with previous studies which showed that the ALP activity peaked at 14 days.35
The mineralization was higher for the preosteodifferentiation group at 1–7 days. However, it was matched at 14 days, and surpassed at 21 days, by the rhBMP-2 group. BMP-2 was a potent osteogenic factor.6,34–37 The Food and Drug Administration approved the use of rhBMP-2 in 2002 and the creation of a Humanitarian Device Exemption for rhBMP-7 in 2004, and thus began the era of rhBMPs for use in spine fusion.57 In the present study, the rhBMP-2 group had the highest mineralization at 21 days than all other groups. Animal study is needed to examine if this translates into more bone regeneration via rh-BMP-2 delivery with the hUCMSC-CPCF construct. Perhaps a drawback of rhBMPs is the cost, currently at about $1300 for 100 μg of rhBMP-2. In contrast, the cost of osteogenic supplements to make the osteogenic media for cell culture was about $4.50 per 500 mL of the osteogenic media. Indeed it was stated that “Given the limited access (with only two companies producing approved BMPs) as well as their relatively high cost, there has been renewed interest in promoting less expensive and potentially unvalidated alternative bone-graft substitutes.”57 This is consistent with another study that also cited “the high production cost of BMP” as a major obstacle to its commercialization for clinical applications.58 The present study showed that the preosteodifferentiation method and the osteogenic media delivery with hUCMSCs in microbeads in CPCF yielded high gene expressions for ALP, OC, collagen I, and osterix, as well as ALP protein synthesis. Further, the hUCMSC mineralization via the preosteodifferentiation method exceeded those of the rhBMP-2 group at 1 and 7 days, and was only slightly lower than the rhBMP-2 group at 21 days. Therefore, the preosteodifferentiation method and the osteogenic media delivery with hUCMSCs in microbeads in CPCF, which did not use rhBMP-2, warrant further investigation to compare with the rhBMP-2 method for bone regeneration in animal models.
Conclusions
The present study investigated hUCMSC delivery via novel injectable calcium phosphate-fiber (CPCF) scaffolds for bone engineering. The effects on osteodifferentiation and mineralization via osteogenic media delivery, preosteodifferentiation, and rhBMP-2 delivery in microbeads in CPCF were determined for the first time. All three methods yielded hUCMSC constructs that underwent osteogenic differentiation and synthesized bone minerals, while being cultured in control media without osteogenic supplements. The preosteodifferentiation method yielded high expressions for ALP, OC, collagen I, and osterix, as well as ALP protein synthesis. Mineralization for the preosteodifferentiation constructs exceeded those of the rhBMP-2 group at 1 and 7 days, whereas the rhBMP-2 group had the highest mineralization at 21 days. These results indicate that all three fabrication methods for the hUCMSC-CPCF constructs may promote bone regeneration in vivo, and warrant further animal study. The osteogenic media delivery and the preosteodifferentiation method with hUCMSCs in microbeads may be promising alternatives to the use of rhBMP-2 in delivering stem cells in CPCF. These results support the use of hUCMSCs as a low-cost alternative to the gold-standard hBMSCs, which require an invasive procedure to harvest. The injectable, stem cell-encapsulating CPCF scaffold may be useful in minimally invasive and other orthopedic and craniofacial applications.
Acknowledgments
We are indebted to Drs. L.C. Chow and S. Takagi at the Paffenbarger Research Center, and Dr. Carl G. Simon at the National Institute of Standards and Technology for discussions and help. This study was supported by NIH R01 grants DE14190 (H.H.K.X.), Maryland Stem Cell Fund (H.H.K.X.), the University of Maryland Dental School, and the State of Kansas (M.S.D.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Lavik E. Langer R. Tissue engineering: current state and perspectives. Appl Microbiol Biotech. 2004;65:1. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 2.Mao J.J. Giannobile W.V. Helms J.A. Hollister S.J. Krebsbach P.H. Longaker M.T. Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salinas C.N. Anseth K.S. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med. 2008;2:296. doi: 10.1002/term.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kretlow J.D. Young S. Klouda L. Wong M. Mikos A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21:3368. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurencin C.T. Ambrosio A.M.A. Borden M.D. Cooper J.A. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Santos E.M. Radin S. Shenker B.J. Shapiro I.M. Ducheyne P. Si-Ca-P xerogels and bone morphogenetic protein act synergistically on rat stromal marrow cell differentiation in vitro. J Biomed Mater Res. 1998;41:87. doi: 10.1002/(sici)1097-4636(199807)41:1<87::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Mao J.J. Vunjak-Novakovic G. Mikos A.G. Atala A. Regenerative Medicine: Translational Approaches and Tissue Engineering, Chapter 1–3. Boston and London: Artech House; 2007. [Google Scholar]
