Abstract
The liver carries out a variety of essential functions regulated in part by autocrine signaling, including hepatocyte-produced growth factors and extracellular matrix (ECM). The local concentrations of autocrine factors are governed by a balance between receptor-mediated binding at the cell surface and diffusion into the local matrix and are thus expected to be influenced by the dimensionality of the cell culture environment. To investigate the role of growth factor and ECM-modulated autocrine signaling in maintaining appropriate primary hepatocyte survival, metabolic functions, and polarity, we created three-dimensional cultures of defined geometry using micropatterned semisynthetic polyethylene glycol–fibrinogen hydrogels to provide a mechanically compliant, nonadhesive material platform that could be modified by cell-secreted factors. We found that in the absence of exogenous peptide growth factors or ECM, hepatocytes retain the epidermal growth factor (EGF) receptor ligands (EGF and transforming growth factor-α) and the proto-oncogenic mesenchymal epithelial transition factor (c-MET) ligand hepatocyte growth factor (HGF), along with fibronectin. Further, hepatocytes cultured in this three-dimensional microenvironment maintained high levels of liver-specific functions over the 10-day culture period. Function-blocking inhibitors of α5β1 or EGF receptor dramatically reduced cell viability and function, suggesting that signaling by both these receptors is needed for in vitro survival and function of hepatocytes in the absence of other exogenous signals.
Introduction
Autocrine growth factor signaling by hepatocytes in the liver is a central mechanism for both physiological homeostasis and pathophysiological response of hepatocytes to stresses including inflammation and surgical resection. Epidermal growth factor receptor (EGFR) ligands, including transforming growth factor-α (TGF-α) and EGF, are produced by hepatocytes as well as nonparenchymal cells, and concentration gradients of these molecules influence a variety of cell behaviors in the intact liver.1 Extracellular matrix (ECM) molecules can also serve autocrine functions; for example, hepatocytes produce plasma fibronectin (FN) while also expressing its receptor α5β1 integrin and the coreceptor syndecan-4.2 FN fills the Space of Disse within the liver sinusoid, offering an adhesive matrix while allowing hepatocytes access to the circulating blood.3 Thus, in vivo, hepatocytes manipulate their microenvironment via production of autocrine factors essential to their survival and function, in dynamic response to external cues.
Exogenous EGF or TGF-α is typically added to serum-free culture media to enhance hepatocyte survival and function, especially in 2D culture formats,4–9 and EGF enhances spontaneous formation of three-dimensional (3D) spheroidal aggregates of adult hepatocytes cultured on minimally adhesive 2D substrates.10–13 Hepatocytes in 3D spheroidal floating aggregates secrete ECM and exhibit long-term maintenance of liver functions11,12,14–19 compared with cells cultured in 2D formats, wherein addition of DMSO or other cell types is required for functional maintenance.20
Unlike insulin and other hormones added to serum-free cell culture media, hepatocytes produce autocrine EGFR ligands in vitro.6,21 The need for additional exogenous EGFR ligands in 2D culture, or in the formation of spheroids from cells seeded on 2D culture substrates, may arise from a failure of autocrine ligands to be retained locally; that is, ligands produced by cells in 2D can readily diffuse into the medium before recapture by cell surface receptors.22
The contribution of autocrine ligands to enhanced maintenance of liver-specific functions in 3D spheroidal cultures of hepatocytes is unclear. Spheroidal cultures are often characterized by a layer of cells with flattened morphology at the outer rim,23,24 a phenomenon that may arise from gradients in diffusible autocrine growth factors (which may accumulate in the interior portion of the spheroid but diffuse freely from the surface into culture medium); from mechanical stresses associated with contraction and compaction of cells against a free surface; or from loss of appropriate ECM, such as FN, at the outer boundaries.
Here, we investigate the roles of autocrine growth factors and ECM in fostering viability, function, and polarization in adult rat hepatocytes using an experimental micromolded gel system that controls the geometry of 3D cell aggregation, presents a diffusion barrier to loss of autocrine factors, provides an initially inert (to cell adhesion) template that can be altered by ECM deposition to engage cells mechanically with the substrate, and provides a mechanically compliant environment that approximates the compliance of liver. Although collagen gels are serviceable for hepatocyte culture20,25 and have been widely used to create micropatterned environments for other cell types,26–28 hepatocyte adhesion to collagen via collagen-binding integrins induces cell signaling and phenotypes that might mask the effects of autocrine factors or drive cells to undesirable phenotypes.25,29,30 To foster engagement of cell adhesion by cell-secreted FN, while dampening adhesive interactions during initial stages following cell seeding, we therefore focused on a semisynthetic gel comprising polyethylene glycol (PEG) linked to denatured fibrinogen (PEG–fibrinogen). Unlike endothelial cells and other αvβ3 integrin-expressing cells, hepatocytes do not bind directly to fibrinogen but may interact with fibrinogen indirectly through FN, which both binds to fibrin and is also recognized by α5β1 receptors on hepatocytes.17,31 As fibrinogen is denatured during the process to form PEG–fibrinogen gels, the tertiary structure, which typically prevents fibrinogen–FN interaction, is lost and the secondary structure of fibrinogen peptides characteristic of the native protein is evident in the PEG-modified state,32 and hence, presumably, it can bind FN. PEG–fibrinogen gels are relatively easy to micromold via UV-crosslinking processes and can be fabricated with mechanical properties comparable to liver33–35; hence, these gels provide an attractive experimental system for controlling hepatocyte aggregation and local retention of ECM produced by hepatocytes. Their permeability and degradation properties can also be tailored over a wide range through inclusion of additional PEG diacrylate with the PEG–fibrinogen macromer in the polymerization process.36
Exploiting these advantages of micropatterned PEG–fibrinogen hydrogels, this study probes the nature and action of autocrine loops in regulating survival and function of primary hepatocytes. Using a combination of immunostaining and function-blocking inhibitors, we demonstrate that primary hepatocytes in micromolded PEG–fibrinogen gels retain autocrine growth factors (EGF, hepatocyte growth factor [HGF], TGF-α) and cell-derived matrix (FN). These factors are necessary and sufficient to maintain hepatocyte differentiation in culture, as determined by cell morphology and metabolic function. This culture system may prove useful for analysis of other aspects of hepatocellular biology or as a platform to study hepatocyte metabolism of pharmacological agents.
Materials and Methods
Culture of primary hepatocytes in micromolded gels
Primary cells were isolated from male Fisher rats weighing between 150 and 250 g as previously described.20 Following isolation, primary hepatocytes were resuspended in serum-free hepatocyte growth medium (HGM; low-glucose DMEM supplemented with 4 mg/L insulin, 100 nM dexamethasone, 0.03 g/L proline, 0.1 g/L l-ornithine, 0.305 g/L niacinamide, 2 g/L d-(+)-galactose, 2 g/L d-(+)-glucose, 1 mM l-glutamine, 50 mg/L gentamicin, 54.4 μg/L ZnCl2, 75 μg/L ZnSO4 · 7H2O, 20 μg/L CuSO4 · 5H2O, 25 μg/L MnSO4) without EGF.37 Cells were plated onto micromolded gels at a density of 100,000 cells/cm2 (720,000 cells/mL) in a 12-well tissue culture dish and spun at 100 g for 3 min; this process was repeated twice. After seeding, gels were transferred to new wells with fresh HGM to remove excess cells. HGM was replaced after 24 h and again after every fourth day of culture. Cells were incubated at 37°C under 5% CO2 and 95% humidity for the time periods indicated; EGF was included in cultures at 10 ng/mL as indicated. For experiments with cyclic arginine–glycine–aspartate (cRGD) peptide, 10 μM of the peptide cyclo(Arg-Gly-Asp-D-Phe-Val) c(RGDfV) was included at the time of plating and maintained during culture (Peptides International). For experiments with EGFR inhibitor, 10 μM of the monoclonal antibody mAb225 against EGFR was included at the time of plating and refreshed at each medium change (kind gift from H. Steve Wiley Lab, PNNL). Twenty-four-well plates adsorbed with collagen I (BioCoat; BD Biosciences) with EGF containing HGM were used as controls in some experiments.
Synthesis of PEG–fibrinogen
PEG was acrylated similar to previously published protocols.38,39 Briefly, acryloyl chloride (Alfa Aesar) was reacted with PEG-diol (6 or 10 kDa; Sigma) at 4× molar excess to available alcohol groups in benzene and under nitrogen pressure in the presence of triethylamine (Sigma) overnight at room temperature with protection from light. The resulting PEG-diacrylate (PEGDA) product was purified by multiple rounds of diethyl ether precipitation, vacuum dried, lyophilized, and stored under nitrogen gas and at −20°C. Acrylation efficiency was determined by H-NMR and ranged from 85% to 95%.
Fibrinogen was PEGylated by reacting PEGDA (10 kDa) with full-length fibrinogen in 4× molar excess to the number of cysteines on fibrinogen at room temperature in 8 M urea in phosphate-buffered saline (PBS), pH 7.4, for 3 h in the presence of tris(2-carboxyethyl)phosphine hydrochloride (Sigma).39,40 The PEGylated fibrinogen product was precipitated in acetone and dialyzed for 24 h over three changes of PBS at 4°C. The fibrinogen content of each batch of material was quantified with a BCA protein assay (Pierce Chemicals), and the PEG content was quantified by weighing the lyophilized product and subtracting out the contributions of PBS and fibrinogen.39 The fibrinogen content was 7.2 ± 0.2 mg/mL and the PEG content was 29 ± 3.3 mg/mL (both mean ± standard error).
