Abstract
The decrease in western equine encephalomyelitis virus (WEEV; Togaviridae, Alphavirus) activity in North America over the past 20–30 years has prompted research to determine if there have been concurrent declines in virulence. Six (WEEV) strains isolated from Culex tarsalis mosquitoes from California during each of the six preceding decades failed to show a consistent declining temporal trend in virus titer using mosquito (C6/36), avian (duck embryo fibroblast), or mammalian (Vero) cells, results similar to our recent in vivo studies using birds and mosquitoes. Titers measured by Vero cell plaque assay were consistently highest on mosquito cell culture, followed by avian and mammalian cell cultures. Similar to previous in vivo results in house sparrows and mice, titers for the IMP181 strain isolated in 2005 were significantly lower in both avian and mammalian cells. Real-time monitoring of changes in cell growth measured by electrical impedance showed consistent differences among cell types, but not WEEV strains. Collectively, these in vitro results failed to explain the decrease in WEEV enzootic and epidemic activity. Results with the IMP181 strain should be verified by additional sequencing, cell growth, and pathogenesis studies using concurrent or 2006 isolates of WEEV from California.
Key Words : Cell culture, Real-time cell electronic sensing system, Western equine encephalomyelitis virus
Introduction
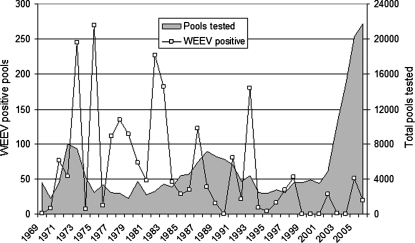
Soon after its isolation in the 1930s (Meyer et al. 1931), western equine encephalomyelitis virus (WEEV; Togaviridae, Alphavirus) was recognized as the etiological agent responsible for large-scale outbreaks of equine neuroinvasive disease throughout western North America (Reisen and Monath 1989). Subsequent research also showed that this virus was a significant cause of neurological illness in humans, especially among very young children residing in the Central Valley of California (Reeves and Hammon 1962). Although most equines now are protected by annual vaccination, the numbers of human cases also have declined and rarely have been reported during the past 20 years. In California, the decline in WEEV human and horse cases has been concurrent with a decline in the frequency of virus detection in mosquitoes tested as part of the California state-wide surveillance program, despite the marked increase in testing effort associated with the West Nile virus epidemic (Fig. 1).
FIG. 1.
Number of pools of mosquitoes positive for western equine encephalomyelitis virus (WEEV) and tested for arboviruses from California, 1969–2007 (Hui et al. 1999, Steinlein et al. 2003).
Recently, we showed that there were minimal differences in virulence for white-crowned sparrows or in the vector competence by Culex tarsalis mosquitoes among six strains of WEEV isolated in California during each decade from 1953 through 2005, although the IMP181 strain isolated in 2005 showed a reduced viremogenic response in house sparrows (Reisen et al. 2008b). We used white-crowned sparrows for these experiments, because this species historically was shown to be highly susceptible to WEEV infection (Hardy and Reeves 1990). Results using mouse mortality/morbidity models have shown variable results. Hardy and coworkers (1997) showed that there were considerable differences in neuroinvasiveness and neurovirulence among several WEEV strains, but virulence was not necessarily aligned temporally or associated with outbreaks of human disease. Interestingly, three strains isolated from southeastern California in 1992 had intermediate to no neurovirulence in mice (including the COAV592 strain used in our experiments). However, they found that young chickens did not show the same patterns depicted by mice, perhaps supporting our results using birds (Reisen et al. 2008b). Two recent experiments using mouse models and an assortment of virus strains produced somewhat conflicting results. Forrester and coworkers (2008) found minor differences in mortality patterns among 10 strains isolated over each of the past six decades, whereas Logue and coworkers (2009) found marked differences among 6 strains, with highest mortality in the McMillan 1941 strain and no mortality in the IMP181 2005 strain. Interestingly, there was variability in titers produced in different cell cultures by these two strains between 12 and 72 h postinfection, with the McMillan strain growing to higher titers in BHK cells and the IMP181 strain growing to higher titers in C6/36 cells; curves were variable in Vero cells (Logue et al. 2009).
