Abstract
Hydrogel-based scaffolds such as alginate have been extensively investigated for cartilage tissue engineering, largely due to their biocompatibility, ambient gelling conditions, and the ability to support chondrocyte phenotype. While it is well established that the viscoelastic response of articular cartilage is essential for articulation and load bearing, the time-dependent mechanical properties of hydrogel-based cartilage scaffolds have not been extensively studied. Therefore, the objective of this study was to characterize the intrinsic viscoelastic shear properties of chondrocyte-laden alginate scaffolds and determine the effects of seeding density and culturing time on these properties. Specifically, the viscoelastic properties (equilibrium and dynamic shear moduli and dynamic phase shift angle) of these engineered cartilage grafts were measured under torsional shear. In addition, the rapid ramp-step shear stress relaxation of the alginate-based cartilage scaffolds was modeled using the quasi-linear viscoelastic (QLV) theory. It was found that scaffold stiffness increased with both culturing time and cell density, whereas viscosity did not change significantly with cell density (30 vs. 60 million/mL). Similar to native cartilage, the energy dissipation of engineered scaffolds under pure shear is highly correlated to the glycosaminoglycan content. In contrast, collagen content was not strongly correlated to scaffold shear modulus, especially the instantaneous shear modulus predicted by the quasi-linear viscoelastic model. The findings of this study provide new insights into the structure–function relationship of engineered cartilage and design of functional grafts for cartilage repair.
Introduction
Articular cartilage, a dense connective tissue covering the articulating ends of bones, is subjected to significant compressive loads and internal shear stresses at the cartilage–subchondral bone interface, and during the rolling and/or sliding motions of diarthrodial joints.1,2 With cartilage trauma and progressive degenerative diseases such as osteoarthritis, the collagen matrix is severely damaged, and the labile proteoglycans are often lost from cartilage. This leads to less effective load support and the eventual destruction of the tissue and of the involved joint. Unfortunately, articular cartilage has a limited ability for self-repair.1–3 Some clinical successes associated with current treatments such as autologous chondrocytes implantation4,5 and osteochondral grafting with mosaicplasty6 have been reported. However, as biological grafts are limited in supply, associated with donor-site morbidity and structural constraints, tissue engineered cartilage grafts have emerged as a promising alternative.7,8
Given the complex loading environment of articular cartilage, current efforts in tissue engineering have focused on matching the mechanical properties of the engineered tissue with those of the native cartilage.9–11 The end-goal of this functional tissue engineering paradigm is to develop grafts capable of meeting the critical long-term mechanical demands in vivo. To this end, the successful engineering of cartilage grafts have to strategically capture, the viscoelastic properties of native cartilage. Articular cartilage can be modeled as a soft hydrated mixture consisting of solid and fluid phases,12 and it exhibits a time-dependent viscoelastic response to applied constant load.2 Consequently, chondrocyte response to physical signals such as stress, strain, fluid flow, electrical current, and voltage within the extracellular matrix (ECM) of native tissue and the tissue-engineered cartilage grafts is expected to be highly dependent on matrix viscoelastic properties.13
Alginate has been widely investigated as scaffold material for cartilage tissue engineering.14–19 Derived from algae, it is a polysaccharide composed of linear block copolymer of D-mannuronic acid and L-guluronic acid.20 Primary advantage of alginate include the well-documented maintenance of cell phenotype and morphology, uniform cell seeding, and matrix elaboration. In addition, it can be fabricated into various sizes and shapes to fit the defect site,21 injected with cells in a minimally invasive manner,22–24 and used for growth factor delivery.20 The mechanical properties of alginate hydrogel crosslinked with divalent cations have largely being investigated under compression.19,25 While valuable information on alginate properties has been obtained, these tests do not fully capture the viscoelastic behaviors of these hydrogels, as they cannot decouple solid matrix intrinsic viscoelastic effects from the flow-dependent viscoelastic effects in such hydrated multiphasic mixtures.12
For hydrogels such as agarose and alginate, which usually have a water content of more than 95%,26,27 the flow-independent and flow-dependent viscosities play equally important roles in the viscoelastic response under compression.28–30 In this context, the torsional shear test may be used to directly extract the intrinsic viscosity of the solid matrix of acellular or cellular hydrogels, and thus allow full characterization of the scaffold viscoelastic properties. Specifically, the equivoluminal torsional shear test can be used to extract the intrinsic viscoelastic properties of the solid matrix that includes polymeric chains and the ECM macromolecules produced by cells.31 Assuming that the material is homogeneous and the stress response to the deformation of embedded cells is negligible, the torsional test imposes, kinematically, a state of pure shear within the sample and with minimal interstitial fluid flow throughout the hydrogel.2,32 Thus, the energy dissipation during pure shear tests on cylindrical specimens comes solely from the internal friction (i.e., the viscosity of the solid matrix), and thus can be separated from the frictional drag caused by the relative motion between the interstitial fluid and the porous-permeable solid matrix.12
The overall objective of this study was to examine temporal changes in the flow-independent intrinsic viscoelastic properties of cell-seeded alginate hydrogels. To this end, we evaluated articular chondrocyte response in alginate hydrogels as a function of seeding density, focusing on characterizing changes in the scaffold viscoelastic properties over time. Specifically, (1) scaffold compressive and shear properties, as well as biochemical contents of these scaffolds at various cell densities (0, 30, and 60 M/mL), were determined at 1, 10, 20, 30, and 40 days; (2) the rapid-step shear (to approximate the Heaviside step-shear loading function) stress–relaxation data of the scaffold were also modeled with the quasi-linear viscoelastic (QLV) model, based on a continuous spectrum proposed by Fung.33 Since the shear response of native cartilage34 and acellular alginate hydrogel35 has been shown to be independent of strain rate, it can be best modeled with the QLV theory, which preserves the benefits of linearity with convolution and allows for relatively simple analysis with Laplace transformation. It is anticipated that findings from these studies will provide new insights into the structure–function relationship of the tissue-engineered cartilage graft and enable the design of optimal hydrogel scaffolds for functional cartilage repair.
Materials and Methods
Cells and cell culture
Primary articular chondrocytes were isolated from the full-thickness cartilage of carpometacarpal joints of 1–4-week-old calves obtained from a local abattoir (Rutland, VT) following published protocols.36 Briefly, the isolated cartilage was minced and then rinsed several times with Dulbecco's modified Eagle's medium (DMEM, Cellgro-Mediatech, Hernodon, VA) supplemented with 200 units/mL penicillin, 200 μg/mL streptomycin, 1% amphotericin B, and 50 μg/mL gentamicin sulfate (Invitrogen, Carlsbad, CA). The cartilage pieces were subsequently digested in 0.25% pronase (Calbiochem, San Diego, CA) for 1 h and 0.05% collagenase (Sigma, St. Louis, MO) for 4 h at 37°C. The resulting cell suspension was filtered through a sterile membrane (∼30 μm pore size), and chondrocytes were then obtained by centrifugation and used immediately for the experiments described below.