Micromolding of PEG–fibrinogen gels
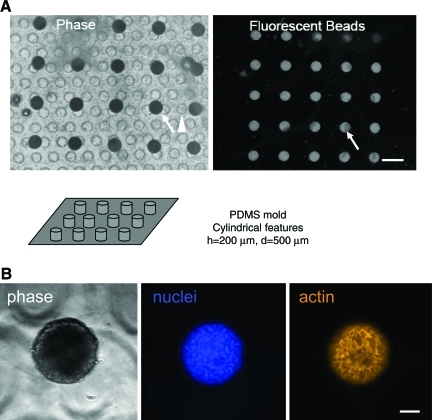
Microwell patterns, a 5 × 7 array of 500-μm-diameter circles, were designed using AutoCAD (Autodesk). A transparency mask was created from the CAD design and printed using a high-resolution printer (PageWorks). The transparency mask was used in photolithography of SU-8 photoresist to create 200 μm high patterns on the silicon wafer master. Micropatterned stamps were made by replica molding of polydimethylsiloxane (PDMS; Sylgard 184) and curing the degassed elastomer mix (10:1, base:curing agent) over the silicon master overnight in a 60°C oven. Polymerized PDMS micropatterned stamps were peeled off the silicon master and used for patterning the PEG–fibrinogen hydrogel; 100 μL of a premixed solution containing PEG–fibrinogen (3.6%, wt/vol), 5% PEG-DA (MW = 6 kDa) and 0.2% Irgacure 2959, was placed on the PDMS stamp, covered with a polycarbonate support scaffold,41 and cured under a handheld long-wave UV lamp, B-100YP, (UVP) for 4 min. Partially polymerized hydrogels were removed from PDMS stamps and cured with feature side up for one additional minute. PEG-DA (Mw = 6 kDa)–micropatterned gels were made in the same manner with 0.2% Irgacure 2959. The resulting polymerized PEG–fibrinogen or PEG-DA micropatterns were hydrated in PBS and UV sterilized before cell seeding. Measurements of PEG–fibrinogen feature sizes done after hydration in PBS indicate that microwells were 522 ± 27 μm in diameter and 213 ± 19 μm deep, measured by confocal imaging of microwells filled with 20-μm-diameter fluorescent beads (Fig. 1).
FIG. 1.
Micromolding of PEG–fibrinogen hydrogels. (A) PEG–fibrinogen gels were polymerized as described in the Materials and Methods section, on top of a polycarbonate support scaffold visible by phase microscopy. After polymerization, fluorescent microbeads were placed on the patterned gel and sonicated for 30 s to allow the beads to settle inside the negative features of the PEG–fibrinogen gel. The gel was subsequently washed with phosphate-buffered saline to remove floating beads and imaged by fluorescence microscopy. Microwells filled with beads are indicated by arrow; support scaffold channels are indicated by arrowhead. For reference, the microbeads have d = 20 μm and support scaffold channels have d = 340 μm. Scale bar = 1 mm. (B) Freshly isolated primary hepatocytes were plated onto molded gels, as described in Materials and Methods, at a density of 100,000 cells/cm2 (720,000 cells/mL) in hepatocyte growth medium lacking growth factors (except for insulin). Three days after plating, cultures were fixed and stained to label the nuclei (blue) and the actin cytoskeleton (orange). Single confocal cross-sections taken within the centermost 25% of the structure are shown. Scale bar = 100 μm. PEG, polyethylene glycol. Color images available online at www.liebertonline.com/tea.
Evaluation of mechanical properties of PEG–fibrinogen hydrogels
Polymer solutions that matched the composition of those used in cellular experiments, of approximately 250 μL volume, were polymerized between 18 mm coverslips. The bottom coverslip was functionalized with aminopropyltriethoxysilane to covalently link the hydrogel for ensuing testing. Several samples from four different PEG–fibrinogen stocks were measured using atomic force microscopy-enabled nanoindentation. An atomic force microscope (MFP-3D; Asylum Research) was used for all mechanical characterization experiments conducted on hydrogels in 1 × PBS at room temperature. Calibration of atomic force microscope cantilevers of spring constant k = 34.60 pN/nm and nominal probe radius R = 25 nm (MLCT; Veeco) was conducted as previously described.42,43
For each measurement of Young's elastic moduli, a grid of 16 × 16 (256 total) indentations to maximum depths of 25 nm were acquired over a 50 μm2 area on each hydrogel to account for material inhomogeniety. Acquired probe deflection-displacement responses were converted offline (Igor Pro; Wavemetrics), to force-depth responses. Young's elastic moduli E were calculated by applying a modified Hertzian model of spherical contact to the loading segment of the force-depth response, as detailed elsewhere,42,43 with the scientific computing software MATLAB (2007a, TheMathWorks). The computed elastic moduli E are reported as average ± standard error of measurement. The elastic modulus of the gels comprising 5% exogenous PEG diacrylate was 17.5 ± 0.3 kPa (mean ± standard error of the mean), which is intermediate to that of estimates for normal and diseased liver as discussed in the text.33–35
Immunofluorescence microscopy
Samples were fixed in 3.7% formaldehyde in PBS for 25 min at room temperature, permeabilized with 0.1% Triton-X for 10 min at 4°C, and washed twice with PBS for 15 min each. For accumulation of growth factors, samples were blocked for 1 h with 10% normal goat serum (Invitrogen) and 1% bovine serum albumin (Sigma). Samples were incubated with primary antibodies overnight at 4°C and then washed thrice with PBS for 20 min each. Samples were then incubated with secondary antibodies, AlexaFluor568 or AlexaFluor488 phalloidin, and 4′,6-diamidino-2-phenylindole (DAPI) or Hoechst nuclear stains for 1 h at room temperature with protection from light before being washed thrice with PBS for 20 min each. Samples were then stored in PBS at 4°C with protection from light. Visualization was done using a Zeiss Observer A1 microscope equipped with a Photometrics Quant EM S12SC camera and BD CARV II spinning disc confocal (Biovision Technologies). Images were acquired using MetaMorph software. Confocal images shown in the figures in this report are single confocal cross-sections taken within the centermost 25% of the tissue structure. Tissue structures were typically 180–200 μm deep in the absence of inhibitors (and comparable for presence or absence of soluble EGF), 120–150 μm deep (when intact) in the presence of cRGD integrin inhibitor, and 100–120 μm deep in the presence of mAb225 EGFR inhibitor. Zeiss SteREO Discovery V12 stereoscope equipped with AxioCam and fluorescent lamp was used for observing accumulation of TGF-α, EGF, and HGF in PEG–fibrinogen microwells. Consistent exposure times were maintained to assess relative fluorescent staining between conditions. For EGF, TGF-α, and HGF immunofluorescence detection, samples incubated with secondary antibody alone were imaged to ensure that the observed fluorescence was not due to an artifact, such as nonspecific secondary antibody binding or autofluorescence.
For visualization of functional bile canalicular networks, samples were washed thrice with Hanks' balanced salt solution and then incubated for 10 min at 37°C with 2 μM 5(and 6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDFDA; Invitrogen) and 16.2 μM Hoechst stain. After incubation, confocal images were taken at days 3 and 6 (representative image from day 6 is depicted in Fig. 5E).
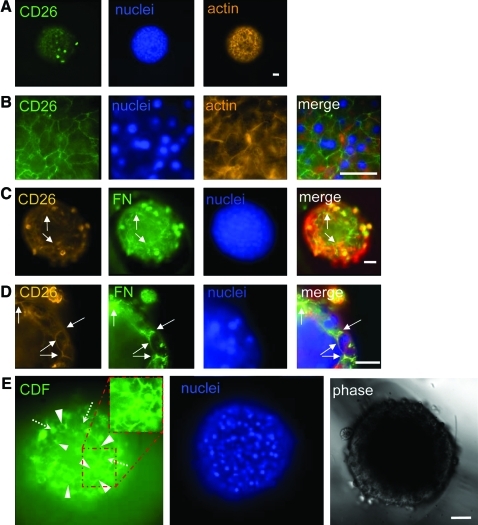
FIG. 5.
Micropatterned PEG–fibrinogen hydrogels support three-dimensional polarization of primary hepatocytes and the formation of a functional bile canalicular network. Cells were plated and cultured in hepatocyte growth medium without EGF as previously described and then fixed and stained for the indicated epitopes. Single confocal cross-sections taken within the centermost 25% of the structure are depicted. (A, B) Cells cultured for 3 days and stained for CD26 (green), nuclei (blue), and the actin cytoskeleton (orange); samples are imaged at 10 × (A) and 63 × (B). (C, D) Cells cultured for 7 days and stained for CD26 (orange), FN (green), and nuclei (blue); samples are imaged at 20 × (C) and 40 × (D). (E) Visualization of functional bile canaliculi by confocal microscopy on day 6 using 5(and 6)-carboxy-2′,7′-dichlorofluorescein (CDF) diacetate at 20 × (with digital zoom of red highlighted region in top right inset). For reference, support scaffold channels have d = 340 μm. Scale bar = 50 μm in each image. Note regions of plasma membrane showing CD26 and FN colocalization (arrows), and concentrated CDF staining in the three-dimensional tissue structure, which is similar to CD26 staining in A and B (arrowheads = bile canaliculi; dotted arrows = intracellular uptake of CDF). Color images available online at www.liebertonline.com/tea.