Due to the variable results among and within previous mouse, avian, and limited cell culture comparisons, we compared the in vitro growth patterns of six WEEV isolates from California on mosquito, avian, and mammalian cell lines to identify phenotypic markers associated with the declines in enzootic and epidemic activity. Based on previous in vivo results, we anticipated to find marked differences among strains in mammalian cell lines that were not duplicated in avian or mosquito cells.
Materials and Methods
Viral strains
WEEV strains were the same as those used previously (Reisen et al. 2008b) and were isolated from pools of Cx. tarsalis mosquitoes collected in California during each decade from 1953 through 2005 (Table 1). Three strains were isolated near Bakersfield in Kern County, whereas BFN3060 was isolated from Butte County near Chico, COA592 from Coachella Valley near the Salton Sea in Riverside County, and IMP181 from near the Salton Sea in Imperial County. Before 1990, isolations were made by suckling mouse intracerebral inoculation, whereas after 1990 all isolations were made in Vero cell culture. Strains were passaged once or twice after initial isolation, stored at −80°C, and then stocks thawed (by us) and passaged once in Vero cells before experimentation.
Table 1.
Mean and Standard Error Peak Viral Titers in Log10 Plaque Forming Units per Milliliter for Each Western Equine Encephalomyelitis Virus Strain on Each Cell Type
| Strain | Year | Vero (48 h) | DEF (24 h) | C6/36 (72 h) | Mean | |||
|---|---|---|---|---|---|---|---|---|
| BFS1703 | 1953 | 4.90 | 0.53 | 5.30 | 0.10 | 6.77 | 0.60 | 5.66a |
| BFS4740 | 1964 | 4.63 | 0.42 | 5.83 | 0.31 | 5.77 | 0.55 | 5.41a |
| BFN3060 | 1971 | 5.17 | 0.15 | 5.40 | 0.10 | 6.27 | 0.21 | 5.61a |
| KERN5547 | 1983 | 5.23a | 0.25 | 5.80 | 0.45 | 6.20 | 0.26 | 5.72a |
| COA592 | 1992 | 5.23 | 0.30 | 5.40 | 0.20 | 6.40 | 0.10 | 5.74a |
| IMP181 | 2005 | 4.67 | 0.15 | 5.70 | 0.46 | 6.33 | 0.06 | 4.98b |
| Mean | 5.00a | 5.28b | 6.32c | |||||
Maxima appeared on different days for different cell types. Row and column means followed by the same letter were not significantly different using a Newman–Keuls multiple comparison test (p > 0.05).
Peak titer at 72 h.
DEF, duck embryo fibroblast.
Growth kinetics
Three cell lines used were green monkey kidney (Vero), duck embryo fibroblast (DEF), and Aedes albopictus (C6/36). Cells were grown in standard 75 cm2 cell culture flasks using buffered minimal essential media (Dulbecco's modified Eagle's medium) with 5% fetal bovine serum (FBS). Cell numbers were estimated with a hemocytometer and then inoculated at a multiplicity of infection of 0.1. Vero and DEF cultures were maintained at 37°C, whereas C6/36 cultures were held at 27°C. At 24 h intervals, a 50 μL aliquot was removed, mixed with 450 μL of viral diluent (phosphate-buffered saline, 15% FBS, antibiotics), and then frozen at −80°C until later titration on Vero cells to estimate viral titer using a standard Vero cell plaque assay.