Alginate hydrogel fabrication
For this study, chondrocytes were cultured in alginate hydrogels. Briefly, a 2% alginate solution was made by solubilizing low-viscosity sodium salt alginic acid (Sigma) in phosphate buffered saline, followed by sterile filtration. The alginate used here has an M/G ratio (mannuronic residue/guluronic residue) of 1.67 and an average molecular weight of ∼50,000 Da. Chondrocytes were mixed with 2% alginate solution at different densities (0, 30, and 60 M/mL), and the cell–alginate suspension was then crosslinked for 30 min in 50 mM CaCl2 and 0.15 M NaCl (Sigma) in a custom Delrin® mold. The resulting samples (10 mm diameter × 1.6 mm thickness) were cultured at 37°C and 5% CO2 in DMEM supplemented with 10% fetal bovine serum (Cellgro-Mediatech), 1% nonessential amino acids, 100 units/mL penicillin, 100 μg/mL streptomycin, and 20 μg/mL ascorbic acid (Sigma). Acellular alginate scaffolds were prepared as controls. The effects of seeding density (0, 30, and 60 M/mL) on cell proliferation, matrix production, and hydrogel mechanical properties were evaluated over 40 days of culture.
Cell proliferation and matrix synthesis
At each time point, the hydrogel samples were first blotted dry and weighed to obtain the wet weight (WW; n = 6), and then lyophilized for 2 days and weighed again to obtain sample dry weight (DW; n = 6). Water content (%) and swelling ratio of the hydrogel samples were then calculated as (WW-DW)/WW × 100% and WW/DW, respectively. The lyophilized samples were subsequently digested for 16 hours at 60°C with 20 μL/mL papain in 0.1 M sodium acetate, 10 mM cysteine HCl, and 50 mM ethylenediaminetetraacetate.
Total DNA content (n = 6) was determined using the PicoGreen dsDNA assay (Molecular Probes, Eugene, OR) following the manufacturer's protocol. Fluorescence was measured using a microplate reader (Tecan, Maennedorf, Switzerland) with excitation and emission wavelengths of 485 and 535 nm, respectively. The fluorescence intensity values were then used to calculate the DNA content of samples from a linear interpolation of the intensities of various DNA standards. The total cell number was obtained using the conversion factor of 7.7 pg DNA/cell.36,37
The glycosaminoglycan (GAG) content of the samples (n = 6) was determined with a modified 1,9-dimethylmethylene blue (DMMB) dye-binding assay38,39 with chondroitin-6-sulfate (Sigma) as standard. To account for the anionic nature of the carboxyl groups on the alginate hydrogel, the pH of the DMMB dye was adjusted to 1.5 with concentrated formic acid (Sigma) so that only the sulfated GAG-DMMB complexes were detected.38 Additionally, the absorbance difference between 540 and 595 nm was used to improve the sensitivity in signal detection.39
The collagen content of the samples (n = 6) was quantified with a simplified hydroxyproline assay.40–42 Specifically, a portion of the digest was hydrolyzed with 2.0 M sodium hydroxide at 120°C for 20 min. The hydrolyzate was then oxidized by a buffered chloramine-T reagent (Sigma) for 25 min before the addition of Ehrlich's reagent. Sample absorbance was measured at 550 nm (Tecan), and the hydroxyproline content was obtained by interpolation along a standard curve of rat tail type I collagen (Invitrogen). The obtained hydroxyproline content was converted to collagen content using a 1:10 conversion ratio of hydroxyproline to collagen.43
For histology, the sample was fixed with acid formalin (4% formaldehyde in 70% ethanol and 5% acetic acid), dehydrated with a graded series of ethanol, embedded in paraffin, and sectioned into 7.5 μm slices. Sample GAG distribution was characterized with Alcian blue staining at pH 1.5, and collagen distribution was ascertained with Picosirius red staining.44 The sections were imaged using the Axiovert 35 microscope (Zeiss, Oberkochen, Germany).
Mechanical properties
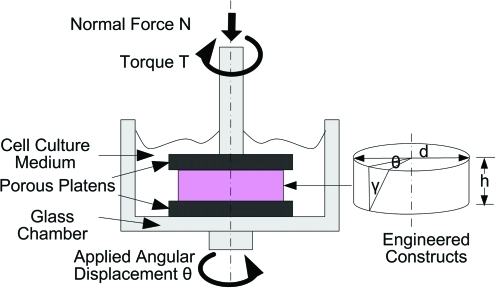
The compressive and shear properties of the chondrocyte–alginate samples (n = 6) were determined at 1, 10, 20, 30, and 40 days. The diameter (d) of each sample was measured with a stereomicroscope (model PP&E 56939; Bausch and Lomb, Rochester, NY), and mechanical tests31 were performed on a shear-strain controlled rheometer (ARES-LS1, TA instruments, New Castle, DE). Briefly, each sample was placed between two flat porous platens and immersed in DMEM to prevent dehydration (Fig. 1). The equilibrium compressive Young's modulus (Eeq) of the sample was calculated at 15% compressive strain (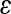 ) as follows:
) as follows:
 |
(1) |
FIG. 1.
Schematic of an alginate hydrogel sample in the torsional shear and compression testing device (ARES-LS1, TA instruments). Color images available online at www.liebertonline.com/tea.
where ΔF is the equilibrium normal force change due to the axial compression, and σz is the normal stress along the axial direction. The 15% compressive strain chosen here is within the physiological range for articular cartilage, and sufficient for the platens to grip the sample surface without slippage. After a 30-min compressors stress relaxation, a step shear strain (γ) of 0.01 radian is applied (Fig. 1). The shear stress was then allowed to equilibrate and shear modulus (Geq) was calculated from the equilibrium torque (Teq) with the torsional shear formula below:
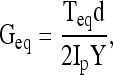 |
(2) |
where Ip is the polar moment of inertia of the cylinder, and is given by Ip = πd4/32. Finally, a dynamic shear test was performed (0.01–10 Hz) on a logarithmic frequency sweep with a specified shear strain amplitude of 0.01 radian. The complex shear modulus was calculated from:
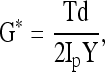 |
(3) |
where, γ is the sinusoidal shear strain and T is the torque response. In general, G* is a complex number and can be expressed as G* = G′ + iG″, where G′ is the storage modulus and G″ is the loss modulus. The magnitude of the complex shear modulus (|G*|) is therefore given by 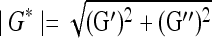 , and the phase shift angle (δ) between the applied strain and the torque response can be calculated from
, and the phase shift angle (δ) between the applied strain and the torque response can be calculated from 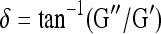 . In general, a high phase shift angle (i.e., δ → 90°) represents a highly viscous material, whereas a low value (δ → 0°) indicates minimal internal energy damping in a material, and δ = 0° defines an elastic material (i.e., no energy dissipation).2
. In general, a high phase shift angle (i.e., δ → 90°) represents a highly viscous material, whereas a low value (δ → 0°) indicates minimal internal energy damping in a material, and δ = 0° defines an elastic material (i.e., no energy dissipation).2
Viscoelastic modeling of the step shear stress relaxation
To mathematically characterize the viscoelastic shear behavior of alginate hydrogel scaffolds, a linear viscoelastic single-spectra relaxation model based on QLV theory was used. The QLV theory was first proposed in 1972 by Fung33 and later used to model a wide variety of biological tissues.45–50 In addition, the model was also combined with the biphasic model,12 which has been used to explain the compressive behavior of articular cartilage.28–30 Therefore, combining experimental findings with the modeling of the shear stress relaxation will provide a complete spectrum of data to augment current understanding of the viscoelastic behavior of engineered scaffolds. Compared to a generalized viscoelastic model, the QLV model is a constitutive assumption that reduces mathematical complexities of rheological modeling via convolution and allows for meaningful analysis with Fourier or Laplace transforms.
For the shear tests, the QLV theory assumes that the shear stress relaxation function is dependent on both shear strain and time, and can be written as
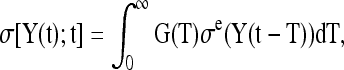 |
(4) |
where σe is the “elastic response” as a function of shear strain γ only, and G(t) is the reduced relaxation function as a function of time only. This separation of variables is an essential step that allows mathematical solutions to be derived. Note that if σe is nonlinearly related to the shear strain γ, the material is quasilinear viscoelastic, whereas a linear relationship is indicative of a linearly viscoelastic material.