Primary antibodies include anti-rat FN at 1:100 (Millipore), anti-rat CD26 at 1:100 (BD Pharmingen), anti-rat TGF-α at 1:500 (Abcam), anti-rat HGF at 1:200 (Abcam), and anti-rat EGF at 1:200 (Abcam). Secondary antibodies at 1:200 and phalloidin dyes at 1:200 were all AlexaFluor conjugates (Invitrogen). Nuclear stains were used at 1 μg/mL for DAPI (Sigma) and 16.2 μM for Hoechst 33342 (Invitrogen).
Quantification of cell viability
Cell viability was determined using the Live/Dead Viability/Cytotoxicity Kit (Invitrogen). Cells were cultured as described above on PEG–fibrinogen gels for the indicated time periods, with or without 10 ng/mL EGF, or in the presence of 10 μM cRGD or 10 μM mAb225, or cultured on adsorbed collagen I in the presence of 10 ng/mL EGF. For PEG–fibrinogen gels, confocal Z-stack images of three to five randomly chosen microwells were taken from each of three to four gel samples per time point using a 10× objective to capture the entire well (10 μm step size in z-stacks). Total cell number was determined by DAPI staining of nuclei and the number of nonviable cells was determined by ethidium bromide nuclear staining. A total of 200–600 nuclei were counted in each microwell to obtain total cell number. For adsorbed collagen samples, in each well, four to six randomly chosen fields (one from each quadrant of the well) were observed using a 10× objective, and all nuclei were counted in each field (300–600 total nuclei per field). The experiments were performed at least three times (for both PEG–fibrinogen and adsorbed collagen samples) and data were statistically analyzed with analysis of variance followed with Tukey's test with alpha = 0.05.
Quantification of albumin and urea production
Culture medium was replaced at 48 h prior to collection on the indicated days. Albumin levels were determined using Rat Albumin ELISA Kit (Bethyl Labs). Urea levels were determined using the QuantiChrom Urea Assay Kit (BioAssay Systems). Samples, standards, and controls were tested in duplicates and experiments were repeated three to six times. Data were normalized to account for sample volumes for both assays. Results are reported as nanograms of product (urea or albumin) per milliliter per day (Fig. 7). The data were normalized to account for cell number at day of sample collection and are presented as micrograms of product (urea or albumin) per 106 cells per day (Supplementary Fig. S5). Cell number per day was calculated based on the total cell counts from the cell viability data for each specific time point. The data were statistically analyzed with analysis of variance followed with Tukey's test with alpha = 0.05.
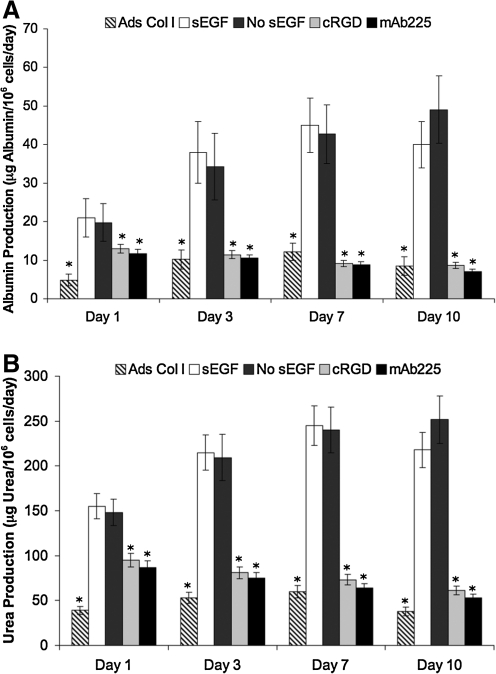
FIG. 7.
PEG–fibrinogen hydrogels promote maintenance of hepatocyte metabolic functions in vitro. Primary hepatocytes were cultured on micromolded PEG–fibrinogen gels or adsorbed collagen I as described in Materials and Methods. Conditioned medium was collected at the indicated time points and metabolites of interest were quantified as described in the Materials and Methods section. Samples, standards, and controls were tested in duplicate. (A) Albumin synthesis; (B) urea synthesis. *Statistically significant difference from sEGF-supplemented PEG–fibrinogen samples at a specific day; p < 0.05, n > 3. Data has been normalized to number of viable cells at each day. For absolute (nonnormalized) rates of secretion, see Supplementary Figure S6.
Results
Micromolding of PEG–fibrinogen gels to create a 3D niche for primary hepatocytes
A PEG–fibrinogen hydrogel formulation was chosen to create niches for primary hepatocytes based on several criteria: amenable to micromolding (to isolate small numbers of hepatocytes); intrinsically nonadhesive for hepatocytes, but possessing specific adhesive domains for cell-secreted matrix, to allow interpretation of effects from retention of cell-secreted matrix; physiologically relevant stiffness (similar to liver) should cellular ECM secretion result in adhesion of the cells with the microwell walls. The precise permeability properties of the hydrogel were a secondary consideration, as the cell aggregate itself provides a means of concentrating autocrine factors locally during initial stages of culture, compared with the case of 2D minimally adhesive surfaces. These criteria were not met by commonly used hydrogels such as collagen and agarose. PEG–fibrinogen hydrogels met these criteria while providing the additional advantage that secreted FN can interact with fibrinogen subunits within the hydrogel, improving sequestration of cell-derived matrix while only providing adhesion upon autocrine FN retention.
The micromolding scheme is presented in Figure 1, wherein arrays of microwells filled with 20 μm fluorescent beads illustrate the appearance of the wells following final processing steps. The dimensions of the cylindrical features (nominal height, h = 200 μm, and nominal diameter, d = 500 μm; actual h = 213 ± 19 μm, d = 522 ± 27 μm) were guided by previous studies on the length scales for self-assembly of isolated hepatocytes (confirmed in these studies; data not shown) and diffusion into 3D cultures.44 Bulk elastic moduli of these micromolded hydrogels were measured via atomic force microscopy-enabled indentation (E = 17.5 ± 0.3 kPa [mean ± standard error of the mean]), which agrees reasonably well with that reported for liver tissue.33–35 PEG–fibrinogen hydrogels can be proteolytically degraded, with degradation rates highly dependent on the content of additional PEG-DA added during polymerization.45 The formulation used here, containing 5% PEG-DA, is in a regime associated with relatively slow degradation, and we saw no evidence of bulk degradation during the course of the experiments.
Primary hepatocytes seeded into microwells in the absence of exogenous EGFR ligands or adhesive matrix molecules aggregated into tissue-like structures over the first 24 h, formed strong adhesions to the gel matrix, and persisted in tissue-like structures over 10 days of culture (Fig. 1B). A modest but noticeable compaction of the tissue structures occurred in the first few days of culture, as expected by increasing cell–cell contacts causing the structures to pull away from some regions of the walls of the microwells, while remaining firmly attached over about 50% of the contact area. Attempts to culture primary hepatocytes on 2D (nonmolded) PEG–fibrinogen gels were unsuccessful; cells did not attach to the gel, instead forming floating spheroids with little retention of FN, in keeping with previous attempts to culture hepatocytes on fibrinogen.17 Similar results were found with micromolded PEG-DA (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/tea) and agarose gels (data not shown), on which, despite intact features, cells did not attach or persist in microwells but rather formed viable floating spheroids.
Primary hepatocytes retain cell-derived FN and growth factors within micropatterned wells
Hepatocytes lack receptors for fibrinogen. Hence, the observation that primary hepatocytes formed adherent tissue-like structures in the wells of PEG–fibrinogen gels in the absence of serum or other sources of exogenous ECM or growth factors suggested that the microwells were capable of retaining autocrine factors, including ECM, necessary for cell survival. To determine if primary hepatocytes were able to retain FN matrix and growth factors produced by cells post-isolation, immunofluorescence microscopy was used to monitor the presence of several key autocrine factors produced by hepatocytes.
