Abstract
During the fight-or-flight response, the sympathetic nervous system stimulates L-type calcium ion (Ca2+) currents conducted by CaV1 channels through activation of β-adrenergic receptors, adenylyl cyclase, and phosphorylation by adenosine 3′,5′-monophosphate–dependent protein kinase [also known as protein kinase A (PKA)], increasing contractility of skeletal and cardiac muscles. We reconstituted this regulation of cardiac CaV1.2 channels in non-muscle cells by forming an autoinhibitory signaling complex composed of CaV1.2Δ1800 (a form of the channel truncated at the in vivo site of proteolytic processing), its noncovalently associated distal carboxyl-terminal domain, the auxiliary α2δ1 and β2b subunits, and A-kinase anchoring protein 15 (AKAP15). A factor of 3.6 range of CaV1.2 channel activity was observed from a minimum in the presence of protein kinase inhibitors to a maximum upon activation of adenylyl cyclase. Basal CaV1.2 channel activity in unstimulated cells was regulated by phosphorylation of serine-1700 and threonine-1704, two residues located at the interface between the distal and the proximal carboxyl-terminal regulatory domains, whereas further stimulation of channel activity through the PKA signaling pathway only required phosphorylation of serine-1700. Our results define a conceptual framework for CaV1.2 channel regulation and identify sites of phosphorylation that regulate channel activity.
INTRODUCTION
The “fight-or-flight” response is a conserved behavior of vertebrates experiencing fear, stress, or intense exercise. Release of epinephrine from the adrenal medulla and norepinephrine from sympathetic nerve endings activates β-adrenergic receptors and leads to an increase in L-type Ca2+ currents in cardiac and skeletal muscle, which in turn increase the force of contraction of skeletal muscle and the beating rate and contractility of the heart (1–3). This regulation of L-type calcium currents occurs through activation of protein kinase A (PKA)–mediated phosphorylation (4–8), but the molecular mechanism is unknown.
CaV1.1 and CaV1.2 channels, which conduct L-type Ca2+ currents in skeletal and cardiac muscle, respectively, are composed of pore-forming α1 subunits, plus auxiliary α2δ and β subunits (9–17). The α1 subunits of CaV1.1 and CaV1.2 channels each exist as two size forms produced by in vivo proteolytic processing of the C terminus in skeletal and cardiac muscle (18–22) and in brain (23, 24). Expression of complementary DNA (cDNA) encoding full-length CaV1.2 channels in non-muscle cells yields only full-length α1 subunits with no evidence of proteolytic processing (25). Deletion of the distal C terminus increases CaV1.2 channel activity (26, 27), and coexpression of the distal C terminus results in formation of a noncovalent autoinhibitory complex with the proximal C terminus of the truncated channel (28). The distal C-terminal domain effectively inhibits Ba2+ currents and reduces coupling efficiency between voltage sensor movement and opening of the pore (21, 28).
CaV1 channels in skeletal and cardiac muscle bind A-kinase anchoring protein 15 (AKAP15) (29–31), and β-adrenergic regulation of these channels requires PKA anchored by AKAP15 (32, 33). The AKAP15-PKA complex interacts with a site located in the proteolytically cleaved distal C terminus, suggesting that the distal C terminus remains associated with the remainder of the channel and mediates PKA-dependent regulation in cardiac myocytes (28, 33, 34).
Physiological regulation of L-type currents by PKA has not been reconstituted in non-muscle cells, impeding detailed analysis of the regulatory properties of calcium channel mutants. Previous studies of PKA-dependent modulation of cloned CaV1.2 channels expressed in non-muscle cells have examined full-length channels [for instance, (35, 36)]. Here we show that PKA-dependent modulation of CaV1.2 channel activity with a dynamic range similar to that seen in cardiac myocytes can be reconstituted in non-muscle cells after optimized expression of the different components of the CaV1.2-AKAP15 autoinhibitory signaling complex. Using this reconstitution system, we have identified sites of protein phosphorylation that are both necessary and sufficient for physiological regulation of CaV1.2 channels in non-muscle cells. These sites are located at the interface between the proximal (PCRD) and the distal (DCRD) C-terminal regulatory domains, well positioned to mediate disinhibition of the auto-inhibitory signaling complex and increase CaV1.2 channel activity in the fight-or-flight response.
RESULTS
Reconstitution of an autoinhibitory CaV1.2 channel signaling complex
The distal C-terminal domain inhibits channel activity when cDNA encoding distal1822–2171 is coexpressed at a 5:1 molar ratio with that encoding CaV1.2 channels truncated at position 1821 (28). For the experiments described here, we cotransfected cDNA encoding CaV1.2 channels truncated at A1800, the exact site of in vivo proteolytic processing (CaV1.2Δ1800) (21), distal1801–2171 (DCT), α2δ1, and β1b to produce a functional auto-inhibitory CaV1.2 channel complex. We assessed channel activity by measuring peak Ba2+ current and by determining the coupling efficiency of pore opening to gating charge movement (28). Gating charge movement was measured by integrating the gating current during depolarization to the reversal potential, and pore opening was measured from tail currents upon repolarization from the reversal potential. The functional properties of CaV1.2Δ1800 channels and their inhibition by DCT were identical to those of CaV1.2Δ1821 and distal1822–2171 [compare (28) and fig. S1].
