Abstract
Bone morphogenetic proteins (BMPs) are widely used as bone graft substitutes in spinal fusion, but are associated with numerous adverse effects. The growth factor Nel-like molecule-1 (Nell-1) is mechanistically distinct from BMPs and can minimize complications associated with BMP therapies. This study evaluates the efficacy of Nell-1 combined with demineralized bone matrix (DBM) as a novel bone graft material for interbody spine fusion using sheep, a phylogenetically advanced animal with biomechanical similarities to human spine. Nell-1+sheep DBM or Nell-1+heat-inactivated DBM (inDBM) (to determine the osteogenic effect of residual growth factors in DBM) were implanted in surgical sites as follows: (1) DBM only (control) (n=8); (2) DBM+0.3 mg/mL Nell-1 (n=8); (3) DBM+0.6 mg/mL Nell-1 (n=8); (4) inDBM only (control) (n=4); (5) inDBM+0.3 mg/mL Nell-1 (n=4); (6) inDBM+0.6 mg/mL Nell-1 (n=4). Fusion was assessed by computed tomography, microcomputed tomography, and histology. One hundred percent fusion was achieved by 3 months in the DBM+0.6 mg/mL Nell-1 group and by 4 months in the inDBM+0.6 mg/mL Nell-1 group; bone volume and mineral density were increased by 58% and 47%, respectively. These fusion rates are comparable to published reports on BMP-2 or autograft bone efficacy in sheep. Nell-1 is an independently potent osteogenic molecule that is efficacious and easily applied when combined with DBM.
Introduction
Spinal fusion, a common surgical procedure for alleviating pain associated with abnormal vertebral movement, frequently requires a bone graft source or substitute to fuse the vertebral bones together. Autogenous bone is considered the gold standard for bone grafting and has been harvested from various sources for a number of years.1,2 However, autograft bone harvest is associated with complications, including donor-site morbidity, increased recovery time, and prolonged hospital stay with increasing healthcare costs.3–10 In addition, for revision surgeries or younger children with small iliac crest anatomy, autogenous bone graft may be very limited in supply or impossible to obtain.11,12 Therefore, the use of osteoinductive growth factors is an important alternative to autologous bone graft.
Bone morphogenetic proteins (BMPs) have been the most consistently effective growth factors for inducing bone. The INFUSE Bone Graft is a commercial product consisting of BMP-2 combined with an absorbable collagen sponge and placed into a tapered lordotic titanium fusion device (LT Cage); it was approved by the U.S. Food and Drug Administration for anterior lumbar interbody fusion.13,14 The product OP-1 (Osteogenic Protein 1), based on BMP-7, is approved for humanitarian use in open tibial shaft fractures and bone fractures refractory to conventional treatments.15
The BMP family of growth factors is highly pleiotropic and critically regulates many developmental processes during embryogenesis besides bone production.16–19
At the molecular level, BMPs are known to upregulate the expression of the master osteogenic transcription factor Runt homology domain transcription factor-2 (Runx2, also known as core binding factor-1 [Cbfa1]).20,21 Runx2 expression is essential for bone formation, and expression of Runx2 in mesenchymal stem cells represents the turning point at which commitment toward an osteochondral progenitor lineage is made.22,23 However, depending on the cell type, BMPs can regulate bone formation in a relatively nonspecific and inefficient way by influencing multiple targets upstream and downstream of Runx2.24 Indeed, BMP has been shown to activate adipogenesis25–27 and osteoclastogenesis,28,29 and can suppress Wnt-mediated osteogenesis in the bone marrow compartment.30 Moreover, the supraphysiological BMP doses necessary for human bone healing31 are accompanied by adverse effects such as cyst formation,32 native bone resorption, heterotopic bone formation, soft tissue swelling, dysphagia, airway compromise, and antibody formation.33–45 Therefore, although BMP exhibits proven osteoinductivity, there is a clinical need for a more specific, mechanistically distinct growth factor with reduced potential for adverse effects.
Nel-like molecule-1 (Nell-1) is a highly conserved secreted protein whose role in bone formation was first described in the context of craniosynostosis.46,47 Nell-1-deficient mice are neonatal lethal, with major skeletal anomalies, indicating that Nell-1 plays a critical role in early bone development.48,49 Further, Nell-1 induces significant in vivo bone formation in animal models.50–53 Importantly, Nell-1 and BMP-2 represent two distinct but complementary pathways of bone formation. In contrast to BMP, which can act both upstream and downstream of Runx2, Nell-1 is a direct downstream target of Runx2,54 suggesting that it acts preferentially on committed osteochondral cells. Indeed, unlike BMP, injection of Nell-1 alone into muscle does not result in ectopic bone formation.55 We have functionally defined the role of Nell-1 in bone formation by demonstrating Nell-1 enhanced bone growth in critical-sized calvarial defect,56 femoral segmental defect (Zara et al., in press), and spinal fusion models,57–59 without observing ectopic bone formation, increased inflammation, or other adverse effects described for BMPs. Therefore, Nell-1 represents an attractive alternative to BMPs, due to its relative specificity to bone formation and lack of undesired sequelae.
The aim of this study was to assess the osteogenicity and optimal dosing of Nell-1 protein in a sheep spinal fusion model. As one of the highest mammalian platforms to study osteogenesis, sheep are excellent bone healing models for both lumbar and thoracic spine.60–63 Further, sheep are an appropriate alternative for evaluating spinal implants due to the quantitative biomechanical similarities of intact sheep and human spines.64,65 As a carrier for the Nell-1 protein, we chose demineralized bone matrix (DBM) in an hyaluronan putty formulation (DBX®) because it is an easily molded, surgeon-friendly, osteoconductive/osteoinductive material.66 It is less compressible than other protein carriers such as absorbable collagen sponges, which would alter local Nell-1 concentrations. We found that Nell-1 protein dose-dependently improved fusion rate in sheep spines from 50% (Nell-1-free control) to 100% (0.6 mg/mL Nell-1) with up to 58% increase in bone volume fraction and 47% increase in bone mineral density in the newly formed bone, as evaluated by microcomputed tomography (μCT), compared to Nell-1-free controls. In addition, by inactivating our DBM-based carrier material with heat, we demonstrated that Nell-1 could function independently of residual growth factors present in bone matrix,67 while maintaining the favorable osteoconductive, form-factor, and material qualities of demineralized bone matrix. Our results quantitatively demonstrate that Nell-1 protein is an excellent inducer of bone tissue that achieved equivalent levels of bone fusion at equivalent time points as previously reported for BMP and autograft,68,69 and shows great promise as an osteoinductive factor that may mitigate adverse effects associated with BMPs.
Materials and Methods
Implant material
To prevent inflammatory and immune responses to xenografts, sheep DBX (Musculoskeletal Transplant Foundation [MTF], Edison, NJ) was prepared as a single lot using a process identical to human DBX production. Cortical bone from the hind limbs of mature female sheep was harvested under sterile conditions in Colorado and shipped to MTF laboratories in New Jersey. The bones were ground down to cortical fragments, sifted to sort out fragments between 200 and 300 μm in diameter, decalcified in HCl, and placed in antibiotic solution. Finally, the fragments were mixed with hyaluronic acid to create a moldable putty. Final calcium content was ∼5%–9%. BMP-2 concentration was estimated at 3.2 pg/mg, which is comparable to the previously reported value of 3.8 pg/mg BMP-2 in human DBX.70 To isolate the osteogenic effects of Nell-1 from the effects of this residual BMP and other osteogenic factors present in demineralized bone, DBM was inactivated (inDBM) by heating to 105°C for 24 h in a gravity oven. Nell-1 protein was produced as previously described56,71 and mixed with DBM or inDBM immediately before surgical implantation.