For soft tissues that are insensitive to strain rate (i.e., the hysteresis loop is relatively uniform with respect to a range of shear rates), Fung proposed a generalized reduced relaxation function,33 which was later written in a simple mathematic form46 as
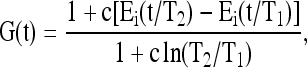 |
(5) |
where Ei(y) is the exponential integral, i.e., 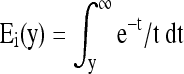 , and c, τ1, and τ2 are the material parameters in the QLV theory. Under dynamic loading, most of the viscoelastic energy dissipation within the solid ECM occurs at the frequency range over [1/τ2, 1/τ1], and its magnitude is indicated by the dissipation parameter c. With pure shear, it has been reported that c = 0.13, τ1 = 0.0004 s, and τ2 = 26.2 s for articular cartilage.49
, and c, τ1, and τ2 are the material parameters in the QLV theory. Under dynamic loading, most of the viscoelastic energy dissipation within the solid ECM occurs at the frequency range over [1/τ2, 1/τ1], and its magnitude is indicated by the dissipation parameter c. With pure shear, it has been reported that c = 0.13, τ1 = 0.0004 s, and τ2 = 26.2 s for articular cartilage.49
In this study, the elastic response σe is further assumed to relate linearly to the shear strain γ, that is, σe(γ) = G0γ, where G0 is the instantaneous shear modulus since G0 = σ(0)/γ(0). The model used for the curve-fitting for the step stress function is assumed to be
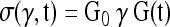 |
(6) |
with G(t) given in Equation (5) as the relaxation function. It has been reported that the parameter τ1 is usually very small for alginate, with a value of 0.001 s.35 Using this value, a three-parameter (G0, c, and τ2) curve fit was used for the step shear stress relaxation data of all samples with a least-squares curve-fitting algorithm computed with Matlab (Mathworks Inc., Natick, MA). The values of G0 (instantaneous shear modulus), c (dissipation coefficient), and τ2 (upper limit of the characteristic time) were then determined.
Statistical analysis
All statistical analyses were performed using the Statistical Analysis Software (SAS, Cary, NC). Data are presented as means ± standard deviation. Two-way analysis of variance was performed on the equilibrium compressive modulus, the equilibrium and dynamic shear properties, the viscoelastic model parameters, and the matrix content to assess the variation of the mechanical and chemical properties of chondrocyte–alginate scaffolds with incubation time and cell density. Fisher's least-significant-difference post-hoc tests were performed at a 95% confidence interval to determine statistical significance.
For structure–function correlations, regression analysis was performed to investigate the contribution of chemical deposition to the viscoelastic properties of the tissue-engineered scaffolds. Specifically, the data of mechanical properties and matrix content of the cell-seeded groups (i.e., both 30 and 60 M/mL group) at days 10, 20, 30, and 40 were used. The variables that accounted for the variation in hydrogel mechanical properties are water content, collagen content, and GAG content, which were first normalized to the WW of scaffolds. First, the nature of the relationship (e.g., linear or nonlinear) between mechanical parameters (Eeq, Geq, |G*|, G0, c, and τ2) and ECM components (collagen, GAG, and water content) were identified by studying the pattern of the residuals between the experimental mechanical data and the predicted values from the linear or nonlinear regression. The nonlinear relations were then transformed into linear relations with transformed parameters. Finally, a forward stepwise linear regression was performed to find the best set of chemical parameters for describing the variation of the mechanical properties among scaffolds (Table 1).51 A combined addition and elimination method was used until only statistically significant associations remained. At each step, the most significant chemical parameter was included and the regression recomputed. Each of the variables previously included was reevaluated and considered for elimination to ensure that it had not become insignificant with the addition of the current variable.
Table 1.
Multiple Regression Results Relating Biochemical Constituents (Normalized to Wet Weight) to the Mechanical Properties of Chondrocytes-Seeded Alginate Scaffolds (n = 6) at Days 10, 20, 30, and 40
| GAG | p | H2O | p | Intercept | p | R2of regression | p | |
|---|---|---|---|---|---|---|---|---|
| ln(Eeq) | — | — | −50.7 ± 8.0 | <0.0001 | 50.5 ± 7.7 | <0.0001 | 0.52 | <0.0001 |
| ln(Geq) | 83.5 ± 30.9 | 0.0104 | −62.9 ± 22.7 | 0.0088 | 22.0 ± 1.4 | 0.0089 | 0.74 | <0.0001 |
| ln(|G*|) | 171.9 ± 26.9 | 0.0002 | −85.0 ± 19.8 | <0.0001 | 82.7 ± 19.2 | <0.0001 | 0.87 | <0.0001 |
| δ | 377.9 ± 31.9 | <0.0001 | — | — | 1.19 ± 0.30 | 0.0003 | 0.79 | <0.0001 |
| ln(G0) | 231.8 ± 24.5 | <0.0001 | — | — | 2.80 ± 0.23 | <0.0001 | 0.71 | <0.0001 |
| c | 11.0 ± 2.0 | <0.0001 | — | — | 0.09 ± 0.02 | <0.0001 | 0.46 | <0.0001 |
Results
Scaffold water content and swelling ratio
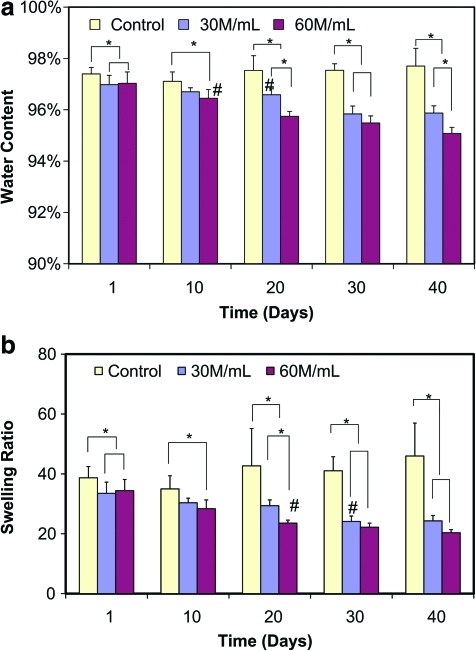
The water content of the acellular alginate hydrogel control exhibited no significant change with incubation time (Fig. 2a), ranging from 97.1 ± 0.4% to 97.7 ± 0.7% during the 40 days of culture. Similarly, the swelling ratio of alginate control remained relatedly constant over time (Fig. 2b). However, significant differences in both water content and swelling ratio were detected between the cellular groups and acellular control at all time points tested (p < 0.05). The water content for cellular groups decreased from 97% on day 1 to 96% on day 40 for the 30 M/mL group (Fig. 2a; p < 0.0001) and from 97% to 95% for the 60 M/mL group (p < 0.0001). A significant decrease in water content compared to day 1 was detected by day 20 and day 10 for the 30 M/mL (p < 0.0001) and 60 M/mL groups (p < 0.05), respectively. A significant difference in water content between the two cell density groups was first observed on day 20 (p < 0.0001), with a lower water content measured for the 60 M/mL group than the 30 M/mL group thereafter. Similarly, swelling ratios of the cellular groups (Fig. 2b) decreased with incubation time and cellular density, with a value of ∼20 measured for cellular alginate hydrogel at day 40. An interactive effect of seeding density and culture time on water content and swelling ratio of engineered scaffolds was also detected (p < 0.0001).