Real-time cell electronic sensing
ACEA Biosciences Inc. (San Diego, CA) recently developed the real-time cell electronic sensing (RT-CES)™ system to constantly monitor cell growth by measuring electrical impedance within standard 96- or 24-well plates (Solly et al. 2004); that is, the greater the number of cells, the greater the impedance as expressed by the Cell Index [(Cell Index = [(impedance without cells/impedance with cells) − 1]) (Xing et al. 2005). This system has been used to monitor the effects of drugs and toxins on cell growth (Xing et al. 2005), and we applied this method to monitor the growth and subsequent death of three cell types following WEEV infection. Approximately, 10,000 cells of each type were added to each of four replicate wells for each viral strain. Two wells served as uninfected controls, whereas two were infected with each viral strain at a multiplicity of infection of 0.1. C6/36 cells grown at 27°C were monitored for 150 h, whereas DEF and Vero cells grown at 37°C were monitored for 100 h.
Results
Growth kinetics
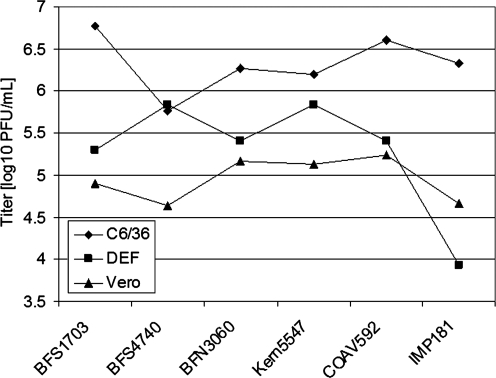
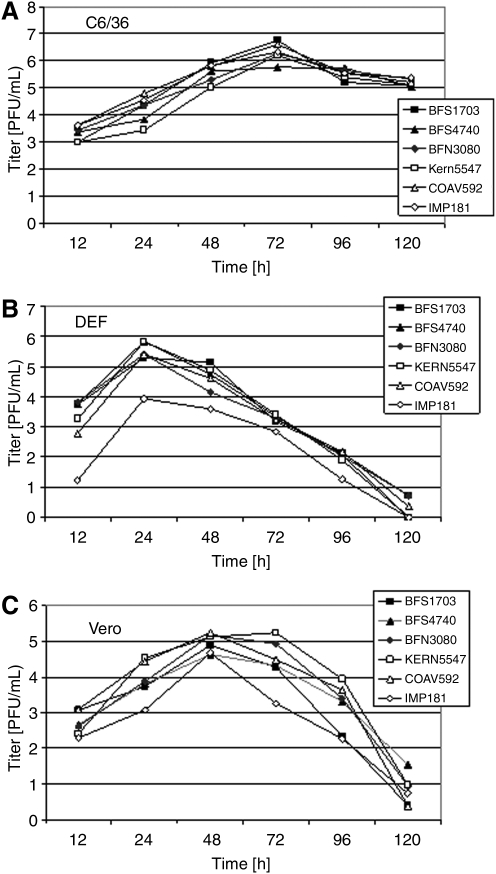
There were consistent differences in the growth of all WEEV strains on different cell types (Table 1 and Fig. 2). At 37°C, all WEEV strains grew faster on DEF cells than on Vero cells, peaking at 24 rather than 48 h, respectively. All strains took longer to grow on C6/36 cells at 27°C, peaking at 72 h. When tested by a two-way analysis of variance (Hintze 2003), mean maximum WEEV titers in log10 plague forming units/mL differed significantly among cell types (F = 80.8, df = 2, 36, p < 0.001), being highest for C6/36 followed by DEF and Vero cells (Table 1). Although there were significant differences among strains (F = 6.73, df = 5, 36, p < 0.001), only the IMP181 strain grew to significantly lower titers (p < 0.05) as determined by the Newman-Keuls multiple-comparison (N-K) test (Hintze 2003) (Table 1). There were significant differences in maximum virus titers among cell types and virus strains as indicated by the significant cell type × viral strain interaction term (F = 5.63, df = 10, 36, p < 0.001) and depicted graphically in Figures 2 and 3.
FIG. 2.