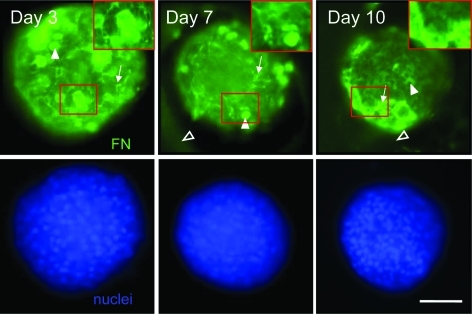
FN—ubiquitously present in liver ECM—is assembled from its soluble form into fibrils in an integrin-dependent manner (α5β1 in the case of hepatocytes).46 In culture, thin (5–25 nm) fibrils forming interconnected networks appear in the extracellular environment early in culture, form networks around cells, and mature into thicker fibril bundles at later stages of culture.46 Immunofluorescence staining, as illustrated by confocal imaging of a single slice in the central tissue region, shows the presence of FN in and around primary hepatocytes seeded and cultured in micromolded PEG–fibrinogen hydrogels, with a staining pattern that suggests formation of fibrils and their maturation over time into fibril bundles (Fig. 2). Hepatocytes were cultured, as described earlier, for 3, 7, and 10 days before being fixed, stained with antibodies against FN, and imaged by confocal microscopy to view the region of tissue approximately halfway between the top and bottom of the tissue structure (75–100 μm below the top of the gel). Culture medium was serum free and did not contain any exogenous matrix molecules or peptide growth factors, except for insulin; hence, observed FN was produced by hepatocytes. FN with a bright staining pattern characteristic of fibrils is present surrounding cells in the central region of the tissue structures by day 3 and persists at days 7 and 10 (Fig. 2, arrows), with a coarsening and thickening of the apparent network by day 10 (Fig. 2). In addition to fibrils, diffuse staining is observed within the gel adjacent to the microwell by day 7 (Fig. 2). The diffuse appearance of FN staining inside the gel indicates that soluble FN secreted by hepatocytes diffuses into the gel and remains associated with it, as anticipated by the known propensity of FN to associate with regions of fibrinogen. We postulate that interactions between FN and fibrinogen in the gel provide a bridge to link hepatocyte tissue structures firmly to the gel, even as the tissue structures appear to contract slightly and draw away from some regions of the microwell wall at later stages of culture (Fig. 2). The gap between the tissue structure and the gel following this slight contraction appears as a dark region between the brightly staining tissue and the diffuse-staining support gel. The retention of soluble FN within the microwell support structure and the assembly of FN fibrils within the tissue aggregate are unique to cultures within PEG–fibrinogen microwells, as cells cultured in PEGDA microwells show diminished fibril assembly within the tissue aggregate and no diffuse staining in the gel wall adjacent to the tissue structure (Supplementary Fig. S1).
FIG. 2.
Primary hepatocytes retain FN matrix within micropatterned wells. Primary hepatocytes were plated as previously described. At 3, 7, and 10 days, cells were fixed and stained for FN (green) and nuclei (blue). Confocal images were taken at 20 × and digital zoom of red highlighted regions is illustrated in top right insets. Single confocal cross-sections taken within the centermost 25% of the structure are depicted. Note intracellular FN (arrowheads), FN fibrils (arrows), and staining for secreted, soluble FN trapped in the walls of the microwell (open arrowheads). For phase images, see Figs. 1 and 6 (day 3), Supplementary Fig. S4 (day 7), and Supplementary Fig. S5 (day 10). Scale bar = 100 μm. FN, fibronectin. Color images available online at www.liebertonline.com/tea.
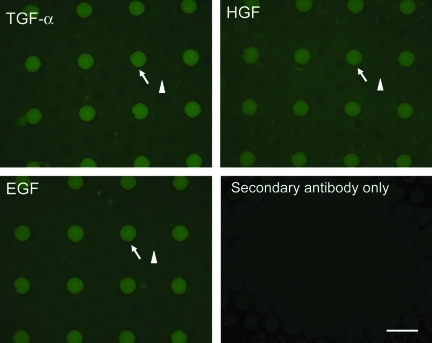
The requirement for growth factor signaling, including HGF and EGFR ligands (including EGF and TGF-α), to maintain primary hepatocyte cultures has been well documented.47–50 The ability of primary hepatocytes to form tissue-like structures on micromolded PEG–fibrinogen gels in the absence of any of these growth factors provided exogenously implicates autocrine signaling. To examine the presence of autocrine growth factors, primary hepatocytes were cultured in serum-free medium devoid of exogenous growth factors and then fixed and stained with antibodies against EGF, HGF, and TGF-α at days 3, 7, and 10. This protocol detects membrane-bound proforms of the growth factors as well as shed factors that become cross-linked to ECM or cell surfaces during fixation. Tissue structures were imaged with low magnification to assess uniformity of stains across multiple wells. All three growth factors are present in the day 3 tissue structures formed by culture of primary adult rat hepatocytes in the microwells (Fig. 3) and expression is sustained through days 7 and 10 (Supplementary Fig. S2). The apparent lack of staining in the gel adjacent to the tissue structure is not surprising, as growth factors are likely consumed locally in autocrine fashion such that only low (undetectable) concentrations escape into the gel.
FIG. 3.
Primary hepatocytes retain autocrine growth factors in micropatterned wells. Cells were cultured as previously described, in the absence of serum or exogenous growth factors (except for insulin). After 3 days, samples were fixed and stained with antibodies against EGF, HGF, and TGF-α. As a negative control, a sample was incubated with secondary antibody alone to confirm that signal was from primary antibody. Fluorescence indicates retention of TGF-α, EGF, and HGF. Microwells filled with cells are indicated by arrow; support scaffold channels are indicated by arrowhead. Support scaffold channels have d = 340 μm. Scale bar = 1 mm (magnification: 35 × ). For days 7 and 10, see Supplementary Figure S2. EGF, epidermal growth factor; HGF, hepatocyte growth factor; TGF, transforming growth factor. Color images available online at www.liebertonline.com/tea.
Autocrine matrix and growth factors are necessary and sufficient to maintain the viability of primary hepatocytes in culture
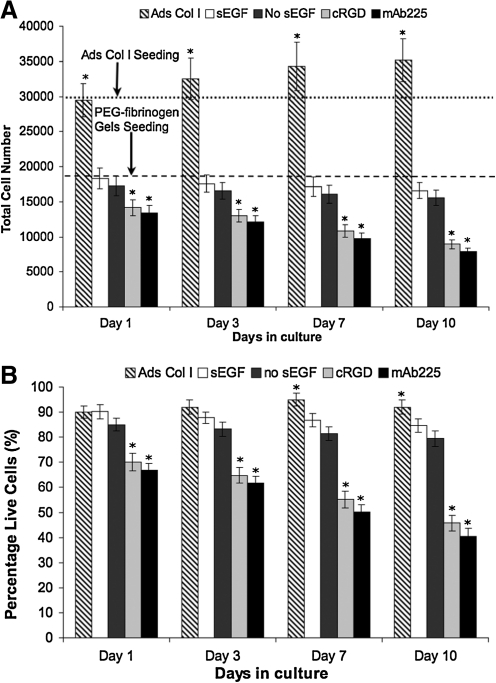
The formation and maintenance of tissue-like aggregates of primary hepatocytes within microwells of nonadhesive PEG–fibrinogen was associated with retention of autocrine factors including FN (Fig. 2), EGF, TGF-α, and HGF (Fig. 3). Previous studies in 2D culture have shown that survival of primary adult rat hepatocytes is diminished but not abolished in the absence of EGFR ligands.51–53 To determine if autocrine signaling from cell-secreted FN and EGFR ligands is sufficient for cell survival the number and viability of primary adult rat hepatocytes cultured on the PEG–fibrinogen gels in the absence of serum or exogenous growth factors was measured as a function of time in culture. Total cell numbers were enumerated by counting all nuclei in 3–5 microwells in 3–4 gel samples (at least 200–600 nuclei were counted in each microwell) and nonviable cells were labeled by uptake of ethidium bromide. In micromolded gel substrates, cell number showed a slight (∼7%) but statistically insignificant decline over the 10 days of culture, with no statistically significant difference between cultures with or without soluble EGF (Fig. 4A). On 2D collagen I-coated substrates in the presence of EGF, a modest (∼10%) but statistically significant increase in cell number was observed over 10 days in culture (Fig. 4A) consistent with other reports for these culture conditions.29 A similar trend was observed in cell viability during the culture period. Cell viability was assessed by the fraction of cells that excluded ethidium bromide versus total cells stained with DAPI at days 1, 3, 7, and 10. Cells plated on adsorbed collagen I supplemented with EGF exhibit a high level of viability (Fig. 4B). Viability of cells in PEG–fibrinogen cultures was 80%–90%, with no statistical difference between cultures supplemented with 10 ng/mL EGF and those without. The fate of dead cells was not assessed, and it is possible that cells appearing as nonviable at early time points persist in culture. These data indicate that the autocrine factors present within the microwells are sufficient to sustain hepatocyte viability in long-term culture.
FIG. 4.
Autocrine signaling is necessary and sufficient for the survival of primary hepatocytes in vitro. (A) Total cell numbers as a function of time in culture. (B) Quantification of cell viability expressed as percentage of viable cells. Freshly isolated primary hepatocytes were plated onto molded gels at a density of 100,000 cells/cm2 in hepatocyte growth medium with 10 ng/mL EGF (white, sEGF), no EGF (dark gray, no EGF), no EGF with 10 μM cRGD peptide (light gray, cRGD), or no EGF with 10 μM mAb225 (black, mAb225). Hepatocytes were cultured on adsorbed collagen I at a density of 100,000 cells/cm2 in hepatocyte growth medium supplemented with 10 ng/mL EGF (diagonal pattern, Ads Col I). At the indicated time points, viable cells were identified by their ability to exclude ethidium bromide and total cell numbers were determined with 4′,6-diamidino-2-phenylindole staining. The horizontal lines depict total number of cells seeded on PEG–fibrinogen gels and adsorbed collagen substrates. For PEG–fibrinogen gels, total number of cells effectively seeded into 35 microwells per culture is based on measurements of the number of nuclei in 20 microwells in four samples at 24 h after plating. *Statistical significance when compared with PEG gel-soluble EGF condition on that specific day; p < 0.05, n > 3. cRGD, cyclic arginine–glycine–aspartate.