Study groups
Eighteen sheep were used to study dose response to Nell-1 protein (Table 1). In all animals, implantation was performed at two lumbar levels, L3/L4 and L5/L6, yielding two data points per animal for a total of 36 lumbar fusions. The two levels have been shown to be biomechanically equivalent64; thus, the two data points per animal are comparable with each other. A 9-mm parallel radiolucent cage (Vertebral Spacer-CR 889.915; Synthes, Monument, CO) filled with 0.4 mL DBM or inDBM was inserted between these lumbar vertebrae. DBM was combined with 0.3 mg/mL Nell-1 (total dose: 0.12 mg; four sheep/eight sites), 0.6 mg/mL Nell-1 protein (total dose: 0.24 mg; four sheep/eight sites), or no protein (four sheep/eight sites). inDBM was combined with 0.3 mg/mL Nell-1 (total dose: 0.12 mg; two sheep/four sites), 0.6 mg/mL Nell-1 protein (total dose: 0.24 mg; two sheep/four sites), or no protein (two sheep/four sites). Contents of cages were randomly assigned to different sheep, thereby blinding the surgeons.
Table 1.
Computed Tomography Assessment of Spinal Fusion Frequency with Indicated Doses of Nell-1 Protein at Each Experimental Time Point
|
A. Native Sheep DBM/Vertebral Spacers (Sacrificed 3 Months Postsurgery) | ||
|---|---|---|
| |
Fused sites/total sites (% fused) |
|
| Implant contents | 2 months | 3 months |
| DBM only (control) | 3/8 (37.5%) | 4/8 (50%) |
| DBM+0.3 mg/mL Nell-1 (total dose: 0.12 mg) | 2/8 (25%) | 7/8 (87.5%) |
| DBM+0.6 mg/mL Nell-1 (total dose: 0.24 mg) | 6/8 (75%) | 8/8 (100%) |
|
B. Heat-Inactivated sheep DBM (inDBM)/Vertebral Spacers (Sacrificed 4 Months Postsurgery) | |||
|---|---|---|---|
| |
Fused sites/total sites (% fused) |
||
| Implant contents | 2 months | 3 months | 4 months |
| inDBM only (control) | 0/4 (0%) | 0/4 (0%) | 2/4 (50%) |
| inDBM+0.3 mg/mL Nell-1 (total dose: 0.12 mg) | 0/4 (0%) | 1/4 (25%) | 4/4 (100%) |
| inDBM+0.6 mg/mL Nell-1 (total dose: 0.24 mg) | 2/4 (50%) | 3/4 (75%) | 4/4 (100%) |
inDBM, inactivated demineralized bone matrix; Nell-1, Nel-like molecule-1.
Sheep surgery model
All sheep surgery protocols were approved by the Colorado State University IULAC. Skeletally mature Rambouillet×Columbian ewes (Three JP; LLC livestock suppliers, La Junta, CO), each weighing 57–102 kg, were perioperatively premedicated with midazolam and ketamine for pain management and cefazolin as an antibiotic. Anesthesia was induced with 5% and maintained with 3% isoflurane gas. Wool was removed from the left lateral lumbar region and the sheep was placed in a right lateral recumbent position on the operating table. The lumbar region was prepared for aseptic surgery with multiple scrubs of povidone-iodine alternated with isopropyl alcohol, followed by sterile draping. A ventrolateral retroperitoneal approach to L3/L4 and L5/L6 through the oblique abdominal muscles to the plane ventral to the transverse processes was made. The disc space of L5/L6 was identified and an annulotomy performed. Using a Midas-Rex burr, the endplate was prepared to a size that would accommodate the cage. Using a vertebral spreader, the disc space was opened and the cage (containing DBM either alone or with protein) was pressed into place. The same procedure was performed at L3/L4, resulting in two implantation sites per animal with an unoperated disc space in between. After implantation, the external abdominal muscular fascia, subcutaneous tissue, and skin were sutured closed. Operative time for each animal was ∼40 min.
Radiographic evaluation and CT imaging
Radiographs of the fusion sites were taken at 0, 2, 3, and (for the inDBM group) 4 months to follow the progression of the fusion. CT images were taken at 2, 3, and (for the inDBM group) 4 months under general anesthesia. Successful spine fusion was defined as ≥50% area of contiguous bridging bone within the implant by CT evaluation. CT evaluation of fusion quality was performed using a 4-point scale described previously, ranging from 0 (no bone formation) to 4 (excellent bone formation).68 Sheep receiving DBM implants were euthanized by barbiturate overdose at 3 months postsurgery; sheep receiving inDBM implants were euthanized after 4 months. The L3/L4 and L5/L6 regions were extracted after sacrifice and fixed in formaldehyde.
μCT and histology
μCT images of the newly developed bone within the cages were collected and analyzed with the Scanco μCT 40 (Brüttisellen, Switzerland). For analysis, a volume of interest centered anteroposteriorly within the cage, with diameter 4.4 mm and height 3 mm, was selected. The bone volume fraction (BV/TV) and bone mineral density (BMD) with respect to the total volume of interest was calculated at a threshold value of 240. For histologic processing, specimens were decalcified with 10% HCl solution, washed under running tap water, and stored in 75% ethanol. Specimens were embedded in paraffin, cut into 5-mm coronal sections at the level of the transverse processes, and mounted on clear slides. Hematoxylin–eosin, Masson's Trichrome, and von Kossa staining were performed as described.72,73
Finite-element analysis
μCT data were converted to DICOM images using Scanco μCT 40 software. Three-dimensional mesh models were reconstructed from DICOM images using Mimics software (Materialise NV, Leuven, Belgium) using a voxel size of 36×36×180 μm. Finite-element analyses (FEA) on the models were performed using ANSYS software (Canonsburg, PA). While fixing the posterior bone surface of the bridging bone within the intervertebral cage as an initial condition, a compressive stress of 0.5 MPa was evenly applied to the anterior surface, and the equivalent tensile stress experienced by each element was calculated and plotted.
Statistical methods
Statistical significance of spinal fusion frequencies was established by χ2 testing at p<0.05. Significance of bone fusion quality scores was established using the one-sided Student's t-test. Significances of BV/TV and BMD measurements were established pairwise using the one-sided Student's t-test and among experimental groups using one-way analysis of variance testing at p<0.05. The Mann–Whitney U-test was also used to compare the control group with the two experimental groups.
Results
Radiograph and CT
Radiography was performed to ensure proper intervertebral cage placement postoperatively and absence of vertebral subsidence throughout the study. Immediately after the procedure, the cage was visible in all sheep spine radiographs (Fig. 1). Axial CT images with sagittal and coronal reconstructions were used to observe bone development within cages. Fusion rates are summarized in Table 1. For DBM-implanted spines evaluated at 3 months postsurgery, only four of eight (50%) Nell-1-free sites exhibited fusion within the central hollow of the cage. However, when DBM/0.3 mg/mL Nell-1 was used, seven of eight (87.5%) sites were fused, and all eight (100%) were fused when DBM/0.6 mg/mL Nell-1 was used. Fusion rate in the DBM/Nell-treated groups, compared to the DBM/Nell-1-free group, was significant to a p-level of 0.002 according to the χ2-test among the three groups. Fused samples exhibited bridging bone within the cages advancing rostrally and caudally to meet at the center. Radiographic scoring of CT images revealed a significant increase in bone quality in DBM/0.6 mg/mL Nell-1 spines at both 2 and 3 months postsurgery (Table 2).
FIG. 1.
(A) Postoperative radiography of sheep spines implanted with DBM plus indicated Nell-1 protein doses. Vertebral spacers are clearly observed at both L3/L4 and L5/L6 axial levels. (B) Sagittal, coronal, and axial CT slices of bone formation within L3/L4 and L5/L6 spacers of spines implanted with DBM plus indicated Nell-1 protein doses. Arrows indicate locations of implants. Nell-1 increased bone formation within the vertebral spacer compared to Nell-1-free controls. CT, computed tomography; DBM, demineralized bone matrix; Nell-1, Nel-like molecule-1.
Table 2.