FIG. 2.
(a) Water content (n = 6) and (b) swelling ratio (n = 6) with time in culture for the acellular control group and the scaffolds seeded with 30 × 106 cells/mL (30 M/mL) and 60 × 106 cells/mL (60 M/mL). *Significant difference between groups (p < 0.05). #The time point when a significant difference within group was first detected from day 1 (p < 0.05). Color images available online at www.liebertonline.com/tea.
Cell proliferation and matrix synthesis
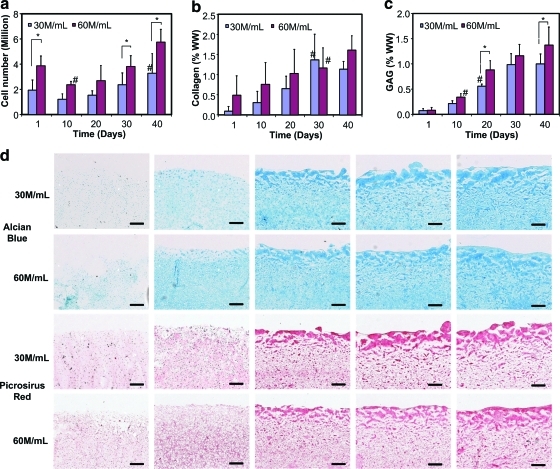
For the chondrocyte–alginate scaffolds, while cell number decreased on day 10 for the 60 M/mL group, it increased thereafter (Fig. 3a). The initial halting of cell growth is likely related to the lack of binding sites on alginate polymer scaffold for cell attachment before the cells have secreted a significant amount of matrix,52 coupled with cell migration out of the scaffold and hydrogel degradation. As expected, significantly higher cell number was measured at days 1, 30, and 40 in the 60 M/mL group (p < 0.05). The total cell number of the scaffolds varied significantly with initial cell density and culture time (p < 0.0001), but had no effect in combination (p = 0.42). In terms of matrix production, sample collagen content at both densities was found to increase significantly with incubation time (Fig. 3b; p < 0.0001, day 1 vs. 30). No significant effect due to seeding density alone (p = 0.064) or its combination with incubation time (p = 0.46) was detected. A significant increase in GAG content over time was found on day 10 for the 60 M/mL group (p = 0.012) and on day 20 for 30 M/mL (p < 0.0001), whereas a significant difference in GAG production was first detected between the two groups on day 20 (p < 0.05). The GAG content of the cell-seeded scaffolds was independent of any interactive effect between seeding density and culture time (p = 0.09). The increase in GAG and collagen deposition with culture time and cell density was further confirmed with histological staining (Fig. 3d).
FIG. 3.
(a) Cell number, (b) collagen, and (c) glycosaminoglycan (GAG) contents of alginate scaffolds (n = 6) seeded with 30 × 106 cells/mL (30 M/mL) and 60 × 106 cells/mL (60 M/mL) over time. *Significant difference between two groups at the same time point (p < 0.05). #The time point when a significant difference within group was first detected from day 1 (p < 0.05). (d) Alcian Blue (for GAG) and Pircrosirus Red (for collagen) staining for alginate constructs seeded with 30 and 60 M/mL chondrocytes at days 1, 10, 20, 30, and 40. Scale bars stand for 250 μm. Color images available online at www.liebertonline.com/tea.
Mechanical properties
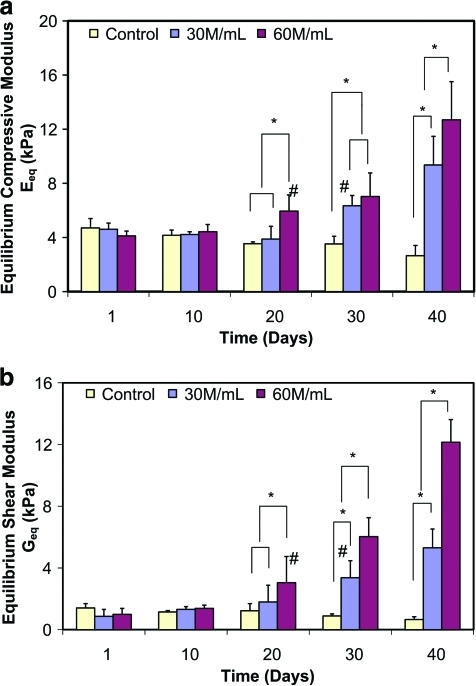
In this study, both compressive and shear properties were determined as a function of cell density and culturing time. The equilibrium compressive modulus (Eeq) and shear modulus (Geq) of the acellular control group remained unchanged with incubation time (Fig. 4a, b; p > 0.05). In contrast, both Eeq and Geq of the cellular groups increased significantly (p < 0.0001). For the 30 M/mL group, significant differences in Eeq and Geq compared to day 1 were not observed until day 30 (p < 0.0001), whereas for the 60 M/mL group, the significant increase in Geq was evident by day 20 (p < 0.0001). Moreover, for the 60 M/mL group, the compressive modulus at day 40 increased about two-fold compared to day 1, whereas the shear modulus increased about 12-fold. A significant difference among the three groups (0 and 30 vs. 60 M/mL) was first observed for both Eeq and Geq on day 20 and thereafter, whereas significant difference between each of these three groups (i.e., 0 vs. 30 vs. 60 M/mL) was detected on day 40 for Eeq and day 30 for Geq, respectively.
FIG. 4.
(a) Equilibrium compressive modulus (Eeq) and (b) shear modulus (Geq) with time in culture for alginate scaffolds (n = 6) seeded with 30 × 106 cells/mL (30 M/mL) and 60 × 106 cells/mL (60 M/mL). *Significant difference between two groups at the same time point (p < 0.05). #The time point when a significant difference within group was first detected from day 1 (p < 0.05). Color images available online at www.liebertonline.com/tea.
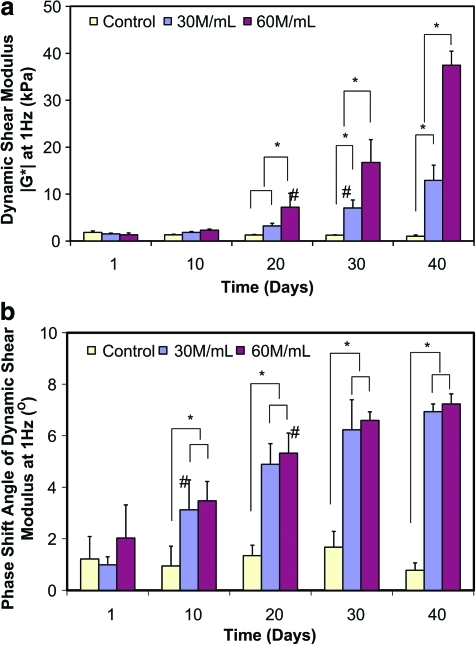
For the cellular groups, both the magnitude of the dynamic shear modulus (|G*|) and the phase shift angle (δ) at 1 Hz increased significantly with time (Fig. 5; p < 0.0001), whereas no significant difference was detected over time for the acellular control (p > 0.05). A significant increase in |G*| was first observed on day 30 for the 30 M/mL group (p < 0.0001) and on day 20 for the 60 M/mL group (p < 0.0001), whereas an increase in δ was first detected on day 10 for both 30 M/mL (p < 0.0001) and 60 M/mL groups (p < 0.05). At 1 Hz, |G*| increased more than 30-fold for the 60 M/mL group, and δ increased about 3.5-fold after 40 days of culture. Significant difference among these three groups was first detected on day 20 for |G*| and day 10 for δ (p < 0.05). In addition, Eeq, Geq, |G*|, and δ were found to be dependent on the interactive effects of seeding density and culture time (p < 0.0001).