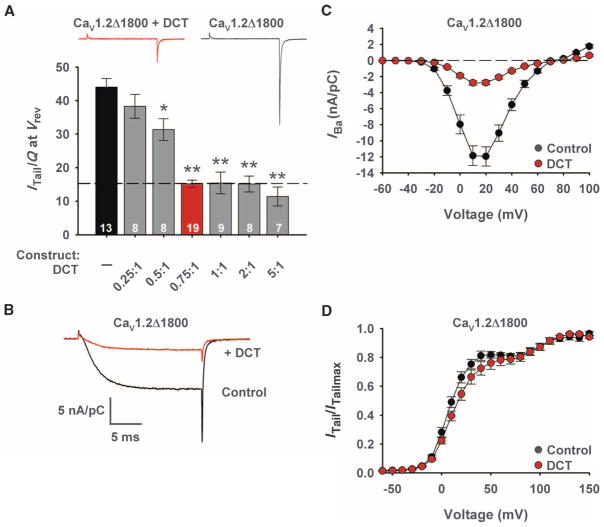
Transfection of cells with increasing ratios of cDNA encoding DCT to cDNA encoding CaV1.2Δ1800 revealed increasing inhibition of channel activity, with maximal inhibition at a ratio of 0.75 (Fig. 1, A and B). Coexpression of the DCT with the truncated channel does not alter the density of CaV1.2 channels on the cell surface, as determined by gating current measurements (28); therefore, reduction of the tail current provides a direct measure of the decrease in the coupling efficiency of the gating charge movement to opening of the pore: 43.6 ± 2.4 nA/pC (n = 13) for CaV1.2Δ1800 channels versus 15.2 ± 1.0 nA/pC for CaV1.2Δ1800 + DCT (n = 19; P < 0.01) (Fig. 1A).
Fig. 1.
Inhibition of CaV1.2Δ1800 channels by the distal C terminus. (A) Coupling efficiency for CaV1.2Δ1800 channels in the presence and absence of the indicated molar ratios of DCT/CaV1.2Δ1800 plasmids expressed in tsA-201 cells. *P < 0.05 or **P < 0.01 versus CaV1.2Δ1800 (ITail, peak tail current; Q, total gating current; Vrev, reversal potential). Red symbols indicate 0.75:1 molar ratio. n values and ± SEM are indicated. Significance was determined by ANOVA. Inset: Representative records for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels. (B) Representative Ba2+ currents through CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels elicited by a test pulse to +10 mV from a holding potential of −80 mV. (C and D) Mean current-voltage (C) and conductance-voltage (D) relationships for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels.
Inward Ba2+ currents conducted by CaV1.2 channels were decreased when DCT was present, similar to previous results (Fig. 1C) (28). However, the shift in voltage-dependent activation to more depolarizing potentials observed with large amounts of distal1822–2171 (28) was barely detectable (but still significant) with smaller amounts of DCT (Fig. 1D). These results suggest that expression of an optimal 0.75:1 molar ratio of the cDNAs encoding DCT and CaV1.2Δ1800 causes a specific reduction in coupling efficiency of gating charge movement to pore opening.
Reduction of basal CaV1.2 channel activity by inhibition of protein kinases
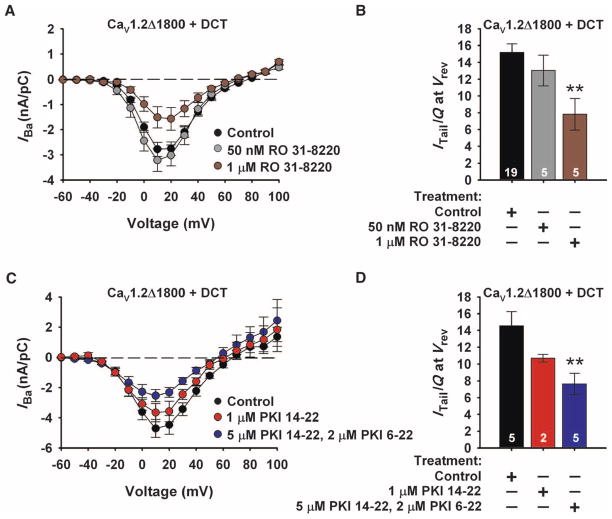
The increase in L-type Ca2+ current caused by basal phosphorylation in unstimulated cardiac myocytes (37) is inhibited by 1 μM RO 31-8220 (38), a potent protein kinase C (PKC) inhibitor (39) that also inhibits PKA and other protein kinases at 1 μM and higher concentrations (40). To determine whether protein kinase inhibition would also decrease L-type current through CaV1.2 channels in tsA-201 human embryonic kidney cells, we applied RO 31-8220 (50 nM or 1 μM) to the extracellular surface of cells transiently transfected with cDNAs encoding CaV1.2Δ1800 + DCT for 5 min before recording channel activity. RO 31-8220 (1 μM) significantly decreased inward Ba2+ currents and coupling efficiency in cells expressing CaV1.2Δ1800 + DCT (Fig. 2, brown). In contrast, 50 nM RO 31-8220, an effective concentration for inhibiting PKC but not PKA (40), had no effect on channel activity. We also observed a significant decrease in basal channel activity when cells expressing CaV1.2Δ1800 + DCT were treated for 1 hour with the specific, membrane-permeant PKA inhibitor myristoylated PKI 14-22 amide (5 μM) before recording channel activity with an intracellular solution containing 2 μM PKI 6-22 amide, the same PKA inhibitor in membrane-impermeant form (Fig. 2, C and D). Collectively, these results indicate that PKA-dependent phosphorylation increases basal CaV1.2 channel activity. The coupling efficiency during PKA inhibition defines the lower limit of CaV1.2 channel activity compared to the maximal coupling efficiency for CaV1.2Δ1800 channels in the absence of DCT.
Fig. 2.
Basal activity of CaV1.2 is reduced by inhibiting kinase activity. (A) Current-voltage relationships for CaV1.2Δ1800 + DCT in the presence and absence of 50 nM or 1 μM RO 31-8220 (RO). (B) Coupling efficiency (nA/pC) for CaV1.2Δ1800 + DCT channels in the presence and absence of 50 nM or 1 μM RO 31-8220. **P < 0.01. Significance was determined by Student’s t test (ITail, peak tail current; Q, total gating current; Vrev, reversal potential). (C) Current-voltage relationships for CaV1.2Δ1800 + DCT in the presence and absence of 1 μM myristoylated PKI 14-22 or 5 μM myristoylated PKI 14-22 with 2 μM PKI 6-22. (D) Coupling efficiency (nA/pC) for CaV1.2Δ1800 + DCT channels in the conditions described in (C). **P < 0.01. Significance was determined by Student’s t test.