Semiquantitative Assessment of Spinal Fusion Adapted from Magin et al. (2001)
|
A. Native Sheep DBM/Vertebral Spacers (Sacrificed 3 Months Postsurgery) | ||
|---|---|---|
| 2 months | 3 months | |
| DBM only | 1.38±1.51 | 2.25±1.39 |
| DBM+0.3 mg/mL Nell-1 | 2.00±0.76 | 2.25±0.46 |
| Significance vs. control | NS | NS |
| DBM+0.6 mg/mL Nell-1 | 2.63±0.52 | 3.38±0.74 |
| Significance vs. control | p<0.03 | p<0.04 |
|
B. Heat-Inactivated Sheep DBM (inDBM)/Vertebral Spacers (Sacrificed 4 Months Postsurgery) | |||
|---|---|---|---|
| 2 months | 3 months | 4 months | |
| inDBM only | 0.75±0.50 | 1.50±0.58 | 2.00±0.00 |
| InDBM+0.3 mg/mL Nell-1 | 1.25±0.50 | 2.25±0.50 | 2.50±0.58 |
| Significance vs. control | NS | p<0.05 | NS |
| inDBM+0.6 mg/mL Nell-1 | 2.25±0.96 | 2.75±0.50 | 3.25±0.50 |
| Significance vs. control | p<0.025 | p<0.009 | p<0.008 |
| Historical radiographic score for autograft68 | 1.75±0.46 | 2.0±0.0 | 2.0±0.0 |
Scores range from 0 (no bone formation) to 4 (excellent bone quality), and are given as mean±SD.
NS, not significant.
For inDBM-implanted spines evaluated at 3 months postsurgery, fusion rates were lower than for DBM-implanted spines at 3 months. However, when evaluated at 4 months postsurgery, all sites were fused when either inDBM/0.3 or 0.6 mg/mL Nell-1 was used, compared to two of four sites fused in the inDBM/Nell-1-free controls. χ2 analysis revealed a significant difference (p=0.01) in fusion rate between the three groups at 4 months (Table 1). Further, bone quality was significantly improved at all time points evaluated when inDBM/0.6 mg/mL Nell-1 was used, and exceeded the historical autograft fusion scores of a previous study using the same scoring method68 (Table 2).
Microcomputed tomography
Sheep containing DBM implants were sacrificed at 3 months postoperation; sheep containing inDBM implants were sacrificed at 4 months postoperation. At sacrifice, vertebral columns were extracted for μCT analysis. Standardized, 3-mm-thick cylindrical regions of bone within the intervertebral cage implant sites were selected for imaging and bone density measurement (Fig. 2). The Nell-1-free spines exhibited minimal, fragmented new bone growth, with very little connectivity between bone fragments and the native bone and among fragments. In contrast, more consolidated bone was present within the cages in Nell-1-treated spines, and complete fusion of vertebrae through the center of the cage was achieved in seven of eight sites containing 0.3 mg/mL Nell-1 and all eight sites containing 0.6 mg/mL Nell-1 (Table 3), confirming our live CT findings.
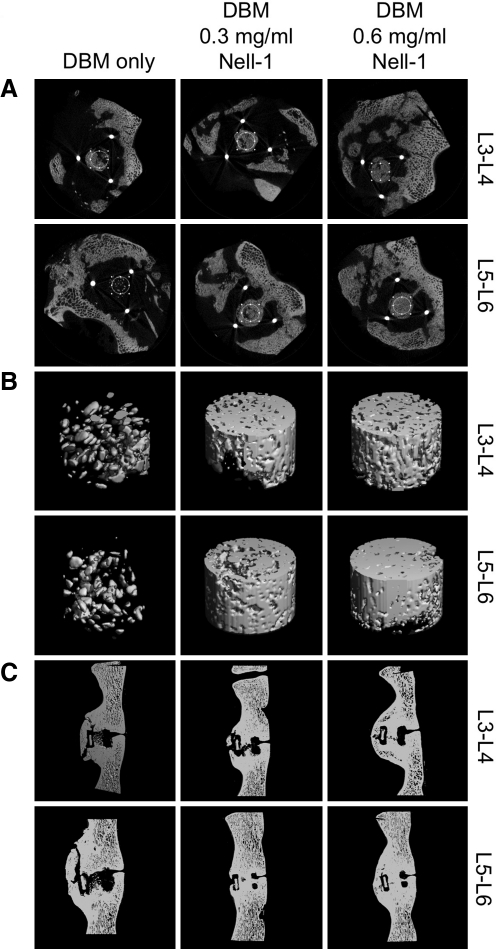
FIG. 2.
Representative μCT images of spinal fusions at L3/L4 and L5/L6 implanted with DBM containing the indicated doses of Nell-1 protein. (A) Axial sections through anteroposterior center of implant. Circles in the two-dimensional transverse sections indicate the region of bone within the spacer selected for three-dimensional reconstructions (B). Threshold value 300. (C) Sagittal reconstructions taken at mediolateral center of implant. Threshold value 180.
Table 3.
Postsacrifice Microcomputed Tomography Assessment of Spinal Fusion Quality
|
A. Native Sheep DBM/Vertebral Spacers (Sacrificed 3 Months Postsurgery) | ||||
|---|---|---|---|---|
| Implant contents | BV/TV (mean±SD) | t-test p (compared to control) | ||
| DBM only (control) | 0.59±0.35 | — | ||
| Fused (4/8) | Nonfused (4/8) | |||
| 0.90±0.12 | 0.29±0.14 | |||
| DBM+0.3 mg/mL Nell-1 | 0.81±0.18 | 0.071 | ||
| Fused (7/8) | Nonfused (1/8) | |||
| 0.81±0.20 | 0.84 | |||
| DBM+0.6 mg/mL Nell-1 | 0.93±0.07 | 0.014a | ||
| Fused (8/8) | ||||
| Implant contents | BMD (mg/mm3) (mean±SD) | t-test p (compared to control) | ||
|---|---|---|---|---|
| DBM only (control) | 307±153 | — | ||
| Fused (4/8) | Nonfused (4/8) | |||
| 438±78 | 176±50 | |||
| DBM+0.3 mg/mL Nell-1 | 394±86 | 0.094 | ||
| Fused (7/8) | Nonfused (1/8) | |||
| 397±93 | 371 | |||
| DBM+0.6 mg/mL Nell-1 | 450±64 | 0.018a | ||
| Fused (8/8) | ||||
|
B. Heat-Inactivated Sheep DBM/Vertebral Spacers (Sacrificed 4 Months Postsurgery) | ||
|---|---|---|
| Implant contents | BV/TV | t-test p (compared to control) |
| inDBM only (control) | 0.58±0.04 | — |
| inDBM+0.3 mg/mL Nell-1 | 0.79±0.14 | 0.023a |
| inDBM+0.6 mg/mL Nell-1 | 0.88±0.07 | 0.0003b |
| Implant contents | BMD (mg/mm3) | t-test p (compared to control) |
|---|---|---|
| inDBM only (control) | 297±21 | — |
| inDBM+0.3 mg/mL Nell-1 | 405±76 | 0.03a |
| inDBM+0.6 mg/mL Nell-1 | 432±36 | 0.0008b |
Fused and unfused subsets within each group are indicated. BV/TV (bone volume/tissue volume) and BMD (bone mineral density) given as mean±SD.
p≤0.05.
p≤0.001.
BMD, bone mineral density.