FIG. 5.
(a) Dynamic shear modulus magnitude (|G*|) and (b) phase shift angle at 1 Hz over time for alginate scaffolds (n = 6) seeded with 30 × 106 cells/mL (30 M/mL) and 60 × 106 cells/mL (60 M/mL). *Significant difference between two groups at the same time point (p < 0.05). #The time point when a significant difference within group was first detected from day 1 (p < 0.05). Color images available online at www.liebertonline.com/tea.
Modeling of step shear stress relaxation response of chondrocyte–alginate scaffolds
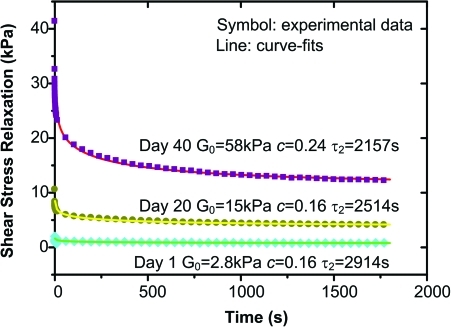
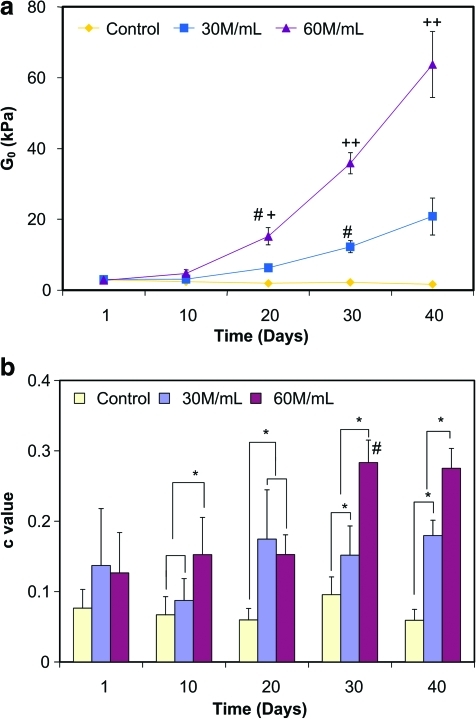
With the QLV model, three curve-fitting parameters (G0, c, and τ2) that characterize the step shear stress relaxation response were obtained, along with representative curve-fits with time shown in Figure 6. Specifically, the instantaneous shear modulus G0 of tissue samples, which represents the ability of the scaffolds to resist the impact of torque force, was found to be about 5 times greater than the equilibrium shear modulus (Geq) at all time points measured. For cellular group, G0 increased with time, with a significant change first observed on day 30 for 30 M/mL group (Fig. 7a; p < 0.0001) and on day 20 for the 60 M/mL group (p < 0.0001). In contrast, no significant change in G0 was evident for the acellular group. A significant difference between the acellular control and 60 M/mL group was first detected on day 20 (p < 0.0001), and starting from day 30, significant differences were found between all three groups. Moreover, the combination of initial cell density and culture time was found to have a significant effect on G0 (p < 0.0001).
FIG. 6.
Representative step shear stress relaxation response (symbols) and curve fits (lines) of experimental results with the QLV model for alginate scaffolds with a cell density of 60 × 106/mL (60 M/mL) at 1 (▪), 20 (•) and 40 (♦) days. Parameters G0, c, and τ2 represent instantaneous shear modulus, energy dissipation coefficient, and upper limit of characteristic time, respectively. QLV, quasi-linear viscoelastic. Color images available online at www.liebertonline.com/tea.
FIG. 7.
(a) The instantaneous shear modulus G0 and (b) the c value calculated from the QLV model for alginate scaffolds (n = 6) seeded with 30 × 106 cells/mL (30 M/mL) and 60 × 106 cells/mL (60 M/mL) over time. +Significant difference from the acellular group at the same time point. ++Significant difference between any two groups. #The time point when a significant difference within group was first detected from day 1 (p < 0.05). *Significant difference between two groups at the same time point (p < 0.05). Color images available online at www.liebertonline.com/tea.
The parameter c, calculated from the QLV model and representing the energy dissipation under constant shear strain, increased significantly over time in the 60 M/mL cellular group only (Fig. 7b, p < 0.0001). In addition, significant differences in c were detected on day 10 between the acellular control and 60 M/mL group (p < 0.05) and on day 20 between the acellular control and 30 M/mL group (p < 0.05). Starting from day 30, significant differences were observed between all three groups (p < 0.05). After 40 days of culture, the highest c value (0.28 ± 0.03) was found in the 60 M/mL group, with c = 0.18 ± 0.02 for the 30 M/mL group and c = 0.06 ± 0.02 for the acellular control. Parameter c was found to be dependent on the interactive effect between initial seeding density and culture time (p < 0.0001).
Finally, no significant change in τ2, the reciprocal of which represents the lower limit of frequency of scaffold shear mechanical response, was found over time and as a function of cell density. At day 1, τ2 was calculated to be 2374 ± 640 s for the acellular control, 2664 ± 888 s for the 30 M/mL group, and 2153 ± 763 s for the 60 M/mL group. By day 40, τ2 = 2274 ± 869 s, 2173 ± 159 s, and 2534 ± 471 s for the acellular, 30, and 60 M/mL groups, respectively.
Correlation of matrix contents with mechanical properties
Regression analysis revealed that all mechanical moduli examined (Eeq, Geq, |G*|, and G0) increased exponentially with increasing collagen and GAG content, and decreased exponentially with scaffold water content, whereas the phase shift angle (δ) and the dissipation parameter c of the QLV model was linearly related to matrix content (collagen and GAG). Therefore, a natural logarithm transformation was applied on Eeq, Geq, |G*|, and G0 before the multiple linear regression was performed. As summarized in Table 1, stepwise linear regression analysis revealed that mechanical properties of alginate scaffolds correlated well with GAG content and/or water content. In contrast, low correlation coefficients for collagen content were found. Specifically, the coefficients (slopes and intercept at zero chemical content) of the linear curve-fitting, the coefficient of the determination (R2, defined as the fraction of the total squared error that is explained by the linear regression model), and the p-values of the linear regression were determined. Both sample water content and GAG content were needed for establishing a strong correlation with the equilibrium shear modulus ln(Geq) (R2 = 0.74) and the magnitude of dynamic shear modulus ln(|G*|) (R2 = 0.87). In contrast, water content alone was sufficient for accounting for the variation in compressive modulus ln(Eeq) (R2 = 0.52), and GAG content alone for phase shift angle δ, instantaneous modulus ln(G0), and parameter c (R2 = 0.79, 0.71, and 0.46, respectively).
Discussion
The objective of this study was to examine changes in the viscoelastic properties of the ECM of the hydrogel-based, tissue-engineered cartilage scaffolds as a function of culture time and seeding density. It was found that the viscoelastic dynamic moduli and viscosity of chondrocyte-laden alginate hydrogel scaffolds increased with incubation time and seeding density. As such, no difference in the phase shift angle of dynamic shear modulus was observed for with increasing cell density. In addition, for the first time, the step shear stress–relaxation response of the tissue-engineered scaffolds has been shown to be well described by the QLV relaxation spectral model. Model parameters such as instantaneous shear modulus G0 and dissipation coefficient c increased over time and with cell seeding density, whereas the upper limit of the scaffold characteristic time had no significant changes. The structure–function relationship (i.e., the contribution of collagen and GAGs production to the dynamic viscoelastic properties of the scaffolds) was also determined. Overall, the results of this study demonstrate that the shear viscoelastic properties correlate strongly with biochemical contents of the scaffolds and that the torsional (pure) shear test is the optimal method for monitoring changes in dynamic viscoelastic properties with cell density and culture time.