Requirement for AKAP15 for PKA-dependent increase in CaV1.2 channel activity
AKAPs act as molecular scaffolds that enable the formation of signaling complexes (41). AKAP15 directly associates with CaV1.1 and CaV1.2 channels through a modified leucine zipper motif and promotes their co-localization with PKA (29–33). Moreover, physiological regulation of CaV1.2 channels by β-adrenergic stimulation in dissociated cardiac myocytes requires PKA anchored to AKAP15 (33). To test the requirement for AKAP15, we applied 5 μM forskolin to cells transfected with cDNA encoding CaV1.2Δ1800 + DCT for 5 min before recording channel activity. No increase in coupling efficiency was observed in the absence of AKAP15 (Fig. 3A). However, titration of the cDNA encoding AKAP15 downward from a molar ratio of 1:1 relative to that encoding CaV1.2Δ1800 revealed a substantial increase in coupling efficiency by forskolin at low amounts of AKAP15 (Fig. 3A, blue). With the optimum amount of AKAP15, forskolin increased CaV1.2Δ1800 + DCT coupling efficiency to 28.0 ± 2.0 nA/pC versus 15.2 ± 1.0 nA/pC for control without forskolin (n = 19 to 20, P < 0.01), with a corresponding increase in inward Ba2+ current (Fig. 3C), suggesting that AKAP15 is required for PKA-dependent CaV1.2 channel regulation. Comparison of the results with forskolin treatment (Fig. 3) to the results with kinase inhibitors (Fig. 2) revealed a factor of 3.6 dynamic range of modulation of CaV1.2 channel activity, similar to that seen in dissociated cardiac myocytes (25, 34, 38). Acute application of 5 μM forskolin to tsA-201 cells expressing CaV1.2Δ1800 + DCT with the optimum amount of AKAP15 gave a similar dynamic range of modulation (Fig. 3B). To ensure maximum activation of PKA in an undisturbed cellular context, we performed all subsequent experiments on intact CaV1.2-expressing cells treated with 5 μM forskolin for 5 min before recording channel activity.
Fig. 3.
Regulation of CaV1.2 channel activity by optimal expression of cDNA encoding AKAP15. (A) Coupling efficiency (nA/pC) for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with and without AKAP15, and 5 μM forskolin. **P < 0.01 versus control without forskolin. Dashed black line indicates mean current for unstimulated CaV1.2Δ1800 + DCT (ITail, peak tail current; Q, total gating current; Vrev, reversal potential). (B) Time course of peak Ba2+ current during perfusion with 5 μM forskolin (Fsk). Pulses to 10 mV before and during application of forskolin. Inset: current traces indicated by a and b. (C) Current-voltage relationships of CaV1.2 channels from (A). (D) Coupling efficiency (nA/pC) for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with PKA Cα catalytic subunit, PKA RIIα regulatory subunit, AKAP15, and 5 μM forskolin. ***P < 0.001 versus control. Dashed black line indicates mean current for unstimulated CaV1.2Δ1800 + DCT. Right, coupling efficiency (nA/pC) of individual experiments in each condition. Red line indicates the maximum current observed with CaV1.2Δ1800 + DCT. (E) Current-voltage relationships of CaV1.2 channels from (D). Significance was determined by ANOVA.
Overexpressed AKAP15 is expected to have a dominant-negative effect on regulation of CaV1.2 channel activity when its concentration exceeds that of endogenous PKA, so that AKAP15 lacking bound PKA occupies the CaV1.2 channel binding sites unproductively. This necessitated the use of low molar ratios of the cDNA encoding AKAP15 to those encoding CaV1.2Δ1800 + DCT. Analysis of coupling efficiency in individual cells indicated that 65% of cells cotransfected with AKAP15 and CaV1.2Δ1800 + DCT showed higher coupling efficiencies when treated with 5 μM forskolin than the maximum observed in its absence (fig. S2, A and B). In contrast, no individual cells had coupling efficiencies in the same range when CaV1.2Δ1800 + DCT channels lacking AKAP15 were treated with 5 μM forskolin (fig. S2A). The mean coupling efficiency in cells responsive to forskolin treatment was 33.5 ± 1.6 nA/pC (n = 13), whereas unresponsive cells had a mean coupling efficiency of 17.9 ± 0.8 nA/pC (n = 7), similar to that observed for CaV1.2Δ1800 + DCT in the absence of forskolin (fig. S2C). These findings show that PKA-dependent modulation of CaV1.2 channel activity can be reconstituted in non-muscle cells, but only in the presence of optimal amounts of AKAP15. To circumvent the dominant-negative effect of AKAP15, we coexpressed cDNA encoding PKA together with those encoding CaV1.2Δ1800 + DCT and AKAP15. Under these conditions, each individual cell exhibited a coupling efficiency in the presence of forskolin higher than that seen in its absence (Fig. 3, D and E). These results suggest that PKA overexpression ensures that an AKAP15-PKA complex associates with each CaV1.2Δ1800 + DCT channel to mediate forskolin-dependent modulation. However, all subsequent experiments were performed in the absence of exogenous PKA to avoid unwanted effects of kinase overexpression that might result from phosphorylation of secondary sites.
Modulation of full-length CaV1.2 channels by AKAP15
Earlier attempts to reconstitute PKA-dependent modulation of CaV1.2 channels in non-muscle cells used full-length channels (CaV1.2FL) and showed only small increases in CaV1.2 channel activity in response to PKA activation [for instance, (31, 35, 36, 42)]. To determine whether the effects of PKA on CaV1.2FL channel activity were enhanced in the presence of optimal amounts of AKAP15, we recorded CaV1.2FL channel activity in the presence and absence of 5 μM forskolin in cells coexpressing AKAP15. In contrast to CaV1.2Δ1800 + DCT, 5 μM forskolin failed to elicit a significant increase in coupling efficiency for CaV1.2FL at AKAP15 cDNA ratios of 0.003:1 and 1:1 (Fig. 4A, gray and blue), although it produced a small but significant increase in inward Ba2+ current (Fig. 4B, blue; 5.0 ± 0.6 nA/pC versus 7.4 ± 0.6 nA/pC, n = 7; P < 0.05). Inhibition of protein kinases with 1 μM RO 31-8220 decreased coupling efficiency of CaV1.2FL from 17.8 ± 1.5 to 7.0 ± 1.0 (Fig. 4A, brown; n = 5; P < 0.01) and also decreased Ba2+ current (Fig. 4B, brown; n = 5; P < 0.05). These results show that regulation of basal activity of CaV1.2FL is similar or greater than CaV1.2Δ1800 + DCT, but up-regulation by PKA is substantially reduced in channels with the distal C terminus covalently attached.