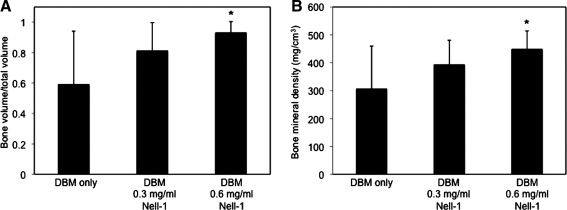
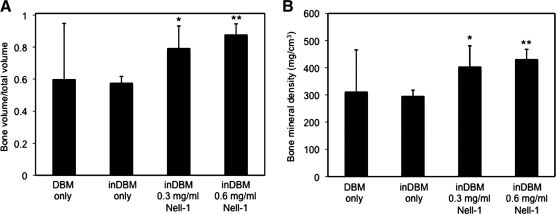
To quantify the amount of bone growth, we measured the bone volume fraction (BV/TV) and bone mineral density (BMD) of the column of bone within the cage (Fig. 3). Implantation sites containing either DBM/0.3 or 0.6 mg/mL Nell-1 exhibited increased BV/TV and BMD in a dose-dependent manner within the cage compared to untreated sites. The DBM/0.6 mg/mL Nell-1 dose increased both parameters significantly as measured by the pairwise Student's t-test, indicating that this is the more optimal dose for bone induction. Notably, the DBM control group exhibited the highest standard deviation in BV/TV and BMD, whereas the DBM/0.6 mg/mL Nell-1 group exhibited the lowest standard deviation. Examining further, we found that BV/TV and BMD of the DBM control group followed a bimodal distribution corresponding to the three fused and five nonfused samples within the group, which contributes to the large standard deviation. The distributions between the DBM control and 0.3 mg/mL groups (Mann–Whitney U=22 [BV/TV], U=23 [BMD], n1=n2=8, p<0.02 two-tailed) and between the DBM control and 0.6 mg/mL groups (Mann–Whitney U=12 [BV/TV], U=15 [BMD], n1=n2=8, p<0.02 two-tailed) differed significantly. Importantly, the fused samples had similar BV/TV and BMD as the fused samples in the DBM/0.3 and 0.6 mg/mL Nell-1 groups, whereas the nonfused samples exhibited an average BV/TV of 0.29 and BMD of 176 mg/cm3—well below the averages for the DBM/Nell-1-treated groups. μCT analysis of the inDBM-implanted samples revealed that the quality of new bone in inDBM Nell-1-free sites was consistently low (BV/TV=0.58±0.04; BMD=297±21 mg/mm3), suggesting that DBM inactivation may have eliminated the high variability observed with native DBM (Table 3). Three-dimensional reconstruction of inDBM Nell-1-free control and Nell-1-treated spines (Fig. 4) revealed disconnected, fragmented bone in Nell-1-free controls contrasting with contiguous, convergent bone in inDBM Nell-1-treated spines, similar to the results observed with native DBM. Quantitative measurements revealed statistically significant increases of BV/TV and BMD in inDBM/Nell-1-treated sites compared to inDBM/Nell-1-free controls (Fig. 5).
FIG. 3.
(A) Bone volume fraction (BV/TV) and (B) bone mineral density measurements of bone segments within vertebral spacers containing native DBM and indicated doses of Nell-1 protein. n = 8 for each group. *p ≤ 0.01.
FIG. 4.
Three-dimensional μCT reconstruction images of spinal fusions at L3/L4 and L5/L6 implanted with inDBM containing the indicated doses of Nell-1 protein. Threshold value 300. Transverse and sagittal views are similar to native DBM (data not shown). inDBM, inactivated DBM.
FIG. 5.
(A) BV/TV and (B) bone mineral density measurements of bone segments within vertebral spacers containing heat-inDBM and indicated doses of Nell-1 protein. DBM measurements were made on samples sacrificed after 3 months; inDBM measurements were made on samples sacrified after 4 months. n = 8 for each group. *p ≤ 0.03, **p ≤ 0.001.
Finite-element analysis
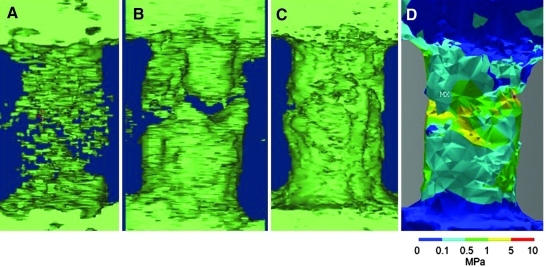
The use of an intervertebral cage between the two vertebral bodies precluded a full ex vivo biomechanical measurement, because newly formed bone is completely confined within the cage, and removing the cage is impossible without damaging or destroying the bone structure within. Thus, we used an FEA approach to perform biomechanical analyses on the harvested spines (Fig. 6). Because the Nell-1-free and DBM/0.3 mg/mL Nell-1 samples did not form a continuous bridge of bone between the vertebral bodies, FEA was impossible except in the DBM/0.6 mg/mL Nell-1 sample; in these samples, all mechanical stress between the two vertebral bodies is borne by the intervertebral cage, not the bridging bone. We reconstructed a three-dimensional model of the continuous bone within the cage in the DBM/0.6 mg/mL Nell-1 sample. Given the relative similarity of human and sheep lumbar spine biomechanical attributes,65 we applied a 0.5 MPa compressive stress to the anterior surface of the bone, which approximates human intradiscal pressure during relaxed standing.74 We confirmed comparatively uniform bone formation, with mechanical stress distributed relatively evenly within a range of 0.1 (light blue) to 5 (yellow) MPa without foci of excessive mechanical stress, and maximal stress levels well below the estimated trabecular bone yield strength of 30 MPa.75
FIG. 6.
Finite-element biomechanical analysis of extracted spines. Three-dimensional meshes of (A) DBM-only and (B) DBM/0.3 mg/mL Nell-1 samples; these did not form a continuous bone bridge and could not be analyzed by finite-element analysis. (C) The DBM/0.6 mg/mL Nell-1 sample formed a continuous bridge of bone. (D) Heat plot of equivalent tensile stress experienced by each element with color key for stress in megapascals (MPa) shown below. No element experienced more than the estimated trabecular bone yield strength of 30 MPa.
Histology
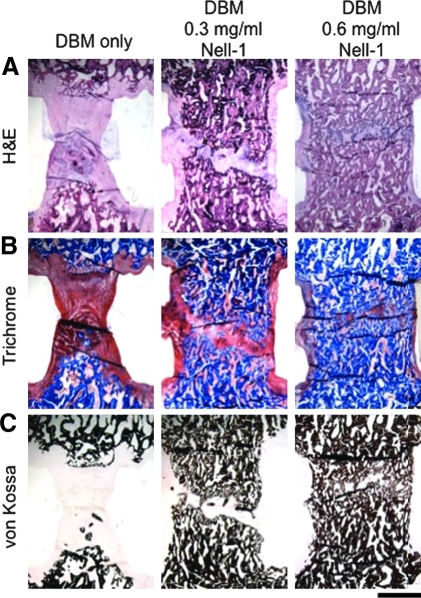
To support our FEA simulations, hematoxylin–eosin, Masson's Trichrome, and von Kossa staining were used to assess overall structure, connective tissue structure, and mineralization, respectively (Fig. 7). In general, histological observations correlated well with μCT measurements. In Nell-1-free samples, the connection between the vertebrae appears to be a pseudarthrosis bridged by fibrous tissue, and only scant, fragmented bone extending from the caudal vertebral body with no bone bridging the two vertebrae was observed. In the DBM/0.3 mg/mL Nell-1 sample, a large amount of mature, trabeculated bone was present between the vertebrae, with a correspondingly smaller pseudarthrosis, although complete bone fusion between the vertebrae was not achieved. Complete bone fusion between vertebrae was achieved at a DBM/Nell-1 dose of 0.6 mg/mL, and the maturity and trabeculation of the new bone within the cage resembled that of the adjacent native bone. Analysis of inDBM spines with 0.3 and 0.6 mg/mL Nell-1 revealed similar evidence of complete fusion between vertebrae compared to the Nell-1-free controls (data not shown).
FIG. 7.
Histological analysis of spinal fusions at L5/L6 at indicated doses of Nell-1 protein. (A) Hematoxylin–eosin, (B) Masson's Trichrome, and (C) von Kossa stains were used to assess overall structure, connective tissue structure, and mineralization, respectively. Blue and black staining represent mineralized bone in Trichrome and von Kossa staining, respectively. Increased bone formation is observed in Nell-1-treated samples. Results are similar at L3/L4 (data not shown). Magnification: 10.8×. Scale bar = 2 mm.