The mechanical properties of the acellular and cell-seeded alginate hydrogel observed here are similar to those of published studies.19,25,35 The compressive properties (Eeq ∼ 12 kPa at day 40) of the chondrocyte–alginate scaffolds are comparable to those reported for low guluronic acid alginate hydrogel seeded with 4 M/mL chondrocytes at day 49 (∼17 kPa) and alginate (medium viscosity) hydrogel seeded with 22 M/mL chondrocytes at day 14 and thereafter (∼8 kPa).19,25 The equilibrium compressive modulus (Eeq ∼ 4 kPa) and equilibrium shear modulus (Geq ∼ 1 kPa) of the 2% acellular alginate are within the same range (Eeq: 3–4 kPa and Geq: 1–2 kPa after 15 h in 0.15 M NaCl) of those reported by LeRoux et al.,35 which utilized an identical mechanical testing configuration and low-viscosity alginate. It is emphasized that the mechanical properties of alginate are highly dependent on its co-polymer ratio (M/G)53 and the testing conditions (e.g., tested in air or in solution,54 at equilibrium or short term54,55). Awad et al. examined the difference in the chondrogenic differentiation potential of human adipose tissue-derived stem cells in alginate, agarose, and fibrous gelatin scaffolds,56 and measured shear properties of low-viscosity alginate hydrogels (Eeq ∼ 1 kPa), which were in the same range of those of this study. The low-viscosity alginate was also proven to be advantageous as an engineered scaffold. Previously, it was found that both mechanical properties and chemical contents of medium-viscosity alginate hydrogel seeded with chondrocytes reached a plateau after 14 days of culture.25 In contrast, they increased with culture time in this study using low-viscosity alginate.
Swelling ratio of engineered scaffolds was measured in the presence of chondrocytes and deposited ECM, which added complexity to understanding this hydrogel property. Seeding cells into alginate had no significant effects on hydrogel mechanical properties at day 1. This indicates that the presence of chondrocytes had a negligible effect on the crosslinking of the alginate, and infers that chondrocytes' initial mechanical properties were similar to the alginate gel. In contrast, the deposited macromolecules, especially proteoglycans, may significantly increase water imbibition.2 As a result, scaffold WW increased several folds over time of culture with a negligible direct contribution from the weight of the ECM molecules. Therefore, the swelling ratio of engineered scaffolds seems to result from a delicate balance between the swelling of both hydrogel polymers and deposited ECM, and the restriction by the crosslinking between alginate chains and possibly ECM molecules.
Although the dynamic shear modulus increased as a function of cell seeding density, no significant difference in the phase shift angle was observed here. One would expect the phase shift angle to increase with cell density, since more collagen and GAG were produced for the high-density cellular group, and energy dissipation would decrease as a result of the increase of the solid content or decrease in water content. One possible explanation is that although the total proteoglycan content for the 60 M/mL group after 40 days of culture is about 1.8 times of that for the 30 M/mL group, the overall density of proteoglycans is only about 1.3 times, as the volume of the scaffolds is about 1.4 times due to differences in scaffold swelling. In addition, matrix production by chondrocytes inside the alginate hydrogel may stretch and uncoil alginate polymer chains, resulting in increased availability of crosslinking sites to actively resist shear deformation. High efficiency in utilizing the crosslinking in mechanical responses tends to decrease the phase shift angle. Therefore, the competing effects of the increasing friction between molecules and the stretching and uncoiling of the alginate and collagen chains determine the relative viscosity of the engineered tissue,32 potentially leading to the insensitivity of the phase shift angle to changes in cell density observed here. This is not too surprising as the fundamental assumption of the QLV theory is that the material is shear rate insensitive.
It was observed that scaffold viscosity increased with the macromolecular deposition inside the tissue-engineered scaffolds. This was indicated by the increase in the magnitude of scaffold phase shift angle δ and dissipation parameter c over time. For the 60 M/mL group, the phase shift angle more than tripled over the 40-day culturing period, whereas the dissipation parameter c more than doubled. Previously, it was reported that the phase shift angle of acellular alginate hydrogel remains essentially unchanged when the alginate concentration ranges from 1% to 3% as a result of the counteracting effects from the increase of the solid content (i.e., DW/WW) and calcium crosslinks.31,35 The fact that phase shift angle δ varied with matrix deposition of cellular groups and did not change with alginate concentration suggests that although the deposition of GAG and collagen increased the stiffness of the tissue-engineered scaffolds, the newly deposited matrix may not have been assembled or linked structurally. Consequently, the hydrogel network cannot yet form a sufficient number of crosslinks, which are as strong as the linkages between guluronic residues of the alginate chains.32 It was also found that the phase shift angle was more highly correlated with the GAG content (R2 = 0.79) than with scaffold water content (R2 = 0.56) or collagen content (R2 = 0.45). This observation is consistent with the finding that the phase shift angle of articular cartilage under pure shear is strongly dependent on the GAG content instead of collagen content inside the tissue.32 Thus, the increase in the viscosity of the tissue-engineered scaffolds may be largely attributed to the interaction (or friction) between proteoglycans, and possibly between proteoglycans and the alginate polysaccharide chains.
In this study, it was found that compared to the compressive test, the pure shear tests appear to be more effective in detecting changes in the cell-seeded hydrogel scaffolds. After 40 days of culture, the equilibrium compressive modulus Eeq of the 60 M cells/mL scaffolds increases only about 3-fold on day 40, whereas the equilibrium shear modulus Geq increases around 12-fold; the dynamic shear modulus |G*| at 1 Hz increases 24-fold, and the instantaneous shear modulus G0 increases 23-fold. These results suggested that the scaffold shear properties may be a more sensitive indicator of the longitudinal variation in hydrogel mechanical properties.
Hydrogels can often be modeled as biphasic materials consisting of two incompressible phases: solid phase and water phase.25,57 They exhibit viscoelasticity when the water or fluid inside the hydrogel gradually flows out under compression. Previously, the linear isotropic biphasic theory12 has been used to accurately describe the mechanical behavior of agarose and to study nutrient transport through the pores of an agarose matrix.25,58 For the alginate hydrogel used in this study, the situation is even more complex because it is an ionotropic hydrogel crosslinked by divalent ions such as calcium. To fully understand the mechanical responses under compression, one has to develop sophisticated constitutive models that take into consideration the following factors: the effect of fixed negative charges59 on the alginate matrix, the affinity of Ca2+ and Na+ to guluronic acid, and the effect of cross-linking density. The approach utilized here (i.e., shear test combined with the QLV model) has special advantages in its ability to determine and fully describe the viscoelastic properties of the solid matrix of the scaffolds, since volume changes are minimized during the equivoluminal pure shear. The QLV model uses several physiologically meaningful parameters (i.e., G0, c, and τ2) to fully describe the viscoelastic behaviors of single-phase biological tissues. Specifically, G0 stands for the shear stiffness of the material upon the impact of a torque force, which is directly related to how tissue would respond during physiological activities such as impact loading from running and jumping. The upper limit characteristic time τ2 is associated with the time necessary for the tissue to fully recover from applied mechanical force or displacement. The dissipation coefficient c is related to what the portion is that the viscosity attributes to tissue stiffness during impact or dynamic loading. With these parameters derived from shear stress relaxation data with the QLV model, one can not only calculate the modulus of the biological tissue at equilibrium or under dynamic loading at various frequencies, but also predict the material response under any arbitrary torque force or shear displacement loading profile. Thus, the pure shear test and the viscoelastic analyses obtained from the QLV model can be considered as essential strategies to directly and fully quantify the viscoelastic behavior of hydrogel-based tissue-engineered scaffolds.