To determine if signaling from either integrin α5β1 or EGFR is necessary for hepatocyte survival, primary hepatocytes were cultured as described above but in the presence of inhibitors. To block α5β1-mediated signaling, cells were cultured with a cRGD peptide to prevent FN and other ECM ligands from binding to this integrin. To block EGFR signaling, cells were cultured in the presence of a monoclonal antibody against EGFR (mAb225), which blocks ligand binding to EGFR, inhibiting autocrine signaling by EGF, TGF-α, and any additional autocrine EGFR ligands such as amphiregulin and HB-EGF that may be present. mAb225 binding induces slow internalization and trafficking of EGFR, but does not result in phosphorylation and activation of the EGFR or any known EGFR-mediated signaling events.54 mAb225 was replenished at each medium change to mitigate effects of receptor-mediated downregulation. The presence of either inhibitor resulted in comparable declines in cell number (Fig. 4A) and cell viability (Fig. 4B) compared with control cultures. Total cell numbers in the presence of inhibitors on day 1 were ∼85% of control values and declined further to ∼50% of control values by day 10 (Fig. 4A), a trend mirrored by cell viability (Fig. 4B). Both inhibitors (i.e., cRGD or EGFR function-blocking antibody) caused a marked decrease in the levels of cell-associated EGF, TGF-α, and HGF as observed by immunofluorescent staining of microwell structures (Supplementary Fig. S3). Compared with control cultures, a faint background staining was observed uniformly on the gels for the EGFR-inhibited case, suggesting that cells are still shedding these factors, and in the absence of EGFR uptake, they are adsorbing to the gel-associated ECM. The apparent loss of tissue-associated staining for growth factors in the inhibited cases may be attributed to the decline in cell number due to decreased viability or due to cross-talk between integrins and growth factors controlling a positive feedback loop of autocrine production, an area for further study.
Micropatterned PEG–fibrinogen hydrogels support formation of functional bile canalicular networks
In vivo, hepatocytes adopt an atypical cell polarity in which the apical cell surface composes the bile canalicular network into which bile is secreted and funneled to the bile duct. To determine the presence of bile canaliculi, cells cultured for 3 days were stained with antibodies to CD26, a marker of the apical surface, and imaged by confocal microscopy. Figure 5A and B show a cross-sectional image of a representative tissue-like structure with apical staining at the cell–cell junctions where canaliculi form. Another aspect of this polarity is the presence of FN at the apical surface: unlike other epithelia, hepatocytes lack a canonical basement membrane; instead, FN is found at apical, basal, and lateral surfaces.55 To determine whether FN was localizing to the apical surface in addition to the general staining seen in Figure 2, cultures were costained with antibodies against FN and CD26. Figure 5C and D show that FN does colocalize with CD26 at discrete regions of the apical domain of the hepatocytes. The functionality of the canalicular network was examined using CDFDA. CDFDA is actively taken up by hepatocytes, deesterified intracellularly to fluorescent CDF, and secreted into the canaliculi, allowing visualization of canalicular domains with intact tight junctions during a 10–15 min period before contraction of canaliculi releases their contents into the medium. Results of this assay are shown in Figure 5E, wherein the CDF is visible in a pattern similar to the CD26 staining. Thus, primary hepatocytes are capable of recapitulating aspects of their complex in vivo morphology in vitro with only autocrine signals to direct them.
Autocrine FN is necessary for formation and maintenance of tissue-like structures
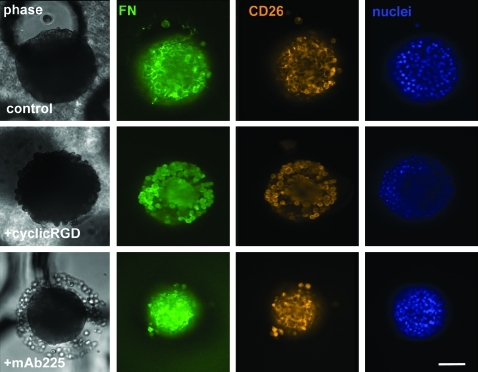
FN is abundant in the liver sinusoid where this ECM protein is in direct contact with the hepatocytes that are responsible for production of plasma FN. Similarly, in micromolded PEG–fibrinogen gels, primary hepatocytes retain FN and create fibrillar networks in the extracellular environment. To determine whether this autocrine ECM is crucial for the survival of primary hepatocytes and the formation or maintenance of tissue-like structures, hepatocytes were cultured in the presence of a cRGD peptide that blocks cell-surface α5β1 integrins from binding FN. Cells were cultured as described above but 10 μM of the cRGD peptide inhibitor was included at the time of plating and subsequent media changes. Cultures were fixed at 3, 7, and 10 days and stained with anti-FN and anti-CD26 antibodies.
Blocking α5β1 integrin prevents the formation of viable tissue-like aggregates such as those seen in control cultures on day 3 and, instead, results in dissociated, rounded cells (Fig. 6) that gradually become more diffuse by days 7 and 10 (Supplementary Figs. S4 and S5), concomitant with significantly reduced viability compared with controls (Fig. 4). In control cultures, abundant fibrillar FN networks are observed in the microwells by day 3 (Figs. 5 and 6, top), whereas cultures with the cRGD inhibitor lack detectable extracellular FN, even though intracellular FN is present. Further, cells cultured in the presence of cRGD fail to organize CD26 into canalicular like-structures at early (Fig. 6) or late (Supplementary Figs. S4 and S5) stages of culture, instead showing diffuse staining throughout the cell body. The rounded, individual cell morphologies revealed by both FN and CD26 staining show that the cells treated with cRGD have little functional cell–cell contact compared with control cultures. This combined failure to localize CD26 and the absence of extracellular FN fibrils is in stark contrast to images of control tissue-like aggregates, which show a consistent colocalization of FN fibrils with bile canalicular structures (Fig. 5C, D).
FIG. 6.
Blocking α5β1 but not EGFR disrupts tissue-like structures. Cells were plated as described above and cultured for 3 days in the absence or presence of 10 μM cRGD peptide or mAb225. After 3 days, cultures were fixed and stained for FN (green), CD26 (orange), and nuclei (blue). Note the absence of FN fibrils or discrete CD26 staining and the loose, dissociated cells in the microwells of cultures with cRGD. Also note the smooth edges of the aggregates, loose cells in the bottom of the well, and staining for FN and CD26 similar to control culture in the microwells of cultures with mAb225. For reference, support scaffold channels have d = 340 μm. Scale bar = 100 μm. For days 7 and 10, see Supplementary Figures S4 and S5. EGFR, epidermal growth factor receptor. Color images available online at www.liebertonline.com/tea.
To determine if EGFR signaling was also crucial to the formation or maintenance of the tissue-like structures, hepatocytes were cultured in the presence of the function-blocking EGFR antibody mAb225. The antibody was added to cultures at the time of plating and subsequent media changes, and then cells were fixed and stained for FN and CD26 at 3, 7, and 10 days. The tissue structures formed under inhibition of EGFR had a much more compact morphology than controls at day 3, with many dead, dissociated cells in the bottom of the microwell (Fig. 6, compare phase and DAPI stains). This highly compact morphology compared with controls (Fig. 6) arose from both reduction in cell number by about 40% compared with controls (Fig. 4) together with a closer spacing of cells than in the RGD-inhibited case (where cells were partially or completely dissociated), as evidenced by the close location of nuclei in EGFR-inhibited cultures; these patterns were accentuated by day 7 (Supplementary Fig. S4) and again by day 10 (Supplementary Fig. S5). Interestingly, the total cell numbers and viabilities as a function of time were comparable for both the integrin-inhibited and EGFR-inhibited cultures (Fig. 4), yet these cultures had strongly divergent morphologies, with RGD-containing cultures lacking a tissue-like structure (Fig. 6 and Supplementary Figs. S4 and S5). The pattern of FN and CD26 staining in EGFR-inhibited cultures appears to be predominantly diffuse in intracellular (FN) and membrane (CD26) staining (Fig. 6 and Supplementary Figs. S4 and S5), without the reticular structures seen in controls; bright, condensed regions of staining appear related to the compactness of the culture rather than functional cell–cell contacts, although occasional CD26 reticular structures are seen in the EGFR-inhibited case.