Discussion
We have demonstrated the ability of Nell-1 protein, delivered from a sheep DBM carrier (DBX), to enhance bone production and achieve solid arthrodesis in a sheep spinal fusion model. We observed callus formation at the surgical sites (Fig. 1); however, calluses were not included in the fusion criteria because they can appear as generalized responses to injury and not as direct effects of growth factors.76 Importantly, our results indicate a positive dose response to the Nell-1 protein, as increases in protein dose increased the amount and quality of new bone formation. FEA simulations demonstrated that the bone formed when DBM/0.6 mg/mL Nell-1 was used did not experience excessive local mechanical stresses under compression; as the bone was tested in isolation, we expect that the intervertebral cage would impart additional mechanical stability to the spine. Finally, by inactivating the endogenous growth factors within DBM with heat, we demonstrated that Nell-1 could increase spinal fusion rate independently of other growth factors present in bone. In both cases, the quality of the bone formed using DBM plus Nell-1 approached or exceeded that of autograft bone in previous studies (Table 2).68
The ultimate goal of this research is to obtain FDA approval for Nell-1-based orthobiologic products for spinal fusion (and, more generally, other bone formation and repair applications). The progression of the phylogenetic complexity of our model systems parallels the preclinical trial history of BMP-2 from dog77 and sheep78,79 to primate80,81 animal models, and finally to clinical reports of successful spinal fusion,14,82 which culminated in FDA approval of INFUSE.13,14 Additionally, in reviewing BMP-2 preclinical and clinical development, it is clear that BMP-2 dose requirements for effective osteoinduction are species-specific. BMP-2 concentration and total dose requirements (i.e., concentration×total implant volume) for inducing consistent bone formation differs substantially between species; for example, the required dose in nonhuman primates is 0.75–2.0 mg/mL, whereas it is 0.02–0.4 mg/mL in rodents.83 In addition, there are fundamental differences between osteogenicity in rodent versus human bone marrow stromal cells.84 Thus, our demonstration of spinal fusion in the sheep spine mediated by DBM/Nell-1 is an essential milestone, and is necessary for supporting our previous work demonstrating Nell-1-mediated spinal fusion in rat models.57,58,85
Clinical advantages of Nell-1 protein
Although BMPs are widely used to induce osteoblastogenesis in spinal fusion and fracture repair, the mechanism of BMP function suggests that it may mediate many other processes besides, or instead of, bone formation. BMPs can activate the transcription factor PPARγ, a key regulator of adipocyte commitment,25–27 and can induce formation of cyst-like bone voids filled with fatty marrow instead of bone.32,86 Besides PPARγ activation, the typical, high BMP-2 doses used in humans also represses endogenous β-catenin-dependent Wnt signaling, which downregulates Runx2 expression and inhibits osteoblast differentiation and intramembranous ossification in the endosteum and bone marrow cavity.87 Since Wnt activity suppresses PPARγ and is essential for intramembranous ossification during bone healing by promoting Runx2,87–89 the anti-osteogenic effects of high BMP-2 doses derive in part from its repression of Wnt signaling. Taken together, BMPs contribute to the differentiation of progenitor cells to nonosteoblast fates, such as adipose and cartilage in addition to or instead of bone, which can compromise the structural stability of newly formed bone.90–92
Our laboratory has already proven the principle that Nell-1 delivered by adenovirus improves spinal fusion rate in rats.85 The major advantages of using soluble Nell-1 protein are the avoidance of immunological complications related to adenoviral gene therapy, and an improved ability to control the therapeutic dose, both by adjusting the amount of Nell-1 protein delivery and designing biomaterials to control the rate and timing of delivery (discussed below). Our lab has successfully used Nell-1 in a variety of bone defect models in rats and mice and demonstrated increased bone production limited to the site of implantation without ectopic bone growth (reviewed in Ref.47). At the molecular level, this process represents the ability of Nell-1 to accelerate bone growth and maturation with increased specificity, as it is transcriptionally regulated by Runx2 and increases Runx2 phosphorylation and activity.54,93 Further, we have found that Nell-1 upregulates Wnt activity and inhibits PPARγ activity (unpublished data). Together, we believe that these mechanistic differences between Nell-1 and BMP function make Nell-1 an exciting alternative osteoinductive growth factor that may avoid many of the reported complications related to BMP-mediated spinal fusion and other bone tissue engineering applications.
Scaffolds and biomaterials for Nell-1 delivery
Currently, autologous bone graft remains the gold standard for spinal fusion because of lack of immunogenicity, but drawbacks include donor-site morbidity, extended recovery time, and dependence on the bone quality of the patient. An excellent alternative strategy is to combine allograft bone with growth factors, which produces an implant material with both osteoconductive and osteoinductive modules, and can produce more consistent results because growth factors can be more accurately dosed. An example of this is the burrito technique, where a BMP-soaked absorbable collagen sponge is wrapped around a core of demineralized bone chips.94 This strategy informs a similar combination of Nell-1 with appropriate scaffold and delivery biomaterials to produce a clinically powerful product.
DBX is a mixture of demineralized bone matrix and hyaluronic acid largely deprived of mineral, but contains residual osteoinductive growth factors, most notably BMPs.70 However, both growth factor content and quantity between different lots of DBM and even within the same lot, and intragroup biological response to these growth factors can be highly variable.95–98 To address this concern in the present study, we prepared a single lot of sheep DBM and confirmed that its BMP content is similar to human DBM, suggesting that our findings would also apply to human DBM. As a platform for Nell-1 delivery, DBM is osteoconductive, with a favorable form factor for molding and handling. In addition, DBM maintains a constant volume, which in turn results in a relatively stable local Nell-1 concentration. This contrasts with carriers such as absorbable collagen sponges, which shrink in volume during compression and can thus increase the local concentration of collagen-bound growth factors to undesirably high levels, which can increase the incidence of adverse effects as observed with BMP-2.
Heating DBM denatures the residual growth factors within, rendering them biologically inactive.67 Although DBM inactivation delayed fusion by only 1 month, removal of growth factor bioactivity significantly reduced the observed variation in the ability of DBM alone to induce fusion. Heat inactivation is therefore a useful strategy for normalizing demineralized bone matrix and for directly assessing Nell-1 osteoinductivity. Overall, these results suggest that nondemineralized allograft bone, with hyaluronan added to maintain the putty-like consistency of DBX, may be a more easily processed and lower-cost carrier for Nell-1. Burst-released Nell-1 promoted spinal fusion in our model when delivered from inactivated DBM, indicating that Nell-1 can function independently of growth factors supplied by the implant material, as long as an osteoconductive platform is present.
Consistency of spinal fusion rate
Importantly, our work showed that Nell-1 treatment increased the frequency of fusion and indicates that DBM containing Nell-1 promoted more consistent fusion than DBM alone. There was a large standard deviation associated with the BV/TV and BMD measurements of spines in the control group. We attribute this to the presence of both fused and nonfused samples in this group, such that a bimodal distribution was obtained for both statistics and informing the need for nonparametric statistical analysis. Indeed, a Mann–Whitney U-test confirmed that BV/TV and BMD differed significantly between the control group and either the 0.3 or 0.6 mg/mL Nell-1-treated groups. Importantly, after decomposing the control group into fused and nonfused subgroups, we found that the BV/TV and BMD of the fused samples within the control group agrees with those of the fused samples of both Nell-1-treated groups. Biologically, this suggests that there is a threshold for bone formation/fusion, beyond which bone formation will progress to completion in the 3-month (for DBM) or 4-month (for inDBM) timeframes of our study. Clinically, these findings are of great translational significance because they imply that while DBM alone is osteoconductive, Nell-1 can significantly increase the predictability and success rate of spinal fusion surgery.
Use of biomaterials to prolong the osteoinductive window
The immediate release of protein into a microenvironment with subsequent rapid diffusion, known as the burst effect, is well documented for BMPs.99 Because the protein diffuses away from the vicinity of the implant microenvironment quickly, supraphysiological doses of the protein are required to maintain biologically active protein levels needed for bone growth over the entire time course of the treatment. The diffusion of a nonspecific osteogenic protein to nearby tissues can contribute to the adverse effects associated with high doses of BMP. Nell-1 protein mixed directly with DBM is expected to exhibit a similar burst effect, since the hyaluronic acid in DBM is soluble for <24 h in vivo,100 suggesting that Nell-1 may be released prematurely. Indeed, we have found that 50%–60% of Nell-1 protein bursts from DBM after 24 h (unpublished data). However, since Nell-1 acts preferentially downstream of Runx2 on committed osteochondral cells,54,86 we expect fewer adverse effects related to excessive or ectopic bone growth. Indeed, we have not observed ectopic bone growth, swelling, or inflammation at the implant site in our various model systems (reviewed in Ref.47).