Fig. 4.
Regulation of full-length CaV1.2 channels. (A) Coupling efficiency (nA/pC) for CaV1.2FL channels in the presence or absence of 1 μM RO 31-8220 or CaV1.2FL channels with AKAP15 (1:1 or 0.003:1 molar cDNA ratio) and 5 μM forskolin. **P < 0.01 (ITail, peak tail current; Q, total gating current; Vrev, reversal potential). (B) Current-voltage relationships for the CaV1.2 channels studied in (A). Significance was determined by Student’s t test.
Phosphorylation of sites at the regulatory interface between the distal and the proximal C-terminal domains of CaV1.2 channels
Successful reconstitution of CaV1.2 channel modulation provided the opportunity to identify phosphorylation sites that are important for PKA-dependent potentiation of L-type calcium current. Ser1928 in the distal C terminus is phosphorylated in response to β-adrenergic stimulation in cardiac myocytes (20, 25, 43). However, its phosphorylation is not required for the increase in L-type channel activity elicited by β-adrenergic stimulation of cardiac myocytes (34, 44). We identified two previously unrecognized phosphorylation sites in the C terminus of the CaV1.1 channel by mass spectrometry (MS) (45), and these sites are conserved in the CaV1.2 at Ser1700 and Thr1704 (Fig. 5A). Ser1700 is predicted to be a substrate for PKA and calcium/calmodulin-dependent protein kinase II (CaMKII), whereas Thr1704 is predicted to be a substrate of casein kinase II (http://www.phosphosite.org). Both of these sites reside at the interface between the PCRD and the DCRD, where introduction of negatively charged phosphates could disrupt ionic interactions and relieve autoinhibition (Fig. 5A) (28). Analysis of in vitro phosphorylation of glutathione S-transferase (GST)–labeled CaV1.2(1670–1731) by MS showed that Ser1700 was a good substrate for both PKA and CaMKII with 27% and 77% phosphorylation, respectively, whereas 10% of Thr1704 was phosphorylated by casein kinase II (Fig. 5B). The extents of protein phosphorylation observed in these in vitro experiments reflect both the amino acid sequence contexts of the substrate sites and the local secondary and tertiary structures of the GST-labeled C-terminal protein, which may not retain native conformation in the absence of the complete CaV1.2 channel and its associated proteins.
Fig. 5.
Phosphorylation of Ser1700 and Ser1928 in CaV1.2 channels. (A) Top: docking model of the PCRD-DCRD complex in ribbon representation. Bottom: amino acid sequence surrounding Ser1700 and Thr1704. A, Ala; I, Ile; S, Ser; G, Gly; D, Asp; L, Leu; T, Thr; E, Glu; R, Arg. (B) Purified GST-CaV1.2(1670–1731) was phosphorylated with indicated kinases, digested with trypsin, and subjected to LC-MS analysis. MH2+ extract ions corresponding to the unphosphorylated (0P) or monophosphorylated (1P) forms of the peptides are indicated. CK2, casein kinase II. (C) Cells expressing CaV1.2Δ1800 + DCT channels were treated as indicated, fixed, probed with antibody against CaV1.2-pS1700, and visualized by indirect immunofluorescence. OA, okadaic acid; CsA, cyclosporin A; Iono, ionomycin. (D) Membranes from cells expressing CaV1.2FL, CaV1.2Δ1800, or CaV1.2Δ1800 + DCT channels were solubilized and separated by SDS-PAGE, and immunoblots were probed with anti-bodies against CaV1.2, CaV1.2-pS1700, or CaV1.2-pS1928. For quantitation, band intensities were normalized to those of the unstimulated control samples (n = 3). (E) Cells expressing CaV1.2Δ1800 + DCT channels, plus cDNA encoding the brain CaMKIIN inhibitor peptide as indicated, were pretreated with ionomycin and autocamtide-2 inhibitory peptide (AIP) and subjected to immunoblot analysis as in (D). For quantitation, band intensities were normalized to those of the unstimulated control samples (n = 3).
We used phosphospecific antibodies and immunocytochemistry to assess PKA phosphorylation of Ser1700 and Ser1928 in CaV1.2 channels expressed in tsA-201 cells. Cells expressing CaV1.2Δ1800 + DCT were treated with 10 μM forskolin to activate adenylyl cyclase and PKA, or 5 μM ionomycin to increase intracellular Ca2+ concentration and thereby activate CaMKII. Cells were fixed and antibody against pS1700 immunoreactivity was visualized by indirect immunofluorescence. We observed an increase in phosphorylation of Ser1700 after treatment with forskolin or ionomycin in the presence of 1 μM okadaic acid to inhibit phosphoprotein phosphatases 1 and 2A (Fig. 5C). When tested alone, neither okadaic acid (10 nM) nor the calcineurin inhibitor cyclosporin A (10 nM) substantially increased phosphorylation of Ser1700 (Fig. 5C). These results indicate that both PKA and CaMKII can phosphorylate Ser1700 of CaV1.2 channels in tsA-201 cells.