PEG–fibrinogen hydrogels promote maintenance of hepatocyte metabolic functions in vitro
Primary hepatocytes rapidly dedifferentiate and lose metabolic functions when grown by standard cell culture techniques, as determined by measuring certain standard metabolic functions of hepatocytes: conversion of ammonia to urea and the production of albumin. As the PEG–fibrinogen gels are capable of recapitulating other aspects of hepatocyte function, such as formation of bile canaliculi and production of FN, their metabolic function was monitored and compared with hepatocytes cultured on tissue culture plastic with adsorbed collagen I. Cells were cultured as described above for 10 days and the production of albumin and urea in the conditioned media was assessed. A comparison of the total daily amount of albumin secreted by cells maintained under various culture conditions reveals that at all time points measured, hepatocytes cultured in the micromolded PEG–fibrinogen gel format produce statistically greater amounts of albumin compared with cells on adsorbed collagen I (Fig. 7A). Interestingly, minimal to no difference is observed in cultures maintained with or without EGF. The presence of inhibitors of integrin α5β1 or EGFR function suppress albumin secretion dramatically to levels <20% of control levels. These trends are further accentuated when albumin production rates (shown in Supplementary Fig. S6A) are normalized to viable cell number (Fig. 7A). Production of urea follows similar trends across treatment conditions (Fig. 7B and Supplementary Fig. S6B). These results are in keeping with results presented elsewhere in this report that the retention of autocrine FN and growth factors allow hepatocytes in vitro to maintain aspects of their in vivo morphology and function.
Discussion
The data presented here implicate autocrine ligands of α5β1 and EGFR as effectors of hepatocyte function in long-term culture by combining immunostaining, illustrating the presence of a subset of known ligands for each receptor type (FN for integrin α5β1 and TGF-α and EGF for EGFR) with function-blocking inhibitors. In the absence of exogenous adhesion ligands and growth factors, primary hepatocytes cultured in micromolded PEG–fibrinogen gels are capable of modifying their microenvironment to maintain their differentiation and metabolic function. The PEG–fibrinogen hydrogel system offered a practical advantage for microwell culture of hepatocytes, in that it is not intrinsically adhesive to hepatocytes, which are not known to express receptors for fibrinogen, but can become adhesive in the presence of cell-secreted ECM.
In comparing behavior of hepatocytes seeded onto 2D PEG–fibrinogen substrates formed by the same gelation process to behavior of cells in microwells, we found that hepatocytes adhered to the gels only when cultured in microwell format. Although hepatocytes did form spheroidal aggregates on the 2D substrates, as they have been observed to do on many minimally adhesive 2D substrates,12,45 these aggregates failed to adhere to the 2D PEG–fibrinogen gel substrate. In the microwell format, the local cell environment fosters accumulation of cell-secreted factors simply because of very high local cell density22 compared with 2D. This phenomenon may be further accentuated if the gel also serves as a diffusion barrier or if it binds factors to provide a local depot for sequestration. The precise permeability properties of these gels were not measured; however, they are expected to be less permeable than PEG–fibrinogen formulations commonly used for cell encapsulation purposes36,39,40 as they were formed with a relatively high ratio of 6 kDa PEG-DA (5%) to PEG–fibrinogen (3.6%). Pure PEG-DA gels formed with 10–20 kDa PEG with 10% polymer content present significant diffusion hindrance to proteins above 20 kDa, including HGF, which has a molecular weight ∼60 kDa56,57; small proteins such as EGF likely diffuse relatively unhindered in the as-polymerized gels.
Thus, the differences in formation of adherent cell aggregates during the first day of culture in the microwell format compared with 2D culture on the same substrate may arise from higher local concentration of small peptide factors such as EGF, because of locally high cell concentrations in microwells compared with 2D. These effects may be coupled with enhanced retention of larger autocrine factors such as HGF and enhanced local concentrations of FN due to reduced permeability of the gel to large proteins. Another factor that may be operative in early stages to facilitate interactions between ECM and the gel in microwell format compared with 2D is attainment of locally high concentrations of proteases. Extracellular proteases may degrade the gel to increase the surface area for gel–ECM interaction in the microwell compared with 2D format. Secreted proteases range in size from about 20 kDa to over 100 kDa; hence, a substantial fraction of proteases would likely exhibit hindered diffusion in the gel compared with culture medium. As reported previously,36 PEG–fibrinogen gels polymerized with high additional PEG-DA macromer content exhibit relatively slow enzymatic degradation compared with pure PEG–fibrinogen gels, but even a modest degree of local remodeling may enhance adhesion of ECM and cells. Although other types of cells encapsulated in or cultured on PEG–fibrinogen gels have been observed to migrate into gels, we did not observe cellular ingrowth into the gels or observe any signs of bulk gel degradation (e.g., swelling or fracturing of the gels) for cells cultured in the microwell format. Adult hepatocytes are not highly migratory13 and proteases tend to act in a highly local fashion; hence, we would not anticipate, nor did we observe, bulk gel degradation for a format where cells are so highly localized.
Previous work has found a role for FN in a variety of developmental, regenerative, and disease processes in the liver. For example, during regeneration after partial hepatectomy, there are elevated levels of FN and α5 and β1 integrin subunits and decreased levels of gap junctions in the plasma membrane of hepatocytes prior to and during regeneration.58 Our work here supports that role, as a blockade of α5β1 hinders viability of hepatocytes. It is also interesting to speculate if this increase in cell–matrix adhesions coincidental to a decrease in cell–cell adhesions during regeneration explains the ability of the cRGD peptide to inhibit formation of tissue-like aggregates as we find that bile canaliculi fail to form in the absence of FN fibrils and α5β1 signaling (Fig. 6 and Supplementary Figs. S4 and S5). In vivo, FN is observed at all hepatocyte plasma membrane domains: sinusoidal, lateral, and apical/canalicular.55,58,59 However, during development and oncogenesis, the distribution of the nonintegrin FN receptor AGp110 correlates with the differentiation state of hepatocytes; AGp110 localization at the apical (canalicular) membrane indicates a differentiated state.60–62 Hence, the loss of metabolic function in the absence of FN fibril formation and α5β1 signaling may be due to dedifferentiation resulting from the lack of cell polarity. It is also possible that more generalized signals from FN are crucial to the survival and differentiation of hepatocytes, without which the cells dedifferentiate and are rendered incapable of maintaining cell–cell contacts and other functions.
Growth factors also play a crucial role in liver development and regeneration, notably, HGF (ligand for proto-oncogenic mesenchymal epithelial transition factor [c-MET]), and EGFR ligands, including TGF-α and EGF. The relationship between these growth factors is complex, with a high level of redundancy amongst EGFR ligands as well as synergy with other growth factor receptors.63,64 For instance, mouse knockouts of TGF-α or EGF show no impairment of liver development and loss of TGF-α does not impair liver regeneration. However, loss of EGFR—the signaling nexus for multiple extracellular ligands—causes impairment of liver regeneration in some models,65,66 though not others,67,68 wherein conflicting results may be attributed to differences in species, strain, and the specificity of silencing EGFR in hepatocytes. Although the effects of EGFR ablation on regeneration are equivocal, loss of c-MET, the receptor for HGF, dramatically impairs both embryonic development and liver regeneration.65,69 In cultures where EGFR autocrine stimulation was blocked by mAb225, we observed a very significant loss of viability compared with control cultures (Fig. 4A, B); thus, in vitro, it appears that c-MET stimulation by autocrine HGF (Fig. 4 and Supplementary Fig. S3) does not completely compensate for the loss of EGFR signaling.
Determining the role of individual autocrine growth factors is further complicated by the potential for synergy or cooperativity with integrins. In a variety of cell types, it has been shown that integrin ligand binding and clustering result in increased growth factor receptor phosphorylation and enhanced signaling in shared downstream pathways.70–73 In light of these facts, it is not surprising that hepatocytes in micromolded PEG–fibrinogen cultures require both integrin as well as growth factor signaling for proper survival and morphology as well as maintenance of differentiated metabolic functions. This system may be useful in further dissecting the intersecting and overlapping pathways between integrin and growth factor receptors that constitute the autocrine signaling network in the liver.
Conclusion
Autocrine matrix and growth factor regulation of primary rat hepatocyte survival and function in the absence of exogenous growth factors and adhesive ligands was studied using micropatterned PEG–fibrinogen hydrogels to provide an appropriate environment for 3D culture. Retention of autocrine-generated FN, TGF-α, EGF, and HGF by hepatocytes was observed in these 3D cultures, which assumed a tissue-like appearance and developed attachment to the walls of the microwells. Hepatocytes cultured in microwells adopted complex polarity including a functional bile canalicular network and maintained their viability and production of urea and albumin. Inhibition of α5β1 integrin binding to FN and inhibition of EGFR signaling in this culture format resulted in decreased hepatocyte survival and metabolic function and a decrease in soluble TGF-α, HGF, and EGF sequestration in the 3D tissue structure. Further, inhibition of α5β1 integrin showed a deficiency in fibrillar FN assembly, a disruption in the formation of tissue-like aggregates, and a failure of cells to polarize. Thus, this report indicates that autocrine matrix and growth factors are necessary and sufficient for maintenance of hepatocyte differentiation and survival in vitro.