In the present study, we found that fusion can occur as early as 2 months postoperation at the highest dose tested of 0.6 mg/mL Nell-1 (Table 1). Thus, one strategy to further accelerate fusion is to optimize the efficiency of Nell-1 delivery by using a biomaterial carrier to reduce the burst effect and maintain a relatively stable local Nell-1 dose over the entire therapeutic timeframe, thus prolonging the effect of the osteoinductive component of the implant. To this end, we have recently developed β-tricalcium phosphate- and chitosan/alginate-based carriers that are electrostatically coupled to Nell-1.71 This will allow us to achieve controlled release of Nell-1, which will reduce the total dose of Nell-1 required by reducing the burst effect and consequently lowering the total dose of Nell-1 protein required. In addition, a greater amount of Nell-1 would remain localized to the implantation site, increasing local Nell-1 concentration and potentially increasing fusion success rate by extending the period over which Nell-1 can continue to stimulate bone regeneration after initial implantation. We predict that controlled Nell-1 delivery could decrease the time required for 100% fusion to under 3 months for DBM and under 4 months for inDBM.
Conclusion
Our study demonstrated significantly increased fusion rate and enhanced bone quality in sheep when Nell-1 protein was delivered from DBM carrier within implanted intervertebral cages. Nell-1 improved fusion rate from 50% (DBM only) to 100% when 0.6 mg/mL Nell-1 was added, and increased bone formation within the cage by up to 58% and bone mineral density by 47% compared to Nell-1-free controls. Meanwhile, heat inactivation of DBM delayed the osteogenic effect of Nell-1 by only 1 month. Most importantly, Nell-1 in DBM induced spine fusion in time frames comparable to historical studies with autograft bone in sheep (Table 2) without evidence of adverse effects such as cyst formation or ectopic bone production. Overall, Nell-1 is a potently osteogenic molecule that is mechanistically distinct from BMP. The fact that Nell-1 acts more specifically on osteochondral cells than BMPs may minimize adverse effects to improve clinical outcomes.
This present study allows us to envision a combined modular Nell-1/allograft bone-based orthobiologic material that will simultaneously reduce undesirable effects while maintaining or increasing efficacy of current therapies. In this formulation, Nell-1 will be released from carriers in a controlled fashion to both reduce the amount of Nell-1 required and to increase the time window for osteoinductive Nell-1 effects. Further studies will be aimed at elucidating the precise mechanism of Nell-1-induced bone formation, and to develop an ideal combination of Nell-1 protein, sustained delivery vehicle, and scaffold biomaterial for spinal fusion.
Acknowledgments
Sheep DBX was donated by the MTF. Nell-1 protein was donated by Bone Biologics, Inc. The authors would like to thank the Translational Pathology Core Laboratory (TPCL) and Surgical Pathology divisions of the UCLA Department of Pathology and Laboratory Medicine for histology processing, and Moon Hae Sunwoo and Wilton Reynoso at MTF for technical assistance. This work was supported by NIH grant R01 DE016107-01 (K.T.) and UC Discovery Grants Bio05-10489 (J.C.W.) and Bio07-10677 (B.M.W.).
Disclosure Statement
Bone Biologics, Inc., licensed Nell-1-related patents from UCLA. C.S., K.T., B.M.W., and X.Z. are founders of Bone Biologics, Inc., and inventors of the related patents. J.C.W. is a scientific consultant for Bone Biologics, Inc. A.A.G. is a member of the Scientific Advisory Board of Bone Biologics, Inc.
References
- 1.Canady J.W. Zeitler D.P. Thompson S.A. Nicholas C.D. Suitability of the iliac crest as a site for harvest of autogenous bone grafts. Cleft Palate Craniofac J. 1993;30:579. doi: 10.1597/1545-1569_1993_030_0579_sotica_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 2.Frodel J.L.J. Marentette L.J. Quatela V.C. Weinstein G.S. Calvarial bone graft harvest. Techniques, considerations, and morbidity. Arch Otolaryngol Head Neck Surg. 1993;119:17. doi: 10.1001/archotol.1993.01880130019002. [DOI] [PubMed] [Google Scholar]
- 3.Vail T.P. Urbaniak J.R. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am. 1996;78:204. doi: 10.2106/00004623-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Laurie S.W. Kaban L.B. Mulliken J.B. Murray J.E. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933. doi: 10.1097/00006534-198406000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Kurz L.T. Garfin S.R. Booth R.E., Jr. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Kline R.M., Jr. Wolfe S.A. Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 1995;95:5. discussion 4–20. [PubMed] [Google Scholar]
- 7.Keen M. Complications of harvesting cranial bone grafts. Plast Reconstr Surg. 1995;96:1753. doi: 10.1097/00006534-199512000-00060. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe S.A. Complications of harvesting cranial bone grafts. Plast Reconstr Surg. 1996;98:567. doi: 10.1097/00006534-199609000-00039. [DOI] [PubMed] [Google Scholar]
- 9.Sandhu H.S. Grewal H.S. Parvateneni H. Bone grafting for spinal fusion. Orthop Clin North Am. 1999;30:685. doi: 10.1016/s0030-5898(05)70120-6. [DOI] [PubMed] [Google Scholar]
- 10.Sawin P.D. Traynelis V.C. Menezes A.H. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 11.Gamradt S.C. Lieberman J.R. Bone graft for revision hip arthroplasty: biology and future applications. Clin Orthop Relat Res. 2003;417:183. doi: 10.1097/01.blo.0000096814.78689.77. [DOI] [PubMed] [Google Scholar]
- 12.Boden S.D. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (Phila Pa 1976) 2002;27:S26. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 13.McKay B. Sandhu H.S. Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine. 2002;27:S66. doi: 10.1097/00007632-200208151-00014. [DOI] [PubMed] [Google Scholar]
- 14.Boden S.D. Zdeblick T.A. Sandhu H.S. Heim S.E. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine (Phila Pa 1976) 2000;25:376. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 15.Grauer J.N. Patel T.C. Erulkar J.S. Troiano N.W. Panjabi M.M. Friedlaender G.E. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine (Phila Pa 1976) 2001;26:127. doi: 10.1097/00007632-200101150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Rivera-Feliciano J. Tabin C.J. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducy P. Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 18.Vogt R.R. Unda R. Yeh L.C. Vidro E.K. Lee J.C. Tsin A.T. Bone morphogenetic protein-4 enhances vascular endothelial growth factor secretion by human retinal pigment epithelial cells. J Cell Biochem. 2006;98:1196. doi: 10.1002/jcb.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F. Lu S. Vida L. Thomson J.A. Honig G.R. Bone morphogenetic protein 4 induces efficient hematopoietic differentiation of rhesus monkey embryonic stem cells in vitro. Blood. 2001;98:335. doi: 10.1182/blood.v98.2.335. [DOI] [PubMed] [Google Scholar]
- 20.Lee K.S. Kim H.J. Li Q.L. Chi X.Z. Ueta C. Komori T., et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tou L. Quibria N. Alexander J.M. Transcriptional regulation of the human Runx2/Cbfa1 gene promoter by bone morphogenetic protein-7. Mol Cell Endocrinol. 2003;205:121. doi: 10.1016/s0303-7207(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 22.Ducy P. Zhang R. Geoffroy V. Ridall A.L. Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 23.Komori T. Yagi H. Nomura S. Yamaguchi A. Sasaki K. Deguchi K., et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 24.Komori T. Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem. 2005;95:445. doi: 10.1002/jcb.20420. [DOI] [PubMed] [Google Scholar]