- 8.Mikos A.G. Herring S.W. Ochareon P. Elisseeff J. Lu H.H. Kandel R. Schoen F.J. Toner M. Mooney D. Atala A. van Dyke M.E. Kaplan D. Vunjak-Novakovic G. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong H.J. Smith M.K. Mooney D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang H.S. Hung S.C. Peng S.T. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 11.Sarugaser R. Lickorish D. Baksh D. Hosseini M.M. Davies J.E. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 12.Can A. Karahuseyinoglu S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 13.Baksh D. Yao R. Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 14.Bailey M.M. Wang L. Bode C.J. Mitchell K.E. Detamore M.S. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 15.Karahuseyinoglu S. Kocaefe C. Balci D. Erdemli E. Can A. Functional structure of adipocytes differentiated from human umbilical cord stroma-derived stem cells. Stem Cells. 2008;26:682. doi: 10.1634/stemcells.2007-0738. [DOI] [PubMed] [Google Scholar]
- 16.Wang L. Singh M. Bonewald L.F. Detamore M.S. Signaling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Regen Med. 2009;3:398. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 17.Weiss M.L. Medicetty S. Bledsoe A.R. Rachakatla R.S. Choi M. Merchav S. Luo Y. Rao M.S. Velagaleti G. Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 18.Jäger M. Degistirici O. Knipper A. Fischer J. Sager M. Krauspe R. Bone healing and migration of cord blood-derived stem cells into a critical size femoral defect after xenotransplantation. J Bone Miner Res. 2007;22:1224. doi: 10.1359/jbmr.070414. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L. Burguera E.F. Xu H.H.K. Amin N. Ryou H. Arola D.D. Fatigue and human umbilical cord stem cell seeding characteristics of calcium phosphate-chitosan-biodegradable fiber scaffolds. Biomaterials. 2010;31:840. doi: 10.1016/j.biomaterials.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducheyne P. Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20:2287. doi: 10.1016/s0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 21.Foppiano S. Marshall S.J. Marshall G.W. Saiz E. Tomsia A.P. The influence of novel bioactive glasses on in vitro osteoblast behavior. J Biomed Mater Res A. 2004;71:242. doi: 10.1002/jbm.a.30159. [DOI] [PubMed] [Google Scholar]
- 22.Deville S. Saiz E. Nalla R.K. Tomsia A.P. Freezing as a path to build complex composites. Science. 2006;311:515. doi: 10.1126/science.1120937. [DOI] [PubMed] [Google Scholar]
- 23.Reilly G.C. Radin S. Chen A.T. Ducheyne P. Differential alkaline phosphatase responses of rat and human bone marrow derived mesenchymal stem cells to 45S5 bioactive glass. Biomaterials. 2007;28:4091. doi: 10.1016/j.biomaterials.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown W.E. Chow L.C. A new calcium phosphate water setting cement. In: Brown P.W., editor. Cements Research Progress. Westerville, OH: American Ceramic Society; 1986. pp. 352–379. [Google Scholar]
- 25.Barralet J.E. Gaunt T. Wright A.J. Gibson I.R. Knowles J.C. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J Biomed Mater Res B. 2002;63:1. doi: 10.1002/jbm.1074. [DOI] [PubMed] [Google Scholar]
- 26.Bohner M. Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Link D.P. van den Dolder J. van den Beucken J.J. Wolke J.G. Mikos A.G. Jansen J.A. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-β1 loaded gelatin microspheres. Biomaterials. 2008;29:675. doi: 10.1016/j.biomaterials.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Shindo M.L. Costantino P.D. Friedman C.D. Chow L.C. Facial skeletal augmentation using hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 1993;119:185. doi: 10.1001/archotol.1993.01880140069012. [DOI] [PubMed] [Google Scholar]
- 29.Friedman C.D. Costantino P.D. Takagi S. Chow L.C. BoneSource hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res B. 1998;43:428. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Simon C.G., Jr. Guthrie W.F. Wang F.W. Cell seeding into calcium phosphate cement. J Biomed Mater Res A. 2004;68:628. doi: 10.1002/jbm.a.20008. [DOI] [PubMed] [Google Scholar]
- 31.Weir M.D. Xu H.H.K. Simon C.G., Jr. Strong calcium phosphate cement-chitosan-mesh construct containing cell-encapsulating hydrogel beads for bone tissue engineering. J Biomed Mater Res A. 2006;77:487. doi: 10.1002/jbm.a.30626. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L. Weir M.D. Xu H.H.K. Human umbilical cord stem cell encapsulation in calcium phosphate scaffolds for bone engineering. Biomaterials. 2010;31:3848. doi: 10.1016/j.biomaterials.2010.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L. Weir M.D. Xu H.H.K. An injectable calcium phosphate—alginate hydrogel—umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karageorgiou V. Tomkins M. Fajardo R. Meinel L. Snyder B. Wade K. Chen J. Vunjak-Novakovic G. Kaplan D.L. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J Biomed Mater Res A. 2006;78:324. doi: 10.1002/jbm.a.30728. [DOI] [PubMed] [Google Scholar]