Immunoblot analysis showed measurable basal phosphorylation of Ser1700 and small increases in antibody against pS1700 immunoreactivity when cells expressing CaV1.2Δ1800 channels were treated with ionomycin in the presence of okadaic acid or with forskolin in the presence of okadaic acid and cyclosporin A (Fig. 5D). A more substantial increase in phosphorylation at Ser1700 was observed for CaV1.2Δ1800 + DCT after treatment with forskolin (factor of 1.8 ± 0.4, P < 0.05, n = 3; Fig. 5D) or ionomycin (factor of 2.5 ± 0.04, P < 0.01, n = 3; Fig. 5D) in the presence of okadaic acid or cyclosporin A or both. A small increase in phosphorylation was also observed with a phosphospecific antibody directed against Ser1928 with both ionomycin and forskolin treatment (Fig. 5D). The Ca2+-dependent increase in phosphorylation of Ser1700 was blocked by inhibition of CaMKII with either CaMK inhibitory protein or autocamtide-2–related inhibitor peptide II (1 μM) (Fig. 5E). In contrast to these results with CaV1.2Δ1800 + DCT, we observed no change in phosphorylation of Ser1700 of CaV1.2FL channels with drug treatment (forskolin: factor of 0.8 ± 0.1, P > 0.2; ionomycin: factor of 1.5 ± 0.7, P > 0.05; Fig. 5D). However, we did observe a robust increase in phosphorylation at Ser1928 in CaV1.2FL channels (Fig. 5D). These results demonstrate a difference in PKA phosphorylation of Ser1700 between CaV1.2FL and CaV1.2Δ1800 + DCT channels. Evidently, Ser1700 is a substrate for basal PKA-dependent phosphorylation of CaV1.2 channels in transfected cells, but the increase in PKA phosphorylation of Ser1700 is blocked by covalent association of the distal C terminus in CaV1.2FL.
Phosphorylation sites required for basal CaV1.2 channel activity
To test the role of individual phosphorylation sites in control of basal CaV1.2 channel activity, we made alanine substitutions for Ser1700, Thr1704, and Ser1928 and recorded channel activity. The S1700A and T1704A mutations had no significant effects on the high activity of CaV1.2Δ1800 channels expressed without DCT (fig. S3). However, both S1700A and T1704A significantly reduced the basal coupling efficiency of CaV1.2Δ1800 + DCT and decreased basal Ba2+ currents (Fig. 6A, gray, blue), whereas S1928A did not have a significant effect (Fig. 6A, orange). The only double-alanine mutation to significantly reduce both basal coupling efficiency and Ba2+ currents of CaV1.2Δ1800 + DCT was S1700A, T1704A (Fig. 6B, cyan). These results indicate that phosphorylation of Ser1700 and Thr1704 increases basal activity of CaV1.2Δ1800 + DCT channels, whereas phosphorylation of Ser1928 does not.
Fig. 6.
Requirements for phosphorylation of Ser1700, Thr1704, and Ser1928 for basal CaV1.2 channel activity. (A and B) Coupling efficiency (nA/pC) and current-voltage relationships for wild-type CaV1.2Δ1800 + DCT channels or channels with alanine substitutions at Ser1700, Thr1704, and Ser1928 individually or simultaneously. *P < 0.05 or **P < 0.01 versus wild-type CaV1.2Δ1800 + DCT channels (ITail, peak tail current; Q, total gating current; Vrev, reversal potential). (C) Coupling efficiency (nA/pC) and current-voltage relationships for CaV1.2Δ1800 + DCT channels with triple-alanine substitution (S1700A, T1704A, and S1928A) or wild-type channels in the presence or absence of 1 μM RO 31-8220. **P < 0.01 versus CaV1.2Δ1800 + DCT channels in control conditions. (D) Coupling efficiency (nA/pC) and current-voltage relationships for CaV1.2FL channels with triple-alanine substitution (S1700A, T1704A, and S1928A) or wild-type channels in the presence or absence of 1 μM RO 31-8220. **P < 0.01 versus wild-type CaV1.2FL channels in control conditions. Significance was determined by ANOVA.
The largest reduction of basal Ba2+ currents and coupling efficiency was observed with CaV1.2Δ1800 + DCT channels containing alanine substitutions at each phosphorylation site (Fig. 6C, bright green); both the low coupling efficiency and the Ba2+ current for the triple-alanine mutant were similar to those observed for wild-type CaV1.2Δ1800 + DCT during inhibition of protein kinase activity (Fig. 6C, brown). Analogous results were observed for CaV1.2FL channels (Fig. 6D). These results show that the basal CaV1.2 channel activity depends on phosphorylation of Thr1704 and Ser1700, but not on phosphorylation of Ser1928.
Phosphorylation sites required for PKA stimulation of CaV1.2 channel activity
We tested the role of phosphorylation of Ser1700, Thr1704, and Ser1928 in PKA-dependent stimulation of CaV1.2 channels by stimulating adenylyl cyclase with 5 μM forskolin. No forskolin-induced stimulation of the triple-alanine mutant was observed (Fig. 7, A and B, bright green) compared to wild-type channels treated with RO 31-8220 (1 μM) (Fig. 7A, brown). These results indicate that both PKA-stimulated activity and basal activity of CaV1.2 channels require phosphorylation of one or more of these three sites.
Fig. 7.
Requirement for phosphorylation of Ser1700, Thr1704, and Ser1928 for PKA-mediated stimulation of CaV1.2 channel activity. (A) Coupling efficiency for the indicated constructs of CaV1.2Δ1800 + DCT in the presence or absence of 1 μM RO 31-8220, AKAP15, and 5 μM forskolin. Dashed black line indicates the mean current in unstimulated CaV1.2Δ1800 + DCT. *P < 0.05 or **P < 0.01 versus AKAP15 and forskolin (ITail, peak tail current; Q, total gating current; Vrev, reversal potential). (B and C) Current-voltage relationships of CaV1.2Δ1800 + DCT from (A) as indicated. Significance was determined by ANOVA.