Mean peak virus titers in log10 plaque forming units per mL for each WEEV strain for each of three cell types, C6/36 Aedes albopictus cells, duck embryo fibroblast (DEF), and Vero cells (means and multiple N-K test results shown in Table 1).
FIG. 3.
Mean virus titers in log10 plaque forming units per mL for each WEEV strain and cell type plotted as a function of time in hours after infection. (A) C6/36 mosquito cells (C6/36), (B) DEF, and (C) Vero mammalian cell (Vero).
Because of the significant interactions among maximum titer and time, and cell type and viral strains, we examined the time series response of each strain over time within cell types (Fig. 3). When blocked by time, there were significant differences in titer among strains on C6/36 cells (F = 3.89, df = 5, 71, p = 0.004), with overall titers of COAV592 and IMP181 greater than KERN5547 and BFS4740 by posteriori N-K test comparison (p < 0.05); these trends remained consistent over time because the strain × time interaction term was not significant (p > 0.05, Fig. 3A). Similarly, there were significant differences among strains grown on DEF cells (F = 48.78, df = 5, 71, p < 0.001), but here overall mean titers of BFS4740 and BFS1703 were significantly greater than IMP181 and COAV592 (p < 0.05). For DEF cells, however, temporal patterns varied significantly among strains as shown by the significant strain × time interaction term (F = 6.43, df = 25, 72, p < 0.001, Fig. 3B). Similar to DEF cells, there were significant differences among WEEV strains grown on Vero cells (F = 13.21, df = 5, 72, p < 0.001), with overall titers for KERN5547 and COA592 greater than IMP181 and BFS1703, and with significant strain × time interaction (F = 2.87, df = 25, 72, p < 0.001, Fig. 3C).
Cell death measured by impedance
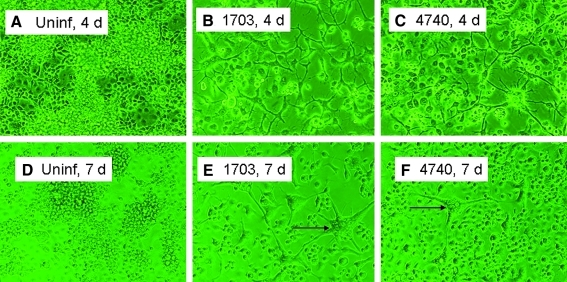
Marked differences in the Cell Index were observed among uninfected cell cultures (Fig. 4). C6/36 cells at 27°C produced a pattern of slow growth, but variable responses to WEEV infection among strains (Fig. 4A). Impedance declined for all strains at ∼45 h and was lowest at ∼72 h, as shown by decreases in the Cell Index. Interestingly, impedance then recovered in all strains and increased again starting at ∼105 h. The timing of the lowest Cell Index was approximately concurrent with the maximum viral titer in C6/36 cell culture measured by plague assay (Fig. 3A), perhaps indicating a loss of impedance due to changes in cell morphology. Subsequent rapid increases in impedance were attributed renewed cell growth, with the strength of the recovery greatest for BFS4740 and COAV592 and least for BFS1703 and IMP181. Changes in impedance agreed with visible changes in cell growth and morphology observed within six-well plate cultures. Figure 5A and D, for example, shows uninfected C6/36 cell growth at 96 and 168 h leading to the increasing Cell Index shown in controls (Fig. 4A), whereas Figure 5B, C and E, F show concurrent changes in morphology and greater spacing among cells infected with WEEV strains BFS1703 and BFS4740, respectively. Infected cells also showed syncitium formation (indicated by arrows in Fig. 5E, F). Interestingly, at 168 h cells infected with BFS4740 (Fig. 5F) seemed to recover and then grow rapidly compared to BFS1703 (Fig. 5E), and these changes were supported temporal patterns of the Cell Index seen in Figure 4A. Viral titers at this time decreased slightly, but then stabilized (Fig. 3A).
FIG. 4.