Supplementary Material
Acknowledgments
The authors appreciate the careful reading of the manuscript by and thoughtful discussions with Prof. Roger D. Kamm (MIT). The authors thank Linda Stockdale (Griffith Lab) for synthesizing PEG-DA; Karel Domansky (Biotechnology Process Engineering Center, MIT) for provision of polycarbonate scaffolds; Rachel Pothier (Griffith Lab) for provision of primary hepatocytes; and Tracey Allen (UROP student, Griffith Lab) for help with albumin and urea assays. A. Zeiger acknowledges generous support by the U.S. National Science and Engineering Defense Graduate Fellowship. The authors are also grateful to Vernella Vickerman and Seok Chung (Kamm lab) for silicon master mold. The authors acknowledge funding from the National Institutes of Health (NIBIB R01EB003805 (to L.G.G.); the NIEHS, through the MIT Center for Environmental Health Sciences (P30ES002109 to L.G.G. and R01ES015241 to L.G.G.); an NRSA postdoctoral fellowship (to S.R.P.); and the National Science Foundation CAREER Award (to K.J.V.V.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Michalopoulos G.K. Liver regeneration. J Cell Physiol. 2007;213:286. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner O.H. Zoremba M. Gressner A.M. Gene expression of syndecans and betaglycan in isolated rat liver cells. Cell Tissue Res. 1996;285:11. doi: 10.1007/s004410050615. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984;51:57. [PubMed] [Google Scholar]
- 4.De Smet K. Beken S. Depreter M. Roels F. Vercruysse A. Rogiers V. Effect of epidermal growth factor in collagen gel cultures of rat hepatocytes. Toxicol In Vitro. 1999;13:579. doi: 10.1016/s0887-2333(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 5.Fremin C. Bessard A. Ezan F. Gailhouste L. Regeard M. Le Seyec J. Gilot D. Pages G. Pouyssegur J. Langouet S. Baffet G. Multiple division cycles and long-term survival of hepatocytes are distinctly regulated by extracellular signal-regulated kinases ERK1 and ERK2. Hepatology. 2009;49:930. doi: 10.1002/hep.22730. [DOI] [PubMed] [Google Scholar]
- 6.Iocca H.A. Isom H.C. Tumor necrosis factor-alpha acts as a complete mitogen for primary rat hepatocytes. Am J Pathol. 2003;163:465. doi: 10.1016/s0002-9440(10)63676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.H. Akaike T. Epidermal growth factor signaling for matrix-dependent cell proliferation and differentiation in primary cultured hepatocytes. Tissue Eng. 2007;13:601. doi: 10.1089/ten.2006.0104. [DOI] [PubMed] [Google Scholar]
- 8.McGowan J.A. Strain A.J. Bucher N.L. DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol. 1981;108:353. doi: 10.1002/jcp.1041080309. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto H. Kimura M. Watanabe N. Ogihara M. Tumor necrosis factor (TNF) receptor-2-mediated DNA synthesis and proliferation in primary cultures of adult rat hepatocytes: The involvement of endogenous transforming growth factor-alpha. Eur J Pharmacol. 2009;604:12. doi: 10.1016/j.ejphar.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Brophy C.M. Luebke-Wheeler J.L. Amiot B.P. Khan H. Remmel R.P. Rinaldo P. Nyberg S.L. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49:578. doi: 10.1002/hep.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawaguchi M. Koide N. Sakaguchi K. Shinji T. Tsuji T. Combination of epidermal growth factor and insulin is required for multicellular spheroid formation of rat hepatocytes in primary culture. Acta Med Okayama. 1992;46:195. doi: 10.18926/AMO/32674. [DOI] [PubMed] [Google Scholar]
- 12.Koide N. Sakaguchi K. Koide Y. Asano K. Kawaguchi M. Matsushima H. Takenami T. Shinji T. Mori M. Tsuji T. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp Cell Res. 1990;186:227. doi: 10.1016/0014-4827(90)90300-y. [DOI] [PubMed] [Google Scholar]
- 13.Powers M.J. Rodriguez R.E. Griffith L.G. Cell-substratum adhesion strength as a determinant of hepatocyte aggregate morphology. Biotechnol Bioeng. 1997;53:415. doi: 10.1002/(SICI)1097-0290(19970220)53:4<415::AID-BIT10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Koide N. Shinji T. Tanabe T. Asano K. Kawaguchi M. Sakaguchi K. Koide Y. Mori M. Tsuji T. Continued high albumin production by multicellular spheroids of adult rat hepatocytes formed in the presence of liver-derived proteoglycans. Biochem Biophys Res Commun. 1989;161:385. doi: 10.1016/0006-291x(89)91609-4. [DOI] [PubMed] [Google Scholar]
- 15.Riccalton-Banks L. Liew C. Bhandari R. Fry J. Shakesheff K. Long-term culture of functional liver tissue: three-dimensional coculture of primary hepatocytes and stellate cells. Tissue Eng. 2003;9:401. doi: 10.1089/107632703322066589. [DOI] [PubMed] [Google Scholar]
- 16.Shinji T. Koide N. Tsuji T. Glycosaminoglycans partially substitute for proteoglycans in spheroid formation of adult rat hepatocytes in primary culture. Cell Struct Funct. 1988;13:179. doi: 10.1247/csf.13.179. [DOI] [PubMed] [Google Scholar]
- 17.Stamatoglou S.C. Hughes R.C. Lindahl U. Rat hepatocytes in serum-free primary culture elaborate an extensive extracellular matrix containing fibrin and fibronectin. J Cell Biol. 1987;105:2417. doi: 10.1083/jcb.105.5.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong J.Z. De Lagausie P. Furlan V. Cresteil T. Bernard O. Alvarez F. Long-term culture of adult rat hepatocyte spheroids. Exp Cell Res. 1992;200:326. doi: 10.1016/0014-4827(92)90179-c. [DOI] [PubMed] [Google Scholar]
- 19.Tostoes R.M. Leite S.B. Miranda J.P. Sousa M. Carrondo M.J. Alves P.M. Perfusion of 3D encapsulated hepatocytes—a synergistic effect enhancing long term functionality in bioreactors. Biotechnol Bioeng. 2011;108:41. doi: 10.1002/bit.22920. [DOI] [PubMed] [Google Scholar]
- 20.Sivaraman A. Leach J.K. Townsend S. Iida T. Hogan B.J. Stolz D.B. Fry R. Samson L.D. Tannenbaum S.R. Griffith L.G. A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr Drug Metab. 2005;6:569. doi: 10.2174/138920005774832632. [DOI] [PubMed] [Google Scholar]
- 21.Cosgrove B.D. Cheng C. Pritchard J.R. Stolz D.B. Lauffenburger D.A. Griffith L.G. An inducible autocrine cascade regulates rat hepatocyte proliferation and apoptosis responses to tumor necrosis factor-alpha. Hepatology. 2008;48:276. doi: 10.1002/hep.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeWitt A. Iida T. Lam H.Y. Hill V. Wiley H.S. Lauffenburger D.A. Affinity regulates spatial range of EGF receptor autocrine ligand binding. Dev Biol. 2002;250:305. [PubMed] [Google Scholar]
- 23.Dvir-Ginzberg M. Elkayam T. Aflalo E.D. Agbaria R. Cohen S. Ultrastructural and functional investigations of adult hepatocyte spheroids during in vitro cultivation. Tissue Eng. 2004;10:1806. doi: 10.1089/ten.2004.10.1806. [DOI] [PubMed] [Google Scholar]
- 24.Peshwa M.V. Wu F.J. Sharp H.L. Cerra F.B. Hu W.S. Mechanistics of formation and ultrastructural evaluation of hepatocyte spheroids. In Vitro Cell Dev Biol Anim. 1996;32:197. doi: 10.1007/BF02722946. [DOI] [PubMed] [Google Scholar]
- 25.Fassett J. Tobolt D. Hansen L.K. Type I collagen structure regulates cell morphology and EGF signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Mol Biol Cell. 2006;17:345. doi: 10.1091/mbc.E05-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson C.M. Vanduijn M.M. Inman J.L. Fletcher D.A. Bissell M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okochi N. Okazaki T. Hattori H. Encouraging effect of cadherin-mediated cell-cell junctions on transfer printing of micropatterned vascular endothelial cells. Langmuir. 2009;25:6947. doi: 10.1021/la9006668. [DOI] [PubMed] [Google Scholar]
- 28.Raghavan S. Nelson C.M. Baranski J.D. Lim E. Chen C.S. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng Part A. 2010;16:2255. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta G. Williams C.M. Alvarez L. Lesniewski M. Kamm R.D. Griffith L.G. Synergistic effects of tethered growth factors and adhesion ligands on DNA synthesis and function of primary hepatocytes cultured on soft synthetic hydrogels. Biomaterials. 2010;31:4657. doi: 10.1016/j.biomaterials.2010.01.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheving L.A. Zhang L. Stevenson M.C. Kwak E.S. Russell W.E. The emergence of ErbB2 expression in cultured rat hepatocytes correlates with enhanced and diversified EGF-mediated signaling. Am J Physiol Gastrointest Liver Physiol. 2006;291:G16. doi: 10.1152/ajpgi.00328.2005. [DOI] [PubMed] [Google Scholar]
- 31.Makogonenko E. Tsurupa G. Ingham K. Medved L. Interaction of fibrin(ogen) with fibronectin: further characterization and localization of the fibronectin-binding site. Biochemistry. 2002;41:7907. doi: 10.1021/bi025770x. [DOI] [PubMed] [Google Scholar]
- 32.Frisman I. Orbach R. Seliktar D. Bianco-Peled H. Structural investigation of PEG-fibrinogen conjugates. J Mater Sci Mater Med. 2010;21:73. doi: 10.1007/s10856-009-3848-4. [DOI] [PubMed] [Google Scholar]