Restoration of Ser1928 had no effect (Fig. 7, A and B, cyan). Restoration of Thr1704 restored basal channel activity but not forskolin-stimulated activity (Fig. 7, A and B, yellow). Restoration of Ser1700 yielded substantial PKA-dependent stimulation of coupling efficiency and Ba2+ currents (Fig. 7, A and B, purple), similar to the effects of forskolin on wild-type CaV1.2Δ1800 + DCT. Together, these results define distinct roles for phosphorylation of Ser1700, Thr1704, and Ser1928 in regulation of CaV1.2 channels: Phosphorylation of Thr1704 contributes primarily to basal regulation of channel function, whereas phosphorylation of Ser1700 is uniquely required for PKA-dependent enhancement of channel activity. A similar pattern emerged when CaV1.2 channels containing only a single-alanine substitution were studied (Fig. 7, A and C). Mutant S1700A lost PKA-dependent modulation of CaV1.2 channel activity but retained basal activity (Fig. 7, A and C, gray-green). In contrast, single-alanine substitutions at Ser1928 or Thr1704 had no significant effect on coupling efficiency or Ba2+ currents (Fig. 7, A and C, blue and orange). Analyses of the coupling efficiency of individual cells supported these conclusions (fig. S2B). Five of eight (62.5%) cells coexpressing CaV1.2Δ1800(T1704A) + DCT with AKAP15 and 5 of 10 (50%) cells coexpressing CaV1.2Δ1800 + DCT(S1928A) with AKAP15 showed a higher coupling efficiency in the presence of 5 μM forskolin than the maximum observed with CaV1.2Δ1800 + DCT in the absence of forskolin (fig. S2D). In contrast, no individual cells showed a coupling efficiency in the same range when cells coexpressing CaV1.2Δ1800(S1700A) + DCT and AKAP15 were treated with 5 μM forskolin. Collectively, these results indicate that phosphorylation of Ser1700 is required for PKA-mediated stimulation of CaV1.2 channels and that phosphorylation of Thr1704 plays a primary role in regulation of basal activity. We did not identify a contribution for phosphorylation of Ser1928 to short-term regulation of CaV1.2 channel activity.
DISCUSSION
Reconstitution of physiological regulation of CaV1.2 channels in non-muscle cells
PKA-mediated regulation of CaV1.2 channels in cardiac myocytes has several enigmatic aspects. The C-terminal domain is proteolytically processed in vivo (20, 46), but is required for regulation of CaV1.2 channels in cardiac myocytes (34). Despite the high concentration of PKA, AKAP anchoring is required for β-adrenergic regulation in ventricular myocytes (33); however, the AKAP15 binding site is located in the proteolytically processed distal C-terminal domain (33). This unexpected set of properties has confounded previous attempts to reconstitute PKA-dependent regulation of CaV1.2 channels in non-muscle cells, as required to define the molecular mechanism of this process. Indeed, only slight PKA-dependent modulation of full-length CaV1.2 channels has been previously observed, even with coexpression ofAKAP150 [for instance, (31, 35, 36, 42)], raising the possibility that key molecular components of the in vivo CaV1.2 channel complex were missing from these reconstituted systems. Our results resolve this conundrum. Here, we show that a reconstituted autoinhibitory CaV1.2 channel signaling complex containing AKAP15 and the noncovalently associated distal C-terminal domain is both necessary and sufficient to reconstitute physiological levels of PKA-dependent regulation of CaV1.2 channels, and we identify key phosphorylation sites that are required for basal regulation of CaV1.2 channel activity and for the PKA-dependent increase of channel activity. Because our reconstitution system incorporates all of the previously described characteristics of regulation of cardiac CaV1.2 channels in vivo, and resolves the apparent paradoxes in the previous in vivo studies, these results provide a conceptual and molecular framework for calcium channel regulation in the fight-or-flight response, in which PKA phosphorylation mediates disinhibition of an autoinhibitory signaling complex containing CaV1.2Δ1800 + DCT and AKAP15.
Requirement for AKAP15 for reconstitution of PKA regulation
AKAP15 was identified biochemically in purified preparations of skeletal muscle CaV1.1 channels (29) and cloned from skeletal and cardiac muscle (30, 31), where it was also designated AKAP18 (31). It anchors PKA to both CaV1.1 and CaV1.2 channels (29–31), and its interaction with the distal C-terminal domain of CaV1.2 channels is required for β-adrenergic regulation through the PKA pathway in ventricular myocytes (33). Consistent with this requirement for AKAP in ventricular myocytes, we find that AKAP15 is required for reconstitution of PKA-dependent modulation in non-muscle cells, supporting the conclusion that our in vitro reconstitution mimics this aspect of PKA regulation in vivo.
Requirement for noncovalent association of the distal C-terminal domain for PKA regulation of CaV1.2 channels
A surprising conclusion from our results is that the distal C-terminal domain is required for CaV1.2 channel regulation, but is effective only when it is noncovalently associated. Most CaV1.2 channels in cardiac and skeletal muscle are truncated near the center of the C terminus (18–20, 46), and about half of CaV1.2 channels in neurons are also truncated (23). The distal C-terminal domain is not degraded and is retained in noncovalent association with the channel (21, 28). Furthermore, the distal C-terminal domain is required for β-adrenergic regulation of truncated CaV1.2 channels in cardiac myocytes (34). Therefore, the requirement for noncovalent association of the distal C-terminal domain observed in our reconstitution system mimics this requirement in cardiac myocytes.
It is unexpected that the distal C-terminal domain is required for anchoring AKAP15 and PKA and yet must be truncated and noncovalently associated to mediate channel regulation. Our biochemical studies provide an explanation for this paradox. When full-length CaV1.2 channels are studied, activation of PKA does not increase phosphorylation of Ser1700 (Fig. 5D). In contrast, activation of PKA increases phosphorylation of Ser1700 substantially for CaV1.2Δ1800 + DCT (Fig. 5D). Evidently, covalent association of the distal C-terminal in the full-length CaV1.2 channel occludes Ser1700 and reduces or prevents its phosphorylation by PKA. These results support the conclusion that the noncovalently associated complex of CaV1.2 channels with DCT is the primary physiological substrate for PKA-dependent enhancement of channel activity.
PKA-mediated enhancement of CaV1.2 channel activity results from disinhibition
The distal C-terminal inhibits the activity of CaV1.2 channels when it is noncovalently associated (28). Our results show that PKA phosphorylation reverses this inhibition and returns the activity of the noncovalently associated complex of CaV1.2Δ1800 + DCT to nearly that observed for CaV1.2Δ1800 alone. These results illustrate an additional essential property of the distal C-terminal domain—it must inhibit CaV1.2 channel activity so that PKA-mediated phosphorylation can relieve that inhibition and increase channel activity. Molecular models suggest that Ser1700 and Thr1704 are located at the interface between the DCRD and the PCRD, where we have shown that charge-neutralizing mutations can block the inhibitory effects of the DCRD (Fig. 5A) (28). These results predict that phosphorylation at Ser1700 and Thr1704 would disrupt the interaction between these two domains and lead to disinhibition of the channel. Thus, PKA-dependent regulation of CaV1.2 channels proceeds through disinhibition of an autoinhibited signaling complex of CaV1.2Δ1800, non-covalently associated DCT, and AKAP15. This mode of regulation by dis-inhibition of an autoinhibited signaling complex is unique for ion channels studied to date.