Cell growth (Cell Index) in control and WEEV-infected cultures plotted as a function of time (hours postinfection) for (A) C6/36 mosquito cells, (B) DEF, and (C) Vero mammalian cells. Cell Index measured by impedance and presented as computer output tracings from the real-time cell electronic sensing system. Scalar values presented for the x and y axes.
FIG. 5.
C6/36 cell growth in control and WEEV-inoculated six-well plates. (A) and (D) are uninfected control cultures at 4 and 7 days, respectively. Panels B, C, and E, F are BFS1703 and BFS4740 strain infected cells at 4 and 7 days postinfection, respectively. Arrows point to syncitium. All photos taken by substage IFA microscopy at 200 × magnification. Color images available online at www.liebertonline.com/vbz.
The rapid DEF cell growth and subsequent decrease in the Cell Index at ∼30 h (Fig. 4B) agreed well with the viral titers monitored in cultures by plaque assay (Fig. 3B). Different from C6/36 cell culture, there was no rebound in the Cell Index, indicating that WEEV killed most of the DEF cells concurrently. Similarly, the apparent timing of Vero cell death at 40–60 h PI (Fig. 4C) agreed relatively well with the timing of WEEV peak titers in flask cultures (Fig. 3C); however, there was considerable variability here among WEEV strains.
Discussion
Similar to our recent in vivo studies (Reisen et al. 2008b), there were no obvious temporal trends in virus titers among strains within cell types. There also did not seem to be spatial separation, because the IMP181 strain frequently was significantly different from the COAV592 strain, isolated from southeastern California during the 1990s, when few human infections were detected by concurrent serosurveys (Reisen et al. 1996, Reisen and Chiles 1997). Similar results were reported in mice using different WEEV isolates (Forrester et al. 2008). Previously, targeted sequencing of the E2 gene from 55 WEEV isolates from California made mostly from Cx. tarsalis mosquitoes from 1938 through 1997 produced four major clades: (A) Central Valley strains isolated since 1978, (B) southern California since 1978 and isolates from the Central Valley from 1978 to 1987, (C) northern California isolates from 1968 to 1971, and (D) early isolates from 1938 to 1961 (Kramer and Fallah 1999). Three of the strains we used were from three different clades and isolated at 20-year increments: BFS1703 from clade D, BFN3060 from clade C, and COAV592 from clade B. Despite their genetic differences and separation in time and space, there were minimal differences in in vitro growth patterns among these strains, including the COAV592 strain shown previously to have low mouse neurovirulence (Hardy et al. 1997). The only strain consistently different was the IMP181 strain, especially when grown on Vero cells, agreeing with previous studies using an in vivo mouse model and BHK hamster cells (Logue et al. 2009). The complete genome of the IMP181 WEEV strain previously was sequenced (Logue et al. 2009). Comparing the phylogenetic relationships of WEEV strains based on the E1-3TR region (Weaver et al. 1997) as well as complete genomes, IMP181 clearly is positioned within clade B of WEEV that is comprised of geographically diverse North American as well as Brazilian and Argentine isolates. Additional sequencing analyses and/or reverse genetic studies are warranted to assess the potential genetic basis for the retarded mammalian in vitro growth observed for the IMP181 strain in these studies. Also needed are genetic and infection studies of additional isolates of WEEV made during the 2000–2010 period to ensure that the IMP181 strain is representative of genomes circulating in California during that period.