In this study, the obtained T2 value (∼2500 s), two orders of magnitude higher than that of articular cartilage (∼26 s), suggested that the relaxation/creep time is much longer for engineered constructs. The value of dissipation coefficient c (∼0.28 for the 60 M/mL group at day 40) is about two times of that native cartilage (∼0.13). This suggested that new synthesized matrix does not contribute much to storage modulus (elastic component) than the loss modulus (viscous component). As both the engineered scaffold and native cartilage are well described with the QLV model, once these parameters (i.e., G0, c, and τ2) are matched, the overall mechanical behaviors of the engineered tissue would be the same as the native cartilage under any shear loading profile when interstitial flow effects are not considered such as in a torsional pure shear test. It is of particular importance for the implantation of engineered tissue; the inability to match these parameters will likely lead to stress concentration and the poor integration between implanted scaffolds and surrounding native tissue under physiological mechanical loading.
In this study, collagen content exhibited relatively low correlation with mechanical properties, whereas for native articular cartilage the shear modulus was found to be highly dependent on collagen content.27 This difference might be attributed to the following: first, the contribution of increased collagen content to scaffold stiffness may appear partially as that of decreased water content; second, collagen content inside the scaffold is relatively low (∼10 times lower than native cartilage) and collagen fibers have not been well assembled and organized (as also indicated by localized Picrosirius Red staining in Fig. 3d) to contribute to scaffold mechanical properties. In this study, the engineered cartilage scaffolds after 40 days of culture still exhibit mechanical moduli (e.g., Geq ∼12 kPa for the 60 M/mL group) more than 10 times lower than those of the native cartilage (Geq∼200 kPa60), although they are comparable to properties achieved in similar studies with alginate hydrogels.25,56
Future studies will investigate the crosslinking of collagen fibrils inside engineered scaffolds as well as the aforementioned swelling of ECM macromolecules and evaluate their effects on scaffold viscoelastic properties. Furthermore, it will be interesting to incorporate growth factors such as transforming growth factor-β into the scaffold to promote biosynthesis, or utilize bioreactor systems that can apply dynamic compression and perfusion culture to investigate how biochemical and biophysical stimuli control the maturation of viscoelastic properties of engineered scaffolds.
Conclusion
In this study, the intrinsic viscoelastic shear properties of tissue-engineered cartilage grafts were extensively characterized. In addition, temporal changes in the structure–function relationships of these alginate-based scaffolds were examined. It was observed that the shear modulus and viscosity of engineered constructs increased with culturing time and with cell seeding. Analysis via the QLV model revealed that the dissipation parameter c, together with the phase shift angle of complex shear modulus of the engineered cartilage tissue, was highly dependent matrix proteoglycan content, which is mainly responsible for shear energy dissipation.
Acknowledgments
This study is supported by Whitaker Foundation Special Development Award, Stanley Dicker and Shelly Ping Liu endowments (Mow), Wallace Coulter Foundation (Lu), and NIH-NIAMS R01 AR055280 (Lu).
Disclosure Statement
No competing financial interests exist.
References
- 1.Mankin H.J. Mow V.C. Buckwalter J.A. Iannotti J.P. Ratcliffe A. Articular cartilage structure, composition, and function. In: Buckwalter J.A., editor; Einhorn T.A., editor; Simon S.R., editor. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. 2nd. Rosemont, IL: American Academy of Orthopaedic Surgeons Publishers; 2000. pp. 443–470. [Google Scholar]
- 2.Mow V.C. Gu W.Y. Chen F.H. Structure and function of articular cartilage and meniscus. In: Mow V.C., editor; Huiskes R., editor. Basic Orthopaedic Biomechanics and Mechano-Biology. 3rd. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 181–258. [Google Scholar]
- 3.Hunziker E.B. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage. 1999;7:15. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 5.Brittberg M. Nilsson A. Lindahl A. Ohlsson C. Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res. 1996;326:270. doi: 10.1097/00003086-199605000-00034. [DOI] [PubMed] [Google Scholar]
- 6.Hangody L. Kish G. Karpati Z. Szerb I. Eberhardt R. Treatment of osteochondritis dissecans of the talus: use of the mosaicplasty technique—a preliminary report. Foot Ankle Int. 1997;18:628. doi: 10.1177/107110079701801005. [DOI] [PubMed] [Google Scholar]
- 7.Skalak R. Fox C.F. New York: Liss; 1988. Tissue Engineering: Proceeding of a workshop held at Granlibakken, Lake Tahoe, California, February 26–29; p. 343. [Google Scholar]
- 8.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 9.Butler D.L. Goldstein S.A. Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 10.Guilak F. Butler D.L. Goldstein S.A. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res. 2001;391(Suppl):S295. [PubMed] [Google Scholar]
- 11.Vunjak-Novakovic G. Goldstein S.A. Biomechanical principles of cartilage and bone tissue engineering. In: Mow V.C., editor; Huiskes R., editor. Basic Orthopaedic Biomechanics and Mechano-biology. 3rd. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 343–408. [Google Scholar]
- 12.Mow V.C. Kuei S.C. Lai W.M. Armstrong C.G. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J Biomech Eng. 1980;102:73. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 13.Mow V.C. Wang C.C. Hung C.T. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- 14.Guo J.F. Jourdian G.W. MacCallum D.K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 15.Hauselmann H.J. Fernandes R.J. Mok S.S. Schmid T.M. Block J.A. Aydelotte M.B., et al. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107((Pt 1)):17. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Masuda K. Sah R.L. Hejna M.J. Thonar E.J. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003;21:139. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 17.Paige K.T. Cima L.G. Yaremchuk M.J. Schloo B.L. Vacanti J.P. Vacanti C.A. De novo cartilage generation using calcium alginate-chondrocyte constructs. Plast Reconstr Surg. 1996;97:168. doi: 10.1097/00006534-199601000-00027. discussion 79–80. [DOI] [PubMed] [Google Scholar]
- 18.Wong M. Siegrist M. Gaschen V. Park Y. Graber W. Studer D. Collagen fibrillogenesis by chondrocytes in alginate. Tissue Eng. 2002;8:979. doi: 10.1089/107632702320934074. [DOI] [PubMed] [Google Scholar]
- 19.Wong M. Siegrist M. Wang X. Hunziker E. Development of mechanically stable alginate/chondrocyte constructs: effects of guluronic acid content and matrix synthesis. J Orthop Res. 2001;19:493. doi: 10.1016/S0736-0266(00)90023-8. [DOI] [PubMed] [Google Scholar]
- 20.Lu H.H. Vo J.M. Chin H.S. Lin J. Cozin M. Tsay R., et al. Controlled delivery of platelet-rich plasma-derived growth factors for bone formation. J Biomed Mater Res A. 2008;86:1128. doi: 10.1002/jbm.a.31740. [DOI] [PubMed] [Google Scholar]