- 25.Hata K. Nishimura R. Ikeda F. Yamashita K. Matsubara T. Nokubi T., et al. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell. 2003;14:545. doi: 10.1091/mbc.E02-06-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin W. Takagi T. Kanesashi S.N. Kurahashi T. Nomura T. Harada J., et al. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell. 2006;10:461. doi: 10.1016/j.devcel.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Sottile V. Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett. 2000;475:201. doi: 10.1016/s0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- 28.Cowan C.M. Aalami O.O. Shi Y-Y. Chou Y-F. Mari C. Thomas R., et al. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko H. Arakawa T. Mano H. Kaneda T. Ogasawara A. Nakagawa M., et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000;27:479. doi: 10.1016/s8756-3282(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 30.Minear S. Leucht P. Miller S. Helms J.A. rBMP represses Wnt signaling and influences skeletal progenitor cell fate specification during bone repair. J Bone Miner Res. 2010;25:1196. doi: 10.1002/jbmr.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker D.H. Wright N.M. Bone morphogenetic proteins and spinal fusion. Neurosurg Focus. 2002;13:1. doi: 10.3171/foc.2002.13.6.4. [DOI] [PubMed] [Google Scholar]
- 32.Sciadini M.F. Johnson K.D. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J Orthop Res. 2000;18:289. doi: 10.1002/jor.1100180218. [DOI] [PubMed] [Google Scholar]
- 33.Haid R.W., Jr. Branch C.L., Jr. Alexander J.T. Burkus J.K. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4527 doi: 10.1016/j.spinee.2004.03.025. discussion 38–39. [DOI] [PubMed] [Google Scholar]
- 34.Poynton A.R. Lane J.M. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine. 2002;27:S40. doi: 10.1097/00007632-200208151-00010. [DOI] [PubMed] [Google Scholar]
- 35.Riew K.D. Wright N.M. Cheng S. Avioli L.V. Lou J. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif Tissue Int. 1998;63:357. doi: 10.1007/s002239900540. [DOI] [PubMed] [Google Scholar]
- 36.Buttermann G.R. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426. doi: 10.1016/j.spinee.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Smucker J.D. Rhee J.M. Singh K. Yoon S.T. Heller J.G. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31:2813. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 38.Usui M. Xing L. Drissi H. Zuscik M. O'Keefe R. Chen D., et al. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J Bone Miner Res. 2008;23:314. doi: 10.1359/JBMR.071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Bergh J.P. ten Bruggenkate C.M. Groeneveld H.H. Burger E.H. Tuinzing D.B. Recombinant human bone morphogenetic protein-7 in maxillary sinus floor elevation surgery in 3 patients compared to autogenous bone grafts. A clinical pilot study. J Clin Periodontol. 2000;27:627. doi: 10.1034/j.1600-051x.2000.027009627.x. [DOI] [PubMed] [Google Scholar]
- 40.Perri B. Cooper M. Lauryssen C. Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7:235. doi: 10.1016/j.spinee.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Shahlaie K. Kim K.D. Occipitocervical fusion using recombinant human bone morphogenetic protein-2. Spine. 2008;33:2361. doi: 10.1097/BRS.0b013e318183971d. [DOI] [PubMed] [Google Scholar]
- 42.Shields L.B.E. Raque G.H. Glassman S.D. Campbell M. Vitaz T. Harpring J., et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 43.Tumialan L.M. Pan J. Rodts G.E., Jr. Mummaneni P.V. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8:529. doi: 10.3171/SPI/2008/8/6/529. [DOI] [PubMed] [Google Scholar]
- 44.Vaidya R. Carp J. Sethi A. Bartol S. Craig J. Les C.M. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen N.F. Smith Z.A. Stiner E. Armin S. Sheikh H. Khoo L.T. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12:40. doi: 10.3171/2009.4.SPINE0876. [DOI] [PubMed] [Google Scholar]
- 46.Ting K. Vastardis H. Mulliken J.B. Soo C. Tieu A. Do H., et al. Human Nell-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14:80. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X. Zara J. Siu R.K. Ting K. Soo C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 2010;89:865. doi: 10.1177/0022034510376401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desai J. Shannon M.E. Johnson M.D. Ruff D.W. Hughes L.A. Kerley M.K., et al. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet. 2006;15:1329. doi: 10.1093/hmg/ddl053. [DOI] [PubMed] [Google Scholar]
- 49.Siu R.K. Zhang X. Ko T. Wu B.M. Ting K. Culiat C.T., et al. Nell-1 deficient mice exhibit abnormal structure in spinal, long bones. ASBMR. 2009;S50 [Google Scholar]
- 50.Aghaloo T. Cowan C.M. Chou Y.F. Zhang X. Lee H. Miao S., et al. Nell-1-induced bone regeneration in calvarial defects. Am J Pathol. 2006;169:903. doi: 10.2353/ajpath.2006.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cowan C.M. Cheng S. Ting K. Soo C. Walder B. Wu B., et al. Nell-1 induced bone formation within the distracted intermaxillary suture. Bone. 2006;38:48. doi: 10.1016/j.bone.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 52.Cowan C.M. Jiang X. Hsu T. Soo C. Zhang B. Wang J.Z., et al. Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts. J Bone Miner Res. 2007;22:918. doi: 10.1359/jbmr.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu S.S. Zhang X. Soo C. Hsu T. Napoli A. Aghaloo T., et al. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007;7:50. doi: 10.1016/j.spinee.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 54.Truong T. Zhang X. Pathmanathan D. Soo C. Ting K. Craniosynostosis-associated gene Nell-1 is regulated by Runx2. J Bone Miner Res. 2007;22:7. doi: 10.1359/jbmr.061012. [DOI] [PubMed] [Google Scholar]
- 55.Cowan C.M. Jiang X. Hsu T. Soo C. Zhang B. Wang J.Z., et al. Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts. J Bone Miner Res. 2007;22:918. doi: 10.1359/jbmr.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aghaloo T. Cowan C.M. Chou Y-F. Zhang X. Lee H. Miao S., et al. Nell-1-induced bone regeneration in calvarial defects. Am J Pathol. 2006;169:903. doi: 10.2353/ajpath.2006.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee M. Li W. Siu R.K. Whang J. Zhang X. Soo C., et al. Biomimetic apatite-coated alginate/chitosan microparticles as osteogenic protein carriers. Biomaterials. 2009;30:6094. doi: 10.1016/j.biomaterials.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W. Lee M. Whang J. Siu R.K. Zhang X. Liu C., et al. Delivery of lyophilized Nell-1 in a rat spinal fusion model. Tissue Eng Part A. 2010;16:2861. doi: 10.1089/ten.tea.2009.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu S.S. Whang J. Zhang X. Wu B. Turner A.S. Seim H.B., et al. NELL-1 promotes bone formation in a sheep spinal fusion model. J Bone Miner Res. 2007;23:S171. doi: 10.1089/ten.tea.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanayama M. Cunningham B.W. Sefter J.C. Goldstein J.A. Stewart G. Kaneda K., et al. Does spinal instrumentation influence the healing process of posterolateral spinal fusion? An in vivo animal model. Spine. 1999;24:1058. doi: 10.1097/00007632-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 61.Sandhu H.S. Toth J.M. Diwan A.D. Seim H.B., 3rd Kanim L.E. Kabo J.M., et al. Histologic evaluation of the efficacy of rhBMP-2 compared with autograft bone in sheep spinal anterior interbody fusion. Spine. 2002;27:567. doi: 10.1097/00007632-200203150-00003. [DOI] [PubMed] [Google Scholar]
- 62.Steffen T. Marchesi D. Aebi M. Posterolateral and anterior interbody spinal fusion models in the sheep. Clin Orthop Relat Res. 2000;371:28. doi: 10.1097/00003086-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Cunningham B.W. Kanayama M. Parker L.M. Weis J.C. Sefter J.C. Fedder I.L., et al. Osteogenic protein versus autologous interbody arthrodesis in the sheep thoracic spine. A comparative endoscopic study using the Bagby and Kuslich interbody fusion device. Spine. 1999;24:509. doi: 10.1097/00007632-199903150-00002. [DOI] [PubMed] [Google Scholar]