The novel RT-CES system (Solly et al. 2004) was used to compare growth curves of different cell types following WEEV and sham inoculation. This system allowed for real-time quantitative monitoring of cell growth and death without the need for subsampling and subsequent plaque assay on Vero cell culture. Results using DEF cells were extremely uniform and agreed well with our plaque assay results. Vero cell culture RT-CES results were variable compared to cell culture and plaque assay, but the trends were generally similar between both systems. However, we currently have no explanation for the more rapid Vero cell growth in wells inoculated with WEEV, except perhaps for the addition of FBS with the virus diluent during infection. Patterns seen with mosquito C6/36 cells at 27°C in the RT-CES system were markedly different from Vero and DEF cells and did not reflect virus titer in plaque assays from flask cultures that peaked at 72 h and then remained relatively constant through 120 h. In contrast, the RT-CES Cell Index increased in parallel with uninfected cells until ∼45 h when all infected cultures showed a synchronous decline. The Cell Index remained near zero until ∼90 h when cultures seemed to recover and the Cell Index increased again. The rate and degree of this recovery differed markedly among WEEV strains, but was consistent among wells infected with the same strains. Cell cultures at this time frequently showed clumping of cells with some syncitium formation and differences in apparent cell growth and density among strains (Fig. 5).
In summary, our in vitro cell culture experiments generally agreed with our in vivo study using birds and mosquitoes (Reisen et al. 2008b) and a parallel study using a mouse infection model (Forrester et al. 2008), and indicated that there has been little temporal change in the virulence of WEEV over time, with the possible exception of decreased virulence of the IMP181 isolate made during 2005 in mice (Logue et al. 2009) and house sparrows (Reisen et al. 2008b). Another strain from southeastern California (COAV592) also previously showed low virulence in mice (Hardy et al. 1997), but in our experiment was not different from other WEEV strains in cell culture. Clearly, further investigation using additional recent isolates of WEEV are warranted to establish if the patterns seen for the IMP181 strain are representative of the WEEV strain circulating in California or North America. Collectively, these data do not seem sufficient to explain the declines in enzootic and epidemic transmission of WEEV seen in California or North America in the past 20–30 years (Reisen and Monath 1989). Historically, outbreaks of WEEV in California have been associated with cool wet spring weather typical of El Niño conditions, flooding on the west side of the Central Valley, and hot summers (Reeves and Hammon 1962). Recently, the amounts of snow and timing of snow melt in spring coupled with reduced overall rainfall (Knowles et al. 2006) may have led to reduced vector mosquito abundance in California during spring (Reisen et al. 2008a) and perhaps WEEV transmission during summer (Barker et al. 2009). In agreement, there has been no documented spillover of WEEV into the rabbit-Aedes cycle since 1983 (Reisen 1984, Reisen et al. 1990).
Acknowledgments
M. Zhang was supported at the Center for Vectorborne Diseases by a grant from the China Scholar Council. This research was supported, in part, by grant R01 AI055607-06A2 from National Institutes of Allergy and Infectious Diseases, NIH. Funding for W.K.R. was provided, in part, from the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, NIH.
Disclosure Statement
No competing financial interests exist.
References
- Barker CM. Reisen WK. Eldridge BF. Park B. Johnson WO. Culex tarsalis abundance as a predictor of western equine encephalomyelitis virus transmission. Proc Mosq Vector Control Assoc Calif. 2009;77:65–68. [Google Scholar]
- Forrester NL. Kenney JL. Deardorff E. Wang E. Weaver SC. Western Equine Encephalitis submergence: lack of evidence for a decline in virus virulence. Virology. 2008;380:170–172. doi: 10.1016/j.virol.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JL. Presser SB. Chiles RE. Reeves WC. Mouse and baby chicken virulence of enzootic strains of western equine encephalomyelitis virus from California. Am J Trop Med Hyg. 1997;57:240–244. doi: 10.4269/ajtmh.1997.57.240. [DOI] [PubMed] [Google Scholar]