- 33.Arena U. Vizzutti F. Corti G. Ambu S. Stasi C. Bresci S. Moscarella S. Boddi V. Petrarca A. Laffi G. Marra F. Pinzani M. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380. doi: 10.1002/hep.22007. [DOI] [PubMed] [Google Scholar]
- 34.Corpechot C. El Naggar A. Poujol-Robert A. Ziol M. Wendum D. Chazouilleres O. de Ledinghen V. Dhumeaux D. Marcellin P. Beaugrand M. Poupon R. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 35.Ganne-Carrie N. Ziol M. de Ledinghen V. Douvin C. Marcellin P. Castera L. Dhumeaux D. Trinchet J.C. Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 36.Dikovsky D. Bianco-Peled H. Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27:1496. doi: 10.1016/j.biomaterials.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 37.Hwa A.J. Fry R.C. Sivaraman A. So P.T. Samson L.D. Stolz D.B. Griffith L.G. Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J. 2007;21:2564. doi: 10.1096/fj.06-7473com. [DOI] [PubMed] [Google Scholar]
- 38.Hern D.L. Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Peyton S.R. Kim P.D. Ghajar C.M. Seliktar D. Putnam A.J. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29:2597. doi: 10.1016/j.biomaterials.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almany L. Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 41.Domansky K. Inman W. Serdy J. Dash A. Lim M.H. Griffith L.G. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10:51. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maloney J.M. Walton E.B. Bruce C.M. Van Vliet K.J. Influence of finite thickness and stiffness on cellular adhesion-induced deformation of compliant substrata. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78:041923. doi: 10.1103/PhysRevE.78.041923. [DOI] [PubMed] [Google Scholar]
- 43.Thompson M.T. Berg M.C. Tobias I.S. Rubner M.F. Van Vliet K.J. Tuning compliance of polyelectrolyte multilayers to modulate cell adhesion. Biomaterials. 2005;26:6836. doi: 10.1016/j.biomaterials.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Powers M.J. Domansky K. Kaazempur-Mofrad M.R. Kalezi A. Capitano A. Upadhyaya A. Kurzawski P. Wack K.E. Stolz D.B. Kamm R. Griffith L.G. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng. 2002;78:257. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 45.Dikovsky D. Bianco-Peled H. Seliktar D. Defining the role of matrix compliance and proteolysis in three-dimensional cell spreading and remodeling. Biophys J. 2008;94:2914. doi: 10.1529/biophysj.107.105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh P. Carraher C. Schwarzbauer J.E. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Block G.D. Locker J. Bowen W.C. Petersen B.E. Katyal S. Strom S.C. Riley T. Howard T.A. Michalopoulos G.K. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalopoulos G.K. Bowen W. Nussler A.K. Becich M.J. Howard T.A. Comparative analysis of mitogenic and morphogenic effects of HGF and EGF on rat and human hepatocytes maintained in collagen gels. J Cell Physiol. 1993;156:443. doi: 10.1002/jcp.1041560303. [DOI] [PubMed] [Google Scholar]
- 49.Michalopoulos G.K. Bowen W.C. Mule K. Luo J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003;11:55. doi: 10.3727/000000003108748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomiya T. Omata M. Imamura H. Fujiwara K. Impaired liver regeneration in acute liver failure: the significance of cross-communication of growth associated factors in liver regeneration. Hepatol Res. 2008;38:S29. doi: 10.1111/j.1872-034X.2008.00423.x. [DOI] [PubMed] [Google Scholar]
- 51.De Smet K. Loyer P. Gilot D. Vercruysse A. Rogiers V. Guguen-Guillouzo C. Effects of epidermal growth factor on CYP inducibility by xenobiotics, DNA replication, and caspase activations in collagen I gel sandwich cultures of rat hepatocytes. Biochem Pharmacol. 2001;61:1293. doi: 10.1016/s0006-2952(01)00612-8. [DOI] [PubMed] [Google Scholar]
- 52.Jansing R. Samsonoff W.A. Effect of epidermal growth factor on cultured adult rat hepatocytes. Tissue Cell. 1984;16:157. doi: 10.1016/0040-8166(84)90040-5. [DOI] [PubMed] [Google Scholar]
- 53.Serra R. Isom H.C. Stimulation of DNA synthesis and protooncogene expression in primary rat hepatocytes in long-term DMSO culture. J Cell Physiol. 1993;154:543. doi: 10.1002/jcp.1041540313. [DOI] [PubMed] [Google Scholar]
- 54.Liao H.J. Carpenter G. Cetuximab/C225-induced intracellular trafficking of epidermal growth factor receptor. Cancer Res. 2009;69:6179. doi: 10.1158/0008-5472.CAN-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enrich C. Evans W.H. Gahmberg C.G. Fibronectin isoforms in plasma membrane domains of normal and regenerating rat liver. FEBS Lett. 1988;228:135. doi: 10.1016/0014-5793(88)80602-1. [DOI] [PubMed] [Google Scholar]
- 56.Cruise G.M. Scharp D.S. Hubbell J.A. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998;19:1287. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 57.Weber L.M. Lopez C.G. Anseth K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J Biomed Mater Res A. 2009;90:720. doi: 10.1002/jbm.a.32134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pujades C. Forsberg E. Enrich C. Johansson S. Changes in cell surface expression of fibronectin and fibronectin receptor during liver regeneration. J Cell Sci. 1992;102(Pt 4):815. doi: 10.1242/jcs.102.4.815. [DOI] [PubMed] [Google Scholar]
- 59.Hughes R.C. Stamatoglou S.C. Adhesive interactions and the metabolic activity of hepatocytes. J Cell Sci Suppl. 1987;8:273. doi: 10.1242/jcs.1987.supplement_8.15. [DOI] [PubMed] [Google Scholar]
- 60.Stamatoglou S.C. Enrich C. Manson M.M. Hughes R.C. Temporal changes in the expression and distribution of adhesion molecules during liver development and regeneration. J Cell Biol. 1992;116:1507. doi: 10.1083/jcb.116.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stamatoglou S.C. Manson M.M. Green J.A. Mayol X. Hughes R.C. Distribution of fibronectin and fibronectin-binding proteins, AGp110 and integrin alpha 5 beta 1, during chemically induced hepatocarcinogenesis in adult rats. J Cell Sci. 1991;100(Pt 3):599. doi: 10.1242/jcs.100.3.599. [DOI] [PubMed] [Google Scholar]
- 62.Torbenson M. Wang J. Choti M. Ashfaq R. Maitra A. Wilentz R.E. Boitnott J. Hepatocellular carcinomas show abnormal expression of fibronectin protein. Mod Pathol. 2002;15:826. doi: 10.1097/01.MP.0000024257.83046.7C. [DOI] [PubMed] [Google Scholar]
- 63.Scheving L.A. Stevenson M.C. Taylormoore J.M. Traxler P. Russell W.E. Integral role of the EGF receptor in HGF-mediated hepatocyte proliferation. Biochem Biophys Res Commun. 2002;290:197. doi: 10.1006/bbrc.2001.6157. [DOI] [PubMed] [Google Scholar]
- 64.Stolz D.B. Michalopoulos G.K. Comparative effects of hepatocyte growth factor and epidermal growth factor on motility, morphology, mitogenesis, and signal transduction of primary rat hepatocytes. J Cell Biochem. 1994;55:445. doi: 10.1002/jcb.240550405. [DOI] [PubMed] [Google Scholar]
- 65.Natarajan A. Wagner B. Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104:17081. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skarpen E. Oksvold M.P. Grosvik H. Widnes C. Huitfeldt H.S. Altered regulation of EGF receptor signaling following a partial hepatectomy. J Cell Physiol. 2005;202:707. doi: 10.1002/jcp.20171. [DOI] [PubMed] [Google Scholar]
- 67.Paranjpe S. Bowen W.C. Tseng G.C. Luo J.H. Orr A. Michalopoulos G.K. RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. Am J Pathol. 2010;176:2669. doi: 10.2353/ajpath.2010.090605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Buren G., 2nd Yang A.D. Dallas N.A. Gray M.J. Lim S.J. Xia L. Fan F. Somcio R. Wu Y. Hicklin D.J. Ellis L.M. Effect of molecular therapeutics on liver regeneration in a murine model. J Clin Oncol. 2008;26:1836. doi: 10.1200/JCO.2007.11.6566. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt C. Bladt F. Goedecke S. Brinkmann V. Zschiesche W. Sharpe M. Gherardi E. Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 70.Cabodi S. Moro L. Bergatto E. Boeri Erba E. Di Stefano P. Turco E. Tarone G. Defilippi P. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem Soc Trans. 2004;32:438. doi: 10.1042/BST0320438. [DOI] [PubMed] [Google Scholar]
- 71.Lee J.W. Juliano R.L. The alpha5beta1 integrin selectively enhances epidermal growth factor signaling to the phosphatidylinositol-3-kinase/Akt pathway in intestinal epithelial cells. Biochim Biophys Acta. 2002;1542:23. doi: 10.1016/s0167-4889(01)00161-6. [DOI] [PubMed] [Google Scholar]
- 72.Miyamoto S. Teramoto H. Gutkind J.S. Yamada K.M. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moro L. Venturino M. Bozzo C. Silengo L. Altruda F. Beguinot L. Tarone G. Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.