Differential regulation of basal and PKA-dependent CaV1.2 channel activity by protein phosphorylation
The basal activity of CaV1.2 channels is decreased by inhibition of PKA and by mutation of Ser1700 and Thr1704 (Figs. 2 and 6). These results implicate phosphorylation of Ser1700 by PKA and Thr1704 by casein kinase II in regulation of basal CaV1.2 channel activity in unstimulated cells. In contrast, the forskolin-dependent increase in CaV1.2 channel activity required phosphorylation of Ser1700, and little or no contribution of phosphorylation of Thr1704 or Ser1928 was detected. Because the response to activation of PKA was completely blocked for mutant S1700A, it is likely that Ser1700 is the primary site of regulation of CaV1.2 channels in response to the β-adrenergic receptor–PKA signaling pathway.
In contrast to Ser1700 and Thr1704 in the proximal C-terminal domain, Ser1928 is located far from the interface between the DCRD and the PCRD. Although Ser1928 is phosphorylated after β-adrenergic activation in ventricular myocytes (25), we found no effect of Ser1928 phosphorylation in regulation of CaV1.2 channels in our reconstituted regulatory system. In this respect, our results fit closely with those observed in vivo, where phosphorylation of Ser1928 was found not to be required for β-adrenergic regulation of CaV1.2 channels in cardiac myocytes in studies using viral transduction or mouse knock-in mutation methods (34, 44). Because Ser1928 is robustly phosphorylated in response to β-adrenergic stimulation, it seems likely that its phosphorylation serves an unidentified regulatory function.
Dual regulatory role of the distal C-terminal domain of CaV1.2 channels
The distal C-terminal domain of CaV1.2 channels has been implicated in regulation of gene expression in heart (47) and brain (48). In cardiac myocytes, some of the proteolytically cleaved distal C-terminal domain is found in the nucleus and can regulate the transcription of CaV1.2 messenger RNA (mRNA) (47). In brain neurons, the distal C-terminal domain is proteolytically processed in response to calcium entry (24). The distal C-terminal protein is localized in the nuclei of a small fraction of brain neurons and can regulate the transcription of neuronal genes (48). The distal C-terminal domain also binds the calcium-dependent phosphatase calcineurin through anchoring by AKAP150 (36), and anchored calcineurin dephosphorylates the transcription factor NFAT (nuclear factor of activated T cells) and regulates gene expression (36).
CaV1.2 channel regulation and disease
Heart failure is one of the most debilitating diseases of the cardiovascular system. Increasing evidence indicates that heart failure involves, at least in part, a maladaptive regulation of calcium signaling in the heart, in which normal β-adrenergic regulation of CaV1.2 channels fails (49, 50). The importance of precise regulation of CaV1.2 channels is also illustrated by Timothy syndrome, a multifaceted disease in which mutations that impair voltage-dependent inactivation cause arrhythmia, developmental abnormalities, and autism spectrum disorder (51). Our results provide the molecular basis for future investigations of the role of misregulation of CaV1.2 channels by the PKA pathway in heart failure, Timothy syndrome, and other diseases.
MATERIALS AND METHODS
Antibodies and cDNA constructs
cDNA constructs (28), antibody against CaV1.2, and antibody against pS1928 (20) were described previously. Phosphospecific antibody against CaV1.2-pS1700 was generated against residues 1694EIRRAIpSGDLTAEEEL in the proximal C terminus (Pacific Immunology). Mutants S1700A, T1704A, and S1928A were constructed as described in the Supplementary Materials. GST-CaV1.2(1670–1731) peptides were expressed and purified from BL21-STAR Escherichia coli.
Electrophysiology
tsA-201 cells were transfected, whole-cell voltage clamp recordings were performed, and data were analyzed as previously described (28) (see Supplementary Materials). All data are expressed as means ± SEM of n cells. Statistical significance was tested with Student’s t test for pairwise analysis, and analysis of variance (ANOVA) followed by Dunnett’s test for comparison of multiple conditions.
Immunofluorescence, immunoblots, and MS
For immunofluorescence experiments, cells were rinsed with phosphate buffered saline (PBS) (pH 7.4) and fixed for 45 min in 4% paraformaldehyde. Indirect immunofluorescence detection was performed with antibody against CaV1.2-pS1700 and biotinylated goat antibody against rabbit. Samples for all images were prepared identically and viewed at a common gain to permit qualitative comparisons. For immunoblot analysis, cells were washed with PBS 24 hours after transfection and treated with drugs as indicated; membranes were collected, solubilized, and separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE); and the resulting blots were probed with the indicated antibodies. For MS, phosphorylated GST-CaV1.2(1670–1731) peptides were digested with trypsin and subjected to liquid chromatography–MS (LC-MS) analysis. See Supplementary Materials and Methods for details.
Supplementary Material
Acknowledgments
We thank K. Konoki, E. Sharp, and J. Hulme for technical assistance and cDNA constructs and R. Westenbroek for advice on anti-phosphopeptide antibody design and immunocytochemistry.
Funding: This work was funded by NIH grants R01-HL085372 (to W.A.C.), N01-HV28179 (to W.A.C. and M.A.E.), and T32-HL007312 (to M.D.F. and M.A.E.) and American Heart Association grant 2009POST2080270 (to M.D.F.).
Footnotes
www.sciencesignaling.org/cgi/content/full/3/141/ra70/DC1
Fig. S1. Inhibition of CaV1.2Δ1800 channels by DCT.
Fig. S2. Modulation of CaV1.2 channel activity in single cells requires coexpression of AKAP15.
Fig. S3. Alanine substitutions have no effect on CaV1.2Δ1800 channel activity in the absence of DCT.