- Hardy JL. Reeves WC. Experimental studies on infection in vertebrate hosts. In: Reeves WC, editor. Epidemiology and Control of Mosquito-Borne Arboviruses in California, 1943–1987. Sacramento, CA: California Mosquito Vector Control Association; 1990. pp. 66–127. [Google Scholar]
- Hintze J. NCSS Statistical Software. Kaysville, UT: 2003. [Google Scholar]
- Hui LT. Husted SR. Myers CM. Ascher MS, et al. Summary of St. Louis encephalitis and western equine encephalomyelitis virus activity in California, 1969–1997. Proc Mosq Vector Control Assoc Calif. 1999;67:61–72. [Google Scholar]
- Knowles N. Dettinger MD. Cayan DR. Trends in snowfall versus rainfall in the Western United States. J Climate. 2006;19:4545–4559. [Google Scholar]
- Kramer LD. Fallah HM. Genetic variation among isolates of western equine encephalomyelitis virus from California. Am J Trop Med Hyg. 1999;60:708–713. doi: 10.4269/ajtmh.1999.60.708. [DOI] [PubMed] [Google Scholar]
- Logue CH. Bosio CF. Welte T. Keene KM, et al. Virulence variation among isolates of western equine encephalitis virus in an outbred mouse model. J Gen Virol. 2009;90:1848–1858. doi: 10.1099/vir.0.008656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KF. Haring CM. Howitt B. The etiology of epizootic encephalomyelitis of horses in the San Joaquin Valley, 1930. Science. 1931;74:227–228. doi: 10.1126/science.74.1913.227. [DOI] [PubMed] [Google Scholar]
- Reeves WC. Hammon WM. Epidemiology of the arthropod-borne viral encephalitides in Kern County, California, 1943–1952. Publ Public Health Univ Calif. 1962;4:1–257. [PubMed] [Google Scholar]
- Reisen WK. Observations on arbovirus ecology in Kern County, California. Bull Soc Vector Ecol. 1984;9:6–16. [Google Scholar]
- Reisen WK. Cayan D. Tyree M. Barker CM, et al. Impact of climate variation on mosquito abundance in California. J Soc Vector Ecol. 2008a;33:89–98. doi: 10.3376/1081-1710(2008)33[89:iocvom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Chiles RE. Prevalence of antibodies to western equine encephalomyelitis and St. Louis encephalitis viruses in residents of California exposed to sporadic and consistent enzootic transmission. Am J Trop Med Hyg. 1997;57:526–529. doi: 10.4269/ajtmh.1997.57.526. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Chiles RE. Lothrop HD. Presser SB. Hardy JL. Prevalence of antibodies to mosquito-borne encephalitis viruses in residents of the Coachella Valley. Am J Trop Med Hyg. 1996;55:667–671. doi: 10.4269/ajtmh.1996.55.667. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Fang Y. Brault AC. Limited interdecadal variation in mosquito (Diptera: Culicidae) and avian host competence for Western equine encephalomyelitis virus (Togaviridae: Alphavirus) Am J Trop Med Hyg. 2008b;78:681–686. [PubMed] [Google Scholar]
- Reisen WK. Hardy JL. Reeves WC. Presser SB, et al. Persistence of mosquito-borne viruses in Kern County, California, 1983–1988. Am J Trop Med Hyg. 1990;43:419–437. doi: 10.4269/ajtmh.1990.43.419. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Monath TP. Western equine encephalomyelitis. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: CRC Press; 1989. pp. 89–138. [Google Scholar]
- Solly K. Wang X. Xu X. Strulovici B. Zheng W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;2:363–372. doi: 10.1089/adt.2004.2.363. [DOI] [PubMed] [Google Scholar]
- Steinlein DB. Husted S. Reisen WK. Kramer VL, et al. Summary of mosquito-borne encephalitis virus surveillance in California: 1998–2002. Proc Mosq Vector Control Assoc Calif. 2003;71:17–27. [Google Scholar]
- Weaver SC. Kang W. Shirako Y. Rumanapf T, et al. Recombinational history and molecular evolution of western equine encephalomyelitis complex alphaviruses. J Virol. 1997;71:613–623. doi: 10.1128/jvi.71.1.613-623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing JZ. Zhu L. Jackson JA. Gabos S, et al. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem Res Toxicol. 2005;18:154–161. doi: 10.1021/tx049721s. [DOI] [PubMed] [Google Scholar]