- 64.Wilke H.J. Kettler A. Claes L.E. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 65.Wilke H.J. Kettler A. Wenger K.H. Claes L.E. Anatomy of the sheep spine and its comparison to the human spine. Anat Rec. 1997;247:542. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 66.Peterson B. Whang P.G. Iglesias R. Wang J.C. Lieberman J.R. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004;86-A:2243. doi: 10.2106/00004623-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 67.Han B. Yang Z. Nimni M. Effects of moisture and temperature on the osteoinductivity of demineralized bone matrix. J Orthop Res. 2005;23:855. doi: 10.1016/j.orthres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Magin M.N. Delling G. Improved lumbar vertebral interbody fusion using rhOP-1: a comparison of autogenous bone graft, bovine hydroxylapatite (Bio-Oss), and BMP-7 (rhOP-1) in sheep. Spine (Phila Pa 1976) 2001;26:469. doi: 10.1097/00007632-200103010-00009. [DOI] [PubMed] [Google Scholar]
- 69.Sandhu H.S. Toth J.M. Diwan A.D. Seim H.B., 3rd Kanim L.E. Kabo J.M., et al. Histologic evaluation of the efficacy of rhBMP-2 compared with autograft bone in sheep spinal anterior interbody fusion. Spine (Phila Pa 1976) 2002;27:567. doi: 10.1097/00007632-200203150-00003. [DOI] [PubMed] [Google Scholar]
- 70.Wildemann B. Kadow-Romacker A. Pruss A. Haas N.P. Schmidmaier G. Quantification of growth factors in allogenic bone grafts extracted with three different methods. Cell Tissue Bank. 2007;8:107. doi: 10.1007/s10561-006-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee M. Li W. Siu R.K. Whang J. Zhang X. Soo C., et al. Biomimetic apatite-coated alginate/chitosan microparticles as osteogenic protein carriers. Biomaterials. 2009;30:6094. doi: 10.1016/j.biomaterials.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X. Kuroda S. Carpenter D. Nishimura I. Soo C. Moats R., et al. Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin Invest. 2002;110:861. doi: 10.1172/JCI15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cowan C.M. Aghaloo T. Chou Y.F. Walder B. Zhang X. Soo C., et al. MicroCT evaluation of three-dimensional mineralization in response to BMP-2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 2007;13:501. doi: 10.1089/ten.2006.0141. [DOI] [PubMed] [Google Scholar]
- 74.Krämer J. Intervertebral Disk Diseases: Causes, Diagnosis, Treatment and Prophylaxis. 3rd. Stuttgart: Thieme; 2008. [Google Scholar]
- 75.Gardeniers J.W.M. Favenesi J.A. Huiskes R. Sloof T.J. Mechanical properties of normal avascular and revascularizing cancellous bone: an animal experiment. Acta Orthop Scand. 1987;58:709. [Google Scholar]
- 76.Donati D. Di Bella C. Lucarelli E. Dozza B. Frisoni T. Aldini N.N., et al. OP-1 application in bone allograft integration: preliminary results in sheep experimental surgery. Injury. 2008;39(Suppl 2):S65. doi: 10.1016/S0020-1383(08)70017-2. [DOI] [PubMed] [Google Scholar]
- 77.Sandhu H.S. Kanim L.E. Kabo J.M. Toth J.M. Zeegan E.N. Liu D., et al. Evaluation of rhBMP-2 with an OPLA carrier in a canine posterolateral (transverse process) spinal fusion model. Spine (Phila Pa 1976) 1995;20:2669. doi: 10.1097/00007632-199512150-00008. [DOI] [PubMed] [Google Scholar]
- 78.Diwan A.D. Sandhu H. Kanim L.E. Histological evaluation of the efficacy of rhBMP-2 when compared to autogenous bone in sheep interbody fusion using titanium cage. 2000 North American Spine Society Annual Meeting: 135–136; 2000. [Google Scholar]
- 79.Sandhu H.S. Kabo J.M. Turner A.S. rhBMP-2 augmentation of titanium fusion cages for experimental anterior lumbar fusion. 1996 North American Spine Society Annual Meeting, 47; 1996. [Google Scholar]
- 80.Boden S.D. Martin G.J., Jr. Horton W.C. Truss T.L. Sandhu H.S. Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J Spinal Disord. 1998;11:95. [PubMed] [Google Scholar]
- 81.Hecht B.P. Fischgrund J.S. Herkowitz H.N. Penman L. Toth J.M. Shirkhoda A. The use of recombinant human bone morphogenetic protein 2 (rhBMP-2) to promote spinal fusion in a nonhuman primate anterior interbody fusion model. Spine (Phila Pa 1976) 1999;24:629. doi: 10.1097/00007632-199904010-00004. [DOI] [PubMed] [Google Scholar]
- 82.Gornet M.F. Burkus K. Dickman C.A. rhBMP-2 with tapered cages: a prospective randomized lumbar fusion study. Read at the 2001 North American Spine Society Annual Meeting; Seattle, WA. 2001. Oct 31-Nov 3. [Google Scholar]
- 83.Boden S.D. Kang J. Sandhu H. Heller J.G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27:2662. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 84.Chamberlain G. Wright K. Rot A. Ashton B. Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS ONE. 2008;3:e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu S.S. Zhang X. Soo C. Hsu T. Napoli A. Aghaloo T., et al. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007;7:50. doi: 10.1016/j.spinee.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 86.Aghaloo T. Jiang X. Soo C. Zhang Z. Zhang X. Hu J., et al. A study of the role of Nell-1 gene modified goat bone marrow stromal cells in promoting new bone formation. Mol Ther. 2007;15:1872. doi: 10.1038/sj.mt.6300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minear S. Leucht P. Miller S. Helms J.A. rBMP represses Wnt signaling and influences skeletal progenitor cell fate specification during bone repair. J Bone Miner Res. 2010;25:1196. doi: 10.1002/jbmr.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takada I. Kouzmenko A.P. Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 89.Kim J.B. Leucht P. Lam K. Luppen C. Ten Berge D. Nusse R., et al. Bone regeneration is regulated by wnt signaling. J Bone Miner Res. 2007;22:1913. doi: 10.1359/jbmr.070802. [DOI] [PubMed] [Google Scholar]
- 90.Isaksson H. van Donkelaar C.C. Ito K. Sensitivity of tissue differentiation and bone healing predictions to tissue properties. J Biomech. 2009;42:555. doi: 10.1016/j.jbiomech.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Colnot C.I. Helms J.A. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev. 2001;100:245. doi: 10.1016/s0925-4773(00)00532-3. [DOI] [PubMed] [Google Scholar]
- 92.Banerjee C. Javed A. Choi J.-Y. Green J. Rosen V. van Wijnen A.J., et al. Differential regulation of the two principal Runx2/Cbfa1 N-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype. Endocrinology. 2001;142:4026. doi: 10.1210/endo.142.9.8367. [DOI] [PubMed] [Google Scholar]
- 93.Bokui N. Otani T. Igarashi K. Kaku J. Oda M. Nagaoka T., et al. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 2008;582:365. doi: 10.1016/j.febslet.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mok J.M. Durrani S.K. Piper S.L. Hu S.S. Deviren V. Berven S.H., et al. Extravasation of rhBMP-2 with use of postoperative drains after posterolateral spinal fusion. Spine (Phila Pa 1976) 2008;33:1668. doi: 10.1097/BRS.0b013e31817b6229. [DOI] [PubMed] [Google Scholar]
- 95.Marusic A. Katavic V. Grcevic D. Lukic I.K. Genetic variability of new bone induction in mice. Bone. 1999;25:25. doi: 10.1016/s8756-3282(99)00095-2. [DOI] [PubMed] [Google Scholar]
- 96.Wildemann B. Kadow-Romacker A. Haas N.P. Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81:437. doi: 10.1002/jbm.a.31085. [DOI] [PubMed] [Google Scholar]
- 97.Bae H.W. Zhao L. Kanim L.E. Wong P. Delamarter R.B. Dawson E.G. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976) 2006;31:1299. doi: 10.1097/01.brs.0000218581.92992.b7. discussion 307–308. [DOI] [PubMed] [Google Scholar]
- 98.Pietrzak W.S. Woodell-May J. McDonald N. Assay of bone morphogenetic protein-2, −4, and −7 in human demineralized bone matrix. J Craniofac Surg. 2006;17:84. doi: 10.1097/01.scs.0000179745.91165.73. [DOI] [PubMed] [Google Scholar]
- 99.Uludag H. D'Augusta D. Palmer R. Timony G. Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 100.Brown T.J. Laurent U.B. Fraser J.R. Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit. Exp Physiol. 1991;76:125. doi: 10.1113/expphysiol.1991.sp003474. [DOI] [PubMed] [Google Scholar]