Materials and Methods
References
Author contributions: W.A.C., T.S., M.D.F., and M.A.E. conceived the research. M.D.F., M.A.E., M.S., T.S., and W.A.C. designed and performed the experiments and analyzed the data. W.A.C., M.D.F., M.A.E., and T.S. wrote the paper.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Reuter H. The dependence of slow inward current in Purkinje fibres on the extra-cellular calcium-concentration. J Physiol. 1967;192:479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsien RW, Giles W, Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac Purkinje fibres. Nat New Biol. 1972;240:181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- 3.Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by β-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- 4.Osterrieder W, Brum G, Hescheler J, Trautwein W, Flockerzi V, Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982;298:576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- 5.Bean BP, Nowycky MC, Tsien RW. β-Adrenergic modulation of calcium channels in frog ventricular heart cells. Nature. 1984;307:371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- 6.Trautwein W, Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- 7.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 8.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi M, Seagar MJ, Jones JF, Reber BF, Catterall WA. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 11.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 12.Ruth P, Röhrkasten A, Biel M, Bosse E, Regulla S, Meyer HE, Flockerzi V, Hofmann F. Primary structure of the β subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989;245:1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 14.Gao T, Puri TS, Gerhardstein BL, Chien AJ, Green RD, Hosey MM. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997;272:19401–19407. doi: 10.1074/jbc.272.31.19401. [DOI] [PubMed] [Google Scholar]
- 15.Striessnig J. Pharmacology, structure and function of cardiac L-type Ca2+ channels. Cell Physiol Biochem. 1999;9:242–269. doi: 10.1159/000016320. [DOI] [PubMed] [Google Scholar]
- 16.Richards MW, Butcher AJ, Dolphin AC. Ca2+ channel β-subunits: Structural insights AID our understanding. Trends Pharmacol Sci. 2004;25:626–632. doi: 10.1016/j.tips.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: A 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc Natl Acad Sci USA. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Jongh KS, Warner C, Colvin AA, Catterall WA. Characterization of the two size forms of the α1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci USA. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 21.Hulme JT, Konoki K, Lin TW, Gritsenko MA, Camp DG, II, Bigelow DJ, Catterall WA. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of Cav1.1 channels in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:5274–5279. doi: 10.1073/pnas.0409885102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brawley RM, Hosey MM. Identification of two distinct proteins that are immunologically related to the α1 subunit of the skeletal muscle dihydropyridine-sensitive calcium channel. J Biol Chem. 1992;267:18218–18223. [PubMed] [Google Scholar]
- 23.Hell JW, Yokoyama CT, Wong ST, Warner C, Snutch TP, Catterall WA. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel α1 subunit. J Biol Chem. 1993;268:19451–19457. [PubMed] [Google Scholar]
- 24.Hell JW, Westenbroek RE, Breeze LJ, Wang KK, Chavkin C, Catterall WA. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci USA. 1996;93:3362–3367. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac Cav1.2 channels during β1-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X, Neely A, Lacerda AE, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J Biol Chem. 1994;269:1635–1640. [PubMed] [Google Scholar]
- 27.Mikala G, Bodi I, Klockner U, Varadi M, Varadi G, Koch SE, Schwartz A. Characterization of auto-regulation of the human cardiac α1 subunit of the L-type calcium channel: Importance of the C-terminus. Mol Cell Biochem. 2003;250:81–89. doi: 10.1023/a:1024910605389. [DOI] [PubMed] [Google Scholar]
- 28.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the Cav1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 30.Gray PC, Johnston BD, Westenbroek RE, Hays LG, Yates JR, III, Scheuer T, Catterall WA, Murphy BJ. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 31.Fraser IDC, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 33.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. β-Adrenergic regulation requires direct anchoring of PKA to cardiac Cav1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci USA. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. β-Adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxy terminus of α1C but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 36.Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kameyama M, Hescheler J, Hofmann F, Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986;407:123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- 38.duBell WH, Rogers TB. Protein phosphatase 1 and an opposing protein kinase regulate steady-state L-type Ca2+ current in mouse cardiac myocytes. J Physiol. 2004;556:79–93. doi: 10.1113/jphysiol.2003.059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis PD, Elliot LH, Harris W, Hill CH, Hurst SA, Keech E, Kumar MK, Lawton G, Nixon JS, Wilkinson SE. Inhibitors of protein kinase C. 2. Substituted bisindolylmaleimides with improved potency and selectivity. J Med Chem. 1992;35:994–1001. doi: 10.1021/jm00084a004. [DOI] [PubMed] [Google Scholar]
- 40.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McConnachie G, Langeberg LK, Scott JD. AKAP signaling complexes: Getting to the heart of the matter. Trends Mol Med. 2006;12:317–323. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Zong X, Schreieck J, Mehrke G, Welling A, Schuster A, Bosse E, Flockerzi V, Hofmann F. On the regulation of the expressed L-type calcium channel by cAMP-dependent phosphorylation. Pflugers Arch. 1995;430:340–347. doi: 10.1007/BF00373908. [DOI] [PubMed] [Google Scholar]
- 43.Mitterdorfer J, Froschmayr M, Grabner M, Moebius FF, Glossmann H, Striessnig J. Identification of PK-A phosphorylation sites in the carboxy terminus of L-type calcium channel α1 subunits. Biochemistry. 1996;35:9400–9406. doi: 10.1021/bi960683o. [DOI] [PubMed] [Google Scholar]
- 44.Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged β-adrenergic stimulation of cardiac L-type calcium channels in Cav1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emrick M, Sadilek M, Konoki K, Catterall WA. β-Adrenergic-regulated phosphorylation of the skeletal muscle CaV1.1 channel in the fight-or-flight response. Proc Natl Acad Sci USA. doi: 10.1073/pnas.1012384107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic processing of the C terminus of the α1C subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem. 2000;275:8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- 47.Schroder E, Byse M, Satin J. L-type calcium channel C terminus autoregulates transcription. Circ Res. 2009;104:1373–1381. doi: 10.1161/CIRCRESAHA.108.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel CaV1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: The beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks AR. Heart failure and sudden cardiac death: Causes and cures. Harvey Lect. 2005;101:39–57. [PubMed] [Google Scholar]
- 51.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Cav1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.