- 35.Benoit D.S. Collins S.D. Anseth K.S. Multifunctional hydrogels that promote osteogenic hMSC differentiation through stimulation and sequestering of BMP2. Adv Funct Mater. 2007;17:2085. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park E.J. Kim E.S. Weber H.P. Wright R.F. Mooney D.J. Improved bone healing by angiogenic factor-enriched platelet-rich plasma and its synergistic enhancement by bone morphogenetic protein-2. Int J Oral Maxillofac Implants. 2008;23:818. [PMC free article] [PubMed] [Google Scholar]
- 37.Young S. Patel Z.S. Kretlow J.D. Murphy M.B. Mountziaris P.M. Baggett L.S. Ueda H. Tabata Y. Jansen J.A. Wong M. Mikos A.G. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyes J.M. Fermanian S. Yang F. Zhou S.Y. Herretes S. Murphy D.B. Elisseeff J.H. Chuck R.S. Metabolic changes in mesenchymal stem cells in osteogenic medium measured by autofluorescence spectroscopy. Stem Cells. 2006;24:1213. doi: 10.1634/stemcells.2004-0324. [DOI] [PubMed] [Google Scholar]
- 39.Nuttelman C.R. Tripodi M.C. Anseth K.S. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. J Biomed Mater Res A. 2006;76:183. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- 40.Xu H.H.K. Takagi S. Quinn J.B. Chow L.C. Fast-setting and anti-washout calcium phosphate scaffolds with high strength and controlled macropore formation rates. J Biomed Mater Res A. 2004;68:725. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 41.Muzzarelli R.A.A. Biagini G. Bellardini M. Simonelli L. Castaldini C. Fraatto G. Osteoconduction exerted by methylpyrolidinone chitosan in dental surgery. Biomaterials. 1993;14:39. doi: 10.1016/0142-9612(93)90073-b. [DOI] [PubMed] [Google Scholar]
- 42.Xu H.H.K. Simon C.G., Jr. Fast setting calcium phosphate-chitosan scaffold: mechanical properties and biocompatibility. Biomaterials. 2005;26:1337. doi: 10.1016/j.biomaterials.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y. Xu H.H.K. Effects of synergistic reinforcement and absorbable fiber strength on hydroxyapatite bone cement. J Biomed Mater Res A. 2005;75:832. doi: 10.1002/jbm.a.30461. [DOI] [PubMed] [Google Scholar]
- 44.Ruhé P.Q. Hedberg E.L. Padron N.T. Spauwen P.H. Jansen J.A. Mikos A.G. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85:75. doi: 10.2106/00004623-200300003-00013. [DOI] [PubMed] [Google Scholar]
- 45.Jansen J.A. Vehof J.W.M. Ruhé P.Q. Kroeze-Deutman H. Kuboki Y. Takita H. Hedberg E.L. Mikos A.G. Growth factor-loaded scaffolds for bone engineering. J Controlled Release. 2005;101:127. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Moreau J.L. Xu H.H.K. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan scaffold. Biomaterials. 2009;30:2675. doi: 10.1016/j.biomaterials.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.Kim K. Dean D. Mikos A.G. Fisher J.P. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules. 2009;10:1810. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y.H. Liu Y. Maye P. Rowe D.W. Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotechnol Prog. 2006;22:1697. doi: 10.1021/bp060274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drury J.L. Mooney D.J. Review. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 51.Drury J.L. Dennis R.G. Mooney D.J. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Shi X. Sitharaman B. Pham Q.P. Liang F. Wu K. Billups W.E. Wilson L.J. Mikos A.G. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials. 2007;28:4078. doi: 10.1016/j.biomaterials.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salinas C.N. Cole B.B. Kasko A.M. Anseth K.S. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007;13:1025. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 54.Nuttelman C.R. Tripodi M.C. Anseth K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68:773. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 55.Markusen J.F. Mason C. Hull D.A. Town M.A. Tabor A.B. Clements M. Boshoff C.H. Dunnill P. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue Eng. 2006;12:821. doi: 10.1089/ten.2006.12.821. [DOI] [PubMed] [Google Scholar]
- 56.Verron E. Khairoun I. Guicheux J. Bouler J.M. Calcium phosphate biomaterials as bone drug delivery systems: a review. Drug Discov Today. 2010;15:547. doi: 10.1016/j.drudis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Bridwell K.H. Anderson P.A. Boden S.D. Vaccaro A.R. Wang J.C. What's new in spine surgery? J Bone Joint Surg Am. 2007;89:1654. [Google Scholar]
- 58.Yeom J. Chang S. Park J.K. Je J.H. Yang D.J. Choi S.K. Shin H.I. Lee S.J. Shim J.H. Cho D.W. Hahn S.K. Synchrotron X-ray bioimaging of bone regeneration by artificial bone substitute of megagen synthetic bone and hyaluronate hydrogels. Tissue Eng Part C Methods. 2010;16:1059. doi: 10.1089/ten.TEC.2009.0759. [DOI] [PubMed] [Google Scholar]