- 21.Hott M.E. Megerian C.A. Beane R. Bonassar L.J. Fabrication of tissue engineered tympanic membrane patches using computer-aided design and injection molding. Laryngoscope. 2004;114:1290. doi: 10.1097/00005537-200407000-00028. [DOI] [PubMed] [Google Scholar]
- 22.Atala A. Cima L.G. Kim W. Paige K.T. Vacanti J.P. Retik A.B., et al. Injectable alginate seeded with chondrocytes as a potential treatment for vesicoureteral reflux. J Urol. 1993;150:745. doi: 10.1016/s0022-5347(17)35603-3. [DOI] [PubMed] [Google Scholar]
- 23.Park D.J. Bong J.P. Park S.Y. Hong K.S. Cartilage generation using alginate-encapsulated autogenous chondrocytes in rabbits. Ann Otol Rhinol Laryngol. 2000;109:1157. doi: 10.1177/000348940010901214. [DOI] [PubMed] [Google Scholar]
- 24.Yang W.D. Chen S.J. Mao T.Q. Chen F.L. Lei D.L. Tao K., et al. A study of injectable tissue-engineered autologous cartilage. Chin J Dent Res. 2000;3:10. [PubMed] [Google Scholar]
- 25.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 26.Likhitpanichkul M. Chow C.C. Guo X.E. Mow V.C. Determination of BPVE Coefficients for Agarose Gels at Various Concentrations from Unconfined Compression. Presented at the Proceedings of the 2006 Summer Bioengineering Conference; ASME, Amelia Island, Florida. 2006. [Google Scholar]
- 27.Olberding J.E. Suh F.J.K. A dual optimization method for the material parameter identification of a biphasic poroviscoelastic hydrogel: potential application to hypercompliant soft tissues. J Biomech. 2006;39:2468. doi: 10.1016/j.jbiomech.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 28.DiSilvestro M.R. Suh F.J.K. A cross-validation of the biphasic poroviscoelastic model of articular cartilage in unconfined compression, indentation, and confined compression. J Biomechanics. 2001;34:519. doi: 10.1016/s0021-9290(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 29.Mak A.F. The apparent viscoelastic behavior of articular cartilage—the contributions from the intrinsic matrix viscoelasticity and interstitial fluid flows. J Biomech Eng. 1986;108:123. doi: 10.1115/1.3138591. [DOI] [PubMed] [Google Scholar]
- 30.Setton L.A. Zhu W.B. Mow V.C. The biphasic poroviscoelastic behavior of articular cartilage: role of the surface zone in governing the compressive behavior. J Biomechanics. 1993;26:581. doi: 10.1016/0021-9290(93)90019-b. [DOI] [PubMed] [Google Scholar]
- 31.Wan L.Q. Jiang J. Arnold D.E. Guo X.E. Lu H.H. Mow V.C. Calcium concentration effects on the mechanical and biochemical properties of chondrocyte-alginate constructs. Cell Mol Bioeng. 2008;1:93. doi: 10.1007/s12195-008-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W.B. Mow V.C. Koob T.J. Eyre D.R. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993;11:771. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 33.Fung Y.C. Stress-strain-history relations of soft tissues in simple elongation. In: Fung Y.C., editor; Perrone N., editor; Anliker M., editor. Biomechanics: Its Foundation and Objectives. Inglewood, N.J.: Prentic Hall Inc.; 1972. pp. 181–208. [Google Scholar]
- 34.Zhu W. Mow V.C. Koob T.J. Eyre D.R. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993;11:771. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 35.LeRoux M.A. Guilak F. Setton L.A. Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. J Biomed Mater Res. 1999;47:46. doi: 10.1002/(sici)1097-4636(199910)47:1<46::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 36.Jiang J. Nicoll S.B. Lu H.H. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338:762. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y.J. Sah R.L. Doong J.Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 38.Enobakhare B.O. Bader D.L. Lee D.A. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243:189. doi: 10.1006/abio.1996.0502. [DOI] [PubMed] [Google Scholar]
- 39.Farndale R.W. Sayers C.A. Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 40.Huszar G. Maiocco J. Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem. 1980;105:424. doi: 10.1016/0003-2697(80)90481-9. [DOI] [PubMed] [Google Scholar]
- 41.Reddy G.K. Enwemeka C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 42.Stegemann H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 43.Vunjak-Novakovic G. Martin I. Obradovic B. Treppo S. Grodzinsky A.J. Langer R., et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 44.Bancroft J.D. Gamble M. Theory and Practice of Histological Techniques. 5th. New York: Churchill Livingstone; 2002. [Google Scholar]
- 45.Spirt A.A. Mak A.F. Wassell R.P. Nonlinear viscoelastic properties of articular cartilage in shear. J Orthop Res. 1989;7:43. doi: 10.1002/jor.1100070107. [DOI] [PubMed] [Google Scholar]
- 46.Woo S.L. Simon B.R. Kuei S.C. Akeson W.H. Quasi-linear viscoelastic properties of normal articular cartilage. J Biomech Eng. 1980;102:85. doi: 10.1115/1.3138220. [DOI] [PubMed] [Google Scholar]
- 47.Woo S.L.Y. Gomez M.A. Akeson W.H. The time and history-dependent viscoelastic properties of the canine medial collateral ligament. J Biomech Eng. 1981;103:293. doi: 10.1115/1.3138295. [DOI] [PubMed] [Google Scholar]
- 48.Woo S.L.Y. Johnson G.A. Smith B.A. Mathematical-modeling of ligaments and tendons. J Biomech Eng. 1993;115:468. doi: 10.1115/1.2895526. [DOI] [PubMed] [Google Scholar]
- 49.Zhu W.B. Lai W.M. Mow V.C. Intrinsic quasilinear viscoelastic behavior of the extracellular matrix of cartilage. Trans Orthop Res Soc. 1986;11:407. [Google Scholar]
- 50.Freeman J.W. Woods M.D. Cromer D.A. Wright L.D. Laurencin C.T. Tissue engineering of the anterior cruciate ligament: the viscoelastic behavior and cell viability of a novel braid-twist scaffold. J Biomater Sci Polym Ed. 2009;20:1709. doi: 10.1163/156856208X386282. [DOI] [PubMed] [Google Scholar]
- 51.Kutner M.H. Nachtsheim C. Neter J. Applied linear regression models. 4th. Boston; McGraw-Hill/Irwin: 2004. [Google Scholar]
- 52.Genes N.G. Rowley J.A. Mooney D.J. Bonassar L.J. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch Biochem Biophys. 2004;422:161. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Martinsen A. Skjakbraek G. Smidsrod O. Alginate as immobilization material .1. Correlation between chemical and physical-properties of alginate gel beads. Biotechnol Bioeng. 1989;33:79. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell J.R. Blanshard J.M.V. Rheological properties of alginate gels. Rheol Acta. 1974;13:180. [Google Scholar]
- 55.Nussinovitch A. Peleg M. Strength-time relationships of agar and alginate gels. J Texture Stud. 1990;21:51. [Google Scholar]
- 56.Awad H.A. Wickham M.Q. Leddy H.A. Gimble J.M. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 57.Goldsmith A.A. Clift S.E. Investigation into the biphasic properties of a hydrogel for use in a cushion form replacement joint. J Biomech Eng. 1998;120:362. doi: 10.1115/1.2798003. [DOI] [PubMed] [Google Scholar]
- 58.Mauck R.L. Hung C.T. Ateshian G.A. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;125:602. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai W.M. Hou J.S. Mow V.C. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 60.Zhu W. Lai W.M. Mow V.C. Intrinsic quasilinear viscoelastic behavior of the extracellular matrix of cartilage. Trans Orthop Res Soc. 1986;11:407. [Google Scholar]